Abstract
Metal-organic frameworks (MOFs) are considered to be the most promising positive anode materials to store charge for electrochromic devices. Nevertheless, a detailed mechanism of the electrochemical and ions storage process has not yet been revealed. Herein, the electrochemical mechanism of the highly porous ZIF-67 films and the electrochromic performance of electrochromic mirrors constructed from ZIF-67 and WO3 electrodes were investigated. The mechanism of the charge storage was revealed in the kinetic analysis of the Li-ion behavior based on the cyclic voltammetry curves and electrochemical impedance spectra. Impressively, the electrochromic mirrors with the self-bleaching effect and self-discharge behavior showed a unique electrochromic performance, such as a high coloration efficiency of 16.47 cm2 C−1 and a maximum reflectance modulation of 30.10% at 650 nm. This work provides a fundamental understanding of MOFs for applications in electrochromic devices and can also promote the exploration of novel electrode materials for high-performance reflective electrochromic devices.
1. Introduction
Electrochromic devices (ECDs) have received widespread attention due to their wide range of applications, including antiglare rearview mirrors [1], smart windows [2,3] and information displays [4,5]. A typical electrochromic (EC) device generally consists of five superimposed layers, an ion electrolyte layer sandwiched between an EC layer and an ion storage layer (counter electrode) that are individually deposited on transparent conductive electrodes [6]. For instance, tungsten oxide (WO3), one of the most typical EC layers, which changes its optical properties reversibly (absorbance/transmittance/reflectance) via redox reactions under an alternating potential or current modulation [7], has been considered as the most promising cathodic electrochromic candidates for EC applications owing to its excellent EC properties, such as high cyclic stability and coloration efficiency [8,9]. In view of the requirement of charge matching and redox balance, another complementary anodic EC ion storage layer based on WO3 is commonly used as an indispensable component in ECDs [10]. Conventionally, a complementary electrochromic device is composed by assembling both WO3 and ion storage layers, such as NiO [11], CeO2 [12] and Prussian blue (iron ferrocyanide) analogues [13], which color under the insertion or extraction of small ions, respectively. Developing ion storage layer materials is an interesting scientific challenge with promising applications in electrochromic devices. Very recently, many researchers have focused their attention on novel multifunctional MOFs [14], supramolecular assemblies formed by the reaction of metal nodes with organic linkers due to the unique features, such as high specific surface area, large pore size and tunable channels for ionic diffusion [15,16,17]. An interesting feature of MOFs is their ability to allow the rapid insertion/extraction of ions and, consequently, offer the possibility to improve their electrochemical performance [18,19]. For instance, a 2-dimensional layered nickel-based MOF has a high specific capacity of 320 mA h g−1 for the storage of Li+ [20]. Highly uniform graphene shell coated 1D NiCo2O4 electrodes, prepared by adjusting the reaction component of ZIF-67, have been reported to enjoy a superior ion storage capacity and cycling stability [21]. Li Z. et al. have demonstrated that the thin-layer structural ZIF-67 electrodes, prepared using a drop-casting method, exhibit a high reversible capacity of 311.6 mA h g−1 and a good cycling stability [22]. Therefore, it is worth exploring the promising ZIF-67 anode materials as an ion storage layer to satisfy the future applications of high performance WO3-based ECDs.
Herein, a novel electrochromic mirror based on ZIF-67 MOFs and WO3 films was constructed. The investigation into the configuration and working mechanism of the electrochromic devices on a basis of Li+-containing electrolyte was performed. A schematic diagram of the mirror mode operation principle is depicted in Figure 1. The obtained reflection-type ECDs with a self-bleaching effect possess a high coloration efficiency of 16.47 cm2 C−1.
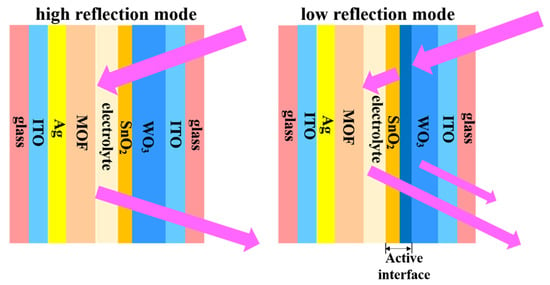
Figure 1.
Schematic illustration of the optical modulation mechanism of the WO3//ZIF-67 ECD.
2. Materials and Methods
2.1. Materials
All of the reagents used were of an analytical grade. Cobalt nitrate hexahydrate (Co(NO3)2·6H2O) and absolute ethyl alcohol (ethanol, analytical grade) were purchased from Aladdin Chemical (Shanghai, China) and Sinopharm Co. Ltd. (Beijing, China), respectively. The conducting transparent indium tin oxide (ITO)-coated glasses were used as the substrate of the WO3 and Ag thin films deposited using the electron beam evaporation technology.
2.2. Synthesis
2.2.1. Preparation of the MOF/Ag films
The approximately 150-nanometer-thick Ag thin films were deposited using the electron beam evaporation technique (MUE-ECO made in ULVAC, Chigasaki, Japan) at a deposition rate of about 0.10 nm/s and a background pressure of less than 2.00 × 10−3 Pa. Subsequently, the MOF films were electrochemically deposited onto the ITO substrates with Ag thin films from a solution containing 40 mL of ethanol, 0.8 M of 2-methylimidazole and 0.1 M of Co(NO3)2·6H2O in a conventional three electrode cell. The Pt sheet, ITO substrate with Ag thin films and SCE were used respectively as counter, working and reference electrodes. The MOF films were obtained by applying a constant voltage of −5 V to the ITO substrates for 10 mins. The electrodeposited films were cleaned using ethanol and deionized water. Finally, the films were dried at 60 °C under air atmosphere for 6 h.
2.2.2. Preparation of the WO3 thin Films with SnO2 Interface
The WO3 thin films were deposited onto the ITO-coated glass with a deposition rate of 0.10 nm/s and a thickness of about 450 nm, at a substrate temperature of 200 °C in a vacuum of less than 2.0 × 10−3 Pa, using an electron beam evaporation technique (MUE-ECO made in ULVAC, Chigasaki, Japan). Subsequently, the SnO2 ultra-thin interfacial layers (thickness: ~5 nm) were grown in situ on the WO3 thin films using the same technique.
2.2.3. Assembly of the Mirrors
The electrochromic mirrors, with an ITO/WO3/SnO2/electrolyte/ZIF-67/Ag/ITO configuration, were assembled by filling an 0.10 M PC-LiClO4 electrolyte into the SnO2/WO3 thin film and the MOF/Ag film in the vacuum, as reported previously [23].
2.3. Measurements
The structure and morphology of the MOF/Ag films were determined using an X-ray diffraction (XRD, D8 Advance, Bruker, Germany) with Cu-Kα radiation (λ = 0.154178 nm), a thermal field-emission scanning electron microscopy (TFESEM, Sirion200, FEI, Hillsboro, OR, USA) and an X-ray photoelectron spectra (XPS) (AXIS UTLTRA DLD, Kratos, Manchester, England). Fourier-transform infrared (FTIR) transmission spectra were obtained using a micro-FTIR spectroscopy (μ-FTIR, Cary660+620, agilent, Santa Clara, CA, USA). In situ optical reflectance spectra were obtained using UV–VIS–IR spectroscopy (Lambda 950, Perkin-Elmer, Waltham, MA, USA) and an electrochemical workstation (CHI660D, Chen hua, Shanghai, China). The cyclic voltammetry measurements were conducted by applying a voltage between 0.00 V and +0.60 V to MOF/Ag films in a three-electrode cell, which employs a platinum sheet, KCl-saturated Hg/HgCl2 and 0.10 M LiClO4-PC electrolyte as a counter electrode, a reference electrode and an electrolyte, respectively. The electrochemical impedance spectra were carried out using an electrochemical workstation (Zennium, IM6) in a frequency range of 10−1–105 Hz. The OCV and cyclic voltammetry measurements of the ECDs were carried out in a two-electrode cell.
3. Results and Discussion
3.1. Characterization of Films
Figure 2a shows XRD patterns of the ZIF-67 powder, ZIF-67/Ag/ITO, Ag/ITO and bared ITO. As can be seen, the peaks located at 31°, 37° and 51° are assigned to In2O3, indium and tin [24]. For the Ag/ITO, several characteristic peaks at 38°, 44° and 64.5° can be observed, corresponding respectively to (111), (200) and (220) planes [25]. This reveals that a single phase with a cubic geometry and space group Fm-3m (225) for Ag is formed. The peaks at 7.40°, 10.35° and 18.03° are respectively indexed to the crystalline ZIF-67 phase (011), (002) and (222) [26], which indicates that the electrodeposited ZIF-67 films enjoy a crystalline characteristic. The average size of the ZIF-67 film is calculated to be about 26.31 nm from the XRD data using Scherrer’s formula. Besides, the two diffraction peaks at 21.01° (114) and 25.32° (233) found in the powder sample are absent in the film sample. The highly porous morphology can be clearly observed in the SEM image in Figure 2b, which is conducive to the insertion of ions. In addition, the elemental compositions of ZIF-67 films can be determined using EDS analysis, as shown in the inset of Figure 2b. Figure 2c shows the FTIR spectra of the ZIF-67 films and powder. In Figure 2c, the absorption peak at 1575 cm−1 is assigned to the C=N band. There is a strong stretching band of O-H at about 3200 cm−1. In addition, blending vibrations of alkane C-H are observed at 1450 cm−1. The peak positions of the film are corresponded basically with that of the powder and are consistent with a previous report [27], which confirms that the film is composed of the ZIF-67 MOFs. In order to further demonstrate the composition details of the resulting samples, an XPS was performed. The high-resolution XPS spectra of Co 2p is shown in Figure 2d. The two major peaks with binding energies of 780.61 eV and 796.17 eV are respectively assigned to Co 2p3/2 and Co 2p1/2, which correspond to the intrinsic peaks for ZIF-67. The other two indistinctive peaks at 785.00 and 801.41 eV are satellite peaks of Co [28]. The results show that the two valence states (Co2+ and Co+) coexist, similar to a previous report [29].
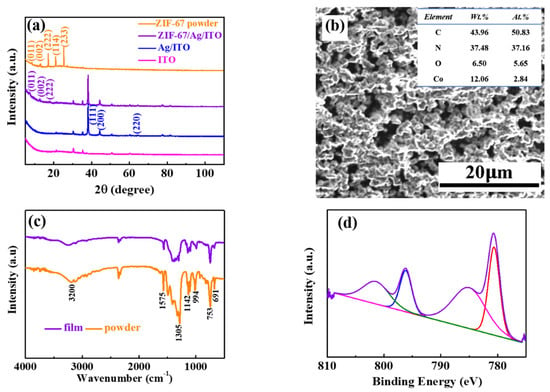
Figure 2.
(a) XRD patterns of ZIF-67 powder, ZIF-67 films on an ITO substrate with a Ag film, Ag thin films on an ITO substrate and bared ITO. (b) SEM image of the ZIF-67 films on an ITO substrate with a Ag film. (c) FTIR spectra of ZIF-67 films and powder. (d) XPS spectra of Co 2p at the initial state.
3.2. Electrochemical Properties of the ZIF-67/Ag Films
The cyclic voltammetry curves of ZIF-67/Ag electrodes at different scan rates, from 5 to 10 mV/s between 0.00 and +0.60 V, are shown in Figure 3a. It can be observed that the peak value of the current density grows accordingly with the increased scan rate. There is no obvious difference for the peak current density between 5 and 6 mV/s, probably due to the saturation of the ion diffusion. It is worth noting that a small shift in the reduction peak with an increasing scan rate can be observed, which indicates the excellent stability of the reaction on the electrode active surface, consistent with a previous report [30]. Moreover, the faradaic and capacitive-controlled processes are identified by the ‘b’ value, which is calculated using the following equation [31]:
where i and v represent, respectively, the peak current and the scan rate. Both ‘a’ and ‘b’ are the adjustable parameters. It is noteworthy that the ‘b’ value determined from the slope of log(i) versus log(v) is close to 0.5 and 1.0, indicating that the current response belongs predominantly to the diffusion controlled and the capacitive processes [32], respectively. As shown in Figure 3b, the ‘b’ value of the ZIF-67/Ag films is calculated to be 0.59 (between 0.5 and 1), indicating that the charge storage is composed of diffusive charges and capacitive charges. Besides, in order to further understand the charge transfer and ion diffusion processes of the ZIF-67/Ag films, electrochemical impedance spectra tests were performed. Figure 3c displays the Nyquist plots measured from the ZIF-67/Ag films with +0.60 V and 0.00 V applied the Li+ electrolyte in the frequency region (100 mHz to 100 kHz). The impedance spectra can be evaluated using the equivalent electrical circuit given in the inset of Figure 3c. The resistance (Rs) of the electrolyte, the interfacial charge-transfer resistance (Rct) and W correspond to the intercept of the real axis in the high frequency region, the diameter of the semicircle and the semi-infinite Warburg element, respectively [33]. The fitted data (Table 1) reveals that the Rct of the ZIF-67/Ag films is respectively estimated to be approximately 82.81 Ω cm−2 and 6.75 Ω cm−2 when 0.00 V and +0.60 V are applied, indicating that the ZIF-67/Ag films at +0.60 V possess a higher conductivity, as stated in a previous report [34]. In addition, the intercalation/extraction capacity of electrodes is a reciprocal function of the absolute value of the imagine resistance at a low frequency [35]. Thus, it can be seen that the extraction of ions is prominent at 0.6 V. Moreover, the relation between the frequency and the phase angle is shown in Figure 3d. In the lower-frequency-limitation region, the phase angle (−62.12°) of +0.60 V is significantly higher than that of 0.00 V (−19.96°), indicating a faster ion diffusion in the electrolyte, in agreement with a previous report [36].
i = avb
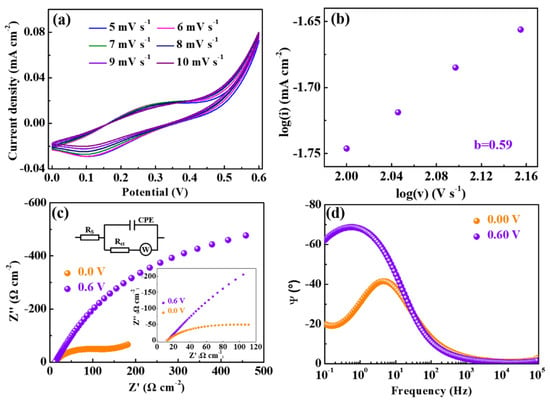
Figure 3.
(a) CV profiles at various scan rates, (b) the power law dependence of the peak current versus the scan rate, (c) Nyquist plots and (d) the impedance phase angle versus the frequency for the ZIF-67/Ag films in 0.10 M PC-LiClO4.

Table 1.
A summary of the Nyquist measurements for the ZIF-67/Ag films in 0.10 M of PC-LiClO4.
3.3. Electrochromic Performance of the WO3//ZIF-67 Mirrors
Figure 4a displays the in situ reflectance spectra for the ECDs at 650 nm, obtained by alternately applying a −1.00 V and 0.00 V voltage both for 40 s and 100 s, respectively. The maximum reflectance modulation (△R, coloration/bleaching at 650 nm) is estimated to be 30.10%. The coloring and bleaching response time (defined as the time required to reach 90% of the reflectance change) is calculated to be 33 s and 30 s, respectively. The lower bleaching time can be attributed to a reduction in the interfacial charge-transfer resistance, as analyzed using the impedance spectra. It is worth noting that no significant change in the regulation of reflectivity of the reflection-type electrochromic devices after nine cycles is observed, indicating a relatively stable optical modulation. In Figure 4b, the devices need 100 s and 300 s, respectively, to reach the peak reflectivity, when a voltage of 0.0 V is applied for 100 s and removed after a voltage of −1.0 V is applied for 40 s. These results may be due to the strong adsorption of ions by the MOF [37], which leads to the spontaneous deintercalation of Li ions at the interface of the WO3/electrolyte, resulting in a corresponding self-bleaching effect. Interestingly, the WO3//ZIF-67 mirrors without ZIF-67 seem to maintain the initial low reflection mode more easily after the voltage is removed, in comparison to the WO3//ZIF-67 mirrors with ZIF-67, as shown in the in situ reflectance spectra in Figure 4c. This result further confirms that the self-bleaching effect is caused by ZIF-67. The coloration efficiency (CE), as one of the most important characteristics of ECDs, represents the ability to achieve optical modulations with changes in energy or charge. The CE, which is defined as the change in optical density per injected charge density at a particular wavelength, is calculated to be 16.47 cm2 C−1 (Figure 4d), larger than the 12.60 cm2 C−1 reported previously [38]. The improved electroactivity reflected by the encapsulated area of the CV curve can be seen after 100 cycles (Figure 4e), suggesting a good electrochemical stability of the mirrors, in line with a previous report [39]. Figure 4f exhibits the OCV experimental results of WO3//ZIF-67 devices. It can be observed obviously that the OCV of the ECDs declines rapidly within 400 s and the decay of the OCV becomes slower after 400 s, which corresponds to the self-bleaching behavior.
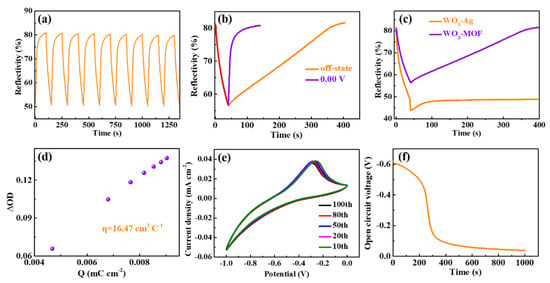
Figure 4.
(a) In situ time-dependent optical reflectance spectra at λ650 nm (−1.00 V/0.00 V, 140 s per cycle) of the WO3//ZIF-67 ECD. (b) In situ time-dependent optical reflectance spectra for WO3//ZIF-67 ECD when a voltage of 0.0 V was applied for 100 s and removed after a voltage of −1.0 V was applied for 40 s. (c) In situ time-dependent optical reflectance spectra for the mirrors with (ITO/WO3/SnO2/electrolyte/ZIF-67/Ag/ITO) and without (ITO/WO3/SnO2/electrolyte/Ag/ITO) ZIF-67. (d) The plots of in situ optical density variation as a function of charge density at λ650 nm, (e) cyclic voltammograms with a potential range from −1.00 to 0.00 V at 50 mV s−1 and (f) OCV curves for the WO3//ZIF-67 ECD.
4. Conclusions
In summary, the electrochemical properties of the electrochemically deposited ZIF-67 films in the Li+-based electrolyte have been analyzed. Both the semi-infinite diffusion and surface-controlled process contributed to the electrochemical reaction of the highly porous ZIF-67 films. The novel electrochromic mirrors constructed from ZIF-67 and WO3 electrodes exhibit a high coloration efficiency of 16.47 cm2 C−1. The self-bleaching effect that originated from the strong adsorption of highly porous ZIF-67 films for ions is demonstrated. The results suggest that the MOF-based electrode materials as an ion storage layer is a promising strategy for promoting the development of reflection-type electrochromic devices.
Author Contributions
Conceptualization, K.T.; methodology, R.J.; validation, L.L.; formal analysis, J.G.; investigation, K.W.; data curation, K.W.; writing—original draft preparation, K.W.; writing—review and editing, H.Z.; supervision, H.Z. and H.C.; project administration, H.C.; funding acquisition, H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the National Natural Science Foundation of China (61974148).
Data Availability Statement
Data available in a publicly accessible repository.
Conflicts of Interest
There are no conflict to declare.
References
- Rosseinsky, D.R.; Mortimer, R.J. Electrochromic systems and the prospects for devices. Adv. Mater. 2001, 13, 783. [Google Scholar] [CrossRef]
- Niklasson, G.A.; Granqvist, C.G. Electrochromics for smart windows: Thin films of tungsten oxide and nickel oxide, and devices based on these. J. Mater. Chem. 2006, 17, 127–156. [Google Scholar] [CrossRef]
- Cheng, W.; Moreno-Gonzalez, M.; Hu, K.; Krzyszkowski, C.; Dvorak, D.J.; Weekes, D.M.; Tam, B.; Berlinguette, C.P. Solution-Deposited Solid-State Electrochromic Windows. iScience 2018, 10, 80–86. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Wang, X.; Zhang, W.; Zheng, W.; Zhang, Y.-M.; Zhang, S.X.-A. A multicolour bistable electronic shelf label based on intramolecular proton-coupled electron transfer. Nat. Mater. 2019, 18, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.C.; Kim, C.-H.; Lodge, T.P.; Frisbie, C.D. Multicolored, Low-Power, Flexible Electrochromic Devices Based on Ion Gels. ACS Appl. Mater. Interfaces 2016, 8, 6252–6260. [Google Scholar] [CrossRef]
- Cai, G.; Wang, J.; Lee, P.S. Next-Generation Multifunctional Electrochromic Devices. Acc. Chem. Res. 2016, 49, 1469–1476. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, M.; Pei, Z.; Li, H.; Wang, Z.; Xue, Q.; Zhi, C. Multifunctional Energy Storage and Conversion Devices. Adv. Mater. 2016, 28, 8344–8364. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, J.; Ke, L.; Liu, X.; Demir, H.V.; Yang, M.F.; Sun, X.W. Electrochromic properties of nanostructured tungsten trioxide (hydrate) films and their applications in a complementary electrochromic device. Electrochim. Acta 2012, 63, 153–160. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, J.; Wang, J.-L.; Xu, J.; Li, H.-H.; Yu, S.-H. Ultrathin W18O49 Nanowire Assemblies for Electrochromic Devices. Nano Lett. 2013, 13, 3589–3593. [Google Scholar] [CrossRef]
- Invernale, M.A.; Seshadri, V.; Mamangun, D.M.D.; Ding, Y.; Filloramo, J.; Sotzing, G. Polythieno[3,4-b]thiophene as an Optically Transparent Ion-Storage Layer. Chem. Mater. 2009, 21, 3332–3336. [Google Scholar] [CrossRef]
- Choi, D.; Lee, M.; Kim, H.; Chu, W.-S.; Chun, D.-M.; Ahn, S.-H.; Lee, C.S. Investigation of dry-deposited ion storage layers using various oxide particles to enhance electrochromic performance. Sol. Energy Mater. Sol. Cells 2018, 174, 599–606. [Google Scholar] [CrossRef]
- Kim, C.-Y.; Cho, S.-G.; Lim, T.-Y. Cycle test and degradation analysis of WO3/PC+LiClO4/CeO2 center dot TiO2 electrochromic device. Sol. Energy Mater. Sol. Cells 2009, 93, 2056–2061. [Google Scholar] [CrossRef]
- Wang, B.; Han, Y.; Chen, Y.; Xu, Y.; Pan, H.; Sun, W.; Liu, S.; Yan, M.; Jiang, Y. Gradient substitution: An intrinsic strategy towards high performance sodium storage in Prussian blue-based cathodes. J. Mater. Chem. A 2018, 6, 8947–8954. [Google Scholar] [CrossRef]
- Wade, C.R.; Li, M.; Dincă, M. Facile Deposition of Multicolored Electrochromic Metal-Organic Framework Thin Films. Angew. Chem. Int. Ed. 2013, 52, 13377–13381. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, M.; Sun, W.; Wang, Y. Bimetal-Organic Framework: One-Step Homogenous Formation and its Derived Mesoporous Ternary Metal Oxide Nanorod for High-Capacity, High-Rate, and Long-Cycle-Life Lithium Storage. Adv. Funct. Mater. 2016, 26, 1098–1103. [Google Scholar] [CrossRef]
- Liu, X.B.; Li, W.X.; Zhao, X.D.; Liu, Y.C.; Nan, C.W.; Fan, L.Z. Two Birds with One Stone: Metal-Organic Framework Derived Micro-/Nanostructured Ni2P/Ni Hybrids Embedded in Porous Carbon for Electrocatalysis and Energy Storage. Adv. Funct. Mater. 2019, 29, 9. [Google Scholar] [CrossRef]
- Meek, S.T.; Greathouse, J.A.; Allendorf, M.D. Metal-Organic Frameworks: A Rapidly Growing Class of Versatile Nanoporous Materials. Adv. Mater. 2011, 23, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Pramanik, A.; Manju, U.; Mahanty, S. Reversible Lithium Storage in Manganese 1,3,5-Benzenetricarboxylate Metal–Organic Framework with High Capacity and Rate Performance. ACS Appl. Mater. Interfaces 2015, 7, 16357–16363. [Google Scholar] [CrossRef]
- Anonymous. Materials: Supercapacitor made from MOF. Nature 2016, 538, 143. [Google Scholar] [CrossRef]
- An, T.; Wang, Y.; Tang, J.; Zhang, L.; Zheng, G. A flexible ligand-based wavy layered metal–organic framework for lithium-ion storage. J. Colloid Interface Sci. 2015, 445, 320–325. [Google Scholar] [CrossRef]
- Jia, Z.; Tan, Y.; Cui, Z.; Zhang, L.; Guo, X. Construction of NiCo2O4@graphene nanorods by tuning the compositional chemistry of metal–organic frameworks with enhanced lithium storage properties. J. Mater. Chem. A 2018, 6, 19604–19610. [Google Scholar] [CrossRef]
- Li, Z.; Huang, X.; Sun, C.; Chen, X.; Hu, J.; Stein, A.; Tang, B. Thin-film electrode based on zeolitic imidazolate frameworks (ZIF-8 and ZIF-67) with ultra-stable performance as a lithium-ion battery anode. J. Mater. Sci. 2017, 52, 3979–3991. [Google Scholar] [CrossRef]
- Xu, C.; Ma, C.; Kong, X.; Taya, M. Vacuum filling process for electrolyte in enhancing electrochromic polymer window assembly. Polym. Adv. Technol. 2009, 20, 178–182. [Google Scholar] [CrossRef]
- Isfahani, V.B.; Memarian, N.; Dizaji, H.R.; Arab, A.; Silva, M. The physical and electrochromic properties of Prussian Blue thin films electrodeposited on ITO electrodes. Electrochim. Acta 2019, 304, 282–291. [Google Scholar] [CrossRef]
- Azizian-Kalandaragh, Y.; Nouhi, S.; Amiri, M. Effect of post-annealing treatment on the wetting, optical and structural properties of Ag/Indium tin oxide thin films prepared by electron beam evaporation technique. Mater. Express 2015, 5, 137–145. [Google Scholar] [CrossRef]
- Gross, A.F.; Sherman, E.; Vajo, J.J. Aqueous room temperature synthesis of cobalt and zinc sodalite zeolitic imidizolate frameworks. Dalton Trans. 2012, 41, 5458–5460. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Shi, Y.; Zhang, M.; Fan, S.; Yin, Z.; Qin, M.; Lian, T.; Li, X. Rational design of cobalt and nitrogen co-doped carbon hollow frameworks for efficient photocatalytic degradation of gaseous toluene. J. Colloid Interface Sci. 2018, 528, 45–52. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Yan, W.; Liu, X.; Yang, X.; Miao, S.; Wang, W.; Wang, A.; Zhang, T. Single-atom dispersed Co–N–C catalyst: Structure identification and performance for hydrogenative coupling of nitroarenes. Chem. Sci. 2016, 7, 5758–5764. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jin, H.; Jiang, X.; Gui, R. Assembly of Black Phosphorus Nanosheets and MOF to Form Functional Hybrid Thin-Film for Precise Protein Capture, Dual-Signal and Intrinsic Self-Calibration Sensing of Specific Cancer-Derived Exosomes. Anal. Chem. 2020, 92, 2866–2875. [Google Scholar] [CrossRef]
- Lv, Y.; Li, Y.; Han, C.; Chen, J.; He, Z.; Zhu, J.; Dai, L.; Meng, W.; Wang, L. Application of porous biomass carbon materials in vanadium redox flow battery. J. Colloid Interface Sci. 2020, 566, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Mitchell, J.B.; Lo, W.C.; Genc, A.; Lebeau, J.; Augustyn, V. Transition from Battery to Pseudocapacitor Behavior via Structural Water in Tungsten Oxide. Chem. Mater. 2017, 29, 3928–3937. [Google Scholar] [CrossRef]
- Li, K.; Shao, Y.; Liu, S.; Zhang, Q.; Wang, H.; Li, Y.; Kaner, R.B. Aluminum-Ion-Intercalation Supercapacitors with Ultrahigh Areal Capacitance and Highly Enhanced Cycling Stability: Power Supply for Flexible Electrochromic Devices. Small 2017, 13, 13. [Google Scholar] [CrossRef]
- Solomon, G.; Mazzaro, R.; You, S.; Natile, M.M.; Morandi, V.; Concina, I.; Vomiero, A. Ag2S/MoS2 Nanocomposites Anchored on Reduced Graphene Oxide: Fast Interfacial Charge Transfer for Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2019, 11, 22380–22389. [Google Scholar] [CrossRef]
- Nobili, F.; Tossici, R.; Marassi, R.; Croce, F.; Scrosati, B. An AC Impedance Spectroscopic Study of LixCoO2 at Different Temperatures. J. Phys. Chem. B 2002, 106, 3909–3915. [Google Scholar] [CrossRef]
- Kim, M.; Oh, I. Superior electric double layer capacitors using micro- and mesoporous silicon carbide sphere. J. Mater. Chem. A 2015, 3, 3944–3951. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Ai, L.; Jiang, J. Mechanistic insight into the interaction and adsorption of Cr(VI) with zeolitic imidazolate framework-67 microcrystals from aqueous solution. Chem. Eng. J. 2015, 274, 238–246. [Google Scholar] [CrossRef]
- Yan, C.; Kang, W.; Wang, J.; Cui, M.; Wang, X.; Foo, C.Y.; Chee, K.J.; Lee, P.S. Stretchable and Wearable Electrochromic Devices. ACS Nano 2014, 8, 316–322. [Google Scholar] [CrossRef]
- Guzel, M.; Karatas, E.; Ak, M. Synthesis and Fluorescence Properties of Carbazole Based Asymmetric Functionalized Star Shaped Polymer. J. Electrochem. Soc. 2016, 164, H49–H55. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).