Two-Dimensional V2O5 Inverse Opal: Fabrication and Electrochromic Application
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Materials
2.2. Fabrication of PS Monolayer Inverse Opal
2.3. Fabrication of 2D-V2O5 IO Structure: Improved “Dynamic-Hard-Template” Infiltration Strategy
2.4. Characterization
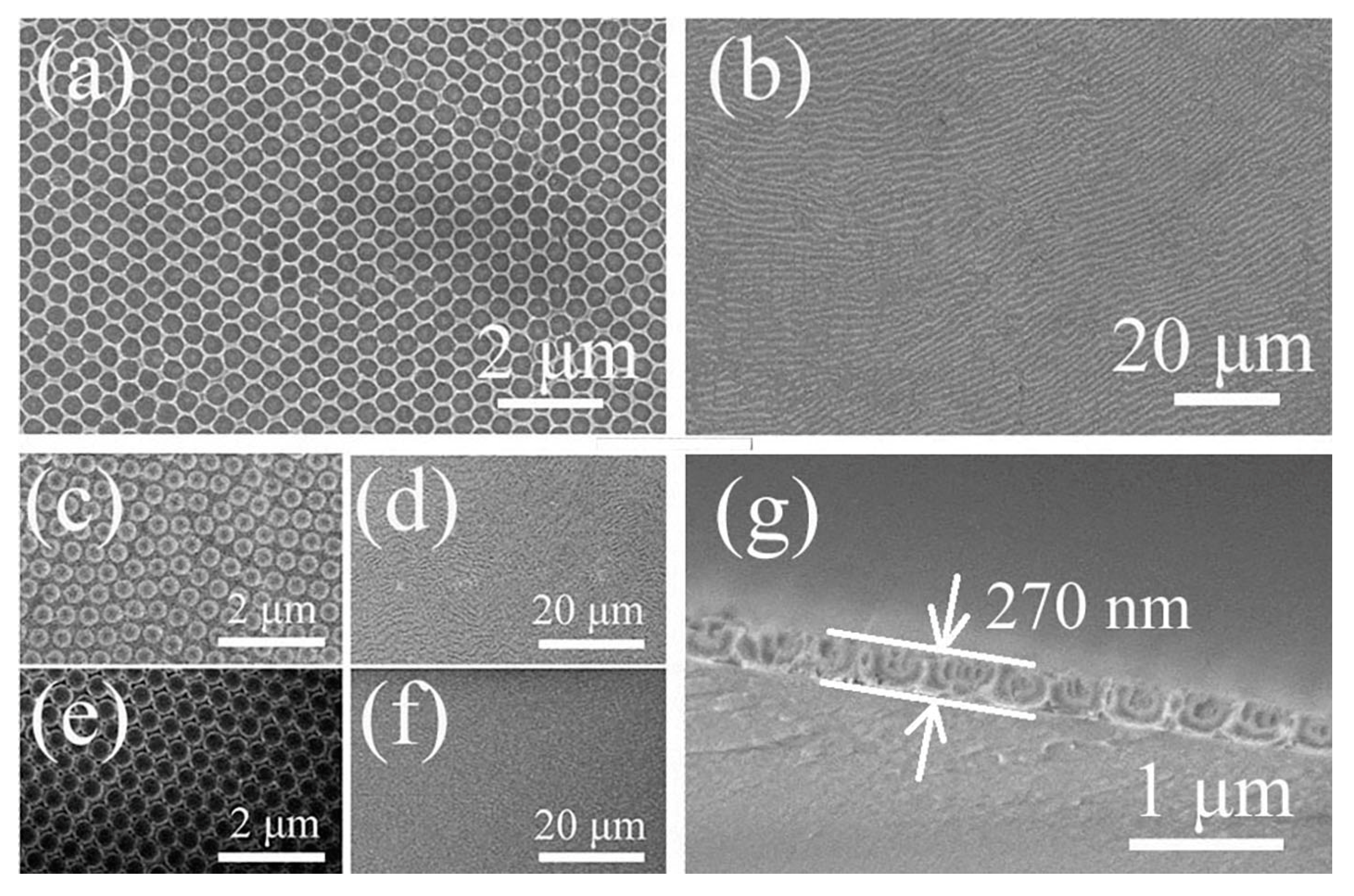
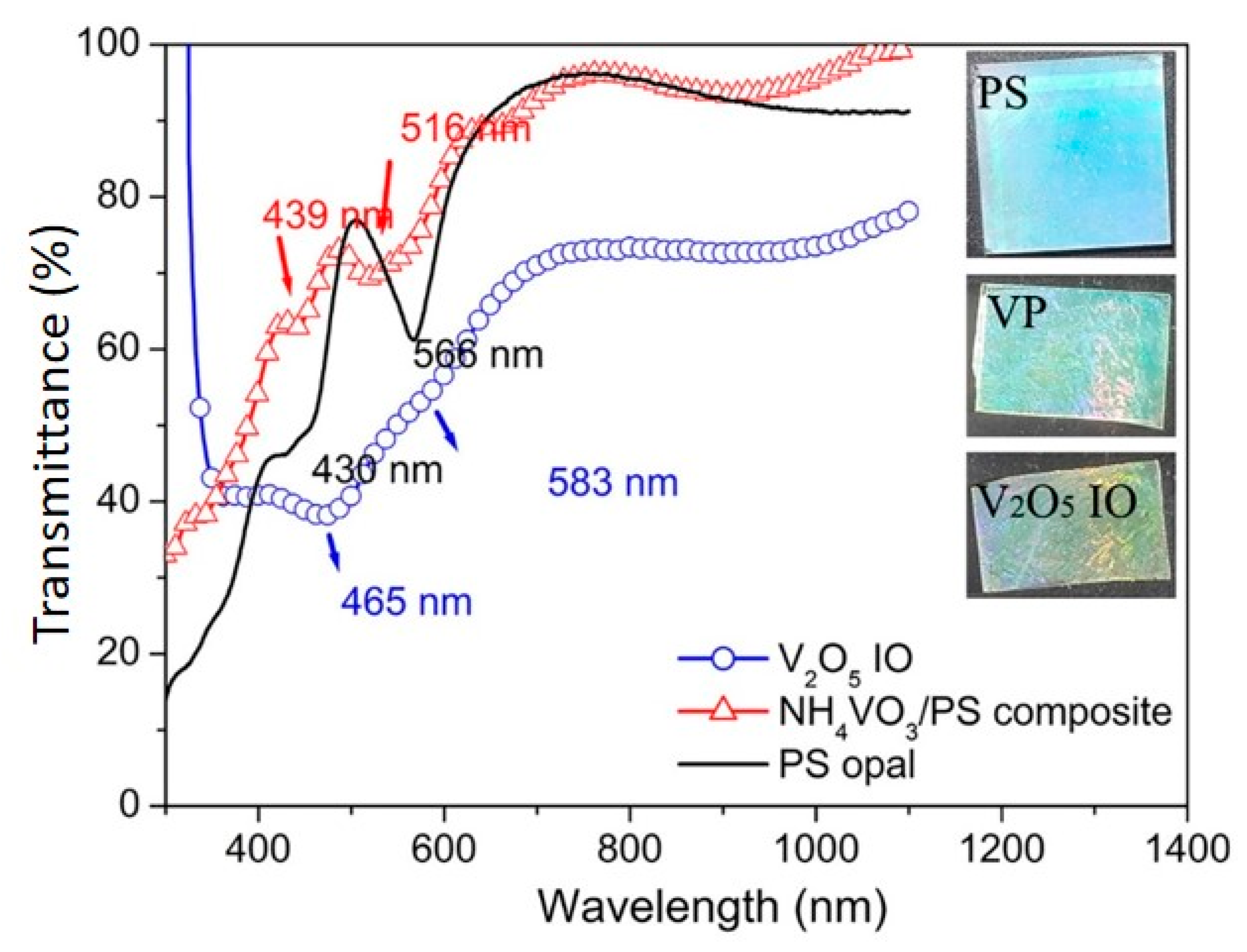
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Steiner, U.; Mahajan, S. Improved electrochromic performance in inverse opal vanadium oxide film. J. Mater. Chem. 2010, 20, 7131–7134. [Google Scholar] [CrossRef]
- Granqvist, C.G. Handbook of Inorganic Electrochromic Devices, 1st ed.; Elsevier: Amsterdan, The Netherlands, 1995. [Google Scholar]
- Salek, G.; Bellanger, B.; Gaudon, I.M.; Rougier, A. Polyol Synthesis of Ti-V2O5 Nanoparticles and Their Use as Electrochromic Films. Inorg. Chem. 2016, 55, 9838–9847. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Yang, H.; Na, L.; Qu, H.; Zhang, X.; Zhao, J.; Li, Y. Versatile displays based on a 3-dimensionally ordered microporous vanadium oxide film for advanced electrochromic devices. J. Mater. Chem. C 2015, 3, 3159–3166. [Google Scholar] [CrossRef]
- Tang, Y.; Rui, X.; Zhang, Y.; Lim, T.M.; Dong, Z.; Hng, H.H.; Chen, X.; Yan, Q.; Chen, Z. Vanadium pentoxide cathode materials for high-performance lithium-ion batteries enabled by a hierarchical nanoflower structure via an electrochemical process. J. Mater. Chem. A 2013, 1, 82–88. [Google Scholar] [CrossRef]
- Zhu, J.; Cao, L.; Wu, Y.; Gong, Y.; Liu, Z.; Hoster, H.E.; Zhang, Y.; Zhang, S.; Yang, S.; Yan, Q.; et al. Building 3D structures of vanadium pentoxide nanosheets and application as electrodes in supercapacitors supercapacitors. Nano Lett. 2013, 13, 5408–5413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Cao, G. Synthesis and Enhanced Intercalation Properties of nanostructured Vanadium Oxides. Chem. Mater. 2006, 18, 2787–2804. [Google Scholar] [CrossRef]
- Marley, P.M.; Horrocks, G.A.; Pelcher, K.E.; Banerjee, S. Transformers: The changing phases of lowdimensional vanadium oxide bronzes. Chem. Commun. 2015, 51, 5181–5198. [Google Scholar] [CrossRef]
- D’Elia, A.; Cepek, C.; de Simone, M.; Macis, S.; Belec, B.; Fanetti, M.; Piseri, P.; Marcelli, A.; Coreno, M. Interplay among work function, electronic structure and stoichiometry in nanostructured VOx films. Phys. Chem. Chem. Phys. 2020, 22, 6282–6290. [Google Scholar] [CrossRef] [Green Version]
- Mattelaer, F.; Geryl, K.; Rampelberg, G.; Dobbelaere, T.; Dendooven, J.; Detavernier, C. Atomic layer deposition of vanadium oxides for thin-film lithium-ion battery applications. RSC Adv. 2016, 6, 114658–114665. [Google Scholar] [CrossRef] [Green Version]
- Braithwaite, J.S.; Catlow, C.R.A.; Gale, J.D.; Harding, J.H. Lithium intercalation into vanadium pentoxide: A theoretical study. Chem. Mater. 1999, 11, 1990–1998. [Google Scholar] [CrossRef]
- Wu, C.; Xie, Y. Promising vanadium oxide and hydroxide nanostructures: From energy storage to energy saving. Energy Environ. Sci. 2010, 3, 1191–1206. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, G. Developments in Nanostructured Cathode Materials for High-Performance Lithium-Ion Batteries. Adv. Mater. 2008, 20, 2251–2269. [Google Scholar] [CrossRef]
- Li, H.; He, P.; Wang, Y.; Hosono, E.; Zhou, H. High-surface vanadium oxides with large capacities for lithium-ion batteries: From hydrated aerogel to nanocrystalline VO2(B), V6O13 and V2O5. J. Mater. Chem. 2011, 21, 10999–11009. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Y.; Liu, J.; Huang, X.; Yuan, C.; Lou, X.W. Recent Advances in Metal Oxide-based Electrode Architecture Design for Electrochemical Energy Storage. Adv. Mater. 2012, 24, 5166–5180. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, P.; Zhu, K.; Wang, J.; Liu, J.; Qiu, J. A general and simple method to synthesize well-crystallized nanostructured vanadium oxides for high performance Li-ion batteries. J. Mater. Chem. A 2015, 3, 9385–9389. [Google Scholar] [CrossRef]
- Kang, W.; Yan, C.; Wang, X.; Foo, C.Y.; Tan, A.W.M.; Chee, K.J.Z.; Lee, P.S. Green synthesis of nanobelt-membrane hybrid structured vanadium oxide with high electrochromic contrast. J. Mater. Chem. C 2014, 2, 4727–4732. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-Z.; Cai, Y.; Jin, J.; Li, Y.; Zheng, X.-F.; Wang, H.-E.; Wu, M.; Chen, L.-H.; Su, B.-L. Annealed vanadium oxide nanowires and nanotubes as high performance cathode materials for lithium ion batteries. J. Mater. Chem. A 2014, 2, 14099–14108. [Google Scholar] [CrossRef]
- Pang, H.; Dong, Y.; Ting, S.L.; Lu, J.; Li, C.M.; Kima, D.-H.; Chen, P. 2D single- or double-layered vanadium oxide nanosheet assembled 3D microflowers: Controlled synthesis, growth mechanism, and applications. Nanoscale 2013, 5, 7790–7794. [Google Scholar] [CrossRef]
- Liu, Y.; Clark, M.; Zhang, Q.; Yu, D.; Liu, D.; Liu, J.; Cao, G. V2O5 Nano-Electrodes with High Power and Energy Densities for Thin Film Li-Ion Batteries. Adv. Energy Mater. 2011, 1, 194–202. [Google Scholar] [CrossRef]
- Uchaker, E.; Zheng, Y.Z.; Li, S.; Candelaria, S.L.; Hu, S.; Cao, G.Z. Better than crystalline: Amorphous vanadium oxide for sodium-ion batteries. J. Mater. Chem. A 2014, 2, 18208–18214. [Google Scholar] [CrossRef]
- Armstrong, E.; McNulty, D.; Geaney, H.; O’Dwyer, C. Electrodeposited Structurally Stable V2O5 Inverse Opal Networks as High Performance Thin Film Lithium Batteries. ACS Appl. Mater. Interfaces 2015, 7, 27006–27015. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.; Osiak, M.; Geaney, H.; Glynn, C.; O’Dwyer, C. 2D and 3D vanadium oxide inverse opals and hollow sphere arrays. CrystEngComm 2014, 16, 10804–10815. [Google Scholar] [CrossRef]
- Rolison, D.R.; Long, J.W.; Lytle, J.C.; Fischer, A.E.; Rhodes, C.P.; McEvoy, T.M.; Bourg, M.E.; Lubers, A.M. Multifunctional 3D nanoarchitectures for energy storage and conversion. Chem. Soc. Rev. 2009, 38, 226–252. [Google Scholar] [CrossRef]
- Caes, S.; Arrebola, J.C.; Krins, N.; Eloy, P.; Gaigneaux, E.M.; Henrist, C.; Cloots, R.; Vertruyen, B. Mesoporous lithium vanadium oxide as a thin film electrode for lithium-ion batteries: Comparison between direct synthesis of LiV2O5 and electrochemical lithium intercalation in V2O5. J. Mater. Chem. A 2014, 2, 5809–5815. [Google Scholar] [CrossRef]
- Stein, A.; Wilson, B.E.; Rudisill, S.G. Design and functionality of colloidal-crystal-templated materials—Chemical applications of inverse opals. Chem. Soc. Rev. 2013, 42, 2763–2803. [Google Scholar] [CrossRef]
- Li, H.; Theriault, J.; Rousselle, B.; Subramanian, B.; Robichaud, J.; Djaoued, Y. Facile fabrication of crack-free large-area 2D WO3 inverse opal films by a ‘dynamic hard-template’ strategy on ITO substrates. Chem. Commun. 2014, 50, 2184–2186. [Google Scholar] [CrossRef]
- Li, H.; Vienneau, G.; Jones, M.; Subramanian, B.; Robichaud, J.; Djaoued, Y. Crack-free 2D-inverse opal anatase TiO2 films on rigid and flexible transparent conducting substrates: Low temperature large area fabrication and electrochromic properties. J. Mater. Chem. C 2014, 2, 7804–7810. [Google Scholar] [CrossRef]
- Li, H.; Djaoued, H.; Robichaud, J.; Djaoued, Y. A pleasant blue-green colored 2D Vanadium dioxide inverse opal monolayer: Large area fabrication and its thermochromic application. J. Mater. Chem. C 2020, 8, 11572–11580. [Google Scholar] [CrossRef]
- Li, H.; Wu, H.; Xiao, J.; Su, Y.; Robichaud, J.; Bruning, R.; Djaoued, Y. A hierarchically porous anatase TiO2 coated-WO3 2D IO bilayer film and its photochromic properties. Chem. Commun. 2016, 52, 892–895. [Google Scholar] [CrossRef]
- Li, H.; Robichaud, J.; Djaoued, Y. A simple way to fabricate pure anatase 2D TiO2 IO monolayer: Structure, color control and its application in electrochromism. RSC Adv. 2021, 11, 8065–8072. [Google Scholar] [CrossRef]
- Baddour-Hadjean, R.; Smirnov, M.B.; Smirnov, K.S.; Kazimirov, V.Y.; Gallardo-Amores, J.M.; Amador, M.E.U.; Arroyo-de Dompablo, J.P. Pereira-Ramos, Lattice Dynamics of β-V2O5: Raman Spectroscopic Insight into the Atomistic Structure of a High-Pressure Vanadium Pentoxide Polymorph. Inorg. Chem. 2012, 51, 3194–3201. [Google Scholar] [CrossRef] [PubMed]
- Urena-Begara, F.; Crunteanub, A.; Raskin, J.-P. Raman and XPS characterization of vanadium oxide thin films with temperature. Appl. Surf. Sci. 2017, 403, 717–727. [Google Scholar] [CrossRef]
- Sahana, M.B.; Sudakar, C.; Thapa, C.; Naik, V.M.; Auner, G.W.; Naik, R.; Padmanabhan, K.R. The effect of titanium on the lithium intercalation capacity of V2O5 thin films. Thin Solid Film 2009, 517, 6642–6651. [Google Scholar] [CrossRef]
- Wang, Y.; Takahashi, K.; Lee, K.; Cao, G.Z. Nanostructured Vanadium Oxide Electrodes for Enhanced Lithium-Ion Intercalation. Adv. Funct. Mater. 2006, 16, 1133–1144. [Google Scholar] [CrossRef]
- Chu, S.; Zhou, L.; Wang, Z. A study of thermal decomposition of ammonium metavanadate. Eng. Chem. Metall. 1991, 12, 69–70. (In Chinese) [Google Scholar]
- Goodenough, J.B. Interpretation of MxV2O5-β and MxV2−yTyO5-β phases. J. Solid State Chem. 1970, 1, 349–358. [Google Scholar] [CrossRef]
- Wen, T.; Lu, Z.; Xu, Z. Phase relationship and electrical conductivity of low valent vanadium-strontium oxide system. J. Inorg. Mater. 1994, 9, 493–496. (In Chinese) [Google Scholar]
- Lu, X.L. Photovoltaic Effect and Application of Vanadium Oxide. Master’s Thesis, University of Electronic Science and Technology of China, Chengdu, China, 2011. (In Chinese). [Google Scholar]
- Zhao, X.K. The factors affecting absorption of IR spectrum. Inn. Mong. Petrochem. Ind. 2007, 12, 179–181. (In Chinese) [Google Scholar]
- Talledo, A.; Granqvist, C.G. Electrochromic vanadium–pentoxide–based films: Structural, electrochemical, and optical properties. J. Appl. Phys. 1995, 77, 4655–4666. [Google Scholar] [CrossRef]


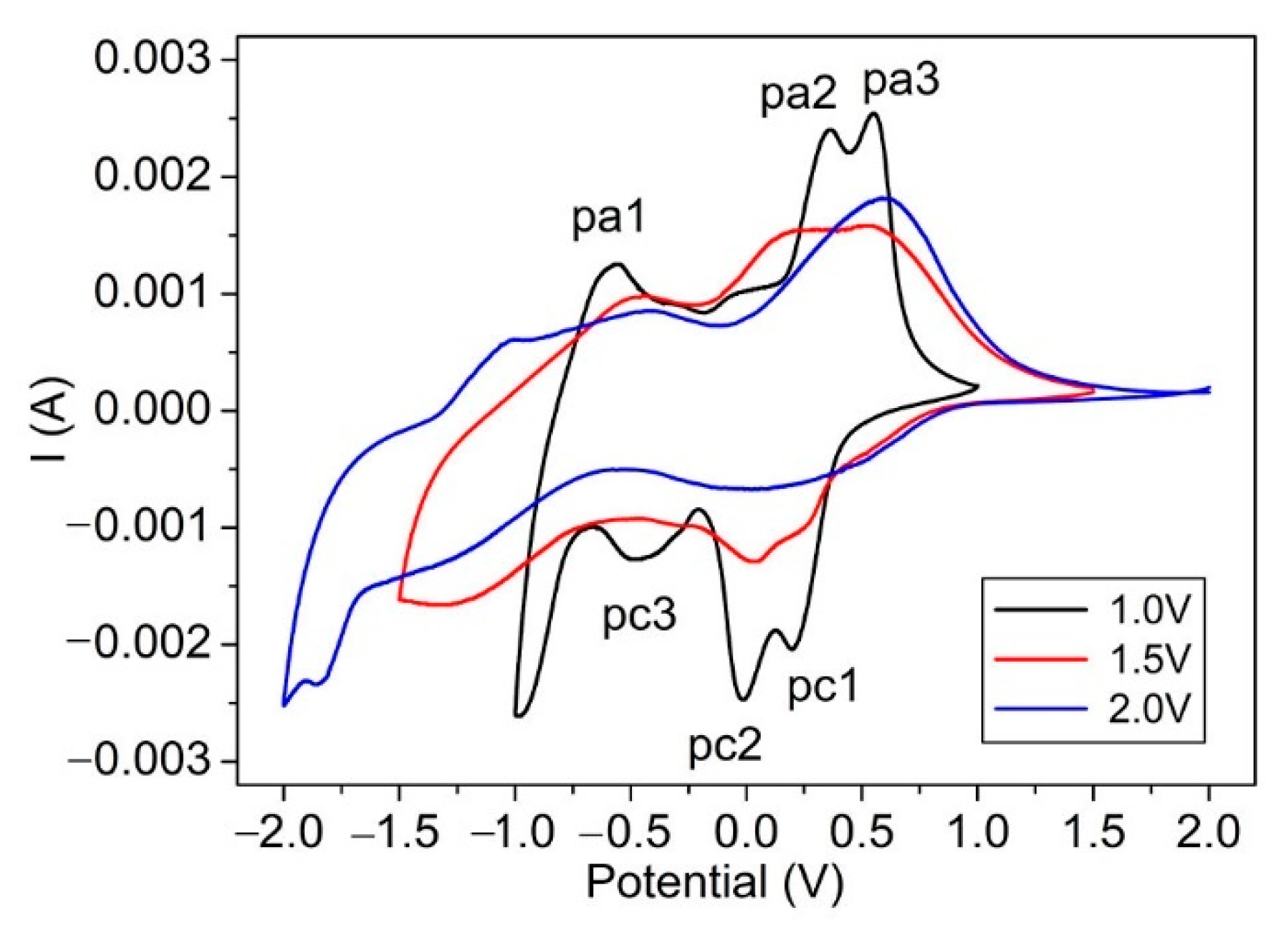

| Voltage (V) | Biggest Contrast | |||
|---|---|---|---|---|
| Wavelength (nm) | ||||
| 1.0 | 495 | −8 | −6.9 | 13.4 |
| 1.5 | 487 | 12.1 | 8.9 | −14.3 |
| 2.0 | 442.5 | 31 | 30.8 | −18.6 |
| Applied Potential (V) | Eg (eV) | Applied Potential (V) | Eg (eV) |
|---|---|---|---|
| 0 | 1.68 | 0 | 1.68 |
| −1.0 | 2.0 | 1.0 | 1.93 |
| −1.5 | 1.86 | 1.5 | 2.15 |
| −2.0 | 1.70 | 2.0 | 2.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Tang, Z.; Liu, Y.; Robichaud, J.; Liang, J.; Jiang, W.; Djaoued, Y. Two-Dimensional V2O5 Inverse Opal: Fabrication and Electrochromic Application. Materials 2022, 15, 2904. https://doi.org/10.3390/ma15082904
Li H, Tang Z, Liu Y, Robichaud J, Liang J, Jiang W, Djaoued Y. Two-Dimensional V2O5 Inverse Opal: Fabrication and Electrochromic Application. Materials. 2022; 15(8):2904. https://doi.org/10.3390/ma15082904
Chicago/Turabian StyleLi, Hua, Zijuan Tang, Yuwei Liu, Jacques Robichaud, Jian Liang, Weihui Jiang, and Yahia Djaoued. 2022. "Two-Dimensional V2O5 Inverse Opal: Fabrication and Electrochromic Application" Materials 15, no. 8: 2904. https://doi.org/10.3390/ma15082904
APA StyleLi, H., Tang, Z., Liu, Y., Robichaud, J., Liang, J., Jiang, W., & Djaoued, Y. (2022). Two-Dimensional V2O5 Inverse Opal: Fabrication and Electrochromic Application. Materials, 15(8), 2904. https://doi.org/10.3390/ma15082904






