Porous Carbon Material Derived from Steam-Exploded Poplar for Supercapacitor: Insights into Synergistic Effect of KOH and Urea on the Structure and Electrochemical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Porous Carbon Materials
2.2. Characterization
2.3. Electrochemical Measurements
3. Results
3.1. Effect of KOH Dosage on the Porous Structure and Electrochemical Performance of Carbons in the Pre-Carbonization Process
3.2. Effect of Urea Dosage on the Porous Structure and Electrochemical Performance of Carbons in the Carbonization Process
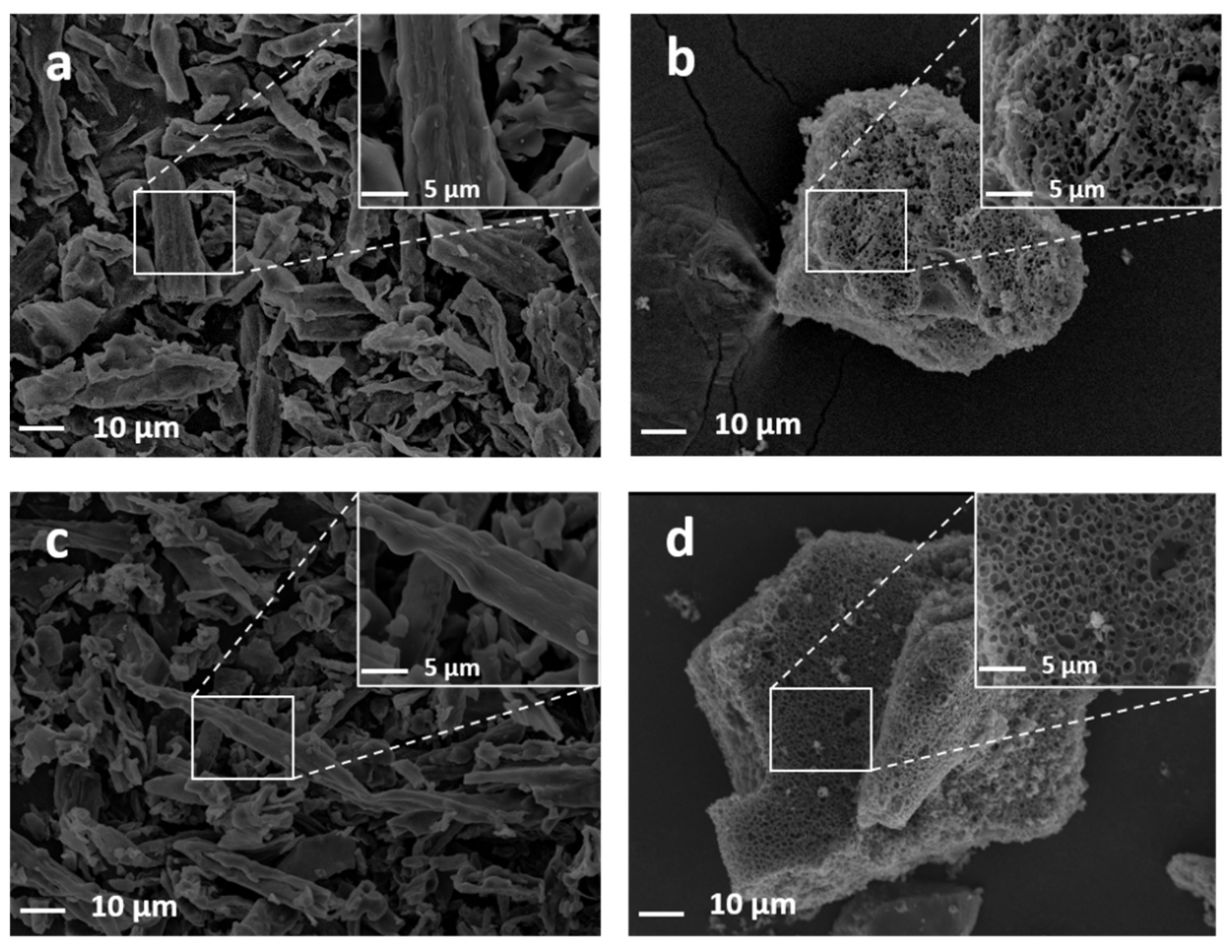
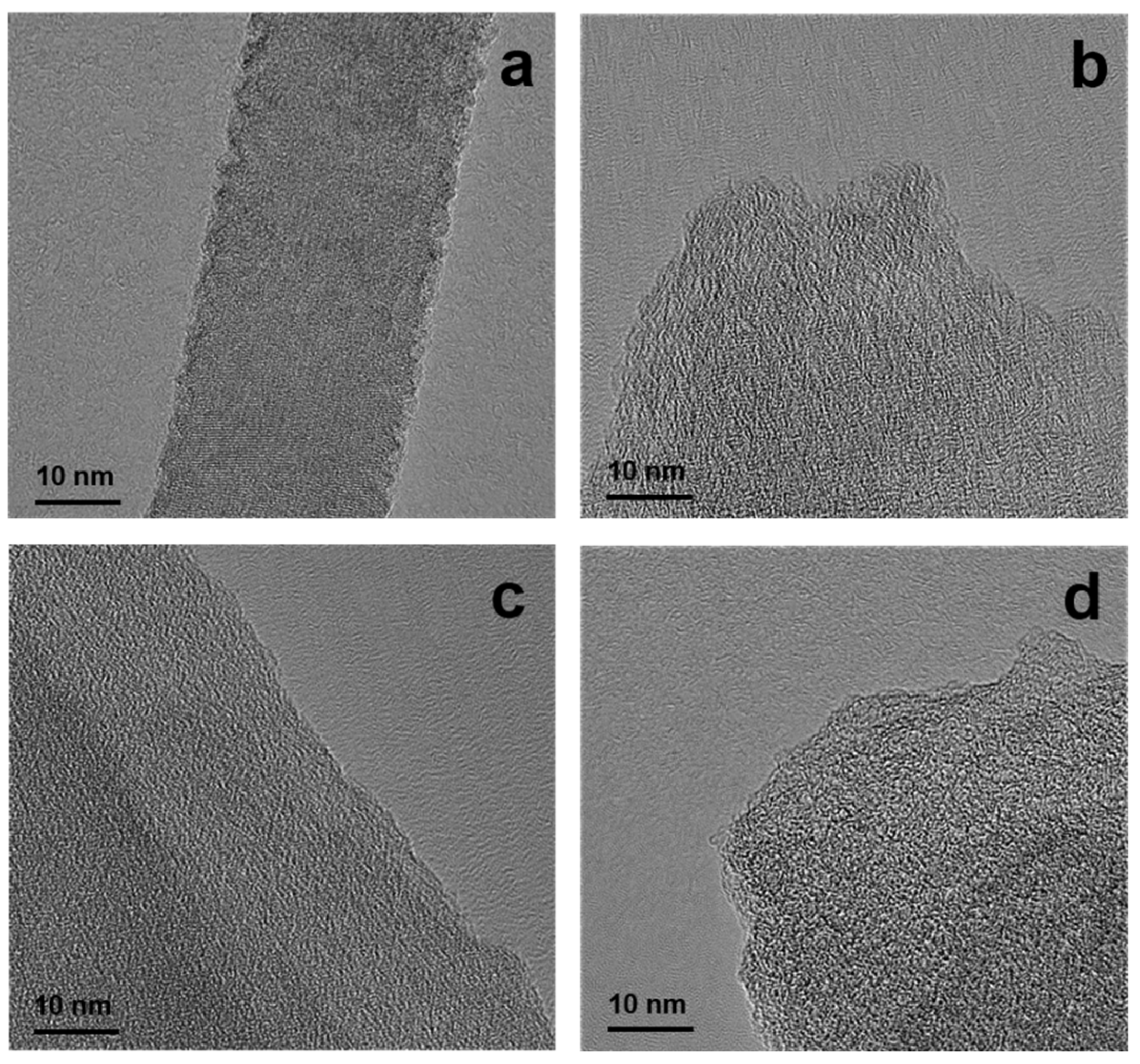
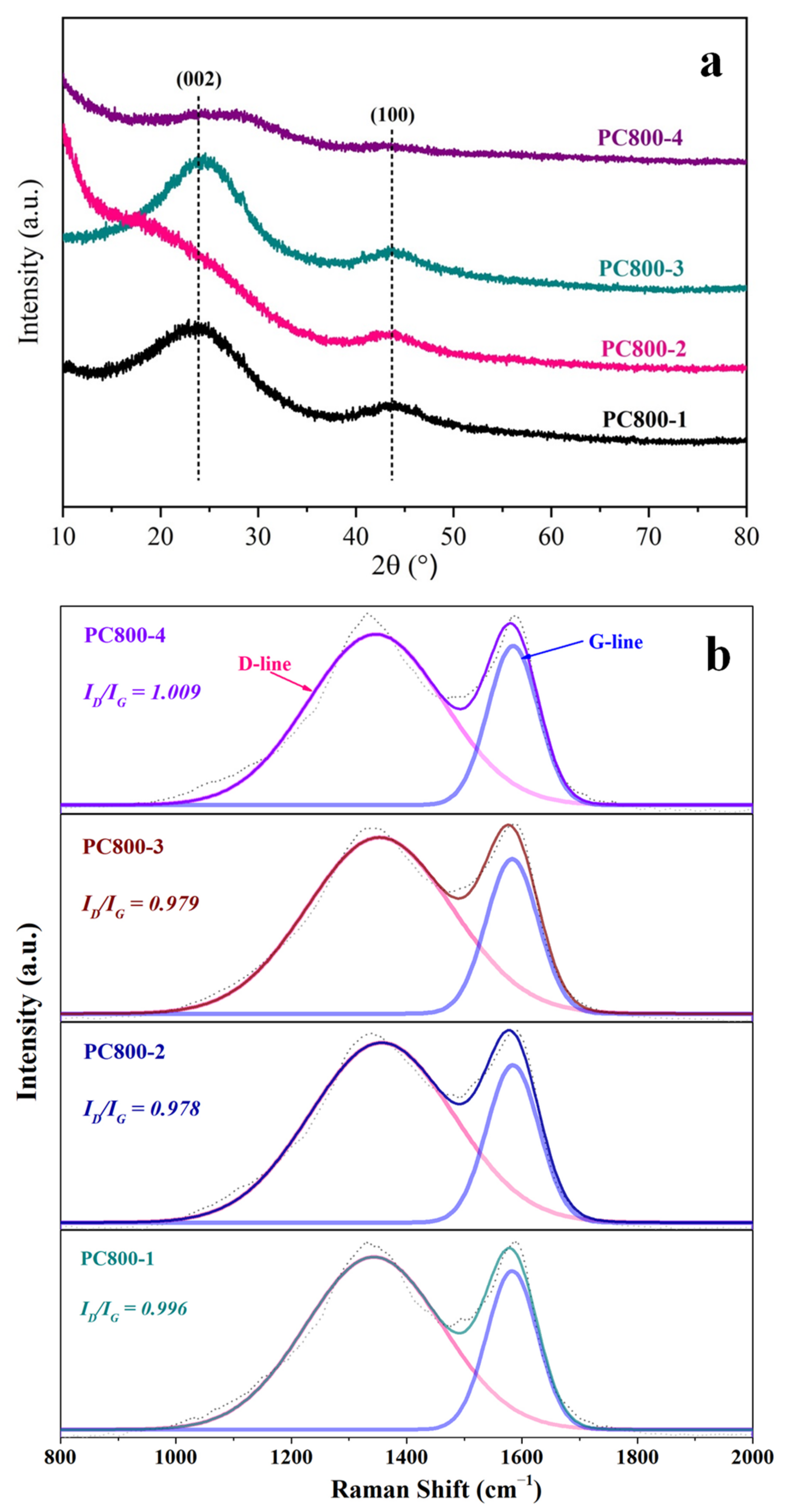
3.3. Morphology and Structure of PCs
3.4. Electrochemical Performance of Carbon Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.F.; Yan, L.; Le, Y.; Lou, X.W.D. Designed formation of hollow particle-based nitrogen-doped carbon nanofibers for high-performance supercapacitors. Energy Environ. Sci. 2017, 10, 1777–1783. [Google Scholar] [CrossRef]
- Jyothibasu, J.P.; Wang, R.-H.; Ong, K.; Ong, J.H.L.; Lee, R.-H. Scalable synthesis of γ-Fe2O3–based composite films as freestanding negative electrodes with ultra-high areal capacitances for high-performance asymmetric supercapacitors. Cellulose 2022, 29, 321–340. [Google Scholar] [CrossRef]
- Chen, J.; Han, Y.; Kong, X.; Deng, X.; Ji, H. The Origin of Improved Electrical Double-Layer Capacitance by Inclusion of Topological Defects and Dopants in Graphene for Supercapacitors. Angew. Chem. Int. Ed. 2016, 55, 13822–13827. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, L.; Hao, B.W.; Lin, J.; Shen, Z.; Xiong, W. High-performance flexible asymmetric supercapacitors based on a new graphene foam/carbon nanotube hybrid film. Energy Environ. Sci. 2014, 7, 3709–3719. [Google Scholar] [CrossRef]
- Miller, E.E.; Hua, Y.; Tezel, F.H. Materials for energy storage: Review of electrode materials and methods of increasing capacitance for supercapacitors. J. Energy Storage 2018, 20, 30–40. [Google Scholar] [CrossRef]
- Jyothibasu, J.P.; Lee, R.H. Green synthesis of polypyrrole tubes using curcumin template for excellent electrochemical performance in supercapacitors. J. Mater. Chem. A 2020, 8, 3186–3202. [Google Scholar] [CrossRef]
- Fei, H.; Saha, N.; Kazantseva, N.; Moucka, R.; Cheng, Q.; Saha, P. A Highly Flexible Supercapacitor Based on MnO2/RGO Nanosheets and Bacterial Cellulose-Filled Gel Electrolyte. Materials 2017, 10, 1251. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Zhang, C.; Su, P.; Shen, Z.; Liu, H.; Wang, G.; Liu, S.; Liu, J. Metal–organic-framework-derived formation of Co-N-doped carbon materials for efficient oxygen reduction reaction. J. Energy Chem. 2020, 40, 137–143. [Google Scholar] [CrossRef] [Green Version]
- González-García, P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew. Sust. Energ. Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Huang, Z.; Qin, C.; Wang, J.; Cao, L.; Ma, Z.; Yuan, Q.; Lin, Z.; Zhang, P. Research on High-Value Utilization of Carbon Derived from Tobacco Waste in Supercapacitors. Materials 2021, 14, 1714. [Google Scholar] [CrossRef]
- Sun, G.; Ling, Q.; Zhu, M.; Kang, K.; Guo, X. Activated carbons prepared by hydrothermal pretreatment and chemical activation of Eucommia ulmoides wood for supercapacitors application. Ind. Crop. Prod. 2018, 125, 41–49. [Google Scholar] [CrossRef]
- Wang, X.; Ding, D.; Liu, Z.; Cheng, J.; Hui, L. Synergistic Effect of Moderate Steam Explosion Pretreatment and Bovine Serum Albumin Addition for Enhancing Enzymatic Hydrolysis of Poplar. BioEnerg. Res. 2021, 14, 534–542. [Google Scholar] [CrossRef]
- Zhao, G.; Li, Y.; Zhu, G.; Shi, J.; Lu, T.; Pan, L. Biomass-Based N, P, and S Self-Doped Porous Carbon for High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 12052–12060. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Wang, M.; Xu, X.; Lu, T.; Sun, C.Q.; Pan, L. Phosphorus-doped 3D carbon nanofiber aerogels derived from bacterial-cellulose for highly-efficient capacitive deionization. Carbon 2018, 130, 377–383. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, J.; Han, J.; Yan, T.; Shi, L. N, P, S co-doped hollow carbon polyhedra derived from MOF-based core-shell nanocomposites for capacitive deionization. J. Mater. Chem. A 2018, 6, 15245–15252. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Zhao, Y.; Deng, Y.; Zhu, J.; Jiang, S.; Wang, R. Flexible all-solid-state supercapacitors based on an integrated electrode of hollow N-doped carbon nanofibers embedded with graphene nanosheets. Electrochim. Acta 2020, 332, 135398. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, R.; Tian, Y.; Sun, Z.; Huang, Z.; Wu, X.; Li, B. Heteroatoms-doped hierarchical porous carbon derived from chitin for flexible all-solid-state symmetric supercapacitors. Chem. Eng. J. 2020, 384, 123263. [Google Scholar] [CrossRef]
- Thommes, M. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Chem. Int. 2016, 38, 25. [Google Scholar] [CrossRef]
- Wu, X.; Yang, D.; Wang, C.; Jiang, Y.; Wei, T.; Fan, Z. Functionalized three-dimensional graphene networks for high performance supercapacitors. Carbon 2015, 92, 26–30. [Google Scholar] [CrossRef]
- Zahoor, A.; Christy, M.; Hwang, Y.J.; Lim, Y.R.; Kim, P.; Nahm, K.S. Improved electrocatalytic activity of carbon materials by nitrogen doping. Appl. Catal. B Environ. 2014, 147, 633–641. [Google Scholar] [CrossRef]
- Bezrodna, T.; Puchkovska, G.; Shimanovska, V.; Chashechnikova, I.; Khalyavka, T.; Baran, J. Pyridine-TiO2 surface interaction as a probe for surface active centers analysis. Appl. Surf. Sci. 2003, 214, 222–231. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Jorio, A.; Souza Filho, A.G.; Saito, R. Raman spectroscopy on isolated single wall carbon nanotubes. Carbon 2002, 40, 2043–2061. [Google Scholar] [CrossRef]
- Das, S.; Irin, F.; Tanvir Ahmed, H.S.; Cortinas, A.B.; Wajid, A.S.; Parviz, D.; Jankowski, A.F.; Kato, M.; Green, M.J. Non-covalent functionalization of pristine few-layer graphene using triphenylene derivatives for conductive poly (vinyl alcohol) composites. Polymer 2012, 53, 2485–2494. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Pan, D.; Li, Y.; Yan, Z.; Xie, J. Nitrogen-Doped 3D Graphene/MWNTs Nanoframework-Embedded Co3O4 for High Electrochemical Performance Supercapacitors. ACS Sustain. Chem. Eng. 2017, 5, 5099–5107. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Cheng, H.; Hu, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-doped graphene quantum dots with oxygen-rich functional groups. J. Am. Chem. Soc. 2012, 134, 15–18. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Leroux, F.; Béguin, F. A high-performance carbon for supercapacitors obtained by carbonization of a seaweed biopolymer. Adv. Mater. 2006, 18, 1877–1882. [Google Scholar] [CrossRef]
- Xu, J.; Tan, Z.; Zeng, W.; Chen, G.; Wu, S.; Zhao, Y.; Ni, K.; Tao, Z.; Ikram, M.; Ji, H. A Hierarchical Carbon Derived from Sponge-Templated Activation of Graphene Oxide for High-Performance Supercapacitor Electrodes. Adv. Mater. 2016, 28, 5222–5228. [Google Scholar] [CrossRef]
- Ma, R.; Song, E.; Zhou, Y.; Zhou, Z.; Liu, G.; Liu, Q.; Liu, J.; Zhu, Y.; Wang, J. Ultrafine WC nanoparticles anchored on co-encased, N-doped carbon nanotubes for efficient hydrogen evolution. Energy Storage Mater. 2017, 6, 104–111. [Google Scholar] [CrossRef]
- Sharifi, T.; Hu, G.; Jia, X.; Wågberg, T. Formation of Active Sites for Oxygen Reduction Reactions by Transformation of Nitrogen Functionalities in Nitrogen-Doped Carbon Nanotubes. ACS Nano 2012, 6, 8904–8912. [Google Scholar] [CrossRef]
- Liang, J.; Du, X.; Gibson, C.; Du, X.W.; Qiao, S.Z. N-doped graphene natively grown on hierarchical ordered porous carbon for enhanced oxygen reduction. Adv. Mater. 2013, 25, 6226–6231. [Google Scholar] [CrossRef]
- Rybarczyk, M.K.; Peng, H.-J.; Tang, C.; Lieder, M.; Zhang, Q.; Titirici, M.-M. Porous carbon derived from rice husks as sustainable bioresources: Insights into the role of micro-/mesoporous hierarchy in hosting active species for lithium–sulphur batteries. Green Chem. 2016, 18, 5169–5179. [Google Scholar] [CrossRef] [Green Version]
- Gong, Y.; Li, D.; Fu, Q.; Zhang, Y.; Pan, C. Nitrogen self-doped porous carbon for high-performance supercapacitors. ACS Appl. Energy Mater. 2020, 3, 1585–1592. [Google Scholar] [CrossRef]
- Xue, D.; Zhu, D.; Xiong, W.; Cao, T.; Wang, Z.; Lv, Y.; Li, L.; Liu, M.; Gan, L. Template-free, self-doped approach to porous carbon spheres with high N/O contents for high-performance supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 7024–7034. [Google Scholar] [CrossRef]
- Yan, X.; Liu, Y.; Fan, X.; Jia, X.; Yu, Y.; Yang, X. Nitrogen/phosphorus co-doped nonporous carbon nanofibers for high-performance supercapacitors. J. Power Sources 2014, 248, 745–751. [Google Scholar] [CrossRef]
- Chen, W.-C.; Wen, T.-C.; Teng, H. Polyaniline-deposited porous carbon electrode for supercapacitor. Electrochim. Acta 2003, 48, 641–649. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Y.; Sun, L.; Wan, P.; Zhang, X.; Qiu, J. Sustainable synthesis of phosphorus- and nitrogen-co-doped porous carbons with tunable surface properties for supercapacitors. J. Power Sources 2013, 239, 81–88. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, F.; Xu, S.D.; Wang, F.Y.; Yu, Q.; Liu, L. Nitrogen-phosphorous co-doped hollow carbon microspheres with hierarchical micro-meso-macroporous shell as efficient electrodes for supercapacitors. J. Mater. Chem. A 2017, 5, 22631–22640. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Q.; Wang, Z.; Li, L.; Jin, Z.; Wang, C.; Zhang, L.; Chen, L.; Su, Z. Nitrogen and Sulfur-Codoped Porous Carbon Nanospheres with Hierarchical Micromesoporous Structures and an Ultralarge Pore Volume for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2020, 12, 8225–8232. [Google Scholar] [CrossRef]
- Cao, M.; Feng, Y.; Tian, R.; Chen, Q.; Chen, J.; Jia, M.; Yao, J. Free-standing porous carbon foam as the ultralight and flexible supercapacitor electrode. Carbon 2020, 161, 224–230. [Google Scholar] [CrossRef]
- Amiri, A.; Conlee, B.; Tallerine, I.; Kennedy, W.J.; Naraghi, M. A novel path towards synthesis of nitrogen-rich porous carbon nanofibers for high performance supercapacitors. Chem. Eng. J. 2020, 399, 125788. [Google Scholar] [CrossRef]
- Meng, Q.; Qin, K.; Ma, L.; He, C.; Liu, E.; He, F.; Shi, C.; Li, Q.; Li, J.; Zhao, N. Flexible and cross-linked N-doped carbon nanofiber network for Silver Network Hybrid for High-Rate Supercapacitor Electrode. ACS Appl. Mater. Interfaces 2017, 9, 30832–30839. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Huang, L.; Xiao, X.; Yao, B.; Yuan, L.; Li, T.; Hu, Z.; Wang, B.; Wan, J.; Zhou, J. Flexible and cross-linked N-doped carbon nanofiber network for high performance freestanding supercapacitor electrode. Nano Energy 2015, 15, 66–74. [Google Scholar] [CrossRef]


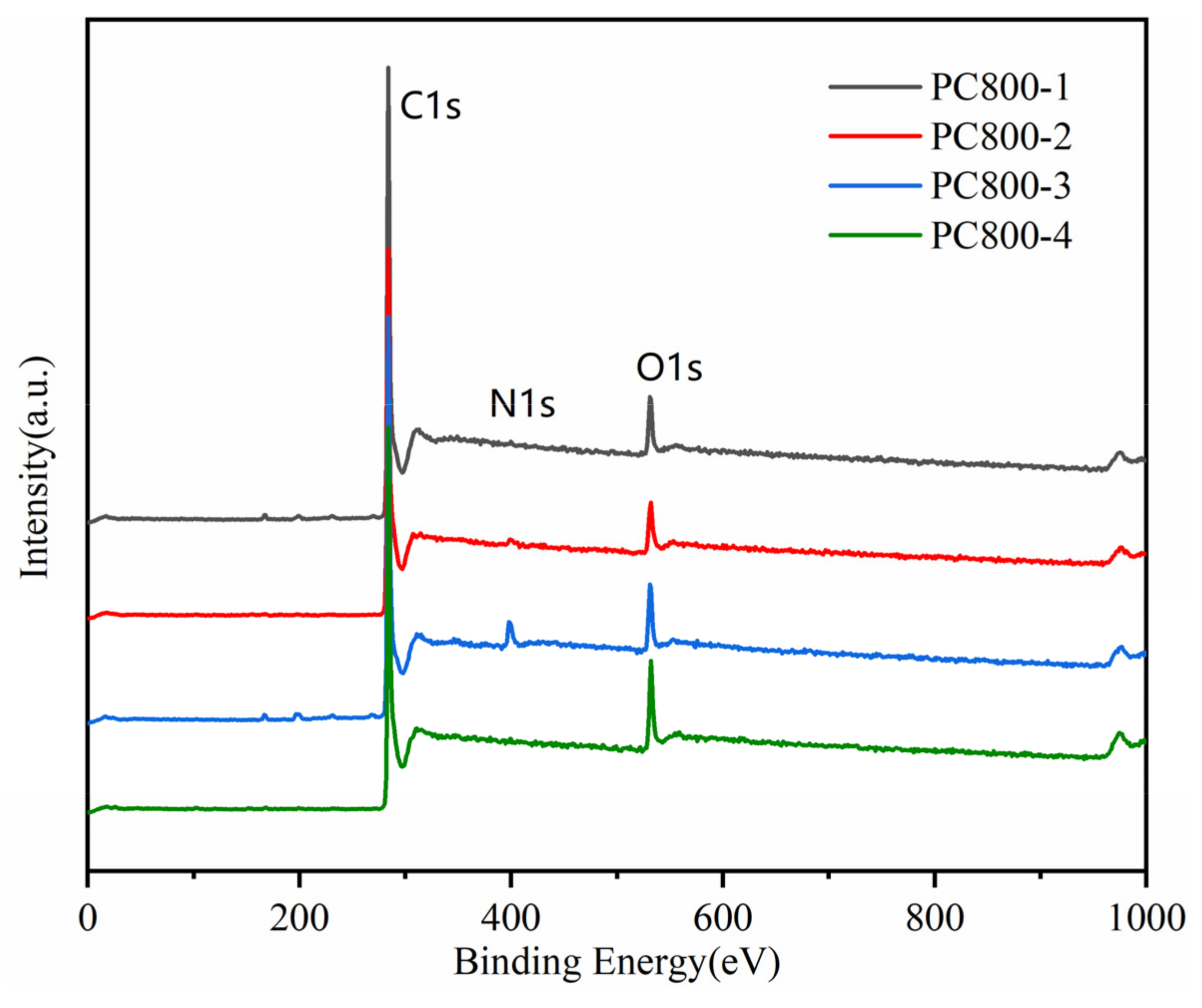
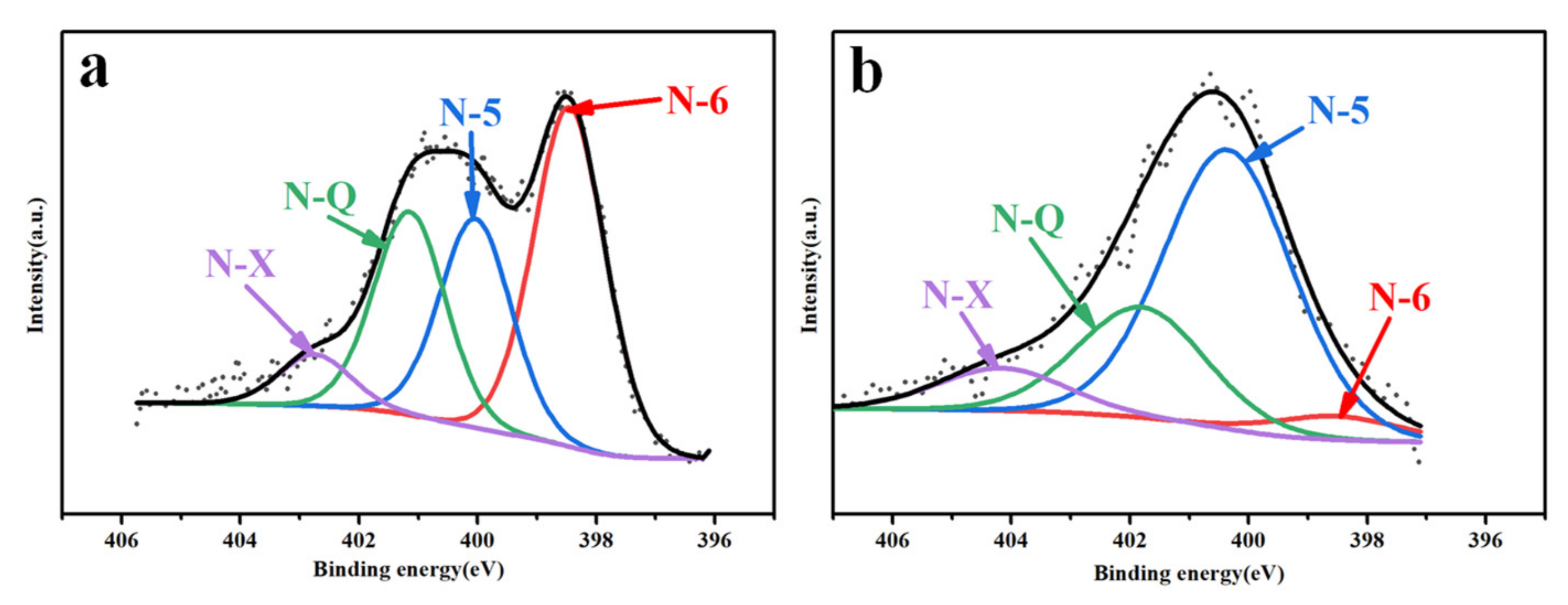

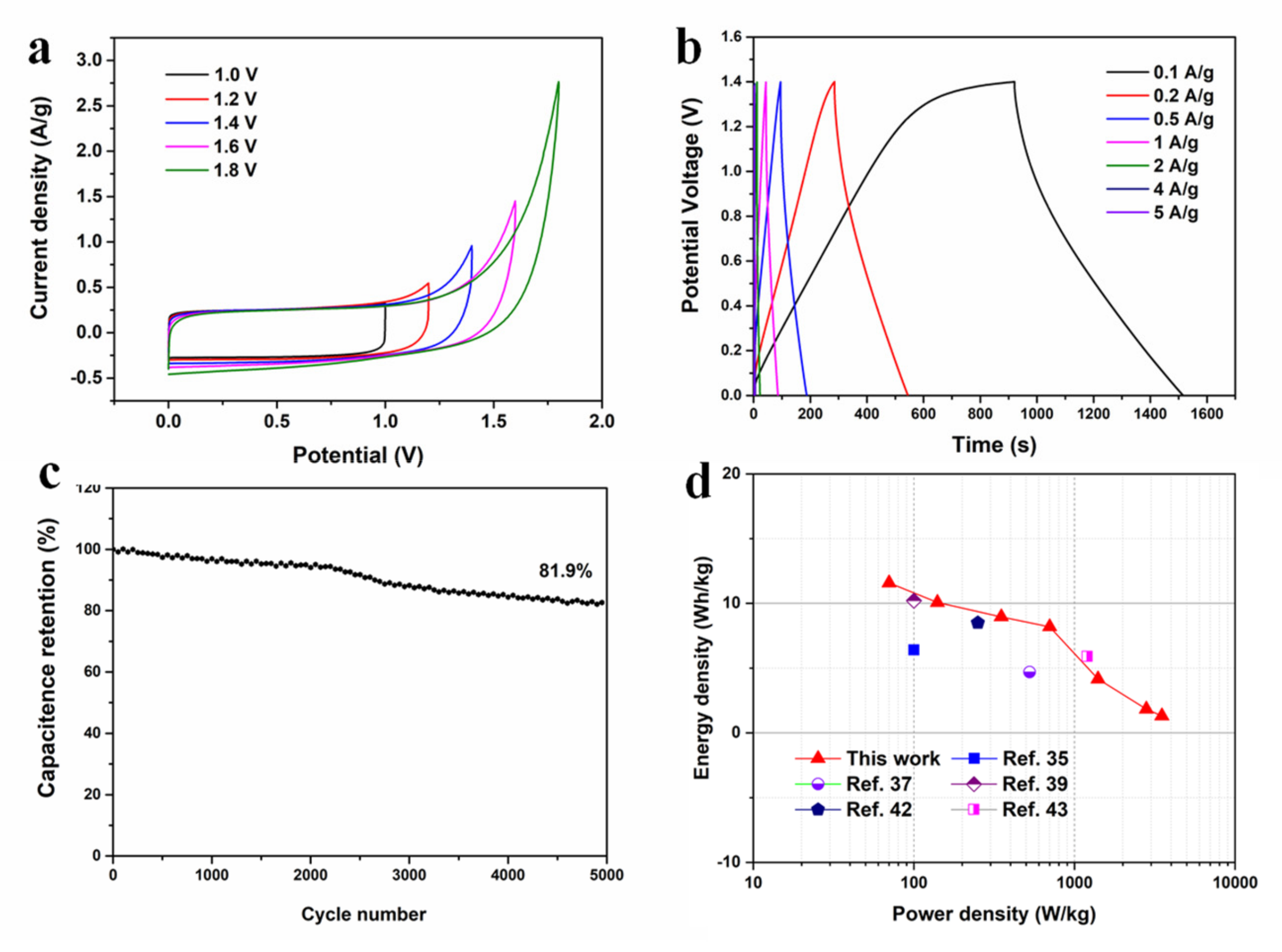
| Samples | SBET (m2/g) | Smicro (m2/g) | Smeso (m2/g) | VT (cm3/g) | Average Pore Size (nm) | Capacitance (F g−1) |
|---|---|---|---|---|---|---|
| KOH-0-SEP-1 | 53 | - | 53 | 0.07 | 4.82 | 95 |
| KOH-0.5-SEP-1 | 1527 | 1233 | 294 | 0.75 | 1.97 | 230 |
| KOH-1-SEP-1 | 3000 | 2012 | 988 | 1.59 | 2.13 | 360 |
| KOH-1.5-SEP-1 | 2400 | 2081 | 319 | 1.14 | 1.89 | 333 |
| KOH-2-SEP-1 | 2309 | 2010 | 299 | 1.15 | 1.99 | 346 |
| Samples | SBET (m2/g) | Smicro (m2/g) | Smeso (m2/g) | VT (cm3/g) | Average Pore Size (nm) | Capacitance (F g−1) |
|---|---|---|---|---|---|---|
| Urea-0-SEP-1 | 1666 | 1511 | 155 | 0.79 | 1.88 | 201 |
| Urea-0.5-SEP-1 | 2527 | 1998 | 528 | 1.25 | 1.98 | 180 |
| Urea-0.75-SEP-1 | 2731 | 2004 | 726 | 1.38 | 2.02 | 193 |
| Urea-1-SEP-1 | 3000 | 2012 | 988 | 1.59 | 2.13 | 262 |
| Urea-1.25-SEP-1 | 3262 | 1307 | 1955 | 1.99 | 2.44 | 351 |
| Urea-1.5-SEP-1 | 2968 | 1397 | 1572 | 1.69 | 2.28 | 278 |
| Samples | C (Atom %) | N (Atom %) | O (Atom %) |
|---|---|---|---|
| PC800-1 | 93.00 | 0.57 | 6.43 |
| PC800-2 | 89.99 | 0.49 | 9.52 |
| PC800-3 | 87.07 | 5.84 | 7.09 |
| PC800-4 | 91.91 | 1.76 | 6.34 |
| Samples | Pyridine-N (%) | Pyrrolidone-N (%) | Graphite-N (%) | Nitrogen Oxide (%) |
|---|---|---|---|---|
| PC800-3 | 1.59 | 3.42 | 0.49 | 0.34 |
| PC800-4 | 0.04 | 0.93 | 0.68 | 0.11 |
| Samples | SBET (m2/g) | Smicro (m2/g) | Smeso (m2/g) | Vmicro (cm3/g) | Vmeso (cm3/g) | VT (cm3/g) |
|---|---|---|---|---|---|---|
| PC800-1 | 200 | 145 | 55 | 0.06 | 0.05 | 0.11 |
| PC800-2 | 1412 | 1226 | 186 | 0.48 | 6.33 | 6.80 |
| PC800-3 | 53 | - | 53 | - | 6.99 | 6.99 |
| PC800-4 | 3282 | 1232 | 2050 | 1.16 | 0.95 | 2.11 |
| Precursor | Doping Element | Capacitance (F g−1) | Electrolyte | Capacitance Retention (%) | Ref. |
|---|---|---|---|---|---|
| Polyacrylonitrile | N, P | 224.9 | 1 M H2SO4 | 70 | [35] |
| Glucose | N, P | 183.8 | 6 M KOH | 93.6 | [37] |
| Formaldehyde | N, P | 200 | 6 M KOH | 91 | [38] |
| Poly acrylic acid | N, S | 309.4 | 1 M H2SO4 | 98.5 | [39] |
| Melamine sponge | N | 238 | 6 M KOH | 99 | [40] |
| Polyacrylonitrile | N | 203.4 | 6 M KOH | >94 | [41] |
| Poplar | N | 319 | 6 M KOH | 103 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, D.; Ma, L.; Li, X.; Liu, Z.; Hui, L.; Zhang, F.; Zhao, Y. Porous Carbon Material Derived from Steam-Exploded Poplar for Supercapacitor: Insights into Synergistic Effect of KOH and Urea on the Structure and Electrochemical Properties. Materials 2022, 15, 2741. https://doi.org/10.3390/ma15082741
Ding D, Ma L, Li X, Liu Z, Hui L, Zhang F, Zhao Y. Porous Carbon Material Derived from Steam-Exploded Poplar for Supercapacitor: Insights into Synergistic Effect of KOH and Urea on the Structure and Electrochemical Properties. Materials. 2022; 15(8):2741. https://doi.org/10.3390/ma15082741
Chicago/Turabian StyleDing, Dayong, Lan Ma, Xin Li, Zhong Liu, Lanfeng Hui, Fengshan Zhang, and Yumeng Zhao. 2022. "Porous Carbon Material Derived from Steam-Exploded Poplar for Supercapacitor: Insights into Synergistic Effect of KOH and Urea on the Structure and Electrochemical Properties" Materials 15, no. 8: 2741. https://doi.org/10.3390/ma15082741
APA StyleDing, D., Ma, L., Li, X., Liu, Z., Hui, L., Zhang, F., & Zhao, Y. (2022). Porous Carbon Material Derived from Steam-Exploded Poplar for Supercapacitor: Insights into Synergistic Effect of KOH and Urea on the Structure and Electrochemical Properties. Materials, 15(8), 2741. https://doi.org/10.3390/ma15082741