The Role of Intermolecular Interaction on Aggregation-Induced Emission Phenomenon and OLED Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods
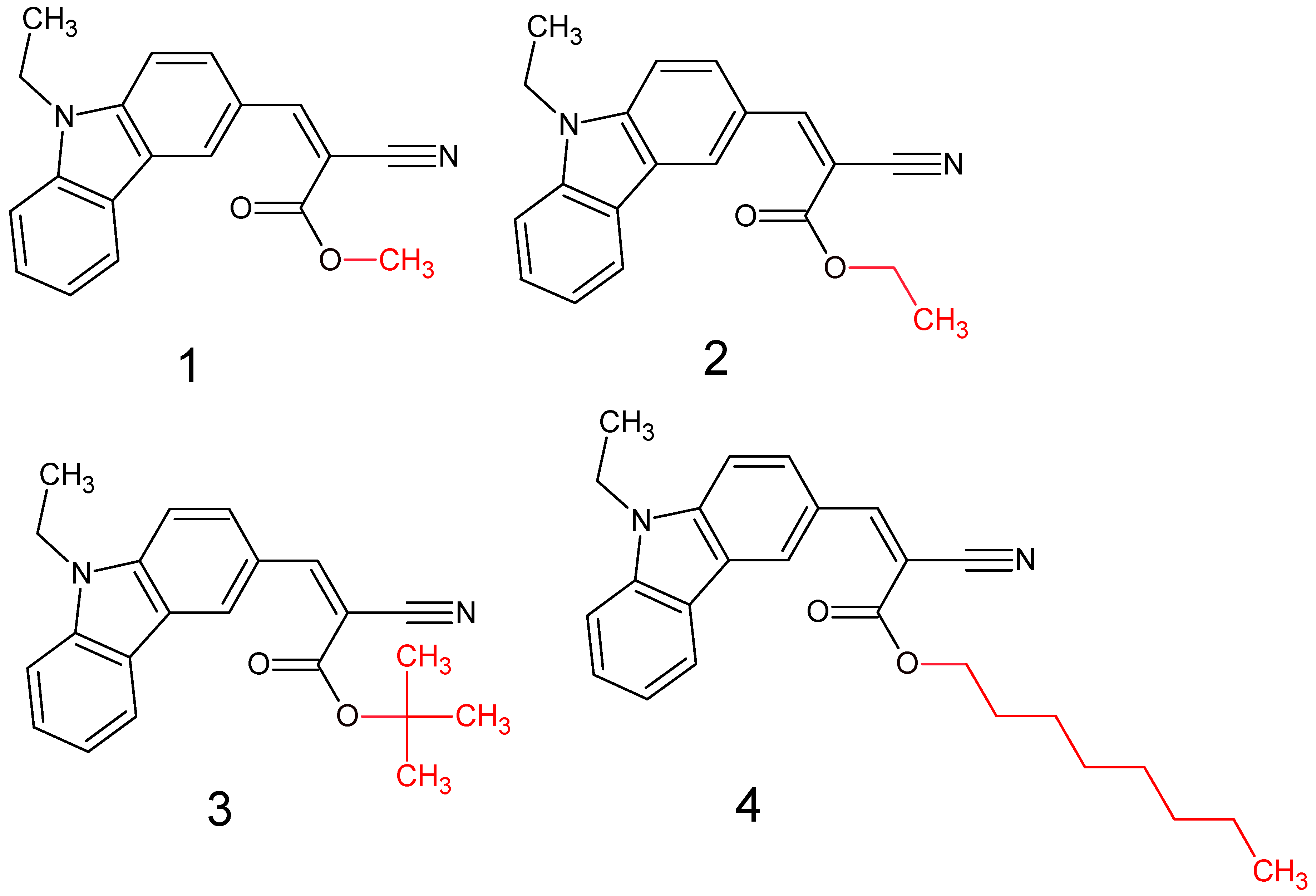
2.2. Synthesis and Characterization
2.2.1. Typical Procedure for the 1–4 Synthesis
2.2.2. Synthesis of 1
2.2.3. Synthesis of 2
2.2.4. Synthesis of 3
2.2.5. Synthesis of 4
3. Results and Discussion
3.1. DFT Calculations
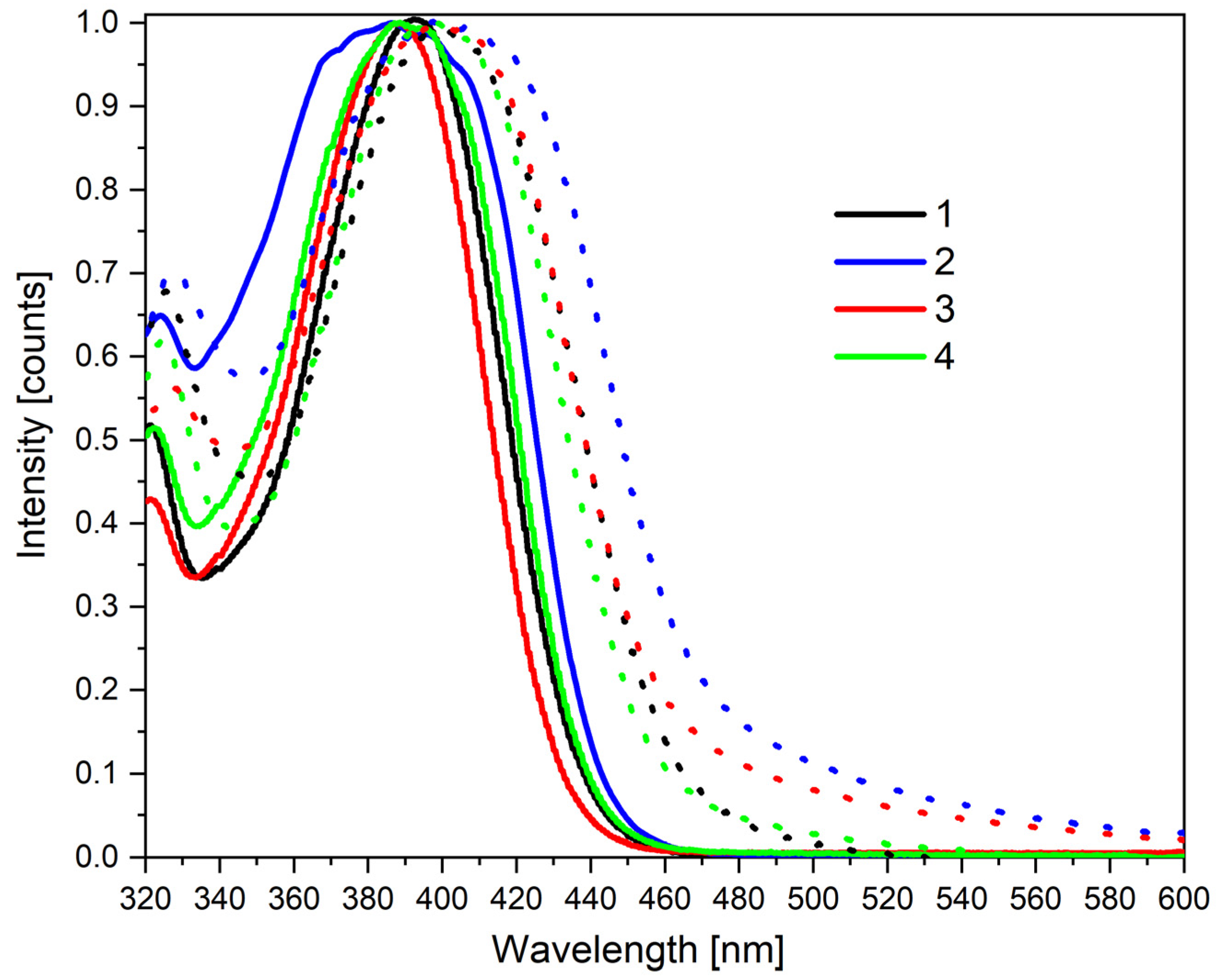
3.2. Photophysical Characterization
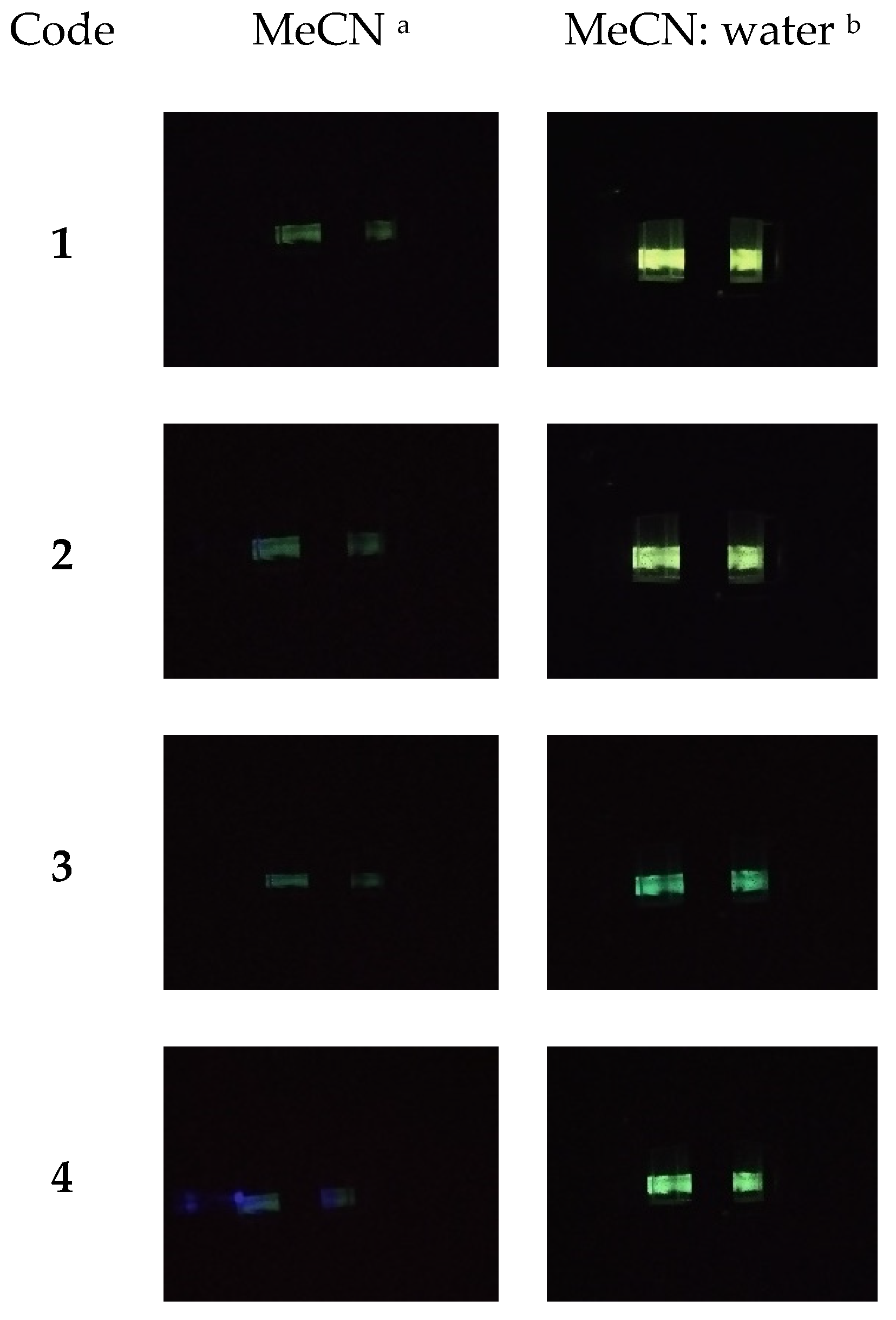
3.3. Aggregation-Induced Emission Investigations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bujak, P.; Kulszewicz-Bajer, I.; Zagorska, M.; Maurel, V.; Wielgus, I.; Pron, A. Polymers for electronics and spintronics. Chem. Soc. Rev. 2013, 42, 8895–8999. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.J.; Han, N.S.; Song, J.K.; Park, S.-R.; Jeon, Y.M.; Suh, M.C. Efficient deep blue fluorescent emitter showing high external quantum efficiency. Dyes. Pigm. 2015, 120, 200–207. [Google Scholar] [CrossRef]
- Deng, L.; Li, J.; Wang, G.-X.; Wu, L.-Z. Simple bipolar host materials incorporating CN group for highly efficient blue electrophosphorescence with slow efficiency roll-off. J. Mater. Chem. C. 2013, 1, 8140–8145. [Google Scholar] [CrossRef]
- D’Andrade, B.W.; Forrest, S.R. White Organic Light-Emitting Devices for Solid-State Lighting. Adv. Mater. 2004, 16, 1585–1595. [Google Scholar] [CrossRef]
- Fresta, E.; Costa, R.D. Beyond traditional light-emitting electrochemical cells—A review of new device designs and emitters. J. Mater. Chem. C. 2017, 5, 5643–5675. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 4332–4353. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Berti, L.; Medintz, I.L. Materials for fluorescence resonance energy transfer analysis: Beyond traditional donor-acceptor combinations. Angew. Chem. Int. Ed. 2006, 45, 4562–4589. [Google Scholar] [CrossRef]
- Borisov, S.M.; Wolfbeis, O.S. Optical Biosensors. Chem. Rev. 2008, 108, 423–461. [Google Scholar] [CrossRef]
- Domaille, D.W.; Que, E.L.; Chang, C.J. Synthetic fluorescent sensors for studying the cell biology of metals. Nat. Chem. Biol. 2008, 4, 168–175. [Google Scholar] [CrossRef]
- Lim, M.H.; Lippard, S.J. Metal-Based Turn-On Fluorescent Probes for Sensing Nitric Oxide. Acc. Chem. Res. 2007, 40, 41–51. [Google Scholar] [CrossRef]
- Giepmans, B.N.G.; Adams, S.R.; Ellisman, M.H.; Tsien, R.Y. The Fluorescent Toolbox for Assessing Protein Location and Function. Science 2006, 312, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.W.H.; Holmes, A.B. Poly(dibenzosilole)s. Adv. Polym. Sci. 2008, 212, 85–98. [Google Scholar] [CrossRef]
- Boudreault, P.L.T.; Blouin, N.; Leclerc, M. Poly(2,7-carbazole)s and Related Polymers. Adv. Polym. Sci. 2008, 212, 99–124. [Google Scholar] [CrossRef]
- Hoeben, F.J.M.; Jonkheijm, P.; Meijer, E.W.; Schenning AP, H.J. About Supramolecular Assemblies of π-Conjugated Systems. Chem. Rev. 2005, 105, 1491–1546. [Google Scholar] [CrossRef] [PubMed]
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Marks, R.N.; Mackay, K.; Friend, R.H.; Burns, P.L.; Holmes, A.B. Light-Emitting Diodes Based on Conjugated Polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Kulkarni, A.P.; Tonzola, C.J.; Babel, A.; Jenekhe, S.A. Electron Transport Materials for Organic Light-Emitting Diodes. Chem. Mater. 2004, 16, 4556–4573. [Google Scholar] [CrossRef]
- Cheruku, S.; D’Olislaeger, L.; Smisdom, N.; Smits, J.; Vanderzande, D.; Maes, W.; Ameloot, M.; Ethirajan, A. Fluorescent PCDTBT Nanoparticles with Tunable Size for Versatile Bioimaging. Materials 2019, 12, 2497. [Google Scholar] [CrossRef]
- Zhou, Z.; Xie, S.; Chen, X.; Tu, Y.; Xiang, J.; Wang, J.; He, Z.; Zeng, Z.; Tang, B.Z. Spiro-Functionalized Diphenylethenes: Suppression of a Reversible Photocyclization Contributes to the Aggregation-Induced Emission Effect. J. Am. Chem. Soc. 2019, 141, 9803–9807. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef]
- Tang, B.Z.; Zhan, X.; Yu, G.; Lee, P.P.S.; Liu, Y.; Zhu, D. Efficient blue emission from siloles. J. Mater. Chem. 2001, 11, 2974–2978. [Google Scholar] [CrossRef]
- Gupta, V.D.; Padalkar, V.S.; Phatangare, K.R.; Patil, V.S.; Umape, P.G.; Sekar, N. The synthesis and photophysical properties of extended styryl fluorescent derivatives of N-ethyl carbazole. Dyes Pigm. 2011, 88, 378–384. [Google Scholar] [CrossRef]
- Telore, R.D.; Satam, M.A.; Sekar, N. Push–pull fluorophores with viscosity dependent and aggregation induced emissions insensitive to polarity. Dyes Pigm. 2015, 122, 359–367. [Google Scholar] [CrossRef]
- Ramkumar, S.; Manoharan, S.; Anandan, S. Synthesis of D-(π-A)2organic chromophores for dye-sensitized solar cells. Dyes Pigm. 2012, 94, 503–511. [Google Scholar] [CrossRef]
- Fitzner, R.; Reinold, E.; Mishra, A.; Mena-Osteritz, E.; Ziehlke, H.; Korner, C. Dicyanovinyl-substituted oligothiophenes: Structure-property relationships and application in vacuum-processed small molecule organic solar cells. Adv. Funct. Mater. 2011, 21, 897–910. [Google Scholar] [CrossRef]
- Torres, E.; Berberan-Santos, M.N.; Brites, M.J. Synthesis, photophysical and electrochemical properties of perylene dyes. Dyes Pigm. 2015, 112, 298–304. [Google Scholar] [CrossRef]
- Li, X.; Kim, S.-H.; Son, Y.-A. Optical properties of donor-π-(acceptor)n merocyanine dyes with dicyanovinylindane as acceptor group and triphenylamine as donor unit. Dyes Pigm. 2009, 82, 293–298. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, S.-Y.; Gwon, S.-Y.; Son, Y.-A.; Bae, J.-S. D–π–A solvatochromic charge transfer dyes containing a 2-cyanomethylene-3-cyano-4,5,5-trimethyl-2,5-dihydrofuran acceptor. Dyes Pigm. 2010, 84, 169–175. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Li, G.; Xiao, H.; Lei, Y.; Huang, X. Aggregation-Induced Fluorescence Emission Properties of Dicyanomethylene-1,4-dihydropyridine Derivatives. J. Phys. Chem. C. 2015, 119, 6737–6748. [Google Scholar] [CrossRef]
- Szłapa, A.; Kula, S.; Błaszkiewicz, U.; Grucela, M.; Schab-Balcerzak, E.; Filapek, M. Simple donor–π–acceptor derivatives exhibiting aggregation-induced emission characteristics for use as emitting layer in OLED. Dyes Pigm. 2016, 129, 80–89. [Google Scholar] [CrossRef]
- Fei, N.; Wei, Q.; Cao, L.; Bai, Y.; Ji, H.; Peng, R.; Huang, L.; Shiyou, H.; Ge, Z. A symmetric nonpolar blue AIEgen as nondoped fluorescent OLED emitter with low efficiency roll-off. Org. Electron. 2020, 78, 105574. [Google Scholar] [CrossRef]
- Asiri, A.M.; Khan, S.A.; Al-Amoudi, M.S.; Alam, K.A. Synthesis, characterization, absorbance, fluorescence and non linear optical properties of some donor acceptor chromophores. Bull. Korean Chem. Soc. 2012, 33, 1900–1906. [Google Scholar] [CrossRef]
- Fujiwara, T.; Nakatsu, K. Cyanovinylheteroaromatics for Organic Nonlinear Optics. Mol. Cryst. Liq. Cryst. 1990, 182, 71–79. [Google Scholar] [CrossRef]
- Wada, T.; Zhang, Y.; Yamakado, M.; Sasabe, H. Linear and Nonlinear Optical Properties of Carbazole-Containing Polymers. Mol. Cryst. Liq. Cryst. 1993, 227, 85–92. [Google Scholar] [CrossRef]
- Wada, T.; Zhang, Y.; Wang, L.; Sasabe, H. Novel Molecules for Photorefractive Application. Mol. Cryst. Liq. Cryst. 1996, 280, 71–78. [Google Scholar] [CrossRef]
- Kruciate, G.; Grigalevicius, S. 2,7(3,6)-Diaryl(arylamino)-substituted Carbazole as Components of OLEDs: A Review of the Last Decade. Materials 2021, 14, 6754. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Yu, P.; Xiao, Y. Non-Doped Deep-Blue OLEDs Based on Carbazole-π-Imidazole Derivatives. Materials 2021, 14, 2349. [Google Scholar] [CrossRef]






| CODE | HOMO | LUMO | BOND LENGTHS |
|---|---|---|---|
| 1 | 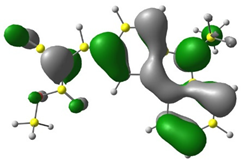 |  |  |
| E = −6.03 [eV] | E = −2.66 [eV] | ||
| 2 |  |  |  |
| E = −6.03 [eV] | E = −2.64 [eV] | ||
| 3 | 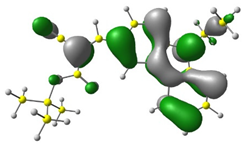 | 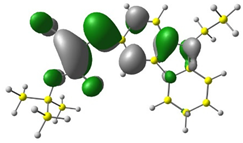 |  |
| E = −6.00 [eV] | E = −2.59 [eV] | ||
| 4 | 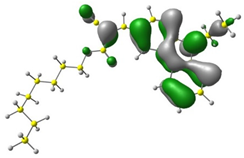 |  |  |
| E = −6.02 [eV] | E = −2.64 [eV] |
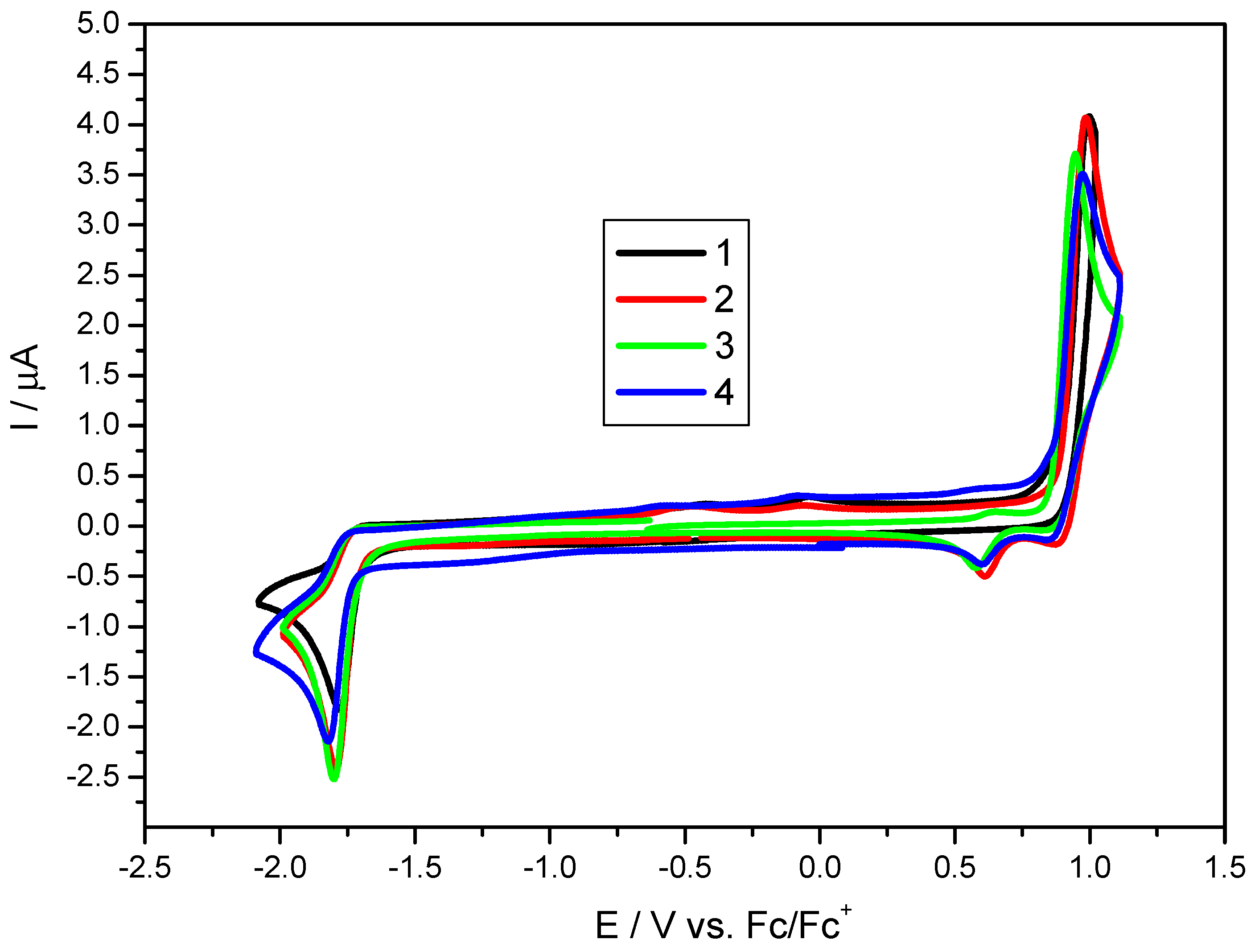
| Code | Eox [V] | Ered [V] | IP (CV) a [eV] | EA (CV) a [eV] | Eg (CV) a [eV] | Eg (Opt) b [eV] | IP (DFT) c [eV] | EA (DFT) c [eV] | Eg (DFT) c [eV] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.89 | −1.71 | −5.99 | −3.39 | 2.6 | 2.83 | −5.84 | −3.00 | 2.84 |
| 2 | 0.88 | −1.72 | −5.98 | −3.38 | 2.6 | 2.8 | −5.82 | −3.03 | 2.80 |
| 3 | 0.86 | −1.72 | −5.96 | −3.38 | 2.58 | 2.87 | −5.80 | −2.98 | 2.82 |
| 4 | 0.87 | −1.74 | −5.97 | −3.36 | 2.61 | 2.82 | −5.84 | −3.02 | 2.81 |
| Code | UV-Vis Absorption λmax [nm] | PL (Photoluminescence) λem [nm] | ||||
|---|---|---|---|---|---|---|
| MeCN a | MeCN b | MeCN: Water c | MeCN a (Φf) | MeCN b (Φf) | MeCN: Water c (Φf) | |
| M1 | 304; 364; 417 | 266; 303; 343; 438 | 270; 340; 380; 437 | 495 (3%) | 504 (3%) | 510 (89%) |
| M2 | 267; 275; 304; 364; 410 | 260; 300; 337; 445 | 234; 301; 365 | 495 (3%) | 505 (3%) | 504 (78%) |
| M3 | 272; 304; 344; 429 | 267; 304; 324; 447 | 210; 376; 410 | 506 (3%) | 500 (3%) | 515 (9%) |
| M4 | 268; 299; 350; 423 | 260; 297; 330; 439 | 273; 305; 359 | 500 (3%) | 505 (3%) | 515 (14%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipek, P.; Karoń, K.; Hellwig, H.; Szłapa-Kula, A.; Filapek, M. The Role of Intermolecular Interaction on Aggregation-Induced Emission Phenomenon and OLED Performance. Materials 2022, 15, 8525. https://doi.org/10.3390/ma15238525
Filipek P, Karoń K, Hellwig H, Szłapa-Kula A, Filapek M. The Role of Intermolecular Interaction on Aggregation-Induced Emission Phenomenon and OLED Performance. Materials. 2022; 15(23):8525. https://doi.org/10.3390/ma15238525
Chicago/Turabian StyleFilipek, Patrycja, Krzysztof Karoń, Hubert Hellwig, Agata Szłapa-Kula, and Michał Filapek. 2022. "The Role of Intermolecular Interaction on Aggregation-Induced Emission Phenomenon and OLED Performance" Materials 15, no. 23: 8525. https://doi.org/10.3390/ma15238525
APA StyleFilipek, P., Karoń, K., Hellwig, H., Szłapa-Kula, A., & Filapek, M. (2022). The Role of Intermolecular Interaction on Aggregation-Induced Emission Phenomenon and OLED Performance. Materials, 15(23), 8525. https://doi.org/10.3390/ma15238525






