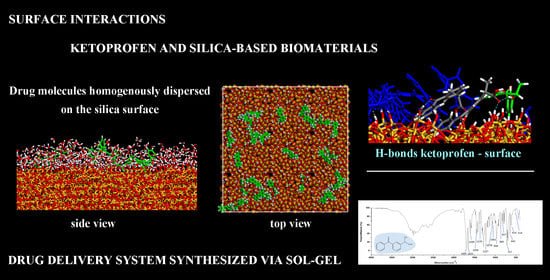
Surface Interactions between Ketoprofen and Silica-Based Biomaterials as Drug Delivery System Synthesized via Sol–Gel: A Molecular Dynamics Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Silica/Ketoprofen (5 and 15 wt. %) Sol–Gel Synthesis
2.2.2. Fourier Transform Infrared Analysis
2.2.3. Molecular Mechanics and Dynamics Methods
3. Results and Discussion
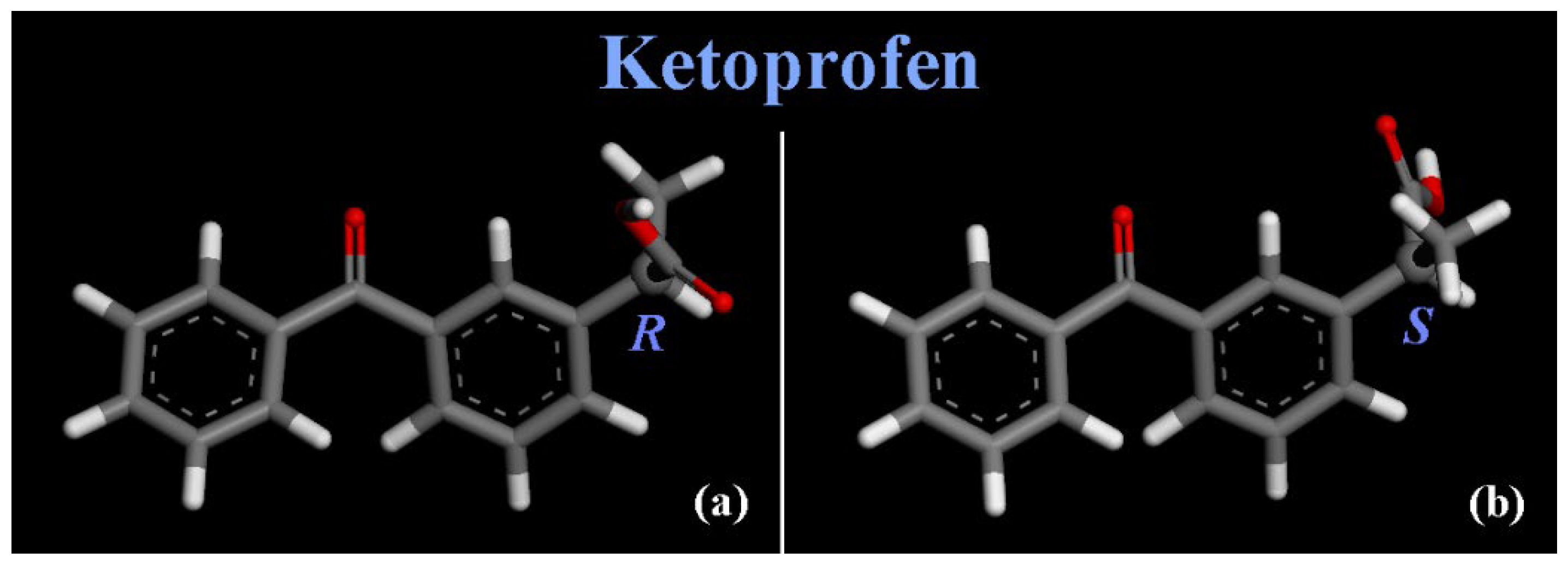
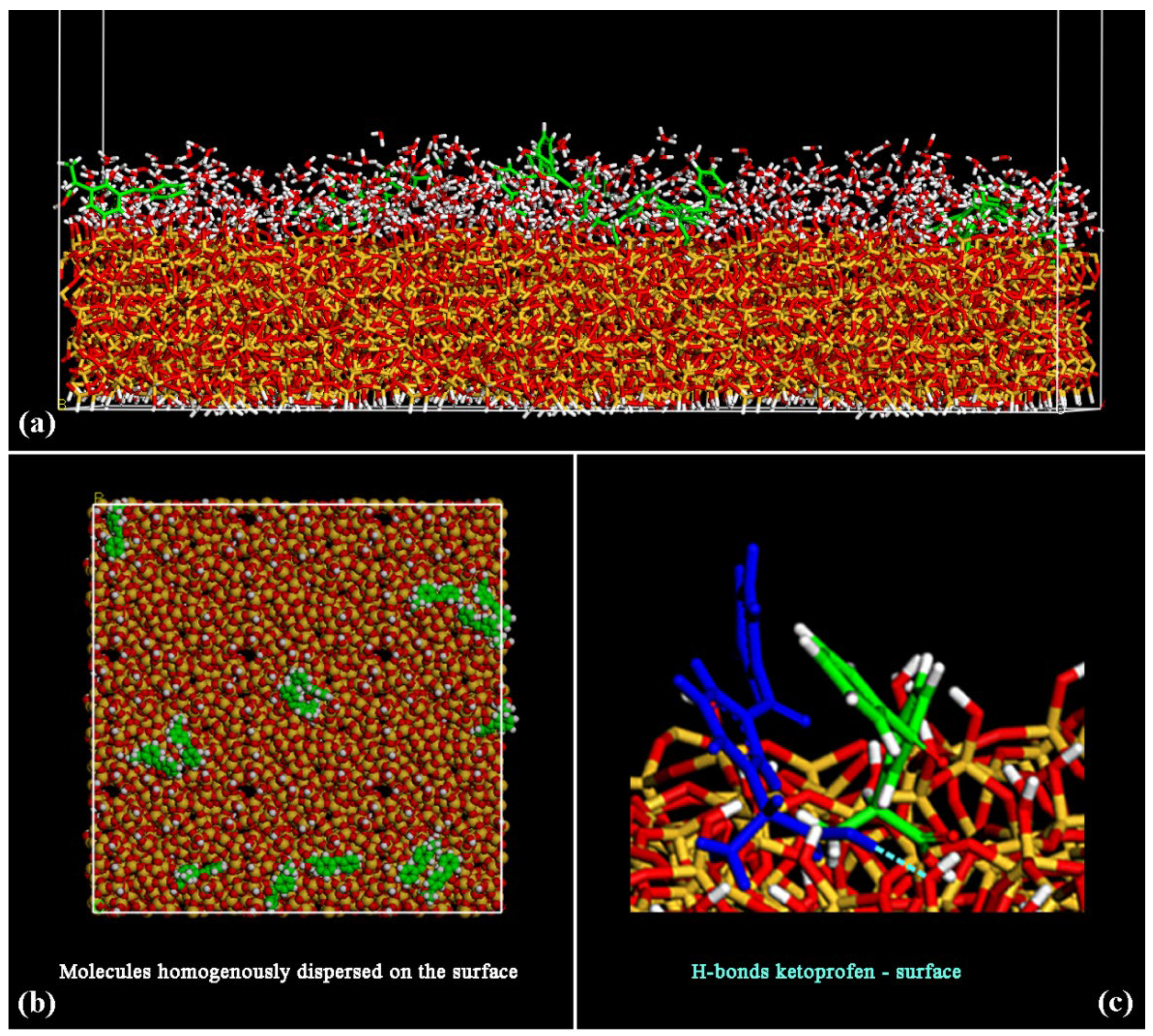
3.1. (R,S) Ketoprofen Molecules on Amorphous SiO2 Surface: Small Drug Concentration
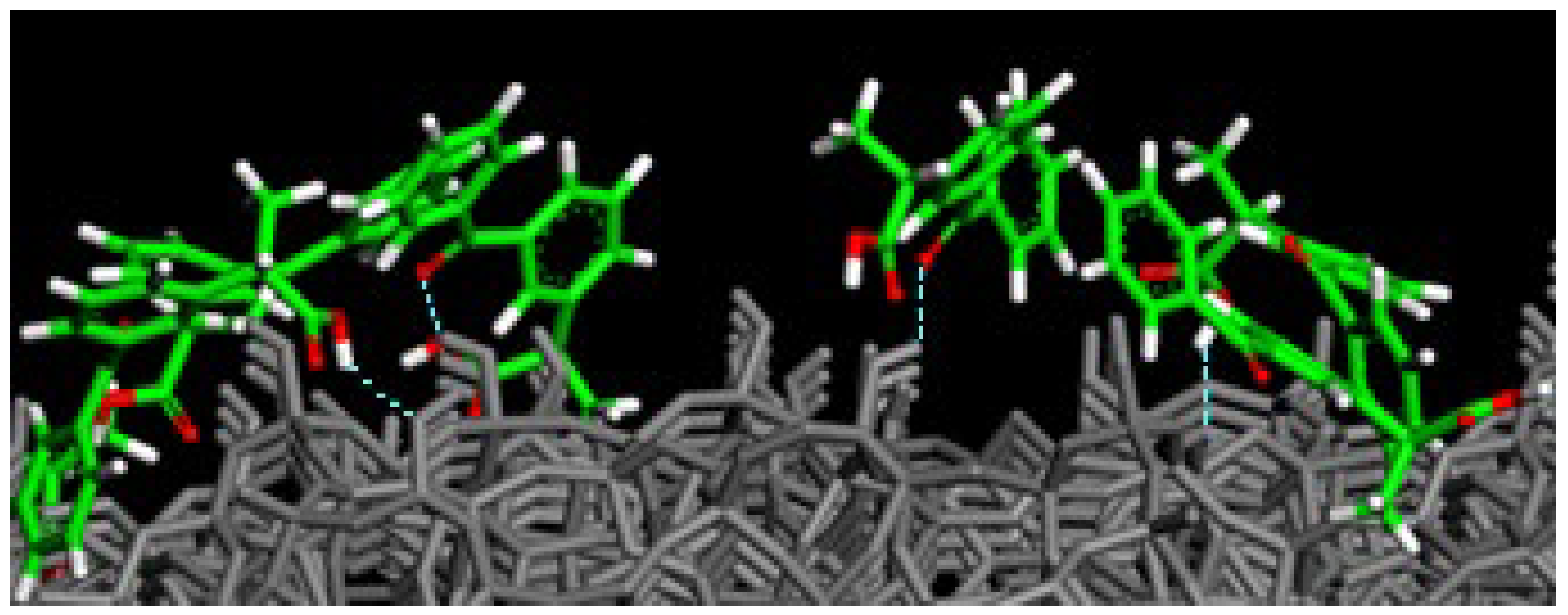
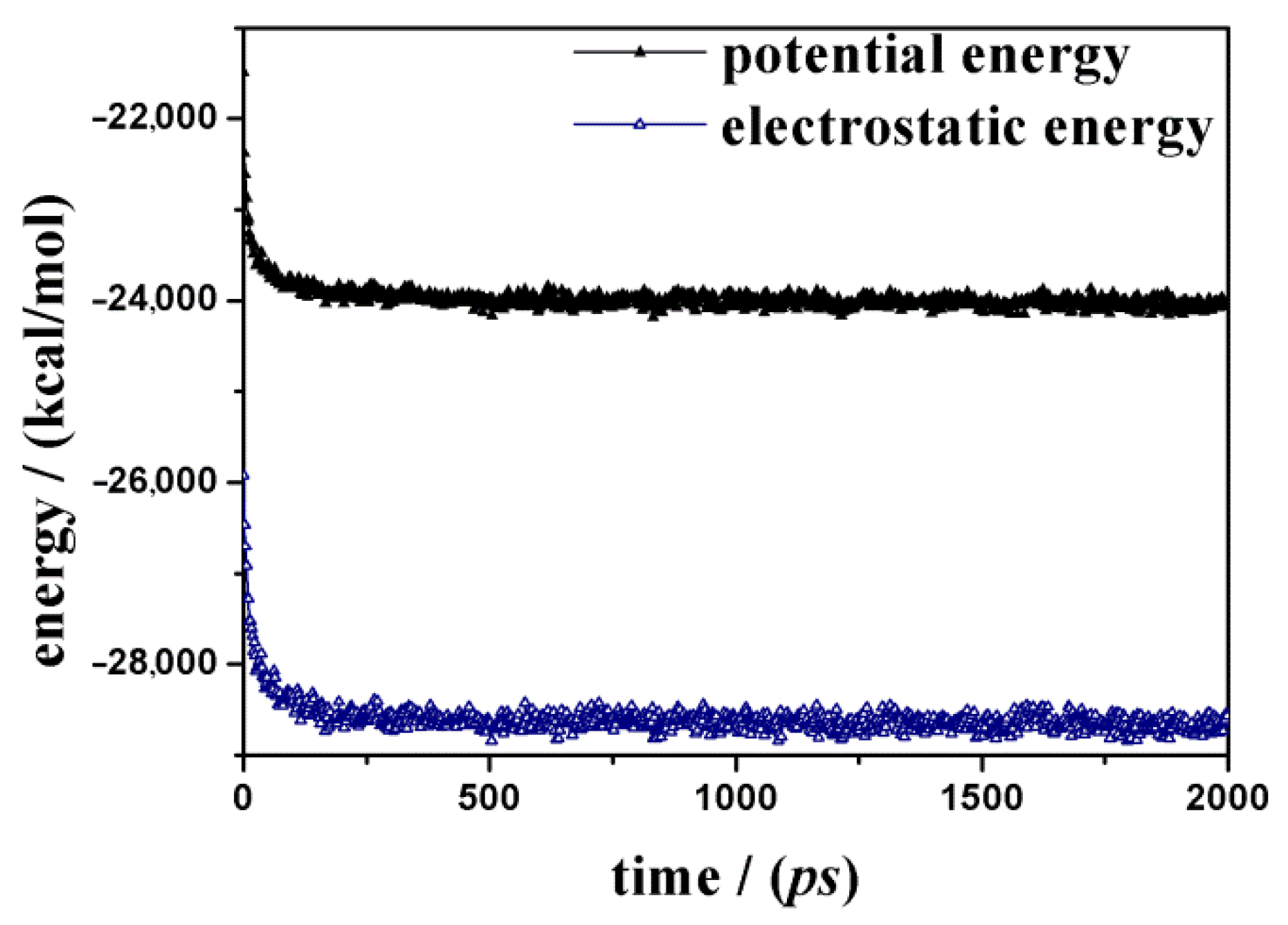
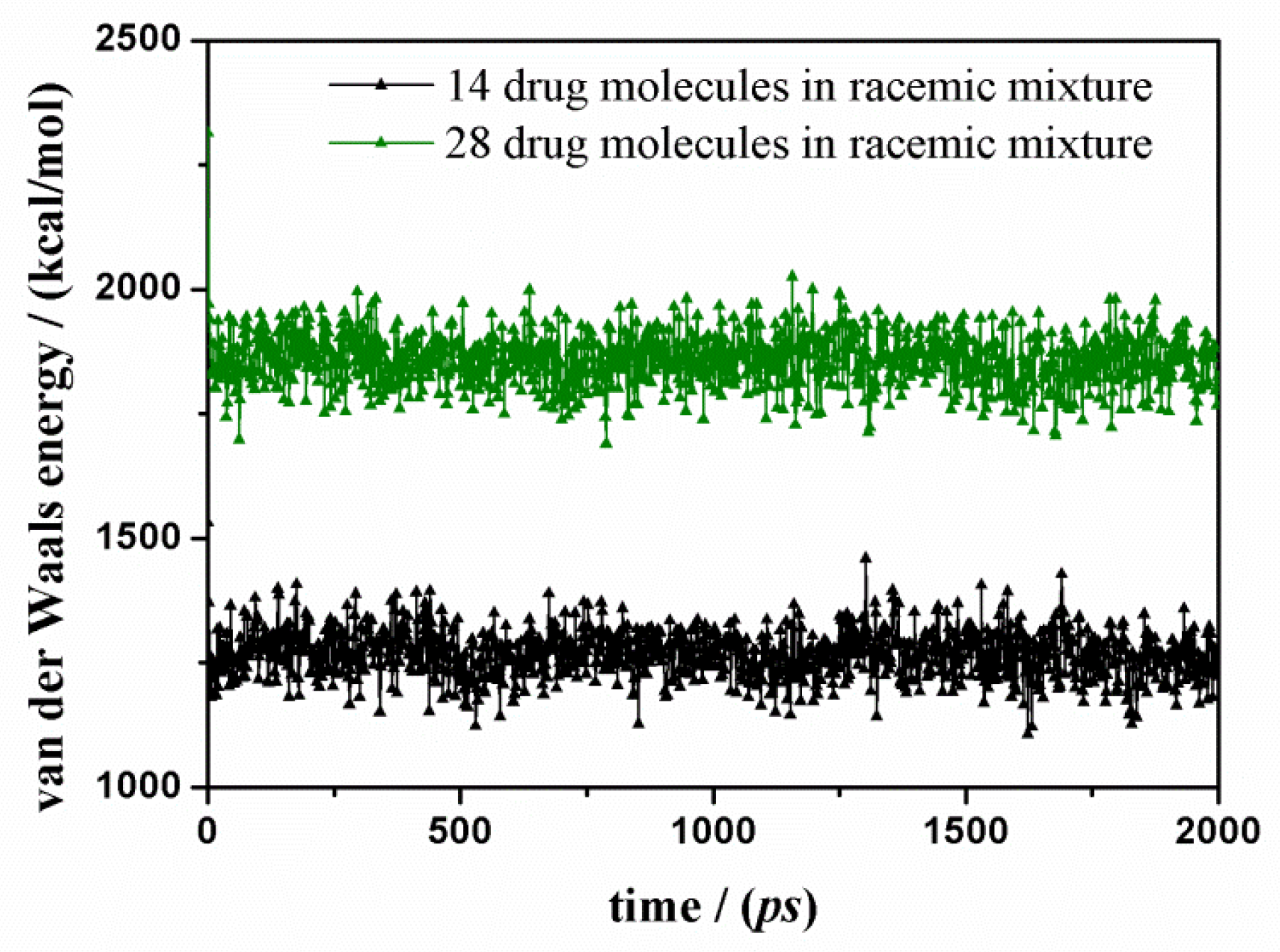
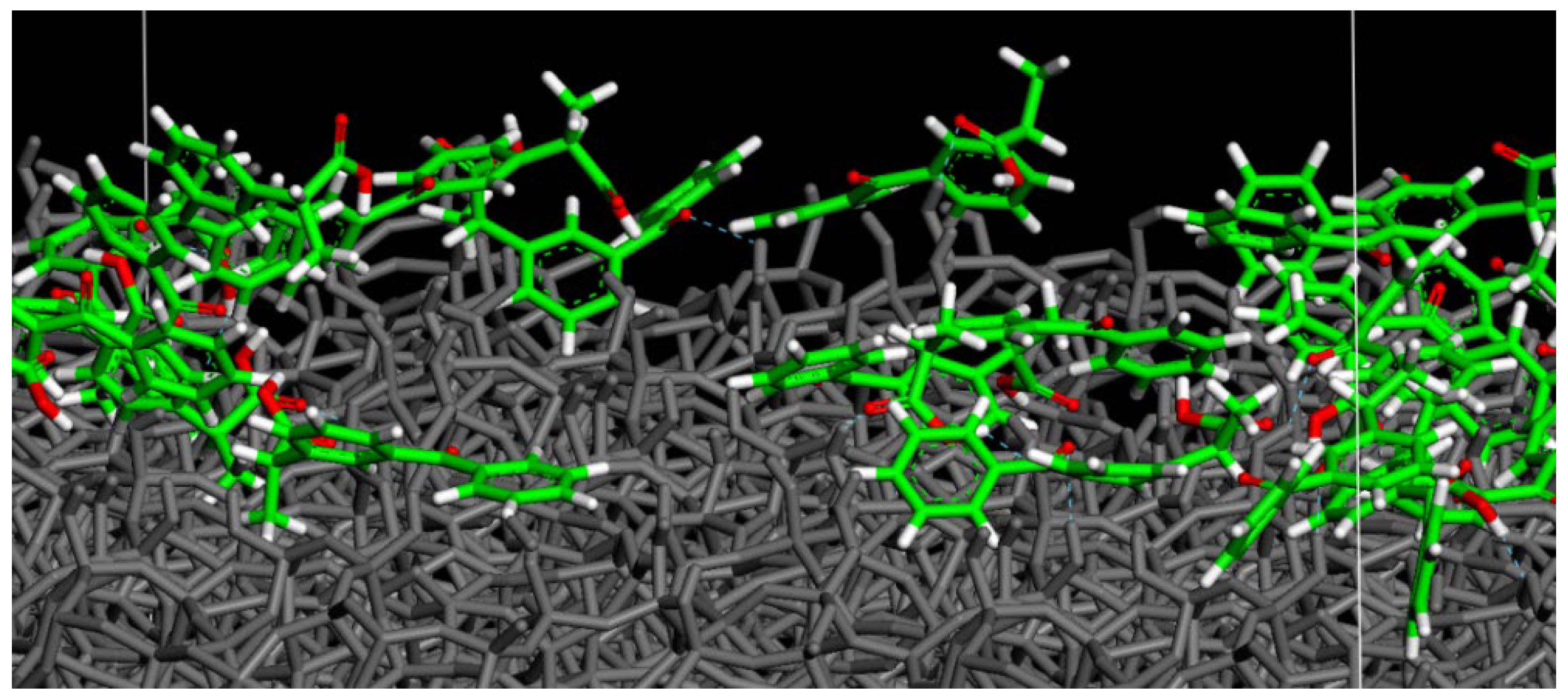
3.2. (R,S) Ketoprofen Molecules on Amorphous SiO2 Surface: Higher Drug Concentration
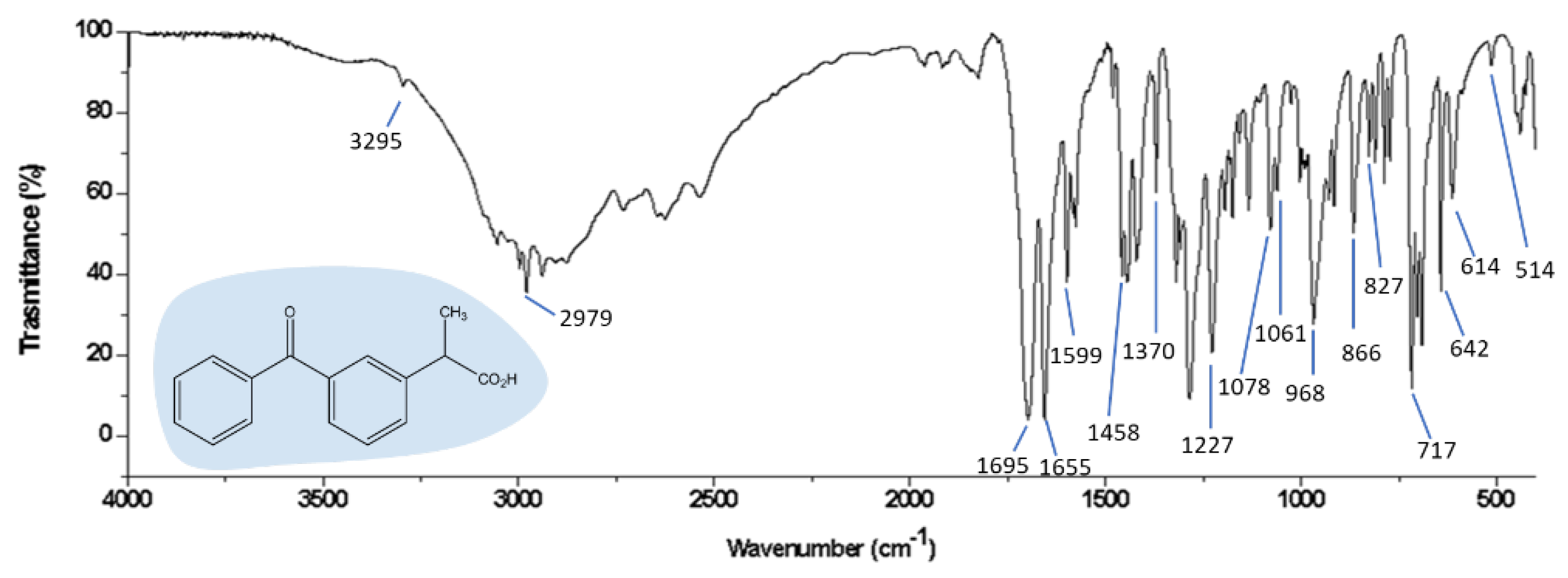
3.3. FTIR Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSN | Mesoporous Silica Nanoparticle |
| MM | Molecular Mechanics |
| NSAID | Nonsteroidal anti-inflammatory drug |
| MSPD | Matrix solid-phase dispersion |
| MD | Molecular Dynamics |
| H-bond | Hydrogen bond |
| NVT ensemble | Number of particles, Volume and Temperature are constant |
References
- Kenchegowda, M.; Rahamathulla, M.; Hani, U.; Begum, M.Y.; Guruswamy, S.; Osmani, R.A.M.; Gowrav, M.P.; Alshehri, S.; Ghoneim, M.M.; Alshlowi, A.; et al. Smart Nanocarriers as an Emerging Platform for Cancer Therapy: A Review. Molecules 2022, 27, 146. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.M.M.; Garcia, A.J.; Guldberg, R.E. Biomaterial strategies for improved intra-articular drug delivery. J. Biomed. Mater. Res. A 2020, 109, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, S.J.; Zhang, F.; Yu, J.Y.; Zhang, X.C.; Yang, G.B.; Liu, X.W. pH-Sensitive Biomaterials for Drug Delivery. Molecules 2020, 25, 5649. [Google Scholar] [CrossRef]
- Zou, Y.D.; Huang, B.T.; Cao, L.H.; Deng, Y.H.; Su, J.C. Tailored Mesoporous Inorganic Biomaterials: Assembly, Functionalization, and Drug Delivery Engineering. Adv. Mater. 2021, 33, 2005215. [Google Scholar] [CrossRef]
- Kumar, A.; Zhou, L.; Zhi, K.; Raji, B.; Pernell, S.; Tadrous, E.; Kodidela, S.; Nookala, A.; Kochat, H.; Kumar, S. Challenges in Biomaterial-Based Drug Delivery Approach for the Treatment of Neurodegenerative Diseases: Opportunities for Extracellular Vesicles. Int. J. Mol. Sci. 2021, 22, 138. [Google Scholar] [CrossRef]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941. [Google Scholar]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Froba, M. Silica-based mesoporous organic-inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef]
- Grela, A.; Kuc, J.; Bajda, T. A Review on the Application of Zeolites and Mesoporous Silica Materials in the Removal of Non-Steroidal Anti-Inflammatory Drugs and Antibiotics from Water. Materials 2021, 14, 4994. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, A.Y.; Zhu, Y.J.; Qi, C. Biodegradable Inorganic Nanostructured Biomaterials for Drug Delivery. Adv. Mater. Interfaces 2020, 7, 2000819. [Google Scholar] [CrossRef]
- Catauro, M.; Melisi, D.; Curcio, A.; Rimoli, M.G. Sol-gel processing of anti-inflammatory entrapment in silica, release kinetics, and bioactivity. J Biomed. Mater. Res. A 2008, 87, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F. Anti-inflammatory entrapment in polycaprolactone/silica hybrid material prepared by sol-gel route, characterization, bioactivity and in vitro release behavior. J. Appl. Biomater. Func. 2013, 11, 172–179. [Google Scholar] [CrossRef]
- Catauro, M.; Verardi, D.; Melisi, D.; Belotti, F.; Mustarelli, P. Novel sol-gel organic-inorganic hybrid materials for drug delivery. J. Appl. Biomater. Biomech. 2010, 8, 42–51. [Google Scholar]
- Catauro, M.; Renella, R.A.; Papale, F.; Vecchio Ciprioti, S. Investigation of bioactivity, biocompatibility and thermal behavior of sol-gel silica glass containing a high PEG percentage. Mat. Sci. Eng. C 2016, 61, 51–55. [Google Scholar] [CrossRef]
- Spohr, E.; Commer, P.; Kornyshev, A.A. Enhancing Proton Mobility in Polymer Electrolyte Membranes: Lessons from Molecular Dynamics Simulations. J. Phys. Chm. B 2002, 106, 10560–10569. [Google Scholar] [CrossRef] [Green Version]
- Shariatinia, Z.; Zahraee, Z. Controlled release of metfotmin from chitosan-based nanocomposite films containing mesoporous MCM-41 nanoparticles as novel drug delivery systems. J. Colloid. Interface Sci. 2017, 501, 60–76. [Google Scholar] [CrossRef]
- Namazi, H.; Ahmadi, H. Improving the proton conductivity and water uptake of polybenzimidazole-based proton exchange nanocomposite membranes with TiO2 and SiO2 nanopartcicles chemically modified surface. J. Power Sources 2011, 196, 2573–2583. [Google Scholar] [CrossRef]
- Abd-Elrahman, A.A.; El Nabarawi, M.A.; Hassan, D.H.; Taha, A.A. Ketoprofen mesoporous silica nanoparticles SBA-15 hard gelatin capsules: Preparation and in vitro/in vivo characterization. Drug Deliv. 2016, 23, 3387–3398. [Google Scholar] [CrossRef] [Green Version]
- Petrescu, M.; Mitran, R.A.; Matei, C.; Radulescu, M.; Berger, D. Silica-Alginate Beads for Intestinal Ketoprofen Delivery. Rev. Chim. 2018, 69, 3416–3422. [Google Scholar] [CrossRef]
- El-Toni, A.M.; Khan, A.; Labis, J.P.; Ibrahim, M.A.; Al-hoshan, M. Synthesis of Magnetic Core-Mesoporous Silica Shell Nanoparticles Using Anionic Surfactant and Their Application for Ketoprofen Control Release. Chem. Lett. 2012, 41, 1357–1359. [Google Scholar] [CrossRef] [Green Version]
- Gañán, J.; Morante-Zarcero, S.; Perez-Quintanilla, D.; Sierra, I. Evaluation of mesoporous imprinted silicas as MSPD selective sorbents of ketoprofen in powder milk. Mater. Lett. 2017, 197, 5–7. [Google Scholar] [CrossRef]
- Sajewicz, M.; Grygierczyk, G.; Gontarska, M.; Kowalska, T. Enantioseparation of S,R-(±)-Ketoprofen on Plain Silica Gel Layers with Achiral Mobile Phase. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 2185–2192. [Google Scholar] [CrossRef]
- Haack, T.; Fattori, R.; Napoletano, M.; Pellacini, F.; Fronza, G.; Raffaini, G.; Ganazzoli, F. Phthalazine PDE IV inhibitors: Conformational study of some 6-methoxy-1,4-disubstituted derivatives. Bioorg. Med. Chem. 2005, 13, 4425–4433. [Google Scholar] [CrossRef]
- Raffaini, G.; Ganazzoli, F.; Malpezzi, L.; Fuganti, C.; Fronza, G.; Panzeri, W.; Mele, A. Validating a strategy for molecular dynamics simulations of cyclodextrin inclusion complexes through single-crystal X-ray and NMR experimental data: A case study. J. Phys. Chem. B 2009, 113, 9110–9122. [Google Scholar] [CrossRef]
- Di Donato, C.; Lavorgna, M.; Fattorusso, R.; Isernia, C.; Isidori, M.; Malgieri, G.; Piscitelli, C.; Russo, C.; Russo, L.; Iacovino, R. Alpha- and Beta-Cyclodextrin Inclusion Complexes with 5-Fluorouracil: Characterization and Cytotoxic Activity Evaluation. Molecules 2016, 21, 1644. [Google Scholar] [CrossRef]
- Loftsson, T.; Saokham, P.; Couto, A.R.S. Self-association of cyclodextrins and cyclodextrin complexes in aqueous solutions. Int. J. Pharm. 2019, 560, 228–229. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J.; Kis, G.L. Spontaneous opalescence of aqueous gamma-cyclodextrin solutions: Complex formation or self-aggregation? J. Pharm. Pharmacol. 1998, 87, 6–778. [Google Scholar] [CrossRef]
- Raffaini, G.; Mazzaglia, A.; Ganazzoli, F. Aggregation behaviour of amphiphilic cyclodextrins: The nucleation stage by atomistic molecular dynamics simulations. Beilstein J. Org. Chem. 2015, 11, 2459. [Google Scholar] [CrossRef] [Green Version]
- Raffaini, G.; Ganazzoli, F.; Mazzaglia, A. Aggregation behavior of amphiphilic cyclodextrins in a nonpolar solvent: Evidence of large-scale structures by atomistic molecular dynamics simulations and solution studies. Beilstein J. Org. Chem. 2016, 12, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swaminathan, S.; Cavalli, R.; Trotta, F.; Ferruti, P.; Ranucci, E.; Gerges, I.; Manfredi, A.; Marinotto, D.; Vavia, P.R. In vitro release modulation and conformational stabilization of a model protein using swellable polyamidoamine nanosponges of β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 183–191. [Google Scholar] [CrossRef]
- Raffaini, G.; Ganazzoli, F. Understanding Surface Interaction and Inclusion Complexes between Piroxicam and Native or Crosslinked beta-Cyclodextrins: The Role of Drug Concentration. Molecules 2020, 25, 2848. [Google Scholar] [CrossRef] [PubMed]
- Pivato, R.V.; Rossi, F.; Ferro, M.; Castiglione, F.; Trotta, F.; Mele, A. beta-Cyclodextrin Nanosponge Hydrogels as Drug Delivery Nanoarchitectonics for Multistep Drug Release Kinetics. ACS Appl. Polym. Mater. 2021, 3, 6562–6571. [Google Scholar] [CrossRef]
- Ozboyaci, M.; Kokh, D.B.; Corni, S.; Wade, R.C. Modeling and simulation of protein-surface interactions: Achievements and challenges. Q. Rev. Biophys. 2016, 49, e4. [Google Scholar] [CrossRef] [Green Version]
- Raffaini, G.; Ganazzoli, F. Surface Topography Effects in Protein Adsorption on Nanostructured Carbon Allotropes. Langmuir 2013, 29, 4883–4893. [Google Scholar] [CrossRef]
- Raffaini, G.; Ganazzoli, F. Protein adsorption on the hydrophilic surface of a glassy polymer: A computer simulation study. Phys. Chem. Chem. Phys. 2006, 8, 2765–2772. [Google Scholar] [CrossRef]
- Raffaini, G.; Ganazzoli, F. A Molecular Dynamics Study of a Photodynamic Sensitizer for Cancer Cells: Inclusion Complexes of gamma-Cyclodextrins with C70. Int. J. Mol. Sci. 2019, 20, 4831. [Google Scholar] [CrossRef] [Green Version]
- Raffaini, G.; Ganazzoli, F. Surface ordering of proteins adsorbed on graphite. J. Phys. Chem. B 2004, 108, 13850–13854. [Google Scholar] [CrossRef]
- Ganazzoli, F.; Raffaini, G. Classical atomistic simulations of protein adsorption on carbon nanomaterials. Curr. Opin. Colloid. Interface Sci. 2019, 41, 11–26. [Google Scholar] [CrossRef]
- Raffaini, G. Surface Chemistry, Crystal Structure, Size and Topography Role in the Albumin Adsorption Process on TiO2 Anatase Crystallographic Faces and Its 3D-Nanocrystal: A Molecular Dynamics Study. Coatings 2021, 11, 420. [Google Scholar] [CrossRef]
- Bronze-Uhle, E.S.; Dias, L.F.G.; Trino, L.D.; Matos, A.A.; de Oliveira, R.C.; Lisboa, P.N. Physicochemical characterization of albumin immobilized on different TiO2 surfaces for use in implant materials. Colloids Surf. A Physicochem. Eng. Asp. 2019, 564, 39–50. [Google Scholar] [CrossRef]
- Pegueroles, M.; Tonda-Turo, C.; Planell, J.A.; Gil, F.J.; Aparicio, C. Adsorption of Fibronectin, Fibrinogen, and Albumin on TiO2: Time-Resolved Kinetics, Structural Changes, and Competition Study. Biointerface 2012, 7, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, S.R.; Moradas-Ferreira, P.; Saramago, B.; Melo, L.V.; Barbosa, M.A. Human serum albumin adsorption on TiO2 from single protein solutions and from plasma. Langmuir 2004, 20, 9745–9754. [Google Scholar] [CrossRef] [PubMed]
- Barinov, N.A.; Prokhorov, V.V.; Dubrovin, E.V.; Klinov, D.V. AFM visualization at a single-molecule level of denaturated states of proteins on graphite. Colloids Surf. B 2016, 146, 777–784. [Google Scholar] [CrossRef]
- Dubrovin, E.V.; Barinov, N.A.; Schaffer, T.E.; Klinov, D.V. In Situ Single-Molecule AFM Investigation of Surface-Induced Fibrinogen Unfolding on Graphite. Langmuir 2019, 35, 9732–9739. [Google Scholar] [CrossRef]
- Nejad, M.A.; Mucksch, C.; Urbassek, H.M. Insulin adsorption on crystalline SiO2: Comparison between polar and nonpolar surfaces using accelerated molecular-dynamics simulations. Chem. Phys. Lett. 2017, 670, 77–83. [Google Scholar]
- Raffaini, G.; Elli, S.; Ganazzoli, F. Computer simulation of bulk mechanical properties and surface hydration of biomaterials. J Biomed. Mater. Res. A 2006, 77A, 618–626. [Google Scholar] [CrossRef]
- Raffaini, G.; Ganazzoli, F. Surface Hydration of Polymeric (Bio)Materials: A Molecular Dynamics Simulation Study. J. Biomed. Mater. Res. Part A 2010, 92, 1382–1391. [Google Scholar] [CrossRef]
- Raffaini, G.; Mele, A.; Caronna, C. Adsorption of Chiral [5]-Aza[5]helicenes on DNA Can Modify Its Hydrophilicity and Affect Its Chiral Architecture: A Molecular Dynamics Study. Materials 2020, 13, 5031. [Google Scholar] [CrossRef]
- Accelrys Inc. InsightII 2000 and Materials Studio Program Packages Distributed by BIOVIA; Accelrys Inc.: San Diego, CA, USA, 2000; Available online: http://www.accelrys.com (accessed on 18 March 2021).
- Young, D. Computational Chemistry: A Practical Guide for Applying Techniques to Real World Problems; John Wiley & Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Vueba, M.L.; Pina, M.E.; Veiga, F.; Sousa, J.J.; Batista de Carvalho, L.A.E. Conformational study of ketoprofen by combined DFT calculations and Raman spectroscopy. J. Pharm. 2006, 307, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catauro, M.; Bollino, F. Release kinetics of anti-inflammatory drug, and characterization and bioactivity of SiO2+PCL hybrid material synthesized by sol-gel processing. J. Appl. Biomater. Funct. Mater. 2014, 12, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Laudisio, G.; Costantini, A.; Fresa, R.; Branda, F. Low Temperature Synthesis, Structure and Bioactivity of 2CaO·3SiO2 Glass. J. Sol-Gel Sci. Technol. 1997, 10, 231–237. [Google Scholar] [CrossRef]
- Catauro, M.; Raucci, M.G.; De Gaetano, F.; Marotta, A. Sol-gel synthesis, characterization and bioactivity of polycaprolactone/SiO2 hybrid material. J. Mater. Sci. 2003, 38, 3097–3102. [Google Scholar] [CrossRef]
- Catauro, M.; Tranquillo, E.; Risoluti, R.; Vecchio Ciprioti, S. Sol-Gel synthesis, spectroscopic and thermal behavior study of SiO2/PEG composites containing different amount of chlorogenic acid. Polymers 2018, 10, 682. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raffaini, G.; Catauro, M. Surface Interactions between Ketoprofen and Silica-Based Biomaterials as Drug Delivery System Synthesized via Sol–Gel: A Molecular Dynamics Study. Materials 2022, 15, 2759. https://doi.org/10.3390/ma15082759
Raffaini G, Catauro M. Surface Interactions between Ketoprofen and Silica-Based Biomaterials as Drug Delivery System Synthesized via Sol–Gel: A Molecular Dynamics Study. Materials. 2022; 15(8):2759. https://doi.org/10.3390/ma15082759
Chicago/Turabian StyleRaffaini, Giuseppina, and Michelina Catauro. 2022. "Surface Interactions between Ketoprofen and Silica-Based Biomaterials as Drug Delivery System Synthesized via Sol–Gel: A Molecular Dynamics Study" Materials 15, no. 8: 2759. https://doi.org/10.3390/ma15082759
APA StyleRaffaini, G., & Catauro, M. (2022). Surface Interactions between Ketoprofen and Silica-Based Biomaterials as Drug Delivery System Synthesized via Sol–Gel: A Molecular Dynamics Study. Materials, 15(8), 2759. https://doi.org/10.3390/ma15082759