Glassy Carbon Electrode Modified with N-Doped Reduced Graphene Oxide Sheets as an Effective Electrochemical Sensor for Amaranth Detection
Abstract
:1. Introduction
2. Experimental Section
2.1. Instruments and Chemicals
2.2. Synthesis of N-rGO Sheets
2.3. Preparing the Electrode
3. Result and Discussion
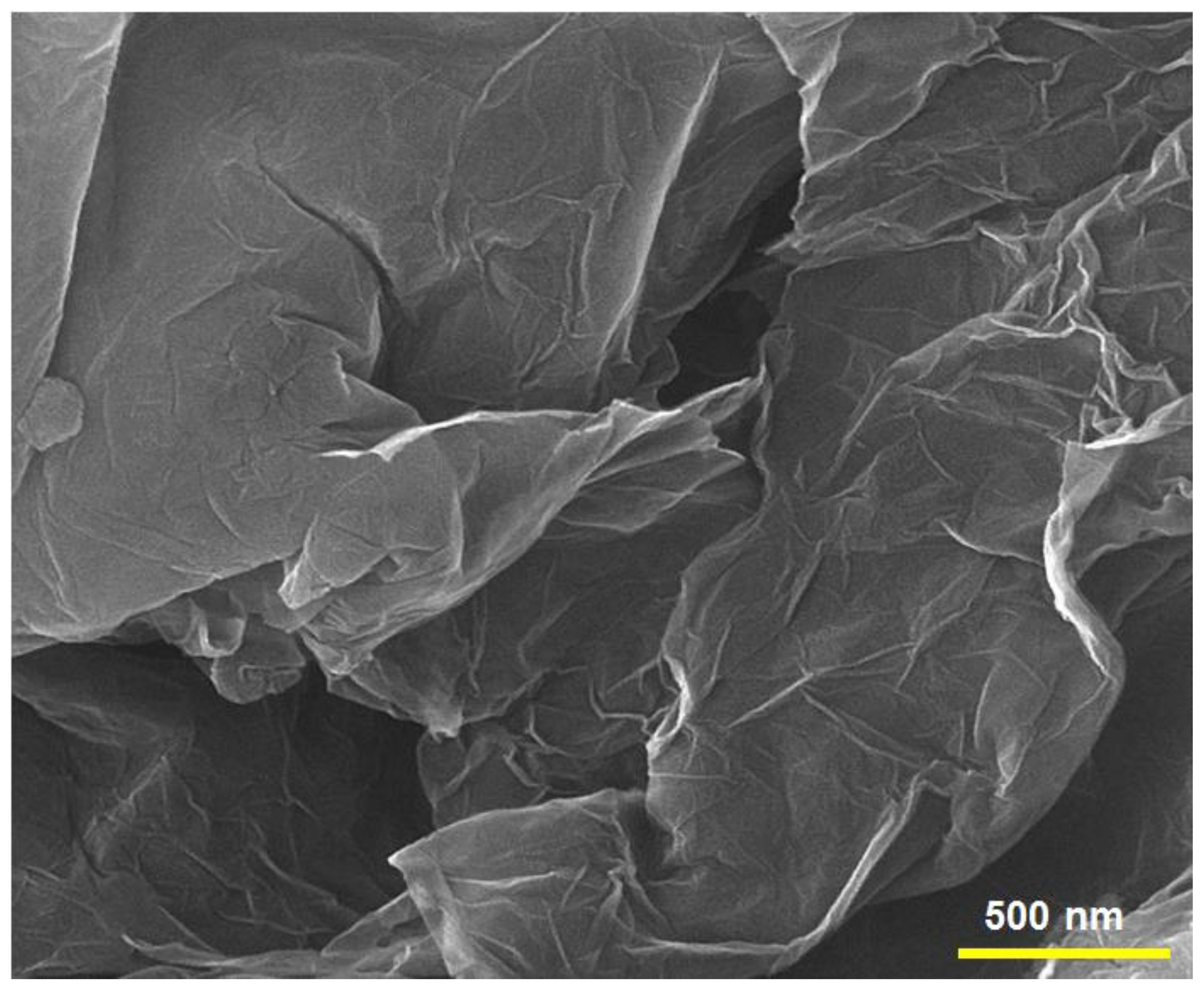
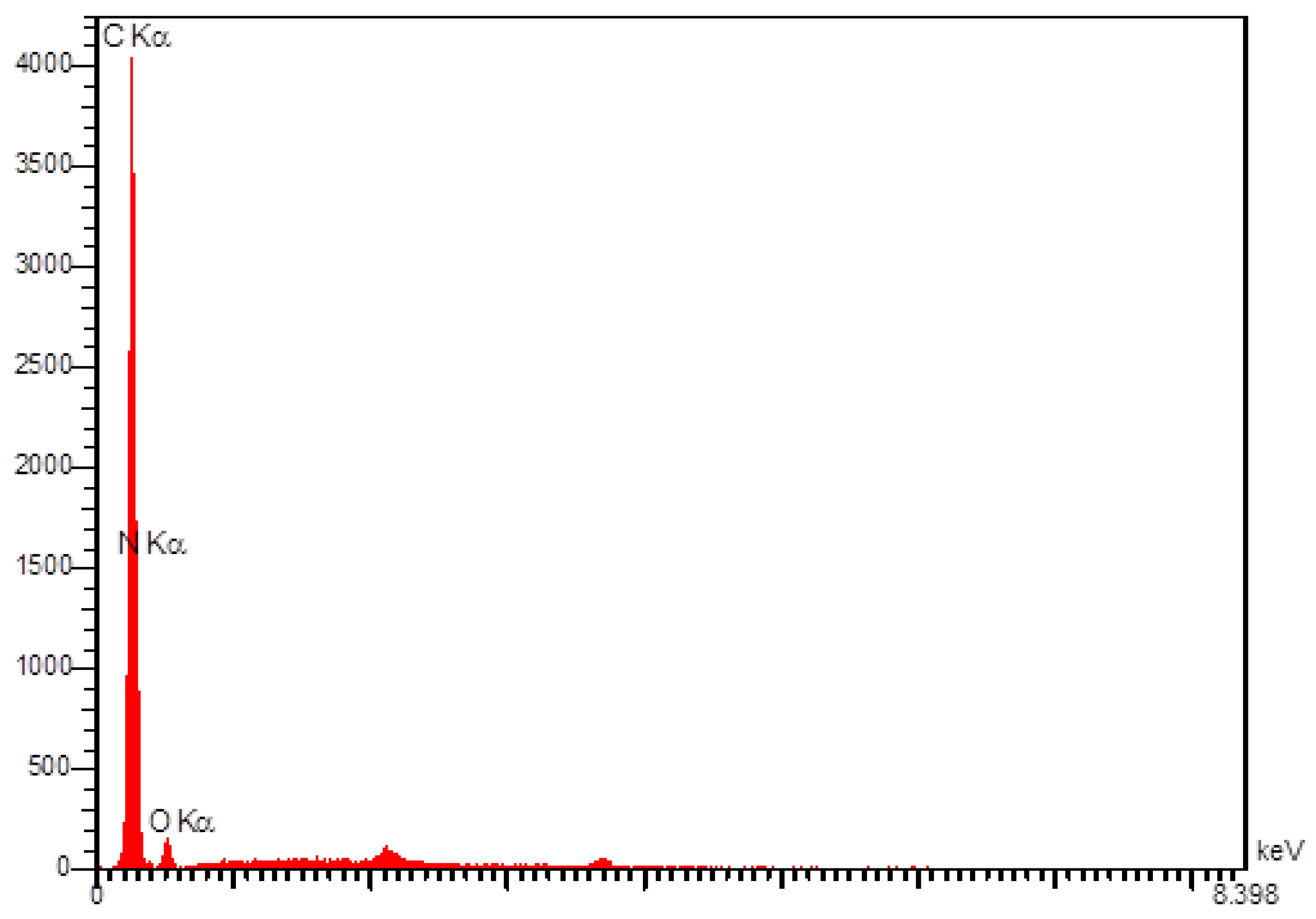
3.1. Characterization of N-rGO Sheets
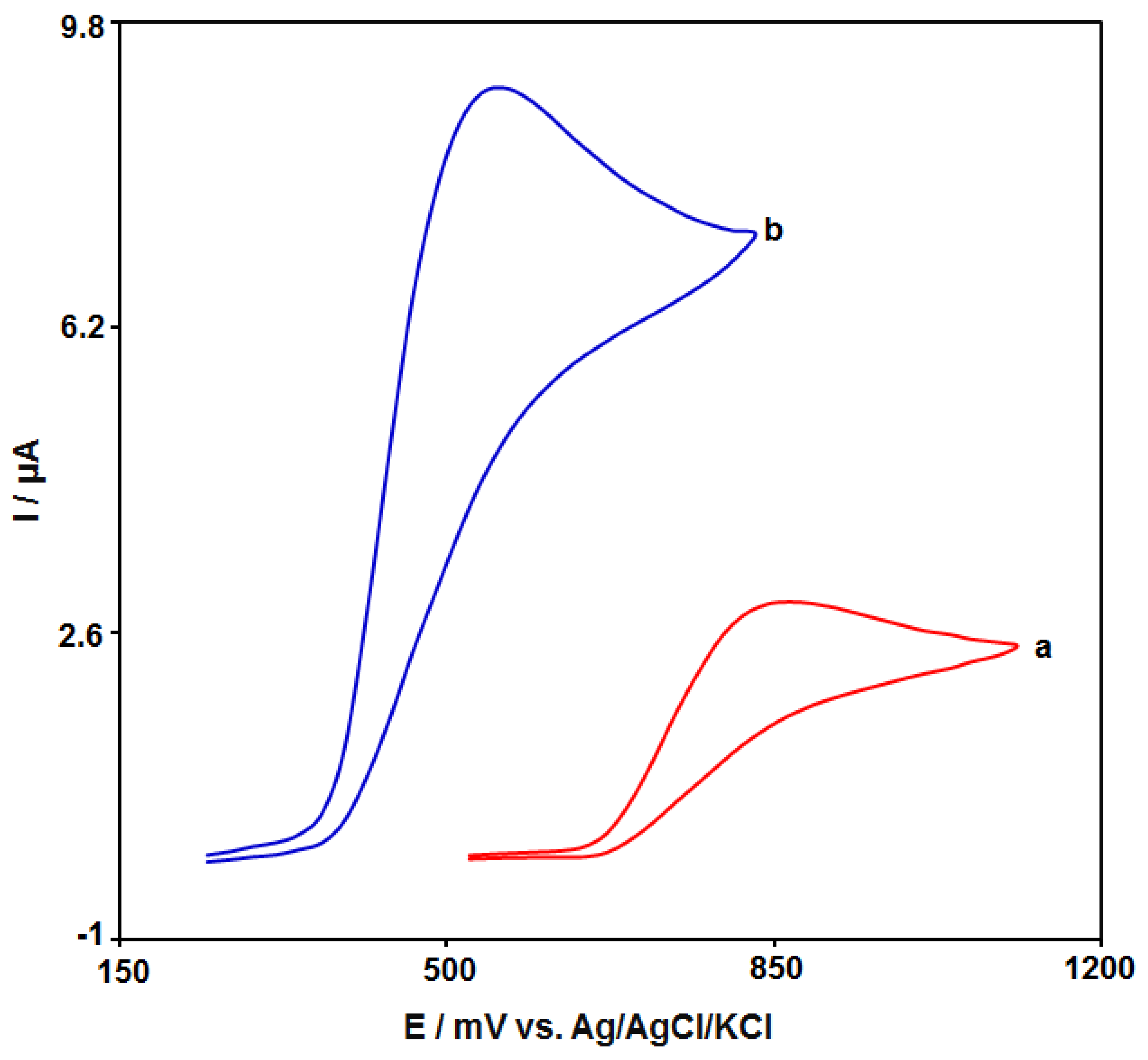
3.2. Electrochemical Behaviour of Amaranth at the Various Surface of Electrodes
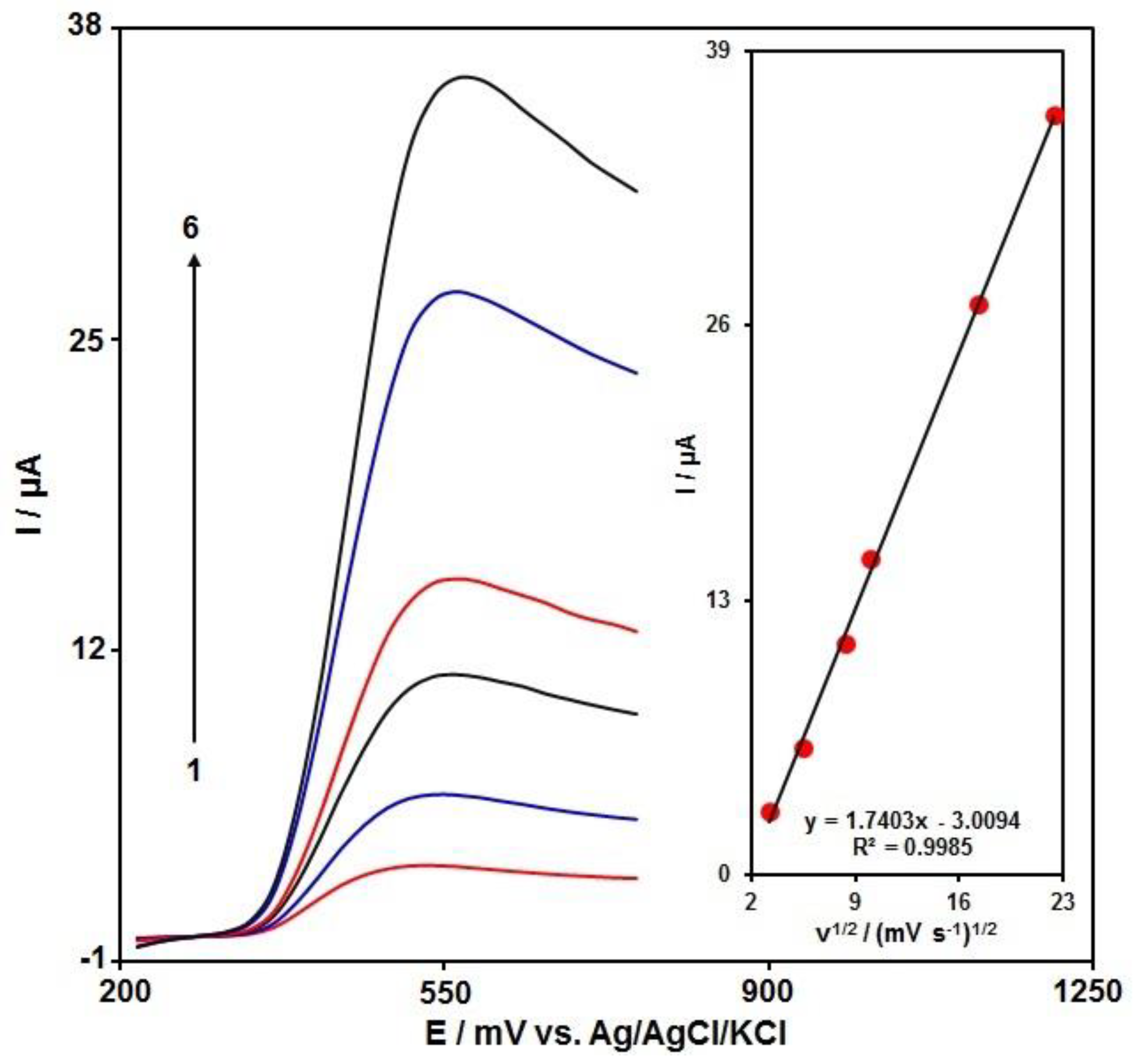
3.3. The Effects of the Scan Rate
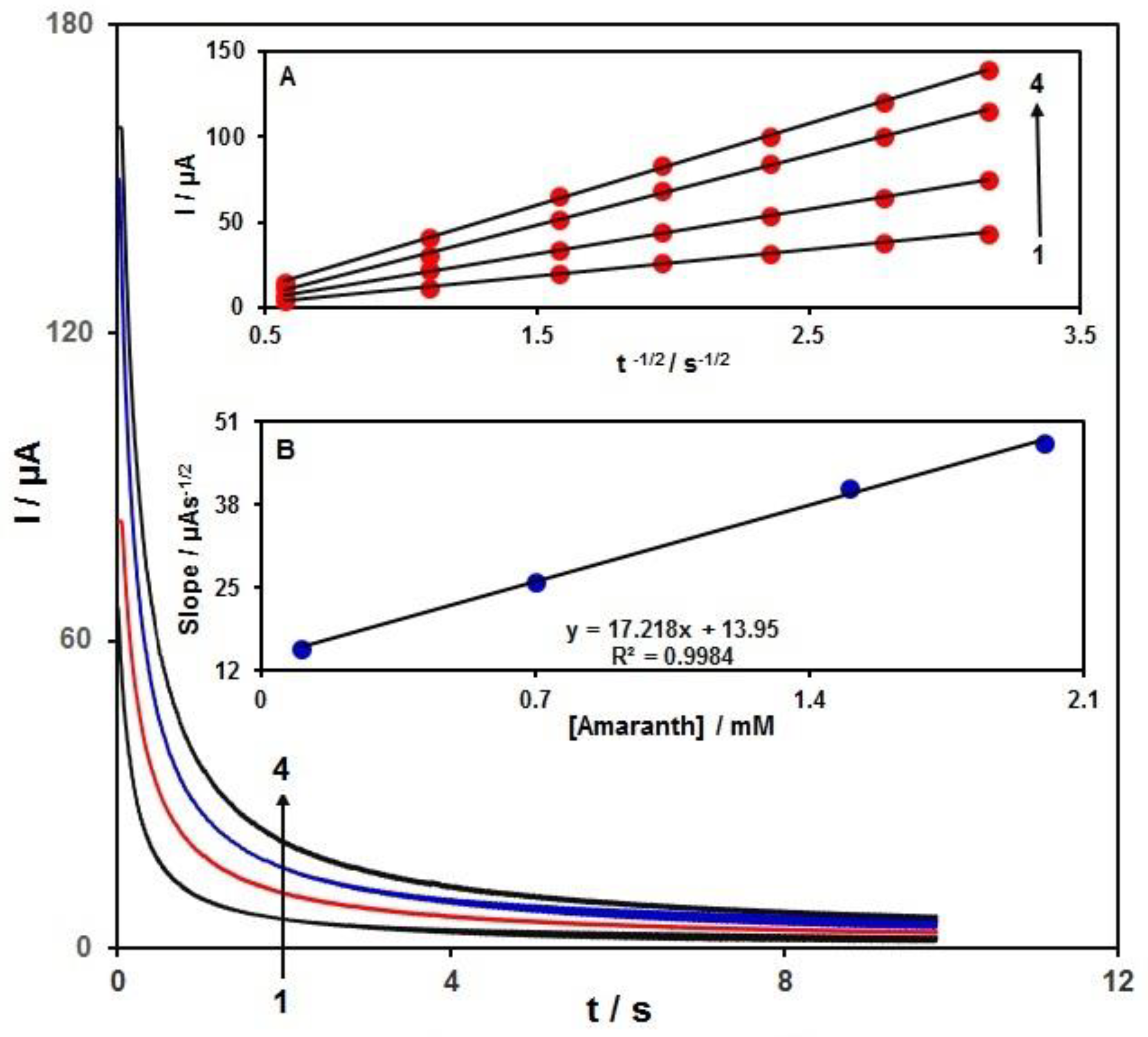
3.4. Chronoamperometric Measurements
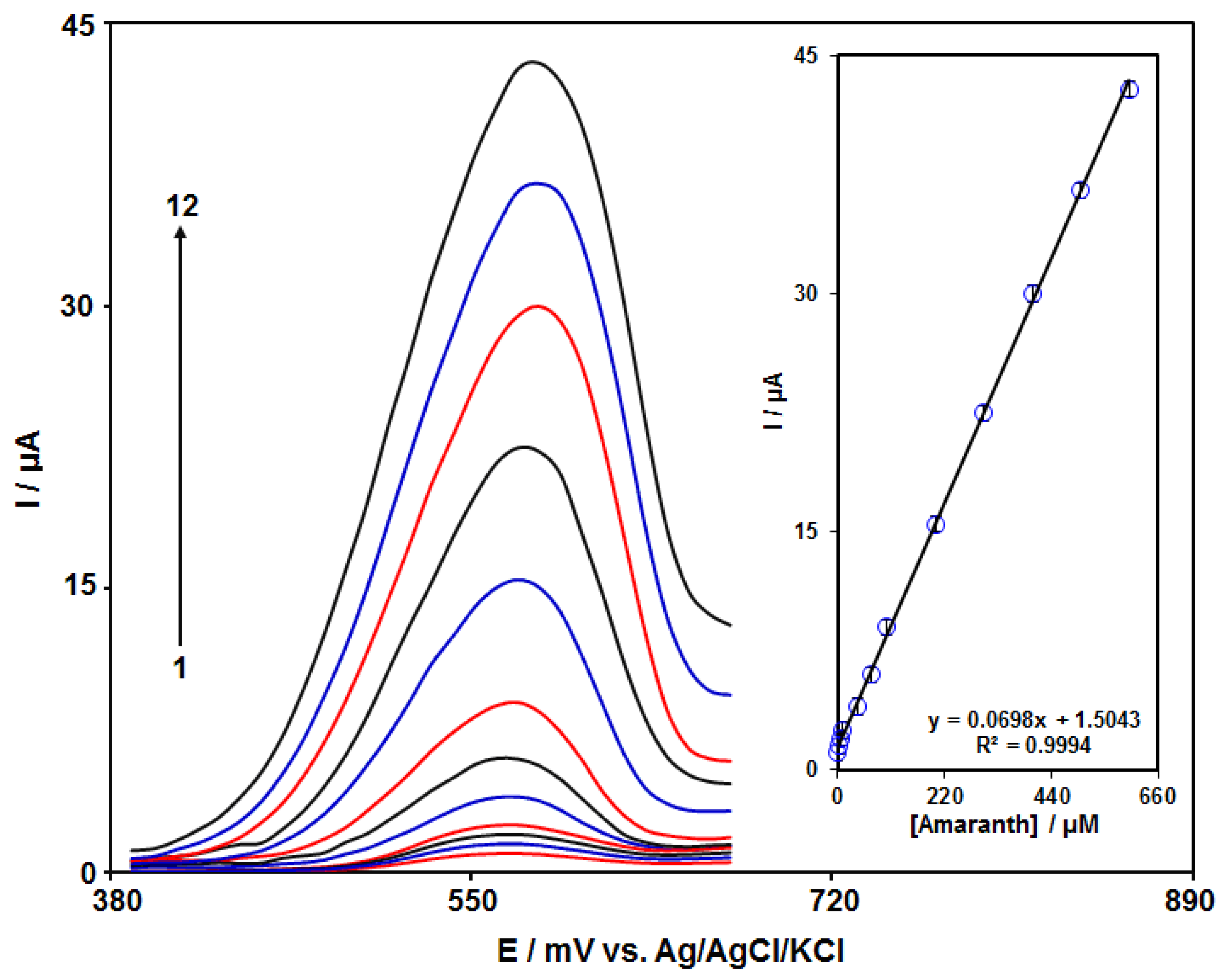
3.5. Calibration Plot and LOD
3.6. Analysing the Real Sample
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akbari, S. A new voltammetric sensor according to graphene quantum dots/ionic liquid modified carbon paste electrode for amaranth sensitive determination. Int. J. Environ. Anal. Chem. 2022, 102, 789–803. [Google Scholar] [CrossRef]
- Han, Q.; Wang, X.; Yang, Z.; Zhu, W.; Zhou, X.; Jiang, H. Fe3O4@rGO doped molecularly imprinted polymer membrane based on magnetic field directed self-assembly for the determination of amaranth. Talanta 2014, 123, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.U.; Li-Ping, G.U.O. Sensitive voltammetric sensor for amaranth based on ordered mesoporous carbon. Chin. J. Anal. Chem. 2013, 41, 681–686. [Google Scholar]
- Li, L.; Zheng, H.; Guo, L.; Qu, L.; Yu, L. A sensitive and selective molecularly imprinted electrochemical sensor based on Pd-Cu bimetallic alloy functionalized graphene for detection of amaranth in soft drink. Talanta 2019, 197, 68–76. [Google Scholar] [CrossRef]
- Jing, S.; Zheng, H.; Zhao, L.; Qu, L.; Yu, L. Electrochemical sensor based on poly (sodium 4-styrenesulfonate) functionalized graphene and Co3O4 nanoparticle clusters for detection of amaranth in soft drinks. Food Anal. Methods 2017, 10, 3149–3157. [Google Scholar] [CrossRef]
- Biswas, S.; Harwansh, R.K.; Kar, A.; Mukherjee, P.K. Validated high-performance thin-layer chromatographic method for the simultaneous determination of quercetin, rutin, and gallic acid in Amaranthus tricolor L. JPC-J. Planar Chroma 2019, 32, 121–126. [Google Scholar] [CrossRef]
- Alves, S.P.; Brum, D.M.; de Andrade, É.C.B.; Netto, A.D.P. Determination of synthetic dyes in selected foodstuffs by high performance liquid chromatography with UV-DAD detection. Food Chem. 2008, 107, 489–496. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, M.J.; Park, H.J.; Kim, H.J.; Cho, S.K.; Jeong, M.H. Simultaneous determination of synthetic food additives in kimchi by liquid chromatography-electrospray tandem mass spectrometry. Food Sci. Biotechnol. 2018, 27, 877–882. [Google Scholar] [CrossRef]
- El-Zomrawy, A.A. Selective and sensitive spectrophotometric method to determine trace amounts of copper metal ions using Amaranth food dye. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 203, 450–454. [Google Scholar] [CrossRef]
- Sha, O.; Zhu, X. Simultaneous ionic liquid aqueous two-phase extraction and spectrophotometric determination of amaranth and brilliant blue in food samples. J. Anal. Chem. 2015, 70, 558–565. [Google Scholar] [CrossRef]
- Yi, J.; Zeng, L.; Wu, Q.; Yang, L.; Xie, T. Sensitive simultaneous determination of synthetic food colorants in preserved fruit samples by capillary electrophoresis with contactless conductivity detection. Food Anal. Methods 2018, 11, 1608–1618. [Google Scholar] [CrossRef]
- Pogacean, F.; Rosu, M.C.; Coros, M.; Magerusan, L.; Moldovan, M.; Sarosi, C.; Porav, A.S.; Stefan-van Staden, R.I.; Pruneanu, S. Graphene/TiO2-Ag based composites used as sensitive electrode materials for amaranth electrochemical detection and degradation. J. Electrochem. Soc. 2018, 165, B3054. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, W.; Wu, D.; Yang, Y.; Feng, X.; Gao, C.; Meng, L.; Shen, X.; Zhang, Y.; Tang, X. An electrochemical method for determination of amaranth in drinks using functionalized graphene oxide/chitosan/ionic liquid nanocomposite supported nanoporous gold. Food Chem. 2022, 367, 130727. [Google Scholar] [CrossRef]
- Shetti, N.P.; Bukkitgar, S.D.; Reddy, K.R.; Reddy, C.V.; Aminabhavi, T.M. ZnO-based nanostructured electrodes for electrochemical sensors and biosensors in biomedical applications. Biosens. Bioelectron. 2019, 141, 111417. [Google Scholar] [CrossRef]
- Payehghadr, M.; Taherkhani, Y.; Maleki, A.; Nourifard, F. Selective and sensitive voltammetric sensor for methocarbamol determination by molecularly imprinted polymer modified carbon paste electrode. Eurasian Chem. Commun. 2020, 2, 982–990. [Google Scholar]
- Eren, T.; Atar, N.; Yola, M.L.; Karimi-Maleh, H. A sensitive molecularly imprinted polymer based quartz crystal microbalance nanosensor for selective determination of lovastatin in red yeast rice. Food Chem. 2015, 185, 430–436. [Google Scholar] [CrossRef]
- Shen, C.; Liu, S.; Li, X.; Yang, M. Electrochemical detection of circulating tumor cells based on DNA generated electrochemical current and rolling circle amplification. Anal. Chem. 2019, 91, 11614–11619. [Google Scholar] [CrossRef]
- Mohanraj, J.; Durgalakshmi, D.; Rakkesh, R.A.; Balakumar, S.; Rajendran, S.; Karimi-Maleh, H. Facile synthesis of paper based graphene electrodes for point of care devices: A double stranded DNA (dsDNA) biosensor. J. Colloid Interface Sci. 2020, 566, 463–472. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Garkani-Nejad, F.; Aghaei Afshar, A.; Beitollahi, H. Voltammetric determination of isoniazid in the presence of acetaminophen utilizing MoS2-nanosheet-modified screen-printed electrode. Micromachines 2022, 13, 369. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Celiesiute, R.; Ramanaviciene, A.; Shirsat, M.D.; Ramanavicius, A. EDTA_PANI/SWCNTs nanocomposite modified electrode for electrochemical determination of copper (II), lead (II) and mercury (II) ions. Electrochim. Acta 2018, 259, 930–938. [Google Scholar] [CrossRef]
- Raoof, J.B.; Ojani, R.; Beitollahi, H.; Hosseinzadeh, R. Electrocatalytic oxidation and highly selective voltammetric determination of L-cysteine at the surface of a 1-[4-(ferrocenyl ethynyl) phenyl]-1-ethanone modified carbon paste electrode. Anal. Sci. 2006, 22, 1213–1220. [Google Scholar] [CrossRef] [Green Version]
- Tajik, S.; Askari, M.B.; Ahmadi, S.A.; Garkani-Nejad, F.; Dourandish, Z.; Razavi, R.; Beitollahi, H.; Di Bartolomeo, A. Electrochemical sensor based on ZnFe2O4/RGO nanocomposite for ultrasensitive detection of hydrazine in real samples. Nanomaterials 2022, 12, 491. [Google Scholar] [CrossRef]
- Yang, L.; Xu, C.; Ye, W.; Liu, W. An electrochemical sensor for H2O2 based on a new Co-metal-organic framework modified electrode. Sens. Actuators B Chem. 2015, 215, 489–496. [Google Scholar] [CrossRef]
- Elobeid, W.H.; Elbashir, A.A. Development of chemically modified pencil graphite electrode based on benzo-18-crown-6 and multi-walled CNTs for determination of lead in water samples. Prog. Chem. Biochem. Res. 2019, 2, 24–33. [Google Scholar]
- Shahsavari, M.; Tajik, S.; Sheikhshoaie, I.; Garkani-Nejad, F.; Beitollahi, H. Synthesis of Fe3O4@ copper (II) imidazolate nanoparticles: Catalytic activity of modified graphite screen printed electrode for the determination of levodopa in presence of melatonin. Microchem. J. 2021, 170, 106637. [Google Scholar] [CrossRef]
- Montazarolmahdi, M.; Masrournia, M.; Nezhadali, A. A new electrochemical approach for the determination of phenylhydrazine in water and wastewater samples using amplified carbon paste electrode. Chem. Methodol. 2020, 4, 732–742. [Google Scholar]
- Karimi-Maleh, H.; Khataee, A.; Karimi, F.; Baghayeri, M.; Fu, L.; Rouhi, J.; Karaman, C.; Karaman, O.; Boukherroub, R. A green and sensitive guanine-based DNA biosensor for idarubicin anticancer monitoring in biological samples: A simple and fast strategy for control of health quality in chemotherapy procedure confirmed by docking investigation. Chemosphere 2022, 291, 132928. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Karimi, F.; Orooji, Y.; Mansouri, G.; Razmjou, A.; Aygun, A.; Sen, F. A new nickel-based co-crystal complex electrocatalyst amplified by NiO dope Pt nanostructure hybrid; a highly sensitive approach for determination of cysteamine in the presence of serotonin. Sci. Rep. 2020, 10, 11699. [Google Scholar] [CrossRef]
- Abrishamkar, M.; Ehsani Tilami, S.; Hosseini Kaldozakh, S. Electrocatalytic oxidation of cefixime at the surface of modified carbon paste electrode with synthesized nano zeolite. Adv. J. Chem. A 2020, 3, 767–776. [Google Scholar]
- Tajik, S.; Shahsavari, M.; Sheikhshoaie, I.; Garkani Nejad, F.; Beitollahi, H. Voltammetric detection of sumatriptan in the presence of naproxen using Fe3O4@ZIF-8 nanoparticles modified screen printed graphite electrode. Sci. Rep. 2021, 11, 24068. [Google Scholar] [CrossRef]
- Miraki, M.; Karimi-Maleh, H.; Taher, M.A.; Cheraghi, S.; Karimi, F.; Agarwal, S.; Gupta, V.K. Voltammetric amplified platform based on ionic liquid/NiO nanocomposite for determination of benserazide and levodopa. J. Mol. Liq. 2019, 278, 672–676. [Google Scholar] [CrossRef]
- Ahmadi, R. Investigating the performance of nano structure C60 as nano-carriers of anticancer cytarabine, a DFT study. Asian J. Green Chem. 2020, 4, 355–366. [Google Scholar]
- Saeidi, S.; Javadian, F.; Sepehri, Z.; Shahi, Z.; Mousavi, F.; Anbari, M. Antibacterial effects of silver nanoparticlesagainst resistant strains of E. coli bacteria. Int. J. Adv. Biol. Biomed. Res. 2016, 4, 96–99. [Google Scholar]
- Karimi-Maleh, H.; Darabi, R.; Shabani-Nooshabadi, M.; Baghayeri, M.; Karimi, F.; Rouhi, J.; Karaman, C. Determination of D&C Red 33 and Patent Blue V Azo dyes using an impressive electrochemical sensor based on carbon paste electrode modified with ZIF-8/g-C3N4/Co and ionic liquid in mouthwash and toothpaste as real samples. Food Chem. Toxicol. 2022, 162, 112907. [Google Scholar]
- Shaikh, U.U.; Tamboli, Q.; Pathange, S.M.; Dahan, Z.A.; Pudukulathan, Z. An efficient method for chemoselective acetylation of activated alcohols using nano ZnFe2O4 as catalyst. Chem. Methodol. 2018, 2, 73–82. [Google Scholar]
- Fazal-ur-Rehman, M.; Qayyum, I. Biomedical scope of gold nanoparticles in medical sciences; an advancement in cancer therapy. J. Med. Chem. Sci. 2020, 3, 399–407. [Google Scholar]
- Karimi-Maleh, H.; Karaman, C.; Karaman, O.; Karimi, F.; Vasseghian, Y.; Fu, L.; Mirabi, A. Nanochemistry approach for the fabrication of Fe and N co-decorated biomass-derived activated carbon frameworks: A promising oxygen reduction reaction electrocatalyst in neutral media. J. Nanostruct. Chem. 2022. [Google Scholar] [CrossRef]
- Sheikhshoaie, I.; Rezazadeh, A.; Ramezanpour, S. Removal of Pb (II) from aqueous solution by gel combustion of a new nano sized Co3O4/ZnO composite. Asian J. Nanosci. Mater. 2018, 1, 271–281. [Google Scholar]
- Abegunde, S.M.; Idowu, K.S.; Sulaimon, A.O. Plant-mediated iron nanoparticles and their applications as adsorbents for water treatment–a review. J. Chem. Rev. 2020, 2, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Karimi-Maleh, H.; Sheikhshoaie, M.; Sheikhshoaie, I.; Ranjbar, M.; Alizadeh, J.; Maxakato, N.W.; Abbaspourrad, A. A novel electrochemical epinine sensor using amplified CuO nanoparticles and an-hexyl-3-methylimidazolium hexafluorophosphate electrode. New J. Chem. 2019, 43, 2362–2367. [Google Scholar] [CrossRef]
- Alavi-Tabari, S.A.; Khalilzadeh, M.A.; Karimi-Maleh, H. Simultaneous determination of doxorubicin and dasatinib as two breast anticancer drugs uses an amplified sensor with ionic liquid and ZnO nanoparticle. J. Electroanal. Chem. 2018, 811, 84–88. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, M.; Mamat, X.; Hu, X.; Hu, G. Hierarchically ordered porous nitrogen doped carbon modified a glassy carbon electrode for voltammetry detection of quercetin. Mater. Res. Bull. 2021, 136, 111131. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Shahsavari, S.; Garkani-Nejad, F. Simultaneous and selective electrochemical sensing of methotrexate and folic acid in biological fluids and pharmaceutical samples using Fe3O4/ppy/Pd nanocomposite modified screen printed graphite electrode. Chemosphere 2022, 291, 132736. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P.; Di Bartolomeo, A.; Şen, F. Enhanced electrochemical performance of MnNi2O4/rGO nanocomposite as pseudocapacitor electrode material and methanol electro-oxidation catalyst. Nanotechnology 2021, 32, 325707. [Google Scholar] [CrossRef]
- Sengar, M.; Saxena, S.; Satsangee, S.; Jain, R. Silver nanoparticles decorated functionalized multiwalled carbon nanotubes modified screen printed sensor for the voltammetric determination of butorphanol. J. Appl. Organomet. Chem. 2021, 1, 95–108. [Google Scholar]
- Karimi-Maleh, H.; Shojaei, A.F.; Tabatabaeian, K.; Karimi, F.; Shakeri, S.; Moradi, R. Simultaneous determination of 6-mercaptopruine, 6-thioguanine and dasatinib as three important anticancer drugs using nanostructure voltammetric sensor employing Pt/MWCNTs and 1-butyl-3-methylimidazolium hexafluoro phosphate. Biosens. Bioelectron. 2016, 86, 879–884. [Google Scholar] [CrossRef]
- Mahmoudi-Moghaddam, H.; Beitollahi, H.; Tajik, S.; Sheikhshoaie, I.; Biparva, P. Fabrication of novel TiO2 nanoparticles/Mn (III) salen doped carbon paste electrode: Application as electrochemical sensor for the determination of hydrazine in the presence of phenol. Environ. Monit. Assess. 2015, 187, 407. [Google Scholar] [CrossRef]
- Prasad, P.; Sreedhar, N.Y. Effective SWCNTs/Nafion electrochemical sensor for detection of dicapthon pesticide in water and agricultural food samples. Chem. Methodol. 2018, 2, 277–290. [Google Scholar]
- Urban, F.; Lupina, G.; Grillo, A.; Martucciello, N.; Di Bartolomeo, A. Contact resistance and mobility in back-gate graphene transistors. Nano Express 2020, 1, 010001. [Google Scholar] [CrossRef]
- Bhardiya, S.R.; Asati, A.; Sheshma, H.; Rai, A.; Rai, V.K.; Singh, M. A novel bioconjugated reduced graphene oxide-based nanocomposite for sensitive electrochemical detection of cadmium in water. Sens. Actuators B Chem. 2021, 328, 129019. [Google Scholar] [CrossRef]
- Yang, S.; Liu, P.; Wang, Y.; Guo, Z.; Tan, R.; Qu, L. Electrochemical sensor using poly-(l-cysteine) functionalized CuO nanoneedles/N-doped reduced graphene oxide for detection of lead ions. RSC Adv. 2020, 10, 18526–18532. [Google Scholar] [CrossRef]
- Beitollahi, H.; Garkani-Nejad, F. Voltammetric determination of vitamin B6 (pyridoxine) at a graphite screen-printed electrode modified with graphene oxide/Fe3O4@SiO2 nanocomposite. Russ. Chem. Bull. 2018, 67, 238–242. [Google Scholar] [CrossRef]
- Barsan, M.M.; Prathish, K.P.; Sun, X.; Brett, C.M. Nitrogen doped graphene and its derivatives as sensors and efficient direct electron transfer platform for enzyme biosensors. Sens. Actuators B Chem. 2014, 203, 579–587. [Google Scholar] [CrossRef]
- Keteklahijani, Y.Z.; Sharif, F.; Roberts, E.P.; Sundararaj, U. Enhanced sensitivity of dopamine biosensors: An electrochemical approach based on nanocomposite electrodes comprising polyaniline, nitrogen-doped graphene, and DNA-functionalized carbon nanotubes. J. Electrochem. Soc. 2019, 166, B1415. [Google Scholar] [CrossRef]
- Jia, H.; Yang, T.; Zuo, Y.; Wang, W.; Xu, J.; Lu, L.; Li, P. Immunosensor for α-fetoprotein based on a glassy carbon electrode modified with electrochemically deposited N-doped graphene, gold nanoparticles and chitosan. Microchim. Acta 2017, 184, 3747–3753. [Google Scholar] [CrossRef]
- Zou, C.E.; Bin, D.; Yang, B.; Zhang, K.; Du, Y. Rutin detection using highly electrochemical sensing amplified by an Au–Ag nanoring decorated N-doped graphene nanosheet. RSC Adv. 2016, 6, 107851–107858. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, M.; Liang, L. Highly efficient in-situ metal-free electrochemical advanced oxidation process using graphite felt modified with N-doped graphene. Chem. Eng. J. 2018, 338, 700–708. [Google Scholar] [CrossRef]
- Di Bartolomeo, A.; Giubileo, F.; Romeo, F.; Sabatino, P.; Carapella, G.; Iemmo, L.; Schroeder, T.; Lupina, G. Graphene field effect transistors with niobium contacts and asymmetric transfer characteristics. Nanotechnology 2015, 26, 475202. [Google Scholar] [CrossRef] [Green Version]
- Salahandish, R.; Ghaffarinejad, A.; Omidinia, E.; Zargartalebi, H.; Majidzadeh, K.; Naghib, S.M.; Sanati-Nezhad, A. Label-free ultrasensitive detection of breast cancer miRNA-21 biomarker employing electrochemical nano-genosensor based on sandwiched AgNPs in PANI and N-doped graphene. Biosens. Bioelectron. 2018, 120, 129–136. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Fu, Q.; Pan, C. Influence of graphene microstructures on electrochemical performance for supercapacitors. Prog. Nat. Sci. Mat. Int. 2015, 25, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Gu, W.; Zhao, L.; Zhang, C.; Peng, W.; Xian, Y. MoS2/Nitrogen-doped graphene as efficient electrocatalyst for oxygen reduction reaction. Electrochim. Acta 2015, 169, 142–149. [Google Scholar] [CrossRef]
- Fakude, C.T.; Arotiba, O.A.; Moutloali, R.; Mabuba, N. Nitrogen-doped graphene electrochemical sensor for selenium (IV) in water. Int. J. Electrochem. Sci. 2019, 14, 9391–9403. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.; Wu, T.; Wang, X.; Huang, G.; Liu, Y.; Qiu, H.; Li, Y.; Wang, W.; Gao, J. Cost-effective reduced graphene oxide-coated polyurethane sponge as a highly efficient and reusable oil-absorbent. ACS Appl. Mater. Interfaces 2013, 5, 10018–10026. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, Z.; Rashidi, A.M.; Samadi, M.T.; Rahmani, A.R. N-doped reduced graphene oxide aerogel for the selective adsorption of oil pollutants from water: Isotherm and kinetic study. J. Ind. Eng. Chem. 2018, 61, 416–426. [Google Scholar] [CrossRef]
- Fan, H.; Li, Y.; Wu, D.; Ma, H.; Mao, K.; Fan, D.; Wei, Q. Electrochemical bisphenol A sensor based on N-doped graphene sheets. Anal. Chim. Acta 2012, 711, 24–28. [Google Scholar] [CrossRef]
- Rahsepar, M.; Foroughi, F.; Kim, H. A new enzyme-free biosensor based on nitrogen-doped graphene with high sensing performance for electrochemical detection of glucose at biological pH value. Sens. Actuators B Chem. 2019, 282, 322–330. [Google Scholar] [CrossRef]
- He, J.L.; Kou, W.; Li, C.; Cai, J.J.; Kong, F.Y.; Wang, W. Electrochemical sensor based on single-walled carbon nanotube-TiN nannocomposites for detecting Amaranth. Int. J. Electrochem. Sci. 2015, 10, 10074–10082. [Google Scholar]
- Wang, P.; Hu, X.; Cheng, Q.; Zhao, X.; Fu, X.; Wu, K. Electrochemical detection of amaranth in food based on the enhancement effect of carbon nanotube film. J. Agric. Food Chem. 2010, 58, 12112–12116. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, S.; Liu, C.; Gu, L.; Chang, J.; Xie, A.; Luo, S. Co3O4-CeO2/Graphene Composite as a Novel Sensor for Amaranth Detection. J. Electrochem. Soc. 2021, 168, 027513. [Google Scholar] [CrossRef]


| Electrochemical Sensor | Method | Linear Range | LOD | Ref. |
|---|---|---|---|---|
| Fe3O4@reduced graphene oxide/GCE | DPV | 0.05–50 ìM | 50 nM | [2] |
| Ordered mesoporous carbon/GCE | DPV | 1.0 × 10−7–3.0 × 10−6 M | 3.2 × 10−8 M | [3] |
| Single-walled carbon nanotube-titanium nitride nanocomposite/GCE | DPV | 0.1–100 ìM | 40 nM | [67] |
| Multi-wall carbon nanotube thin film/GCE | DPV | 40 nM–0.8 ìM | 35 nM | [68] |
| Co3O4-CeO2/graphene nanocomposite modified electrode | DPV | 2–96 μM | 0.1591 μM | [69] |
| N-rGO/GCE | DPV | 0.1–600.0 µM | 0.03 μM | This Work |
| Sample | Spiked (µM) | Found (µM) | Recovery (%) | R.S.D. (%) |
|---|---|---|---|---|
| Tap Water | 0 | - | - | - |
| 5.0 | 4.9 | 98.0 | 3.2 | |
| 6.0 | 6.2 | 103.3 | 1.7 | |
| 7.0 | 7.1 | 101.4 | 2.4 | |
| 8.0 | 7.8 | 97.5 | 2.6 | |
| Apple Juice | 0 | - | - | - |
| 4 | 4.1 | 102.5 | 1.7 | |
| 6 | 5.8 | 96.7 | 3.5 | |
| 8 | 8.1 | 101.2 | 2.6 | |
| 10 | 9.9 | 99.0 | 2.7 | |
| Orange Juice | 0 | - | - | - |
| 5 | 4.8 | 96.0 | 3.6 | |
| 7.0 | 7.3 | 104.3 | 2.8 | |
| 9.0 | 9.1 | 101.1 | 3.0 | |
| 11.0 | 10.9 | 99.1 | 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moradpour, H.; Beitollahi, H.; Nejad, F.G.; Di Bartolomeo, A. Glassy Carbon Electrode Modified with N-Doped Reduced Graphene Oxide Sheets as an Effective Electrochemical Sensor for Amaranth Detection. Materials 2022, 15, 3011. https://doi.org/10.3390/ma15093011
Moradpour H, Beitollahi H, Nejad FG, Di Bartolomeo A. Glassy Carbon Electrode Modified with N-Doped Reduced Graphene Oxide Sheets as an Effective Electrochemical Sensor for Amaranth Detection. Materials. 2022; 15(9):3011. https://doi.org/10.3390/ma15093011
Chicago/Turabian StyleMoradpour, Hediyeh, Hadi Beitollahi, Fariba Garkani Nejad, and Antonio Di Bartolomeo. 2022. "Glassy Carbon Electrode Modified with N-Doped Reduced Graphene Oxide Sheets as an Effective Electrochemical Sensor for Amaranth Detection" Materials 15, no. 9: 3011. https://doi.org/10.3390/ma15093011
APA StyleMoradpour, H., Beitollahi, H., Nejad, F. G., & Di Bartolomeo, A. (2022). Glassy Carbon Electrode Modified with N-Doped Reduced Graphene Oxide Sheets as an Effective Electrochemical Sensor for Amaranth Detection. Materials, 15(9), 3011. https://doi.org/10.3390/ma15093011








