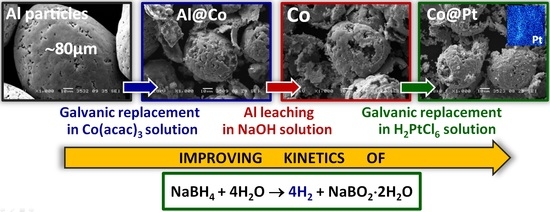
Magnetically Recovered Co and Co@Pt Catalysts Prepared by Galvanic Replacement on Aluminum Powder for Hydrolysis of Sodium Borohydride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalysts Preparation
2.2. Catalytic Tests in NaBH4 Hydrolysis
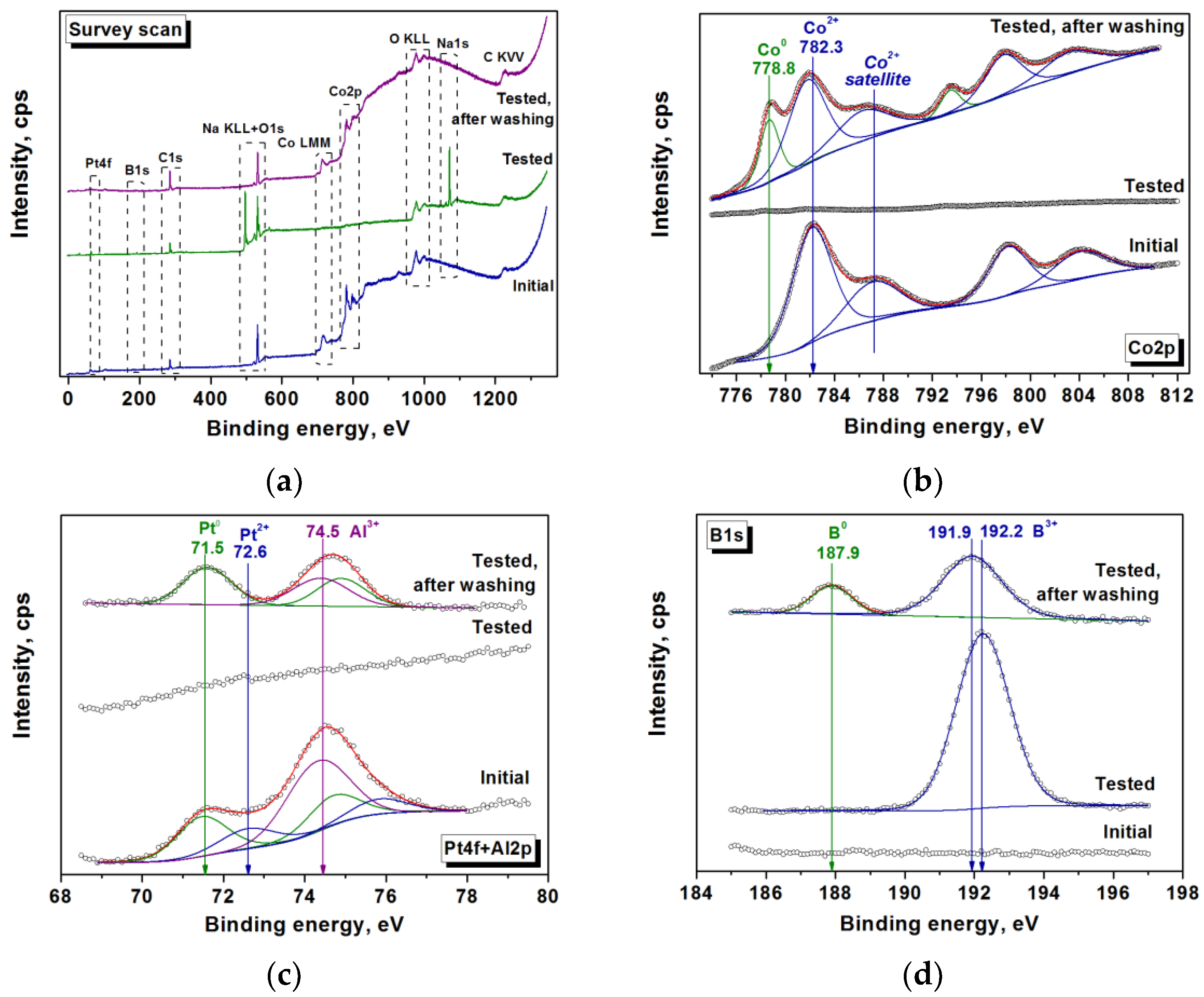
2.3. Catalysts Characterization
3. Results and Discussion
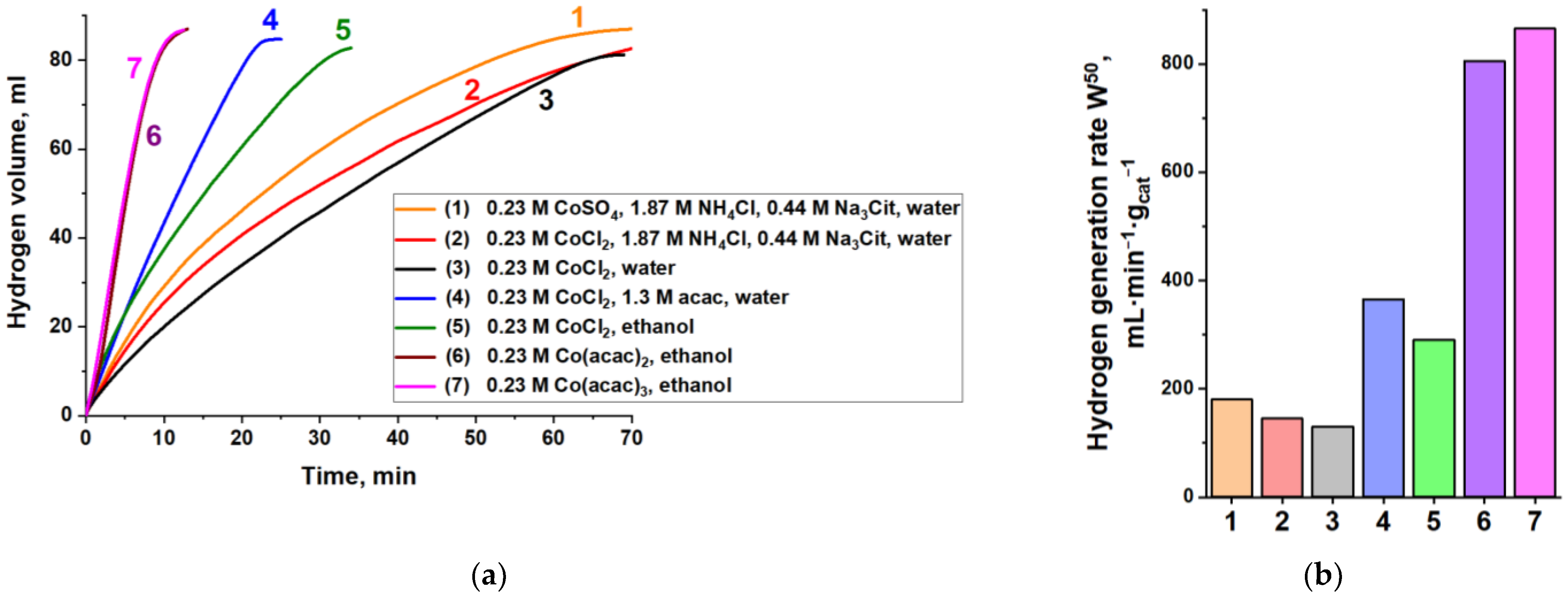
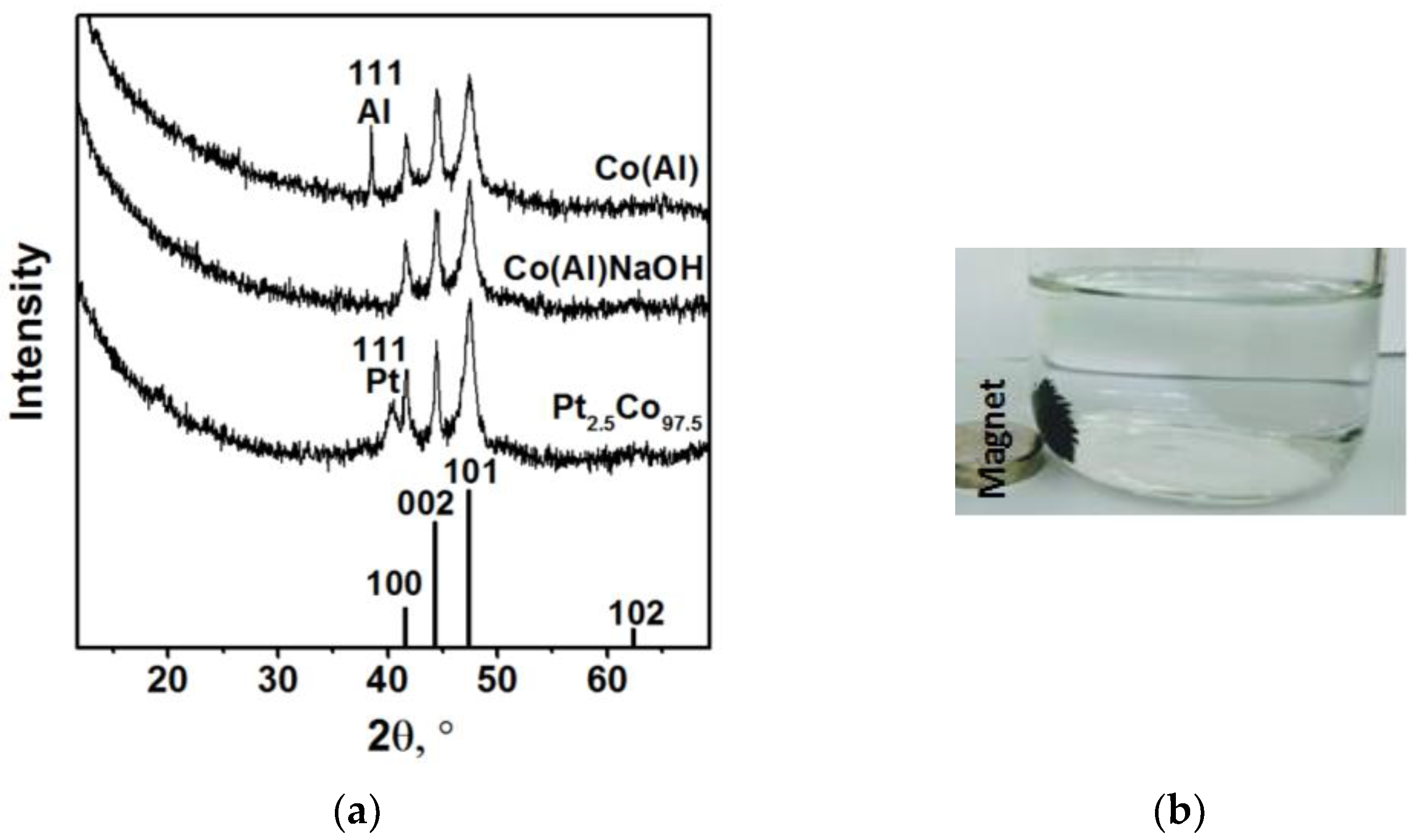
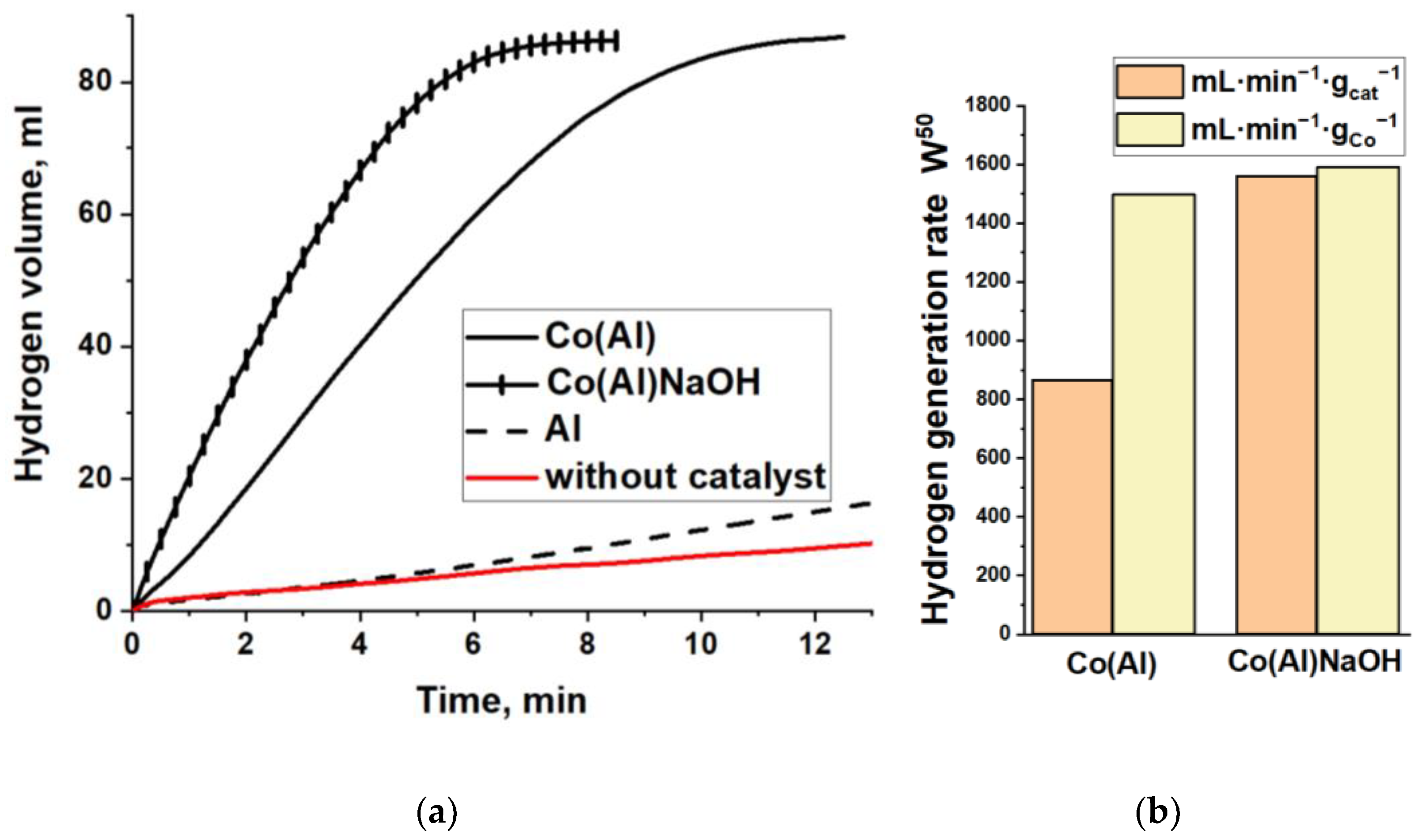
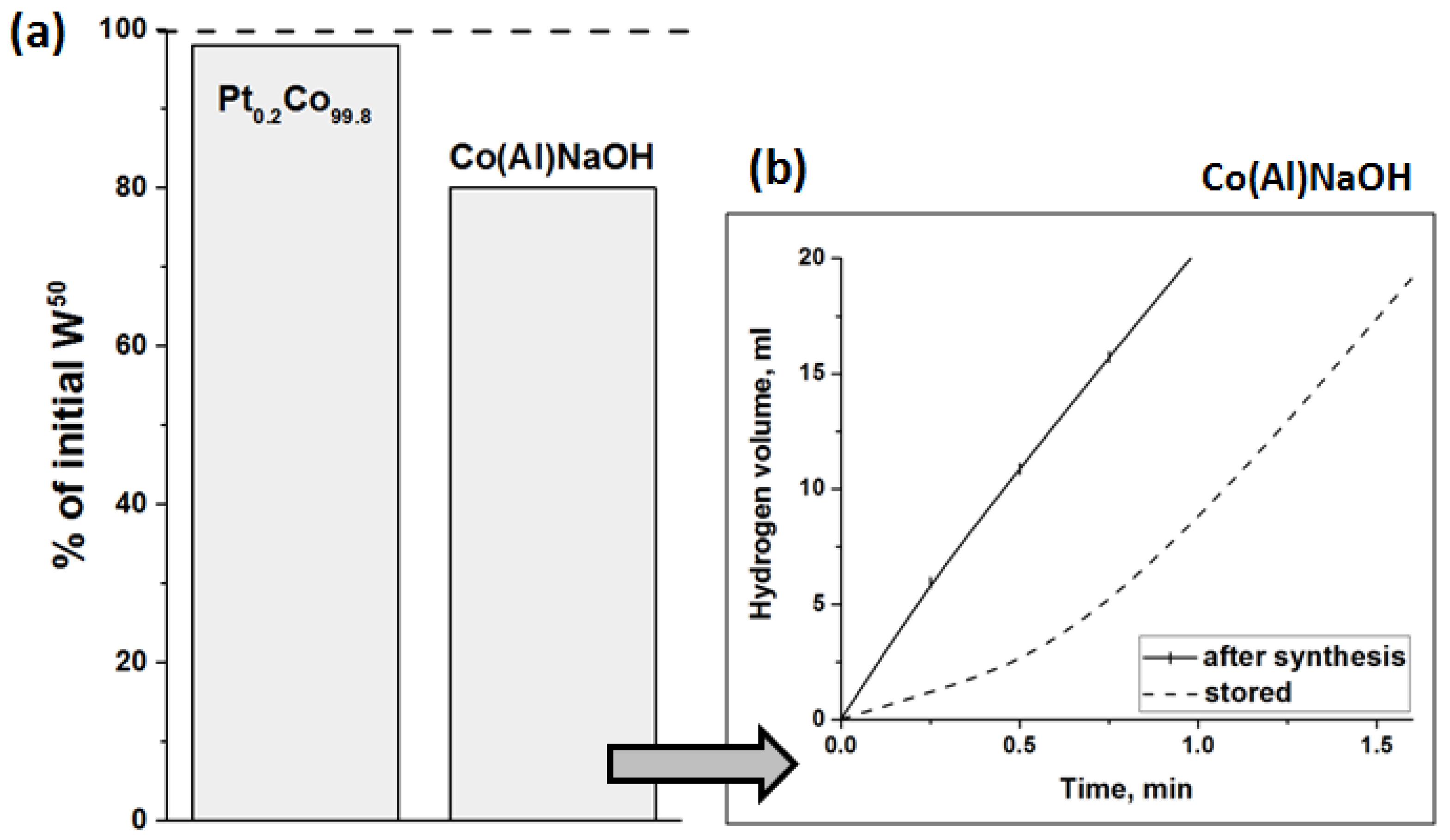
3.1. Synthesis and Investigation of Cobalt-Based Catalysts
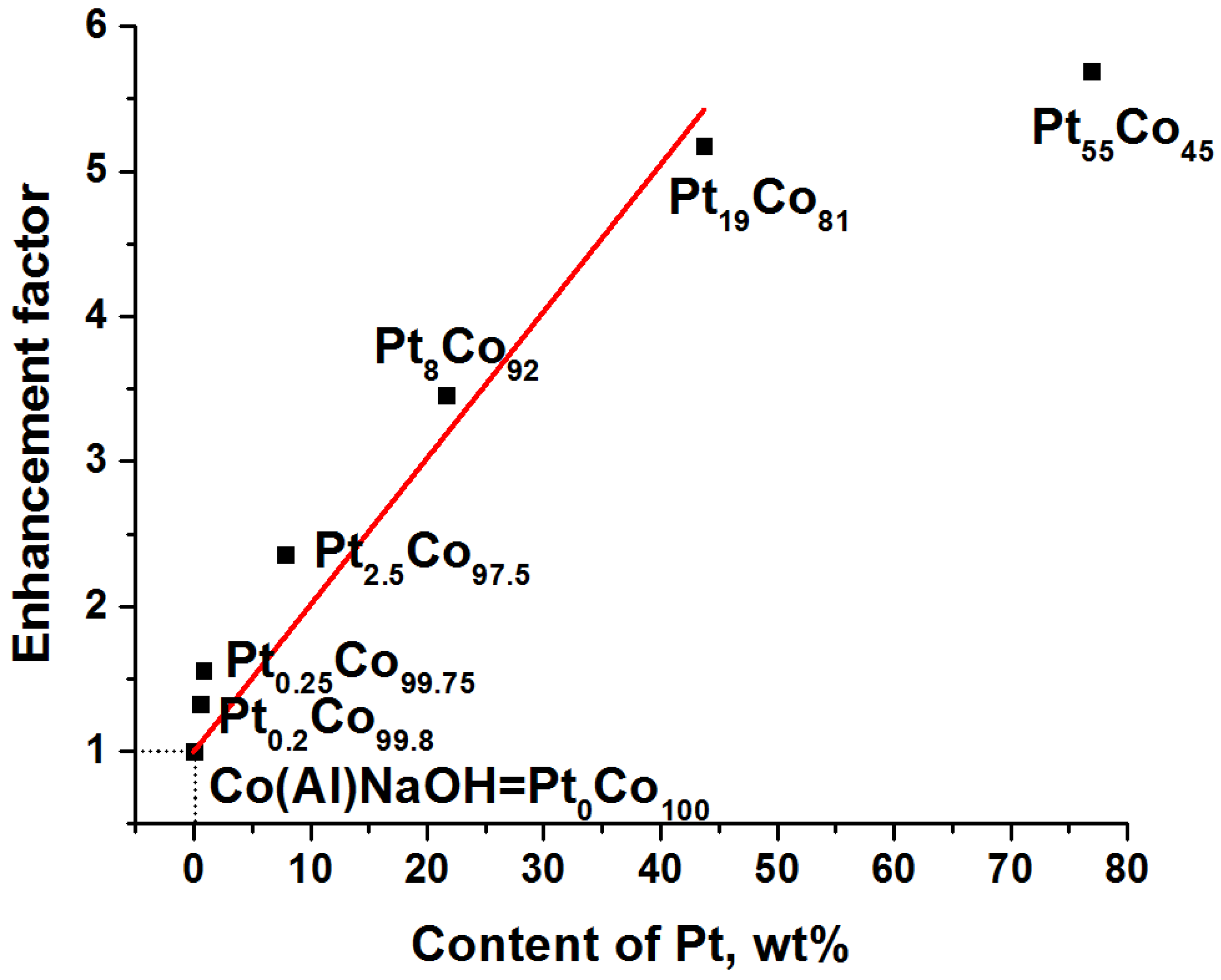
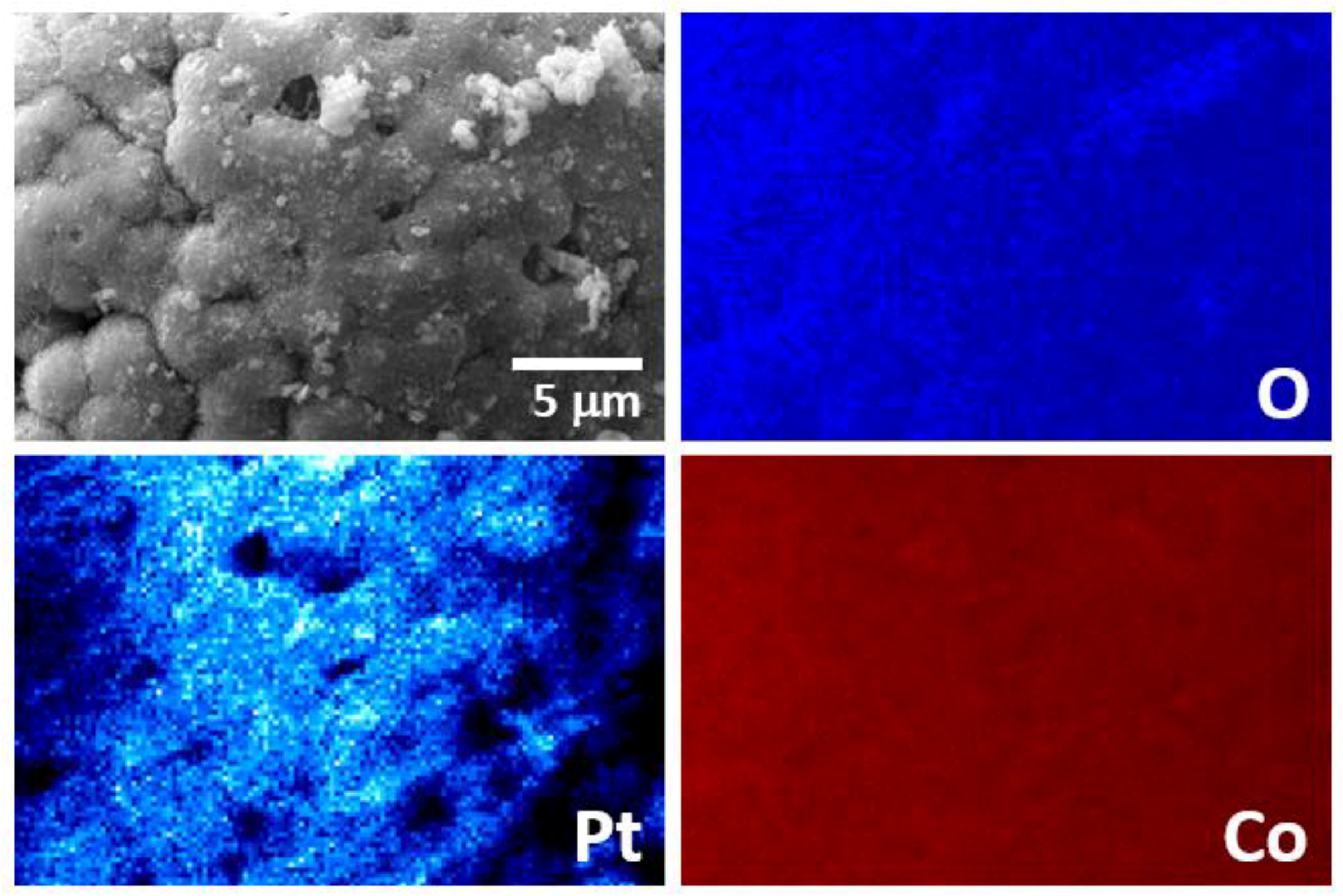
3.2. Co@Pt Catalysts
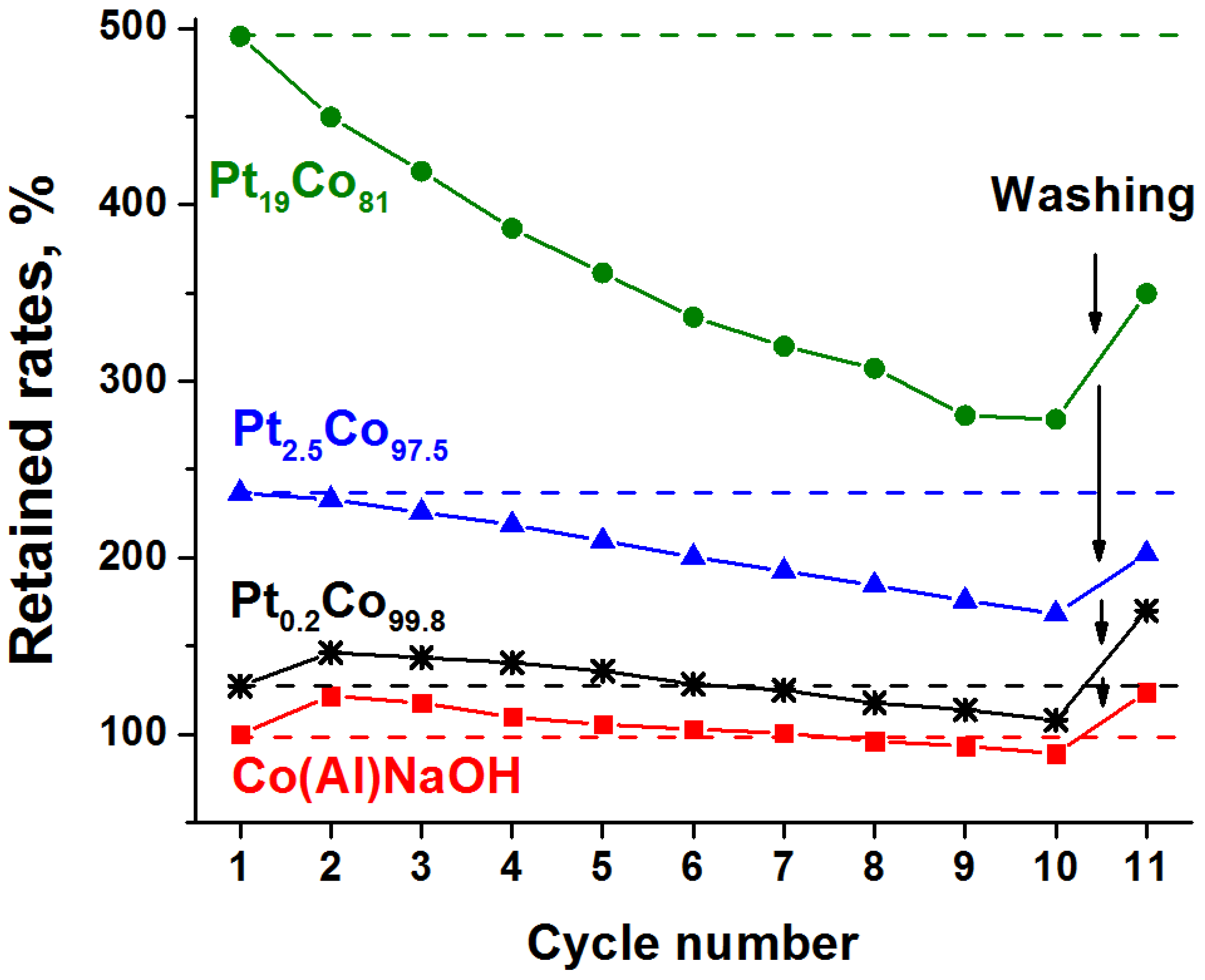
3.3. Catalysts Cycling Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelhamid, H.N. A Review on Hydrogen Generation from the Hydrolysis of Sodium Borohydride. Int. J. Hydrog. Energy 2021, 46, 726–765. [Google Scholar] [CrossRef]
- Wang, C.; Astruc, D. Recent Developments of Nanocatalyzed Liquid-Phase Hydrogen Generation. Chem. Soc. Rev. 2021, 50, 3437–3484. [Google Scholar] [CrossRef] [PubMed]
- Urgnani, J.; Torres, F.J.; Palumbo, M.; Baricco, M. Hydrogen Release from Solid State NaBH4. Int. J. Hydrog. Energy 2008, 33, 3111–3115. [Google Scholar] [CrossRef]
- Mao, J.; Gregory, D.H. Recent Advances in the Use of Sodium Borohydride as a Solid State Hydrogen Store. Energies 2015, 8, 430–453. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y.; Guo, Q. Research Progress on Catalysts for Hydrogen Generation through Sodium Borohydride Alcoholysis. Int. J. Hydrog. Energy 2022, 47, 5929–5946. [Google Scholar] [CrossRef]
- Simagina, V.I.; Ozerova, A.M.; Komova, O.V.; Netskina, O.V. Recent Advances in Applications of Co-B Catalysts in NaBH4-Based Portable Hydrogen Generators. Catalysts 2021, 11, 268. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Singh, S.K.; Yan, J.-M.; Zhang, X.-B.; Xu, Q. Liquid-Phase Chemical Hydrogen Storage: Catalytic Hydrogen Generation under Ambient Conditions. ChemSusChem 2010, 3, 541–549. [Google Scholar] [CrossRef]
- Demirci, U.B. About the Technological Readiness of the H2 Generation by Hydrolysis of B(−N)−H Compounds. Energy Technol. 2018, 6, 470–486. [Google Scholar] [CrossRef] [Green Version]
- Netskina, O.V.; Ozerova, A.M.; Komova, O.V.; Odegova, G.V.; Simagina, V.I. Hydrogen Storage Systems Based on Solid-State NaBH4/CoxB Composite: Influence of Catalyst Properties on Hydrogen Generation Rate. Catal. Today 2015, 245, 86–92. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P.; Suda, S. Solid Sodium Borohydride as a Hydrogen Source for Fuel Cells. J. Alloys Compd. 2009, 468, 493–498. [Google Scholar] [CrossRef]
- Sun, H.; Meng, J.; Jiao, L.; Cheng, F.; Chen, J. A Review of Transition-Metal Boride/Phosphide-Based Materials for Catalytic Hydrogen Generation from Hydrolysis of Boron-Hydrides. Inorg. Chem. Front. 2018, 5, 760–772. [Google Scholar] [CrossRef]
- Patel, N.; Miotello, A. Progress in Co–B Related Catalyst for Hydrogen Production by Hydrolysis of Boron-Hydrides: A Review and the Perspectives to Substitute Noble Metals. Int. J. Hydrog. Energy 2015, 40, 1429–1464. [Google Scholar] [CrossRef]
- Brack, P.; Dann, S.E.; Upul Wijayantha, K.G. Heterogeneous and Homogenous Catalysts for Hydrogen Generation by Hydrolysis of Aqueous Sodium Borohydride (NaBH4) Solutions. Energy Sci. Eng. 2015, 3, 174–188. [Google Scholar] [CrossRef] [Green Version]
- Demirci, U.B.; Miele, P. Reaction Mechanisms of the Hydrolysis of Sodium Borohydride: A Discussion Focusing on Cobalt-Based Catalysts. Comptes Rendus Chim. 2014, 17, 707–716. [Google Scholar] [CrossRef]
- Demirci, U.B.; Miele, P. Cobalt-Based Catalysts for the Hydrolysis of NaBH4 and NH3BH3. Phys. Chem. Chem. Phys. 2014, 16, 6872–6885. [Google Scholar] [CrossRef]
- Muir, S.S.; Yao, X. Progress in Sodium Borohydride as a Hydrogen Storage Material: Development of Hydrolysis Catalysts and Reaction Systems. Int. J. Hydrog. Energy 2011, 36, 5983–5997. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Sequeira, C.A.C. Sodium Borohydride as a Fuel for the Future. Renew. Sustain. Energy Rev. 2011, 15, 3980–4001. [Google Scholar] [CrossRef]
- Li, M.; Deng, H.; Zhang, Y.; Hou, C. A Small Hybrid Power System of Photovoltaic Cell and Sodium Borohydride Hydrolysis-Based Fuel Cell. Micromachines 2021, 12, 278. [Google Scholar] [CrossRef]
- Avrahami, I.; Shvalb, N.; Sasson, M.; Nagar, Y.; Dahan, O.; Dayee, I.; Schechter, A. Hydrogen Production On-Demand by Hydride Salt and Water Two-Phase Generator. Int. J. Hydrog. Energy 2020, 45, 15270–15280. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, M.J.; Kang, S.; Kim, T. Development of a High-Storage-Density Hydrogen Generator Using Solid-State NaBH4 as a Hydrogen Source for Unmanned Aerial Vehicles. Appl. Energy 2019, 251, 113331. [Google Scholar] [CrossRef]
- Zakhvatkin, L.; Zolotih, M.; Maurice, Y.; Schechter, A.; Avrahami, I. Hydrogen Production on Demand by a Pump Controlled Hydrolysis of Granulated Sodium Borohydride. Energy Fuels 2021, 35, 11507–11514. [Google Scholar] [CrossRef]
- Jung, E.S.; Kim, H.; Kwon, S.; Oh, T.H. Fuel Cell System with Sodium Borohydride Hydrogen Generator for Small Unmanned Aerial Vehicles. Int. J. Green Energy 2018, 15, 385–392. [Google Scholar] [CrossRef]
- Okumus, E.; Boyaci San, F.G.; Okur, O.; Turk, B.E.; Cengelci, E.; Kilic, M.; Karadag, C.; Cavdar, M.; Turkmen, A.; Yazici, M.S. Development of Boron-Based Hydrogen and Fuel Cell System for Small Unmanned Aerial Vehicle. Int. J. Hydrog. Energy 2017, 42, 2691–2697. [Google Scholar] [CrossRef]
- Cavaliere, S.; Hannauer, J.; Demirci, U.B.; Akdim, O.; Miele, P. Ex Situ Characterization of N2H4-, NaBH4- and NH3BH3-Reduced Cobalt Catalysts Used in NaBH4 Hydrolysis. Catal. Today 2011, 170, 3–12. [Google Scholar] [CrossRef]
- Cui, Q.; Sha, Y.; Chen, J.; Gu, Z. Galvanic Synthesis of Hollow Non-Precious Metal Nanoparticles Using Aluminum Nanoparticle Template and Their Catalytic Applications. J. Nanopart. Res. 2011, 13, 4785–4794. [Google Scholar] [CrossRef]
- Yiping, L.; Hadjipanayis, G.C.; Sorensen, C.M.; Klabunde, K.J. Magnetic and Structural Properties of Ultrafine Co-B Particles. J. Magn. Magn. Mater. 1989, 79, 321–326. [Google Scholar] [CrossRef]
- Lefterova, E.; Dragieva, I.; Tchanev, V.; Mehandjiev, D.; Mikhov, M. Crystallographic Phases in Nanosized Ferromagnetic Particles Obtained by Two Different Methods. J. Magn. Magn. Mater. 1995, 140–144, 457–458. [Google Scholar] [CrossRef]
- Farkaš, B.; Santos-Carballal, D.; Cadi-Essadek, A.; de Leeuw, N.H. A DFT+U Study of the Oxidation of Cobalt Nanoparticles: Implications for Biomedical Applications. Materialia 2019, 7, 100381. [Google Scholar] [CrossRef]
- Varón, M.; Ojea-Jimenez, I.; Arbiol, J.; Balcells, L.; Martínez, B.; Puntes, V.F. Spontaneous Formation of Hollow Cobalt Oxide Nanoparticles by the Kirkendall Effect at Room Temperature at the Water-Air Interface. Nanoscale 2013, 5, 2429–2436. [Google Scholar] [CrossRef]
- Paladini, M.; Arzac, G.M.; Godinho, V.; Hufschmidt, D.; de Haro, M.C.C.J.; Beltrán, A.M.; Fernández, A. The Role of Cobalt Hydroxide in Deactivation of Thin Film Co-Based Catalysts for Sodium Borohydride Hydrolysis. Appl. Catal. B Environ. 2017, 210, 342–351. [Google Scholar] [CrossRef]
- Sahiner, N.; Seven, F. A Facile Synthesis Route to Improve the Catalytic Activity of Inherently Cationic and Magnetic Catalyst Systems for Hydrogen Generation from Sodium Borohydride Hydrolysis. Fuel Process. Technol. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Liu, C.H.; Chen, B.H.; Hsueh, C.L.; Ku, J.R.; Tsau, F.; Hwang, K.J. Preparation of Magnetic Cobalt-Based Catalyst for Hydrogen Generation from Alkaline NaBH4 Solution. Appl. Catal. B Environ. 2009, 91, 368–379. [Google Scholar] [CrossRef]
- Shih, Y.J.; Su, C.C.; Huang, Y.H.; Lu, M.C. SiO2-Supported Ferromagnetic Catalysts for Hydrogen Generation from Alkaline NaBH4 (Sodium Borohydride) Solution. Energy 2013, 54, 263–270. [Google Scholar] [CrossRef]
- Duan, S.; Han, G.; Su, Y.; Zhang, X.; Liu, Y.; Wu, X.; Li, B. Magnetic Co@g-C3N4 Core-Shells on RGO Sheets for Momentum Transfer with Catalytic Activity toward Continuous-Flow Hydrogen Generation. Langmuir 2016, 32, 6272–6281. [Google Scholar] [CrossRef]
- Guo, Y.; Qian, J.; Iqbal, A.; Zhang, L.; Liu, W.; Qin, W. Pd Nanoparticles Immobilized on Magnetic Carbon Dots@Fe3O4 Nanocubes as a Synergistic Catalyst for Hydrogen Generation. Int. J. Hydrog. Energy 2017, 42, 15167–15177. [Google Scholar] [CrossRef]
- Chen, B.; Chen, S.; Bandal, H.A.; Appiah-Ntiamoah, R.; Jadhav, A.R.; Kim, H. Cobalt Nanoparticles Supported on Magnetic Core-Shell Structured Carbon as a Highly Efficient Catalyst for Hydrogen Generation from NaBH4 Hydrolysis. Int. J. Hydrog. Energy 2018, 43, 9296–9306. [Google Scholar] [CrossRef]
- Baye, A.F.; Abebe, M.W.; Appiah-Ntiamoah, R.; Kim, H. Engineered Iron-Carbon-Cobalt (Fe3O4@C-Co) Core-Shell Composite with Synergistic Catalytic Properties towards Hydrogen Generation via NaBH4 Hydrolysis. J. Colloid Interface Sci. 2019, 543, 273–284. [Google Scholar] [CrossRef]
- Didehban, A.; Zabihi, M.; Babajani, N. Preparation of the Efficient Nano-Bimetallic Cobalt-Nickel Catalysts Supported on the Various Magnetic Substrates for Hydrogen Generation from Hydrolysis of Sodium Borohydride in Alkaline Solutions. Polyhedron 2020, 180, 114405. [Google Scholar] [CrossRef]
- Soltani, M.; Zabihi, M. Hydrogen Generation by Catalytic Hydrolysis of Sodium Borohydride Using the Nano-Bimetallic Catalysts Supported on the Core-Shell Magnetic Nanocomposite of Activated Carbon. Int. J. Hydrog. Energy 2020, 45, 12331–12346. [Google Scholar] [CrossRef]
- Bandal, H.A.; Jadhav, A.R.; Kim, H. Cobalt Impregnated Magnetite-Multiwalled Carbon Nanotube Nanocomposite as Magnetically Separable Efficient Catalyst for Hydrogen Generation by NaBH4 Hydrolysis. J. Alloys Compd. 2017, 699, 1057–1067. [Google Scholar] [CrossRef]
- Faghihi, M.; Akbarbandari, F.; Zabihi, M.; Pazouki, M. Synthesis and Characterization of the Magnetic Supported Metal-Organic Framework Catalysts (CuCoBTC@MAC and CuBTC@MAC) for the Hydrogen Production from Sodium Borohydride. Mater. Chem. Phys. 2021, 267, 124599. [Google Scholar] [CrossRef]
- Prasad, D.; Patil, K.N.; Sandhya, N.; Chaitra, C.R.; Bhanushali, J.T.; Samal, A.K.; Keri, R.S.; Jadhav, A.H.; Nagaraja, B.M. Highly Efficient Hydrogen Production by Hydrolysis of NaBH4 Using Eminently Competent Recyclable Fe2O3 Decorated Oxidized MWCNTs Robust Catalyst. Appl. Surf. Sci. 2019, 489, 538–551. [Google Scholar] [CrossRef]
- Tang, M.; Xia, F.; Gao, C.; Qiu, H. Preparation of Magnetically Recyclable CuFe2O4/RGO for Catalytic Hydrolysis of Sodium Borohydride. Int. J. Hydrog. Energy 2016, 41, 13058–13068. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.; Li, Q.; Li, F.; Zhao, S.; Xia, X. Hydrogen Generation from Hydrolysis of NaBH4 Based on High Stable NiB/NiFe2O4 Catalyst. Int. J. Hydrog. Energy 2017, 42, 3971–3980. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X. Catalytic Hydrolysis of Sodium Borohydride for Hydrogen Production Using Magnetic Recyclable CoFe2O4-Modified Transition-Metal Nanoparticles. ACS Appl. Nano Mater. 2021, 4, 11312–11320. [Google Scholar] [CrossRef]
- Tsai, C.W.; Chen, H.M.; Liu, R.S.; Lee, J.-F.; Chang, S.M.; Weng, B.J. Magnetically Recyclable Fe@Co Core-Shell Catalysts for Dehydrogenation of Sodium Borohydride in Fuel Cells. Int. J. Hydrog. Energy 2012, 37, 3338–3343. [Google Scholar] [CrossRef]
- Papaderakis, A.; Mintsouli, I.; Georgieva, J.; Sotiropoulos, S. Electrocatalysts Prepared by Galvanic Replacement. Catalysts 2017, 7, 80. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Ruditskiy, A.; Xia, Y. 25th Anniversary Article: Galvanic Replacement: A Simple and Versatile Route to Hollow Nanostructures with Tunable and Well-Controlled Properties. Adv. Mater. 2013, 25, 6313–6333. [Google Scholar] [CrossRef]
- Wang, Z.L. Transmission Electron Microscopy of Shape-Controlled Nanocrystals and Their Assemblies. J. Phys. Chem. B 2000, 104, 1153–1175. [Google Scholar] [CrossRef]
- Su, Y.; Xu, H.; Wang, J.; Luo, X.; Xu, Z.L.; Wang, K.; Wang, W. Nanorattle Au@PtAg Encapsulated in ZIF-8 for Enhancing CO2 Photoreduction to CO. Nano Res. 2019, 12, 625–630. [Google Scholar] [CrossRef]
- Zabielaitė, A.; Balčiūnaitė, A.; Stalnionienė, I.; Lichušina, S.; Šimkūnaitė, D.; Vaičiūnienė, J.; Šimkūnaitė-Stanynienė, B.; Selskis, A.; Tamašauskaitė-Tamašiūnaitė, L.; Norkus, E. Fiber-Shaped Co Modified with Au and Pt Crystallites for Enhanced Hydrogen Generation from Sodium Borohydride. Int. J. Hydrog. Energy 2018, 43, 23310–23318. [Google Scholar] [CrossRef]
- Scofield, J.H. Hartree-Slater Subshell Photoionization Cross-Sections at 1254 and 1487 EV. J. Electron. Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 5–80. ISBN 9781498754293. [Google Scholar]
- Kuntyi, O.I.; Zozulya, G.I.; Shepida, M.V. Nanoscale Galvanic Replacement in Non-Aqueous Media: A Mini-Review. Vopr. Khimii Khimicheskoi Tekhnologii 2020, 2020, 5–15. [Google Scholar] [CrossRef]
- Niu, K.Y.; Kulinich, S.A.; Yang, J.; Zhu, A.L.; Du, X.W. Galvanic Replacement Reactions of Active-Metal Nanoparticles. Chem.—Eur. J. 2012, 18, 4234–4241. [Google Scholar] [CrossRef] [PubMed]
- Ita, B.I. Synthesis and X-ray Diffraction Studies of Al2O3 Using Aluminium Acetylacetonate (AAA) Precursor via a Sol-Gel Route. Glob. J. Pure Appl. Sci. 2001, 7, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.; Wang, Y.; Luo, Y. Nanocrystalline P-Type Transparent Cu-Al-O Semiconductor Prepared by Chemical-Vapor Deposition with Cu(Acac)2 and Al(Acac)3 Precursors. Appl. Phys. Lett. 2000, 76, 3959–3961. [Google Scholar] [CrossRef]
- Hafshejani, L.D.; Tangsir, S.; Koponen, H.; Riikonen, J.; Karhunen, T.; Tapper, U.; Lehto, V.P.; Moazed, H.; Naseri, A.A.; Hooshmand, A.; et al. Synthesis and Characterization of Al2O3 Nanoparticles by Flame Spray Pyrolysis (FSP)—Role of Fe Ions in the Precursor. Powder Technol. 2016, 298, 42–49. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Yang, J.; Tang, M. Synthesis and Characterization of Ceramic Precursor Aluminum-Containing Polycarbosilane and Its Pyrolysis. J. Inorg. Organomet. Polym. Mater. 2007, 17, 569–575. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Cherepanova, S.V.; Tsybulya, S.V. In Situ XRD Investigation of Co3O4 Reduction. Z. Krist. Suppl. 2009, 2009, 329–334. [Google Scholar] [CrossRef]
- Cherepanova, S.V.; Bulavchenko, O.A.; Tsybulya, S.V. Structure of Nanocrystalline Particles of Metallic Cobalt Formed during the Reduction of Co3O4 Oxide. J. Struct. Chem. 2008, 49, 512–516. [Google Scholar] [CrossRef]
- Li, D.; Zhu, S.; Gao, X.; Jiang, X.; Liu, Y.; Meng, F. Anchoring Sea-Urchin-like Co(OH)2 Microspheres on Nickel Foam as Three-Dimensional Free-Standing Electrode for High-Performance Supercapacitors. Ionics 2021, 27, 789–799. [Google Scholar] [CrossRef]
- Wang, L.; Fu, J.; Zhang, Y.; Liu, X.; Yin, Y.; Dong, L.; Chen, S. Mesoporous β-Co(OH)2 Nanowafers and Nanohexagonals Obtained Synchronously in One Solution and Their Electrochemical Hydrogen Storage Properties. Prog. Nat. Sci. Mater. Int. 2016, 26, 555–561. [Google Scholar] [CrossRef]
- Simagina, V.I.; Komova, O.V.; Ozerova, A.M.; Netskina, O.V.; Odegova, G.V.; Kellerman, D.G.; Bulavchenko, O.A.; Ishchenko, A.V. Cobalt Oxide Catalyst for Hydrolysis of Sodium Borohydride and Ammonia Borane. Appl. Catal. A Gen. 2011, 394, 86–92. [Google Scholar] [CrossRef]
- Ozerova, A.M.; Simagina, V.I.; Komova, O.V.; Netskina, O.V.; Odegova, G.V.; Bulavchenko, O.A.; Rudina, N.A. Cobalt Borate Catalysts for Hydrogen Production via Hydrolysis of Sodium Borohydride. J. Alloys Compd. 2012, 513, 266–272. [Google Scholar] [CrossRef]
- Simagina, V.I.; Ozerova, A.M.; Komova, O.V.; Odegova, G.V.; Kellerman, D.G.; Fursenko, R.V.; Odintsov, E.S.; Netskina, O.V. Cobalt Boride Catalysts for Small-Scale Energy Application. Catal. Today 2015, 242, 221–229. [Google Scholar] [CrossRef]
- Doherty, S.; Knight, J.G.; Alharbi, H.Y.; Paterson, R.; Wills, C.; Dixon, C.; Šiller, L.; Chamberlain, T.W.; Griffiths, A.; Collins, S.M.; et al. Efficient Hydrolytic Hydrogen Evolution from Sodium Borohydride Catalyzed by Polymer Immobilized Ionic Liquid-Stabilized Platinum Nanoparticles. ChemCatChem 2022, 14, e202101752. [Google Scholar] [CrossRef]
- Biswas, K.; Chattopadhyay, S.; Jing, Y.; Che, R.; De, G.; Basu, B.; Zhao, D. Polyionic Resin Supported Pd/Fe2O3 Nanohybrids for Catalytic Hydrodehalogenation: Improved and Versatile Remediation for Toxic Pollutants. Ind. Eng. Chem. Res. 2019, 58, 2159–2169. [Google Scholar] [CrossRef]
- Zheng, F.; Alayoglu, S.; Guo, J.; Pushkarev, V.; Li, Y.; Glans, P.A.; Chen, J.L.; Somorjai, G. In-Situ x-Ray Absorption Study of Evolution of Oxidation States and Structure of Cobalt in Co and CoPt Bimetallic Nanoparticles (4 nm) under Reducing (H2) and Oxidizing (O2) Environments. Nano Lett. 2011, 11, 847–853. [Google Scholar] [CrossRef]
- Alayoglu, S.; Beaumont, S.K.; Zheng, F.; Pushkarev, V.V.; Zheng, H.; Iablokov, V.; Liu, Z.; Guo, J.; Kruse, N.; Somorjai, G.A. CO2 Hydrogenation Studies on Co and CoPt Bimetallic Nanoparticles under Reaction Conditions Using TEM, XPS and NEXAFS. Top. Catal. 2011, 54, 778–785. [Google Scholar] [CrossRef] [Green Version]
- Simagina, V.I.; Komova, O.V.; Netskina, O.V. Nanosized Cobalt Catalysts for Hydrogen Storage Systems Based on Ammonia Borane and Sodium Borohydride. In Metal Nanopowders: Production, Characterization, and Energetic Applications; Gromov, A.A., Teipel, U., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2014; pp. 199–226. ISBN 9783527680696. [Google Scholar]
- Netskina, O.V.; Komova, O.V.; Mukha, S.A.; Simagina, V.I. Aqueous-Alkaline NaBH4 Solutions: The Influence of Hydride Decomposition on Catalytic Properties of Co3O4. Catal. Commun. 2016, 85, 9–12. [Google Scholar] [CrossRef]
- Paladini, M.; Godinho, V.; Arzac, G.M.; Jiménez De Haro, M.C.; Beltrán, A.M.; Fernández, A. Tailor-Made Preparation of Co-C, Co-B, and Co Catalytic Thin Films Using Magnetron Sputtering: Insights into Structure-Composition and Activation Effects for Catalyzed NaBH4 Hydrolysis. RSC Adv. 2016, 6, 108611–108620. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.J.; Liu, X.; Li, Y.; Li, F.; Deng, Z.; Chen, Y. Ultrasonication-Assisted and Gram-Scale Synthesis of Co-LDH Nanosheet Aggregates for Oxygen Evolution Reaction. Nano Res. 2020, 13, 79–85. [Google Scholar] [CrossRef]
- Khassin, A.A.; Yurieva, T.M.; Kaichev, V.V.; Bukhtiyarov, V.I.; Budneva, A.A.; Paukshtis, E.A.; Parmon, V.N. Metal-Support Interactions in Cobalt-Aluminum Co-Precipitated Catalysts: XPS and CO Adsorption Studies. J. Mol. Catal. A Chem. 2001, 175, 189–204. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Li, Y.; Su, Y. Octahedral Core–Shell Bimetallic Catalysts M@UIO-67 (M = Pt–Pd Nanoparticles, Pt–Pd Nanocages): Metallic Nanocages That Enhanced CO2 Conversion. Appl. Mater. Today 2020, 19, 100609. [Google Scholar] [CrossRef]
- Alderucci, V.; Pino, L.; Antonucci, P.L.; Roh, W.; Cho, J.; Kim, H.; Cocke, D.L.; Antonucci, V. XPS Study of Surface Oxidation of Carbon-Supported Pt Catalysts. Mater. Chem. Phys. 1995, 41, 9–14. [Google Scholar] [CrossRef]
- Demirci, U.B.; Miele, P. Cobalt in NaBH4 Hydrolysis. Phys. Chem. Chem. Phys. 2010, 12, 14651–14665. [Google Scholar] [CrossRef] [PubMed]
- Netskina, O.V.; Kochubey, D.I.; Prosvirin, I.P.; Malykhin, S.E.; Komova, O.V.; Kanazhevskiy, V.V.; Chukalkin, Y.G.; Bobrovskii, V.I.; Kellerman, D.G.; Ishchenko, A.V.; et al. Cobalt-Boron Catalyst for NaBH4 Hydrolysis: The State of the Active Component Forming from Cobalt Chloride in a Reaction Medium. Mol. Catal. 2017, 441, 100–108. [Google Scholar] [CrossRef]
- Arzac, G.M.; Rojas, T.C.; Fernández, A. Boron Compounds as Stabilizers of a Complex Microstructure in a Co-B-Based Catalyst for NaBH4 Hydrolysis. ChemCatChem 2011, 3, 1305–1313. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, Y.; Nitta, Y.; Imanaka, T.; Teranishi, S. Surface Characterisation of Nickel Boride and Nickel Phosphide Catalysts by X-ray Photoelectron Spectroscopy. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1979, 75, 2027–2039. [Google Scholar] [CrossRef]
- Patel, N.; Guella, G.; Kale, A.; Miotello, A.; Patton, B.; Zanchetta, C.; Mirenghi, L.; Rotolo, P. Thin Films of Co-B Prepared by Pulsed Laser Deposition as Efficient Catalysts in Hydrogen Producing Reactions. Appl. Catal. A Gen. 2007, 323, 18–24. [Google Scholar] [CrossRef]
- Goff, H.M.; Hines, J.; Griesel, J.; Mossman, C. Synthesis, characterization, and use of a cobalt(II) complex as an NMR shift reagent: An integrated laboratory experiment. J. Chem. Educ. 1982, 59, 422–423. [Google Scholar] [CrossRef]
- Hung, A.J.; Tsai, S.F.; Hsu, Y.Y.; Ku, J.R.; Chen, Y.H.; Yu, C.C. Kinetics of sodium borohydride hydrolysis reaction for hydrogen generation. Int. J. Hydrog. Energy 2008, 33, 6205–6215. [Google Scholar] [CrossRef]
- Andrieux, J.; Demirci, U.B.; Miele, P. Langmuir-Hinshelwood kinetic model to capture the cobalt nanoparticles-catalyzed hydrolysis of sodium borohydride over a wide temperature range. Catal. Today 2011, 170, 13–19. [Google Scholar] [CrossRef]
- Retnamma, R.; Novais, A.Q.; Rangel, C.M. Kinetics of hydrolysis of sodium borohydride for hydrogen production in fuel cell applications: A review. Int. J. Hydrog. Energy 2011, 36, 9772–9790. [Google Scholar] [CrossRef]


| Sample | Phase Composition | CSR (nm) |
|---|---|---|
| Al | Al | >150 |
| Co(Al) | Co Al | 15 130 |
| Co(Al)NaOH | Co | 12 |
| Pt2.5Co97.5 | Co Pt | 30 10 |
| Sample | Content, at% | |||||
|---|---|---|---|---|---|---|
| Co | Pt | B | O | Na | Al | |
| Initial | 14.9 | 0.1 | 0 | 40.0 | 0 | 2.0 |
| Tested | 0.2 | 0 | 8.3 | 33.8 | 11.4 | 0 |
| Tested, after washing | 5.8 | 0.05 | 4.3 | 32.4 | 0 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozerova, A.M.; Skobelkina, A.A.; Simagina, V.I.; Komova, O.V.; Prosvirin, I.P.; Bulavchenko, O.A.; Lipatnikova, I.L.; Netskina, O.V. Magnetically Recovered Co and Co@Pt Catalysts Prepared by Galvanic Replacement on Aluminum Powder for Hydrolysis of Sodium Borohydride. Materials 2022, 15, 3010. https://doi.org/10.3390/ma15093010
Ozerova AM, Skobelkina AA, Simagina VI, Komova OV, Prosvirin IP, Bulavchenko OA, Lipatnikova IL, Netskina OV. Magnetically Recovered Co and Co@Pt Catalysts Prepared by Galvanic Replacement on Aluminum Powder for Hydrolysis of Sodium Borohydride. Materials. 2022; 15(9):3010. https://doi.org/10.3390/ma15093010
Chicago/Turabian StyleOzerova, Anna M., Anastasia A. Skobelkina, Valentina I. Simagina, Oksana V. Komova, Igor P. Prosvirin, Olga A. Bulavchenko, Inna L. Lipatnikova, and Olga V. Netskina. 2022. "Magnetically Recovered Co and Co@Pt Catalysts Prepared by Galvanic Replacement on Aluminum Powder for Hydrolysis of Sodium Borohydride" Materials 15, no. 9: 3010. https://doi.org/10.3390/ma15093010
APA StyleOzerova, A. M., Skobelkina, A. A., Simagina, V. I., Komova, O. V., Prosvirin, I. P., Bulavchenko, O. A., Lipatnikova, I. L., & Netskina, O. V. (2022). Magnetically Recovered Co and Co@Pt Catalysts Prepared by Galvanic Replacement on Aluminum Powder for Hydrolysis of Sodium Borohydride. Materials, 15(9), 3010. https://doi.org/10.3390/ma15093010