The Impact of Autologous Platelet Concentrates on the Periapical Tissues and Root Development of Replanted Teeth: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Focused Question and Registration
2.2. Eligibility Criteria
2.3. Literature Search
2.4. Data Extraction
2.5. Quality Assessment
3. Results
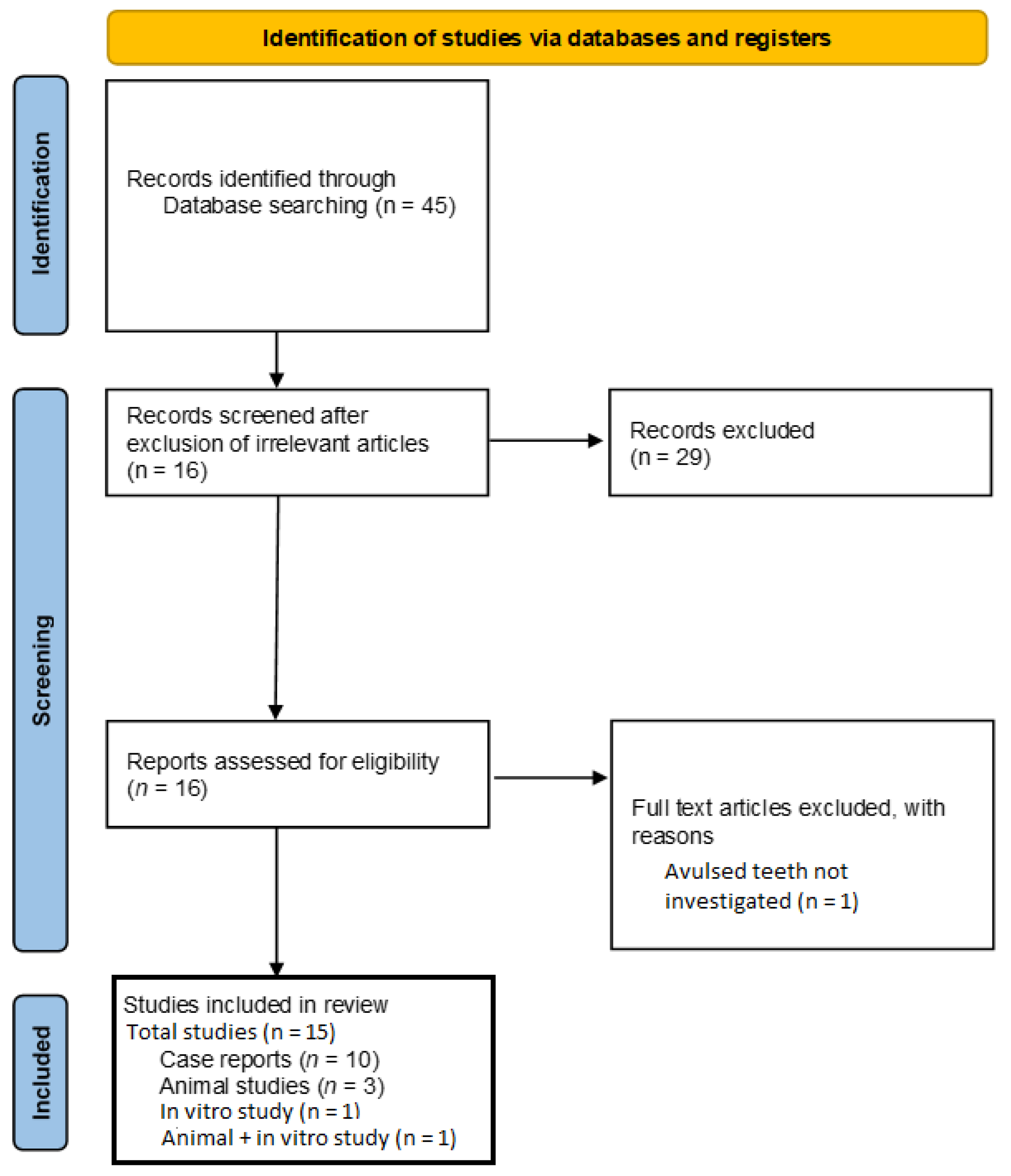
3.1. Results of the Literature Search
3.2. General Characteristics of the Case Reports
3.3. General Characteristics of Animal Studies
3.4. General Characteristics of In Vitro Experiments
3.5. Outcomes of the Included Studies
3.6. Overall Quality of Included Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andreasen, F.M.; Kahler, B. Pulpal response after acute dental injury in the permanent dentition: Clinical implications—A review. J. Endod. 2015, 41, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.D.M.; Dutra, K.L.; Kuntze, M.M.; Bortoluzzi, E.A.; Flores-Mir, C.; Felippe, W.T.; Porporatti, A.L.; De Luca Canto, G. Incidence of Root Resorption after the Replantation of Avulsed Teeth: A Meta-analysis. J. Endod. 2018, 44, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Longo, D.L.; Fumes, A.C.; Küchler, E.C.; Paula-Silva, F.W.G.; Nelson-Filho, P.; Silva, L.A.B. Efficiency of different storage media for avulsed teeth in animal models: A systematic review. Dent. Traumatol. 2018, 34, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moazami, F.; Mirhadi, H.; Geramizadeh, B.; Sahebi, S. Comparison of soymilk, powdered milk, Hank’s balanced salt solution and tap water on periodontal ligament cell survival. Dent. Traumatol. 2012, 28, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Coste, S.C.; Silva, E.F.E.; Santos, L.C.M.; Barbato Ferreira, D.A.; Côrtes, M.I.S.; Colosimo, E.A.; Bastos, J.V. Survival of replanted permanent teeth after traumatic avulsion. J. Endod. 2020, 46, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, M.Z.; Motamedi, M.R.K. Effect of calcium hydroxide on inflammatory root resorption and ankylosis in replanted teeth compared with other intracanal materials: A review. Restor. Dent. Endod. 2019, 44, e32. [Google Scholar] [CrossRef]
- Panzarini, S.R.; Nonato, C.C.; Gulinelli, J.L.; Poi, W.R.; Sonoda, C.K.; Saito, C.T.M.H.; Marão, H.F. Effect of the treatment of root surface-adhered necrotic periodontal ligament with propolis or fluoride in delayed rat tooth replantation. Clin. Oral Investig. 2014, 18, 1329–1333. [Google Scholar] [CrossRef]
- Najeeb, S.; Siddiqui, F.; Khurshid, Z.; Zohaib, S.; Zafar, M.S.; Ansari, S.A. Effect of bisphosphonates on root resorption after tooth replantation–a systematic review. Dent. Traumatol. 2017, 33, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Tuna, E.B.; Arai, K.; Tekkesin, M.S.; Seymen, F.; Gencay, K.; Kuboyama, N.; Maeda, T. Effect of fibroblast growth factor and enamel matrix derivative treatment on root resorption after delayed replantation. Dent. Traumatol. 2015, 31, 49–56. [Google Scholar] [CrossRef]
- Martínez-Zapata, M.J.; Martí-Carvajal, A.; Solà, I.; Bolibar, I.; Angel Expósito, J.; Rodriguez, L.; García, J. Efficacy and safety of the use of autologous plasma rich in platelets for tissue regeneration: A systematic review. Transfusion 2009, 49, 44–56. [Google Scholar] [CrossRef]
- Prakash, S.; Thakur, A. Platelet concentrates: Past, present and future. J. Maxillofac. Oral Surg. 2011, 10, 45–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giudice, A.; Esposito, M.; Bennardo, F.; Brancaccio, Y.; Buti, J.; Fortunato, L. Dental extractions for patients on oral antiplatelet: A within-person randomised controlled trial comparing haemostatic plugs, advanced-platelet-rich fibrin (A-PRF+) plugs, leukocyte- and platelet-rich fibrin (L-PRF) plugs and suturing alone. Int. J. Oral Implantol. (Berl.) 2019, 12, 77–87. [Google Scholar]
- Metlerska, J.; Fagogeni, I.; Nowicka, A. Efficacy of autologous platelet concentrates in regenerative endodontic treatment: A systematic review of human studies. J. Endod. 2019, 45, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Meschi, N.; Castro, A.B.; Vandamme, K.; Quirynen, M.; Lambrechts, P. The impact of autologous platelet concentrates on endodontic healing: A systematic review. Platelets 2016, 27, 613–633. [Google Scholar] [CrossRef]
- Panda, S.; Mishra, L.; Arbildo-Vega, H.I.; Lapinska, B.; Lukomska-Szymanska, M.; Khijmatgar, S.; Parolia, A.; Bucchi, C.; Fabbro, M.D. Effectiveness of Autologous Platelet Concentrates in Management of Young Immature Necrotic Permanent Teeth—A Systematic Review and Meta-Analysis. Cells 2020, 9, 2241. [Google Scholar] [CrossRef]
- Torabinejad, M.; Turman, M. Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: A case report. J. Endod. 2011, 37, 265–268. [Google Scholar] [CrossRef]
- Behnaz, M.; Izadi, S.S.; Mashhadi Abbas, F.; Dianat, O.; Sadeghabadi, S.; Akbarzadeh, T.; Haeri, A.; Kazem, M.; Younessian, F. The impact of platelet-rich fibrin (PRF) on delayed tooth replantation: A preliminary animal study. Aust. Endod. J. 2021, 47, 457–466. [Google Scholar] [CrossRef]
- Moher, D.; Altman, D.G.; Liberati, A.; Tetzlaff, J. PRISMA statement. Epidemiology 2011, 22, 128. [Google Scholar] [CrossRef] [Green Version]
- Nagendrababu, V.; Chong, B.S.; PMcCabe, P.; Shah, P.K.; Priya, E.; Jayaraman, J.; Pulikkotil, S.J.; Dummer, P.M.H. Guidelines for reporting the quality of clinical case reports in Endodontics: A development protocol. Int. Endod. J. 2019, 52, 775–778. [Google Scholar] [CrossRef] [Green Version]
- Nagendrababu, V.; Kishen, A.; Chong, B.S.; Priya, E.; Duncan, H.F.; Rôças, I.N.; Jayaraman, J.; De Figueiredo, J.A.P.; Siqueira, J.F.; Bjørndal, L.; et al. Preferred Reporting Items for study Designs in Endodontology (PRIDE): Guiding authors to identify and correct reporting deficiencies in their manuscripts prior to peer review. Int. Endod. J. 2020, 53, 589–590. [Google Scholar] [CrossRef] [Green Version]
- Hiremath, H.; Kulkarni, S.; Sharma, R.; Hiremath, V.; Motiwala, T. Use of platelet-rich fibrin as an autologous biologic rejuvenating media for avulsed teeth-an in vitro study. Dent. Traumatol. 2014, 30, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Zhang, M.; Liu, N.-X.; Lv, X.; Zhang, J.; Chen, F.-M.; Chen, Y.-J. The combined use of cell sheet fragments of periodontal ligament stem cells and platelet-rich fibrin granules for avulsed tooth reimplantation. Biomaterials 2013, 34, 5506–5520. [Google Scholar] [CrossRef] [PubMed]
- Moradian, H.; Rafiee, A.; Ayatollahi, M. Design and Fabrication of a Novel Transplant Combined with Human Bone Marrow Mesenchymal Stem Cells and Platelet-rich Fibrin: New Horizons for Periodontal Tissue Regeneration after Dental Trauma. Iran. J. Pharm. Res. 2017, 16, 1370–1378. [Google Scholar] [PubMed]
- Tözüm, T.F.; Keçeli, H.G.; Serper, A.; Tuncel, B. Intentional replantation for a periodontally involved hopeless incisor by using autologous platelet-rich plasma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e119–e124. [Google Scholar] [CrossRef] [PubMed]
- Demir, B.; Demiralp, B.; Güncü, G.N.; Uyanik, M.O.; Cağlayan, F. Intentional replantation of a hopeless tooth with the combination of platelet rich plasma, bioactive glass graft material and non-resorbable membrane: A case report. Dent. Traumatol. 2007, 23, 190–194. [Google Scholar] [CrossRef]
- Johns, D.A.; Shivashankar, V.Y.; Maroli, R.K.; Vidyanath, S. Novel management of avulsed tooth by pulpal and periodontal regeneration. J. Endod. 2013, 39, 1658–1662. [Google Scholar] [CrossRef]
- Patel, G.K.; Gujjari, S.K.; Annapoorna, B.M.; Kumar, S.C.V. Management of chronic luxated central incisor with hopeless prognosis. J. Indian Soc. Periodontol. 2013, 17, 670–675. [Google Scholar] [CrossRef]
- Priya, M.H.; Tambakad, P.B.; Naidu, J. Pulp and Periodontal Regeneration of an Avulsed Permanent Mature Incisor Using Platelet-rich Plasma after Delayed Replantation: A 12-month Clinical Case Study. J. Endod. 2016, 42, 66–71. [Google Scholar] [CrossRef]
- Ryana, H.K.; Srinath, R.; Prakash, S. Surgical Re-entry of an Intentionally Replanted Periodontally Compromised Tooth Treated with Platelet Rich Fibrin (PRF): Hopeless to Hopeful. J. Clin. Diagn. Res. 2016, 10, Zd01-4. [Google Scholar] [CrossRef]
- Deshpande, N.M.; Shah, D.; Wadekar, S. Maintenance of cell viability in extraoral conditions for a case of intentional replantation to retrieve a separated endodontic instrument. J. Conserv. Dent. 2019, 22, 207–212. [Google Scholar] [CrossRef]
- Suresh, N. Entitled “THE MAGIC WAND”: A novel treatment option for delayed replantation of an avulsed permanent tooth using injectable platelet-rich fibrin. J. Indian Soc. Periodontol. 2021, 25, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, B.; Huang, C.; Ye, R. Intentional Replantation of a Second Premolar with Internal Resorption and Root Fracture: A Case Report. J. Contemp. Dent. Pract. 2021, 22, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Reichert da Silva Assunção, L.; Colenci, R.; Do-Amaral, C.C.F.; Sonoda, C.K.; Bomfim, S.R.M.; Okamoto, R.; Golim, M.D.A.; Deffune, E.; Percinoto, C.; de Oliveira, S.H.P. Periodontal tissue engineering after tooth replantation. J. Periodontol. 2011, 82, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Yang, K.-I.; Lee, K.-H.; Choi, S.-H.; Kim, B.-O.; Park, J.-C.; Yu, S.-J. Effects of platelet-rich plasma on tooth replantation in dogs: A histologic and histomorphometric analysis. J. Periodontal. Implant. Sci. 2018, 48, 224–235. [Google Scholar] [CrossRef]
- Singh, B.; Goldberg, L.J. Autologous platelet-rich plasma for the treatment of pattern hair loss. Am. J. Clin. Dermatol. 2016, 17, 359–367. [Google Scholar] [CrossRef]
- Carter, M.J.; Fylling, C.P.; Parnell, L.K.S. Use of platelet rich plasma gel on wound healing: A systematic review and meta-analysis. Eplasty 2011, 11, e38. [Google Scholar]
- Nevins, M.; Kao, R.T.; McGuire, M.K.; McClain, P.K.; Hinrichs, J.E.; McAllister, B.S.; Reddy, M.S.; Nevins, M.L.; Genco, R.J.; Lynch, S.E.; et al. Platelet-derived growth factor promotes periodontal regeneration in localized osseous defects: 36-month extension results from a randomized, controlled, double-masked clinical trial. J. Periodontol. 2013, 84, 456–464. [Google Scholar] [CrossRef]
- Ripamonti, U.; Petit, J.C.; Teare, J. Cementogenesis and the induction of periodontal tissue regeneration by the osteogenic proteins of the transforming growth factor-beta superfamily. J. Periodontal. Res. 2009, 44, 141–152. [Google Scholar] [CrossRef]
- Chen, F.M.; Zhao, Y.-M.; Wu, H.; Deng, Z.-H.; Wang, Q.-T.; Zhou, W.; Liu, Q.; Dong, G.-Y.; Li, K.; Wu, Z.-F.; et al. Enhancement of periodontal tissue regeneration by locally controlled delivery of insulin-like growth factor-I from dextran-co-gelatin microspheres. J. Control. Release 2006, 114, 209–222. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Agwan, M.A.S.; Ansari, S.A.; Zafar, M.S.; Matinlinna, P.J. Regenerative potential of platelet rich fibrin (PRF) for curing intrabony periodontal defects: A systematic review of clinical studies. Tissue Eng. Regen. Med. 2017, 14, 735–742. [Google Scholar] [CrossRef]
- Flores, M.T.; Andersson, L.; Andreasen, J.O.; Bakland, L.K.; Malmgren, B.; Barnett, F.; Bourguignon, C.; DiAngelis, A.; Hicks, L.; Sigurdsson, A.; et al. Guidelines for the management of traumatic dental injuries. II. Avulsion of permanent teeth. Dent. Traumatol. 2007, 23, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.D. Intentional replantation techniques: A critical review. J. Endod. 2018, 44, 14–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasjleti, C.E.; Caffesse, R.G.; Castelli, W.A.; Lopatin, D.E.; Kowalski, C.J. Effect of lyophilized autologous plasma on periodontal healing of replanted teeth. J. Periodontol. 1986, 57, 568–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Pan, S.; Dangaria, S.J.; Gopinathan, G.; Kolokythas, A.; Chu, S.; Geng, Y.; Zhou, Y.; Luan, X. Platelet-rich fibrin promotes periodontal regeneration and enhances alveolar bone augmentation. BioMed Res. Int. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Lekovic, V.; Milinkovic, I.; Aleksic, Z.; Jankovic, S.; Stankovic, P.; Kenney, E.B.; Camargo, P.M. Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects. J. Periodontal Res. 2012, 47, 409–417. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Rao, N.S.; Agarwal, E.; Bajaj, P.; Kumari, M.; Naik, S.B. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of 3-wall intrabony defects in chronic periodontitis: A randomized controlled clinical trial. J. Periodontol. 2012, 83, 1499–1507. [Google Scholar] [CrossRef]
- Andersson, L.; Andreasen, J.O.; Day, P.F.; Heithersay, G.; Trope, M.; DiAngelis, A.J.; Kenny, D.J.; Sigurdsson, A.; Bourguignon, C.; Flores, M.T.; et al. International Association of Dental Traumatology guidelines for the management of traumatic dental injuries: 2. Avulsion of permanent teeth. Dent. Traumatol. 2012, 28, 88–96. [Google Scholar] [CrossRef]
- Nishioka, M.; Shiiya, T.; Ueno, K.; Suda, H. Tooth replantation in germ-free and conventional rats. Dent. Traumatol. 1998, 14, 163–173. [Google Scholar] [CrossRef]
- Andreasen, J.O.; Borum, M.K.; Jacobsen, H.L.; Andreasen, F.M. Replantation of 400 avulsed permanent incisors. 1. Diagnosis of healing complications. Endod Dent. Traumatol. 1995, 11, 51–58. [Google Scholar] [CrossRef]
- Shinohara, J.; Shibata, T.; Shimada, A.; Komatsu, K. The biomechanical properties of the healing periodontium of replanted rat mandibular incisors. Dent. Traumatol. 2004, 20, 212–221. [Google Scholar] [CrossRef]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cell Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Latalski, M.; Walczyk, A.; Fatyga, M.; Rutz, E.; Szponder, T.; Bielecki, T.; Danielewicz, A. Allergic reaction to platelet-rich plasma (PRP). Med. (Baltim.) 2019, 98, e14702. [Google Scholar] [CrossRef] [PubMed]
| Study | Patient Details (Gender, Age) | Tooth (n) | Apex (Open/Close) | Reason for Replantation | Type of Autologous Platelet and Location | Other Treatment | Clinical Parameters Assessed | Follow Up | Outcome (s) |
|---|---|---|---|---|---|---|---|---|---|
| Tözüm et al., 2005 | Male, 42 years | Mand central incisor | Closed | Periodontitis | PRP, socket | RCT, periodontal therapy | Periodontal probing, radiography, mobility | 18 months | Mobility decreased to Grade I from Grade III after 12 months of PRP therapy. 3–3.5 mm of new alveolar bone detected. |
| Demir et al., 2007 | Male, 45 years | Mand central incisor | Closed | Periodontitis | PRP, socket | RCT, periodontal therapy, GTR with PTFE and BG graft | Periodontal probing, radiography | 12 months | Bone fill detected radiographically. Pocket depths reduced from 8-7-10 (distal-median-mesial) on the buccal side and 7-7.9 mm on the lingual side to 3-3-4 and 3-3.4 mm respectively. |
| Johns et al., 2011 | Male, 15 years | Max central incisor | Closed | Avulsion (8 h storage in milk) | PRF, intracanal | RCT, root resection | Radiography, mobility | 24 months | PT results positive. Normal mobility and thick radiopacity surrounding area of root resection. |
| Torabinejad & Turman 2011 | Male, 11 years | Max second premolar | Open | Accidental extraction (immediately replanted); pulpal necrosis. | PRP + MTA, intracanal | Antibiotic paste, RCT | Pulp status, Radiography | 5.5 months | Continued root development and apical closure; complete resolution of PA radiolucency; return of pulpal sensitivity. |
| Patel et al., 2013 | Male, 16 years | Max central incisor | Not stated | Mobile (extrusive luxation) | PRP (mixed with nano HA), socket | RCT, periodontal therapy | Radiography | 6 months | Reduction in radiolucency. |
| Priya et al., 2015 | Male, 11 years | Max central incisor | Open | Avulsion (>8 h extra-oral dry time) | PRP, intracanal | Antibiotic paste, splinting, RCT | Radiography | 12 months | Complete resolution of PA radiolucency. Normal mobility. |
| Ryana et al., 2016 | Male, 23 years | Max central incisor | Closed | Periodontitis (post trauma) | PRF, socket | RCT, xenograft, Type I collagen membrane | Radiography | 12 months | 87% new bone detected radiographically. |
| Deshpande et al., 2019 | Female, 23 years | Mand first molar | Closed | Separated instrument in root canal | PRF, socket | RCT, root resection | Periodontal examination, radiography | 2 years | No periodontal pathologies detected clinically or radiographically. |
| Suresh 2019 | Female, 21 years | Max central incisor | Closed | Avulsion (>4 days extra-oral time) | PRF, socket | RCT, splinting | Radiography, mobility | 1 year | No outcomes reported. |
| Yang et al., 2021 | Male, 20 years | Max second premolar | Closed | Vertical root fracture | PRF, socket | RCT, endodontic surgery | Radiography | 2 years | Reduction in PA radiolucency |
| Study | Type of Study | Animals (n)/In Vitro Model | Teeth Analyzed | Groups (n) | Site of APC | Extra-Oral Time/Tooth Storage | Periodontal Variables Assessed | Duration of Study | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Assuncao et al., 2011 | Animal | Adult dogs (n = 4) | Premolars (n = 64) | 1: No treatment 2: PPP 3: PRP/Ca 4: PRP/Thr/Ca 5: BMMCs/PRP/Ca (n not stated) | Socket | 30 min | Histologic, histomorphometric, and immunohistochemical analysis (OP, TRAP, Type III Col, laminin) | 120 days | Only PRP/Thr/Ca group did not exhibit root resorption. All other groups displayed replacement or inflammatory resorption. |
| Zhao et al., 2013 | In vitro/Animal | In vitro: hPDLCSs Animal: Dogs (n = 6) | Incisors (n = 36) | In vitro: hPDLSC + PRF No treatment Animal: 1: Dog PDLSCs + PRF 2: DogThe format of n is not uniform, there are both regular and italic, please unify the full textPDLCSs 3: Dog PRF 4: Dog PDLSCs | Socket | 2 h | In vitro: ALP, BSP, Col-1, OCN Animal: Histology—bone and PDL regeneration | In vitro: 7 days Animal: 8 weeks | PRF/PDLSC grafts induced higher PDL proflieration and regeneration of bone and PDL tissue. |
| Hiremath et al., 2014 | In vitro | PDL cells on extracted human teeth | Unspecified (n = 30) | 1: PRF + PPP (40 min dry) 2: PPP (40 min dry) 3: 0 min, no treatment 4: 1 h, no treatment | 0 min, 40 min, 1 h | PDL cell count | PRF + PPP stimulated higher PDL cell proliferation, | ||
| Yang et al., 2018 | Animal | Mongrel dogs (n = 6; breed and gender not stated) | 36 roots from premolars and incisors (n = 36) | 1: Saline (PDL and cementum intact) (n = 12) 2: Saline (PDL and cementum removed) (n = 12) 3: PRP (PDL and cementum removed) (n = 12) | Intracanal | 5 min | Histological, histomorphometric analysis | 8 weeks | PRP reduced ankylosis and promoted PDL- and cementum-like tissue formation significantly more than Group 2. |
| Behnaz et al., 2021 | Animal | Adult mongrel dogs (n = 2) | Incisors and premolars (n = 16) | 1: No treatment (n = 8) 2: PRF (n = 8) | Socket | 60 min | Histological examination | 8 weeks | Less inflammatory resorption in PRF group. No significant difference in periodontal tissues between groups. |
| Section/Topic | Item Number | Tözüm et al., 2005 | Demir et al., 2007 | Johns et al., 2011 | Torabinejad & Turman 2011 | Patel et al., 2013 | Priya et al., 2015 | Ryana et al., 2016 | Deshpande et al., 2019 | Suresh 2019 | Yang et al., 2021 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | 1a | No | Yes | No | Yes | No | Yes | No | No | No | Yes |
| 1b | Yes | Yes | Yes | Yes | Not clear | Yes | Yes | Yes | Yes | Yes | |
| Keywords | 2a | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Abstract | 3a | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes |
| 3b | Yes | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | |
| 3c | Yes | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | |
| 3d | Yes | No | Yes | Yes | No | Yes | Yes | Yes | No | Yes | |
| 3e | Yes | No | No | Yes | No | Yes | Yes | Yes | No | Yes | |
| Introduction | 4a | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Informed consent | 5a | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | No |
| Case report information | 6a | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 6b | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| 6c | No | No | No | No | No | No | No | No | No | No | |
| 6d | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| 6e | Yes | No | Yes | No | No | Yes | No | Yes | No | No | |
| 6f | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| 6g | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| 6h | No | No | No | No | No | No | No | No | No | No | |
| 6i | Yes | No | Yes | Yes | No | No | No | No | No | No | |
| 6j | No | No | Yes | Yes | No | Yes | No | Yes | No | No | |
| 6k | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | |
| 6l | No | No | No | No | No | No | No | No | No | No | |
| 6m | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | No | |
| 6n | No | No | No | No | No | No | No | No | No | No | |
| 6o | No | No | No | Yes | No | No | No | No | No | No | |
| 6p | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | |
| 6q | No | Yes | Yes | No | Yes | No | Yes | No | No | Yes | |
| 6r | No | No | No | No | No | No | No | No | No | No | |
| 6s | No | No | No | No | No | No | No | No | No | No | |
| Discussion | 7a | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| 7b | No | No | No | No | No | Yes | No | No | No | No | |
| 7c | No | Yes | No | Yes | No | Yes | No | No | No | No | |
| 7d | Yes | Yes | No | Yes | No | Yes | Yes | Yes | No | Yes | |
| Patient perspective | 8a | Yes | Yes | No | Yes | Yes | No | No | No | No | No |
| Conclusion | 9a | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes |
| 9b | Yes | Not clear | No | Yes | Yes | Yes | Yes | Yes | No | Yes | |
| Funding details | 10a | No | No | No | No | Yes | No | No | Yes | No | No |
| Conflict of interest | 11a | No | No | Yes | Yes | Yes | Yes | No | Yes | No | Yes |
| Quality of clinical images and radiographs | 12a | No | No | No | No | No | No | No | No | No | No |
| 12b | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| 12c | No | No | No | No | No | No | No | No | No | No | |
| 12d | No | No | No | No | No | No | No | No | No | No | |
| 12e | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| 12f | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| 12g | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| 12h | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| 12i | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Overall quality | High | Medium | High | High | Low | High | Medium | High | Low | Medium | |
| Section/Topic | Item Number | Assuncao et al., 2011 | Zhao et al., 2013 | Yang et al., 2018 | Behnaz et al., 2021 |
|---|---|---|---|---|---|
| Title | 1a | No | No | Yes | No |
| 1b | Yes | Yes | Yes | Yes | |
| Keywords | 2a | No | Yes | No | No |
| Abstract | 3a | Yes | Yes | Yes | Yes |
| 3b | Yes | Yes | Yes | Yes | |
| 3c | Yes | Yes | Yes | Yes | |
| 3d | Yes | Yes | Yes | Yes | |
| 3e | Yes | Yes | Yes | Yes | |
| 3f | Yes | Yes | Yes | Yes | |
| Introduction | 4a | Yes | Yes | Yes | Yes |
| 4b | No | No | No | No | |
| 4c | Yes | Yes | Yes | Yes | |
| 4d | Yes | Yes | Yes | Yes | |
| Materials and Methods | 5a | Yes | Yes | Yes | Yes |
| 5b | No | No | No | Yes | |
| 5c | No | No | No | Yes | |
| 5d | No | No | No | No | |
| 5e | No | No | Yes | Yes | |
| 5f | No | No | Yes | Yes | |
| 5g | Yes | Yes | Yes | Yes | |
| 5h | Yes | Yes | Yes | Yes | |
| 5i | No | No | No | Yes | |
| 5j | Yes | Yes | Yes | Yes | |
| 5k | Yes | Yes | Yes | Yes | |
| 5l | No | No | No | Yes | |
| Results | 6a | Yes | No | Yes | Yes |
| 6b | Yes | Yes | Yes | Yes | |
| 6c | No | No | No | No | |
| 6d | Yes | Yes | Yes | Yes | |
| Discussion | 7a | Yes | Yes | Yes | Yes |
| 7b | No | No | No | No | |
| 7c | Yes | Yes | Yes | Yes | |
| 7d | Yes | Yes | Yes | Yes | |
| Conclusion(s) | 8a | Yes | Yes | Yes | Yes |
| 8b | Yes | Yes | Yes | Yes | |
| Funding and support | 9a | Yes | Yes | Yes | Yes |
| Conflicts of interest | 10a | Yes | Yes | Yes | Yes |
| Quality of images | 11a | Yes | Yes | Yes | Yes |
| 11b | No | No | No | No | |
| 11c | No | No | No | No | |
| 11d | Yes | Yes | Yes | Yes | |
| 11e | Yes | Yes | Yes | Yes | |
| 11f | Yes | Yes | Yes | Yes | |
| 11g | Yes | Yes | Yes | Yes | |
| 11h | Yes | Yes | Yes | Yes | |
| Overall quality | Medium | High | High | High | |
| Topic | Item Number | Zhou et al., 2013 | Hiremath et al., 2014 |
|---|---|---|---|
| Title | 1a | No | Yes |
| 1b | Yes | Yes | |
| Keywords | 2a | Yes | Yes |
| Abstract | 3a | Yes | Yes |
| 3b | Yes | Yes | |
| 3c | Yes | Yes | |
| 3d | Yes | Yes | |
| 3e | Yes | Yes | |
| Introduction | 4a | Yes | Yes |
| 4b | Yes | Yes | |
| Materials and Methods | 5a | Yes | No |
| 5b | No | No | |
| 5c | Yes | Yes | |
| 5d | Yes | No | |
| 5e | Yes | Yes | |
| 5f | No | No | |
| 5g | No | No | |
| 5h | Yes | Yes | |
| 5i | No | No | |
| 5j | Yes | No | |
| Results | 6a | Yes | Yes |
| 6b | No | No | |
| 6c | Yes | Yes | |
| Discussion | 7a | Yes | Yes |
| 7b | Yes | Yes | |
| 7c | No | No | |
| 7d | No | No | |
| 7e | Yes | Yes | |
| Conclusion(s) | 8a | Yes | No |
| 8b | Yes | Yes | |
| Funding and support | 9a | Yes | Yes |
| Conflicts of interest | 10a | Yes | Yes |
| Quality of images | 11a | Yes | No |
| 11b | Yes | No | |
| 11c | Yes | No | |
| 11d | Yes | No | |
| 11e | Yes | Yes | |
| 11f | Yes | No | |
| 11g | Yes | No | |
| 11h | Yes | Yes | |
| Overall quality | High | Medium |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khurshid, Z.; Asiri, F.Y.I.; Najeeb, S.; Ratnayake, J. The Impact of Autologous Platelet Concentrates on the Periapical Tissues and Root Development of Replanted Teeth: A Systematic Review. Materials 2022, 15, 2776. https://doi.org/10.3390/ma15082776
Khurshid Z, Asiri FYI, Najeeb S, Ratnayake J. The Impact of Autologous Platelet Concentrates on the Periapical Tissues and Root Development of Replanted Teeth: A Systematic Review. Materials. 2022; 15(8):2776. https://doi.org/10.3390/ma15082776
Chicago/Turabian StyleKhurshid, Zohaib, Faris Yahya I. Asiri, Shariq Najeeb, and Jithendra Ratnayake. 2022. "The Impact of Autologous Platelet Concentrates on the Periapical Tissues and Root Development of Replanted Teeth: A Systematic Review" Materials 15, no. 8: 2776. https://doi.org/10.3390/ma15082776
APA StyleKhurshid, Z., Asiri, F. Y. I., Najeeb, S., & Ratnayake, J. (2022). The Impact of Autologous Platelet Concentrates on the Periapical Tissues and Root Development of Replanted Teeth: A Systematic Review. Materials, 15(8), 2776. https://doi.org/10.3390/ma15082776










