NiO–Ba0.95Ca0.05Ce0.9Y0.1O3−δ as a Modified Anode Material Fabricated by the Tape Casting Method
Abstract
:1. Introduction
2. Experiments
2.1. Preparation of Ni–5CBCY Cermet Using the Tape Casting Method
- Pre-mix all the reagents listed in Table 1 and prepare the suspension components (~0.5 h).
- Intensively homogenise all of the reagents by grinding in a ball mill placed on high-speed rollers (~48 h).
- Further homogenise the prepared slurry from stage II at a reduced rotation speed, degassing the slurry (~48 h).
- Transfer the slurries to the laboratory tape caster device and begin the tape casting process for NiO–5CBCY. The gap between the cast squeegee and tape-casting table surface was 2 mm. The green tape was cast at a rate of 2 cm/s on a polytetrafluoroethylene (PTFE) surface (~0.5 h).
- Dry the casted NiO–5CBCY tape in an air-conditioned room (~24 h).
2.2. Analytical Methods for Evaluating the Physicochemical Properties of the NiO–5CBCY and Ni–5CBCY Samples
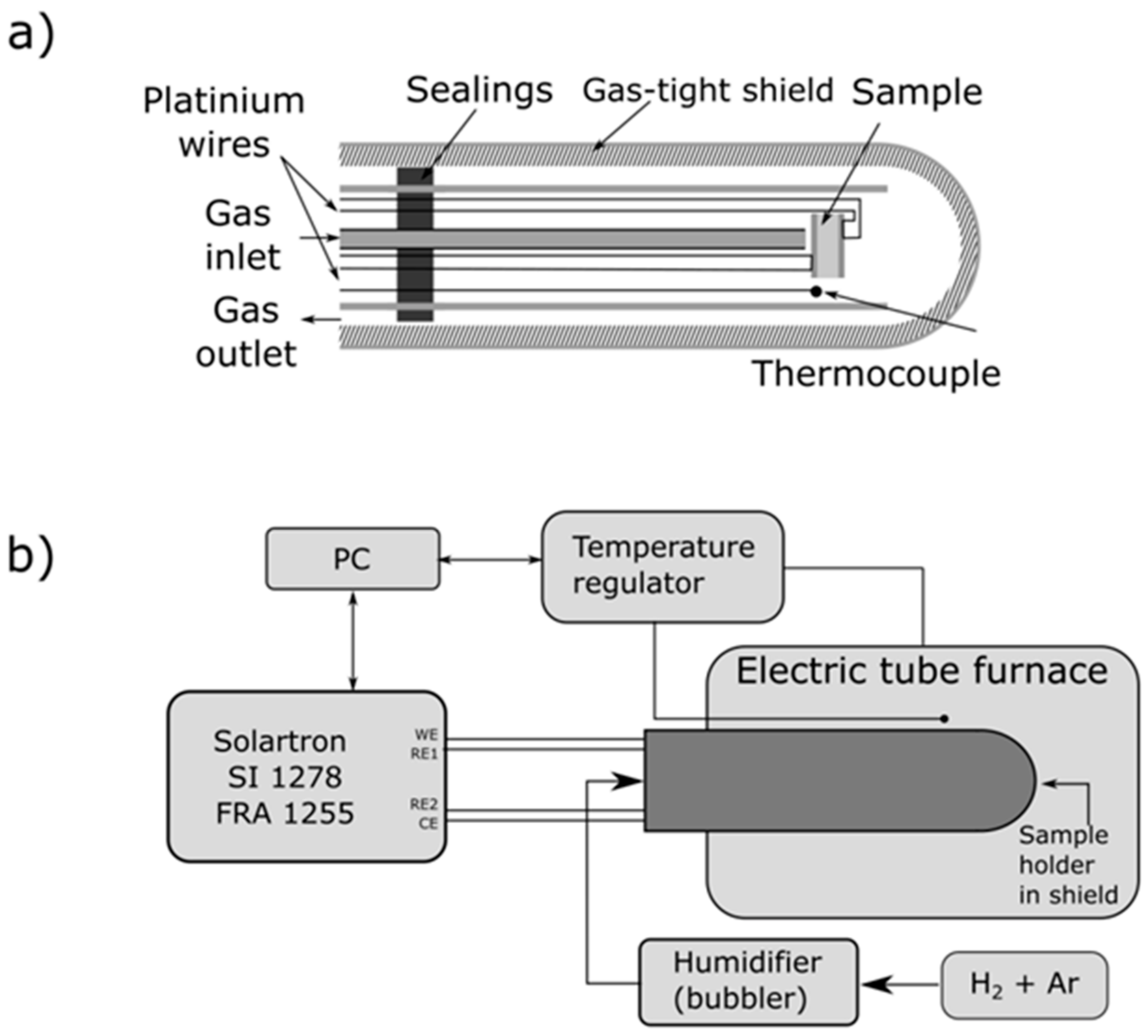
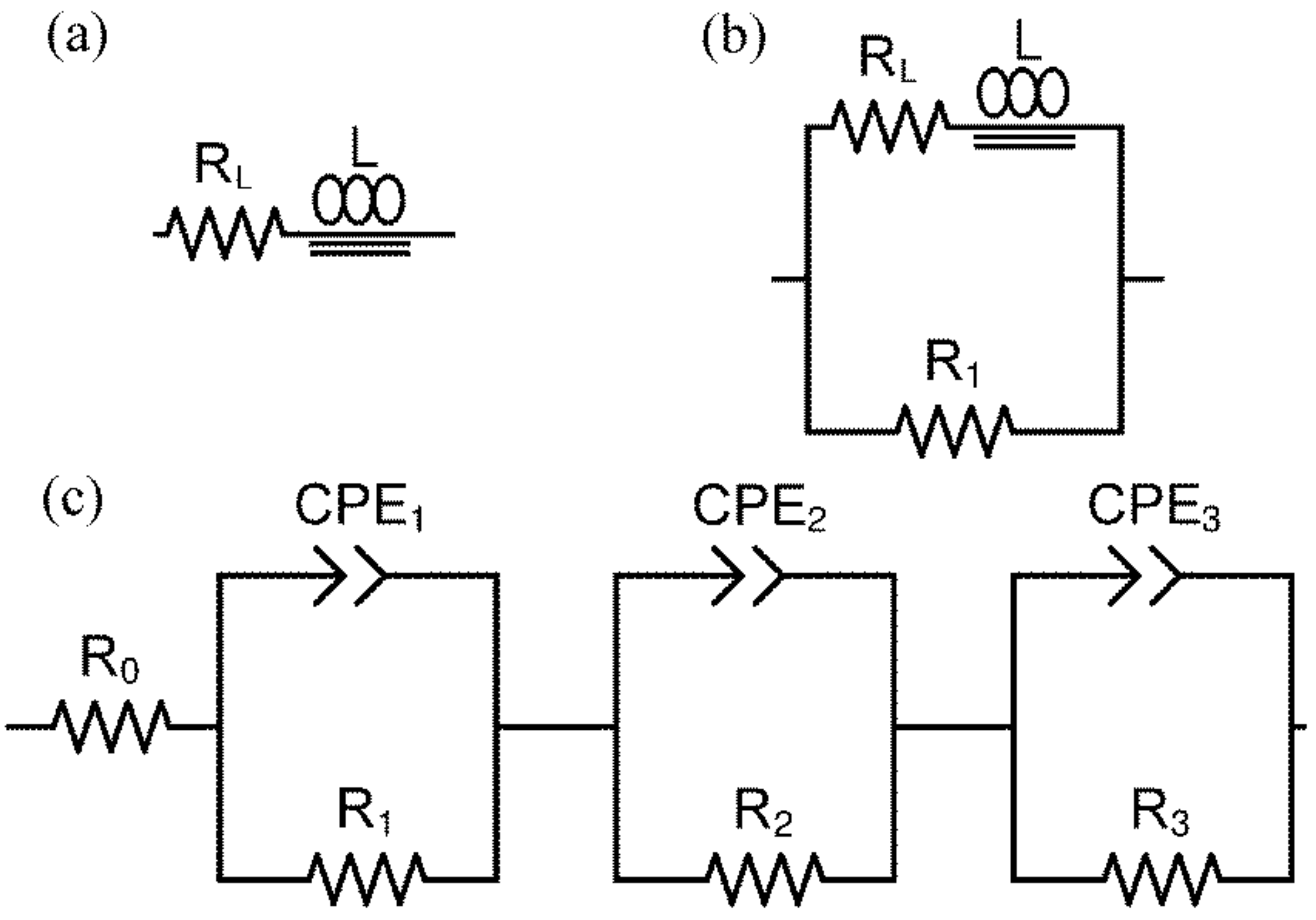
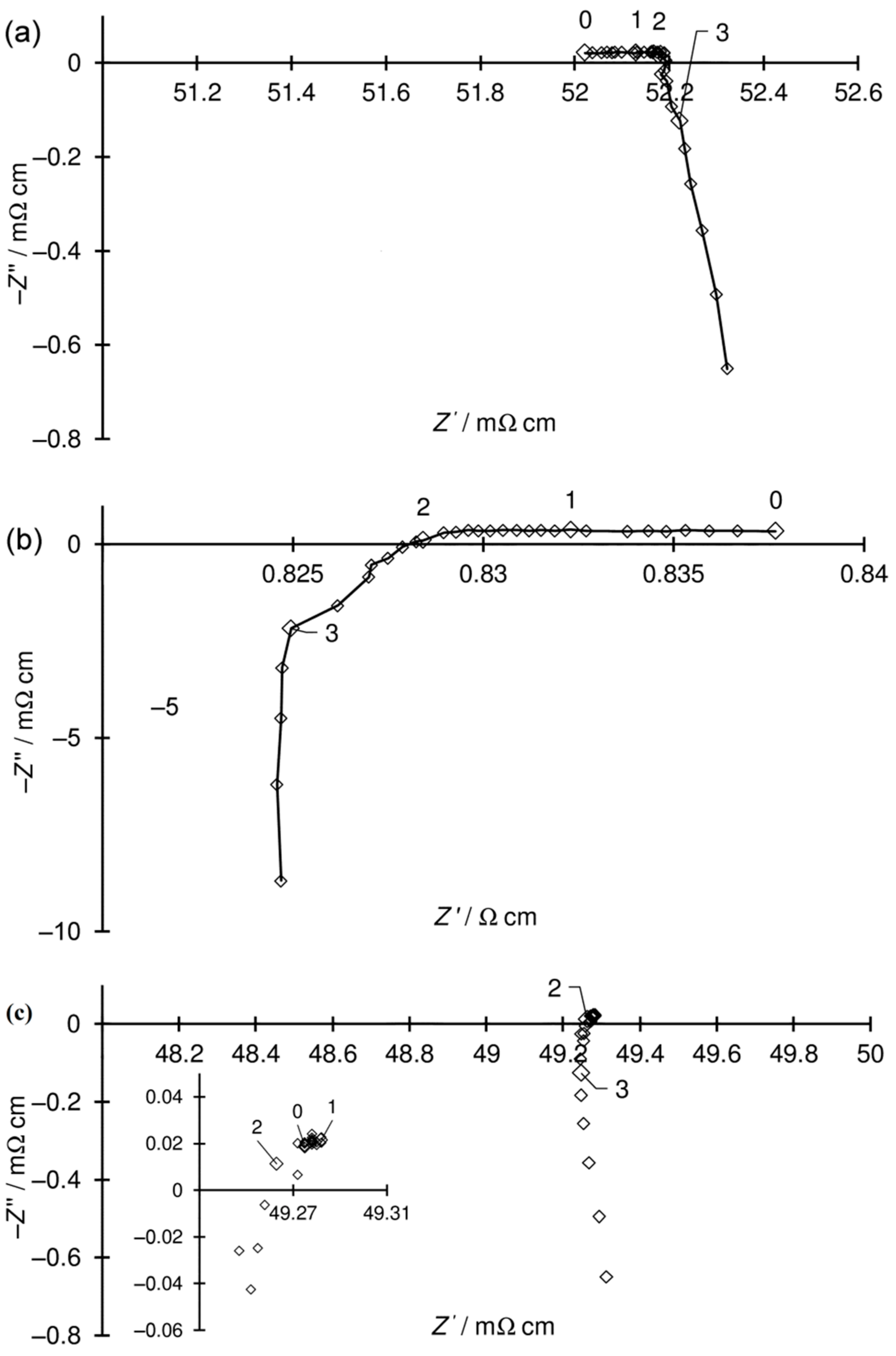
2.3. Electrical Measurements of the Ni–5CBCY Samples Using the AC Four-Wire Probe Method and Electrochemical Impedance Spectroscopy
3. Results
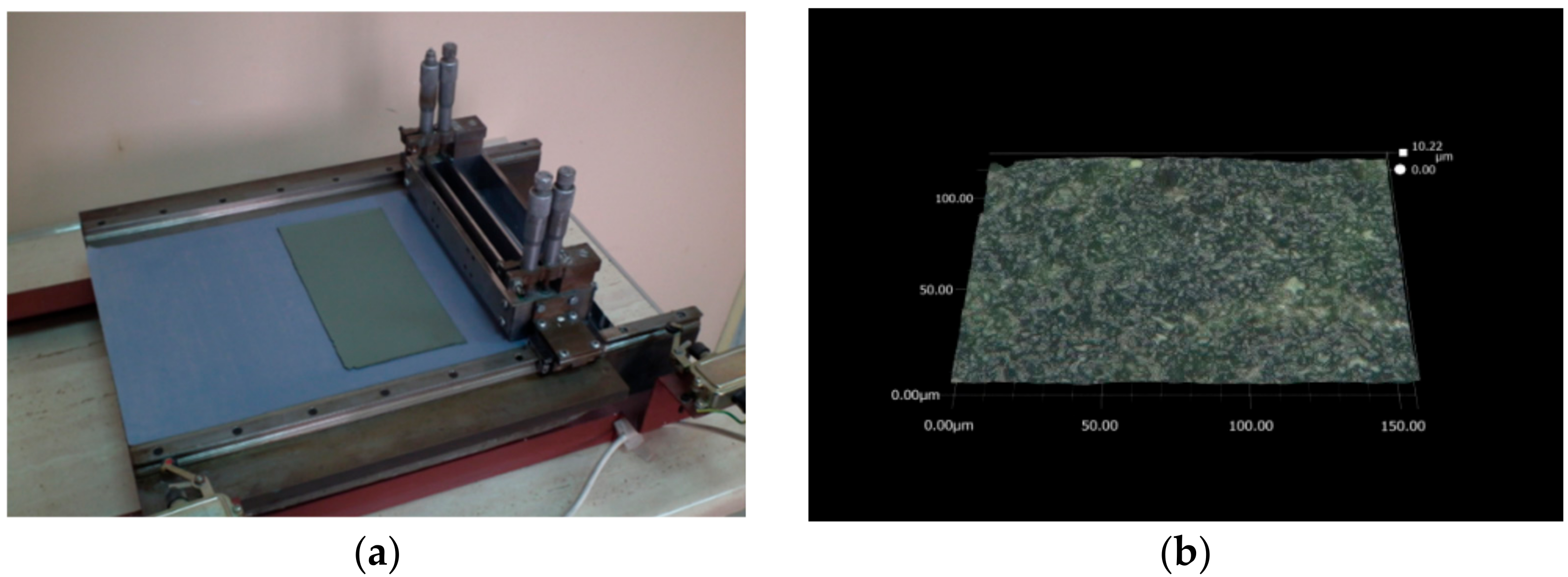
3.1. Optical Microscopy Characterisation of Green NiO–5CBCY Tape Obtained via the Tape Casting Method
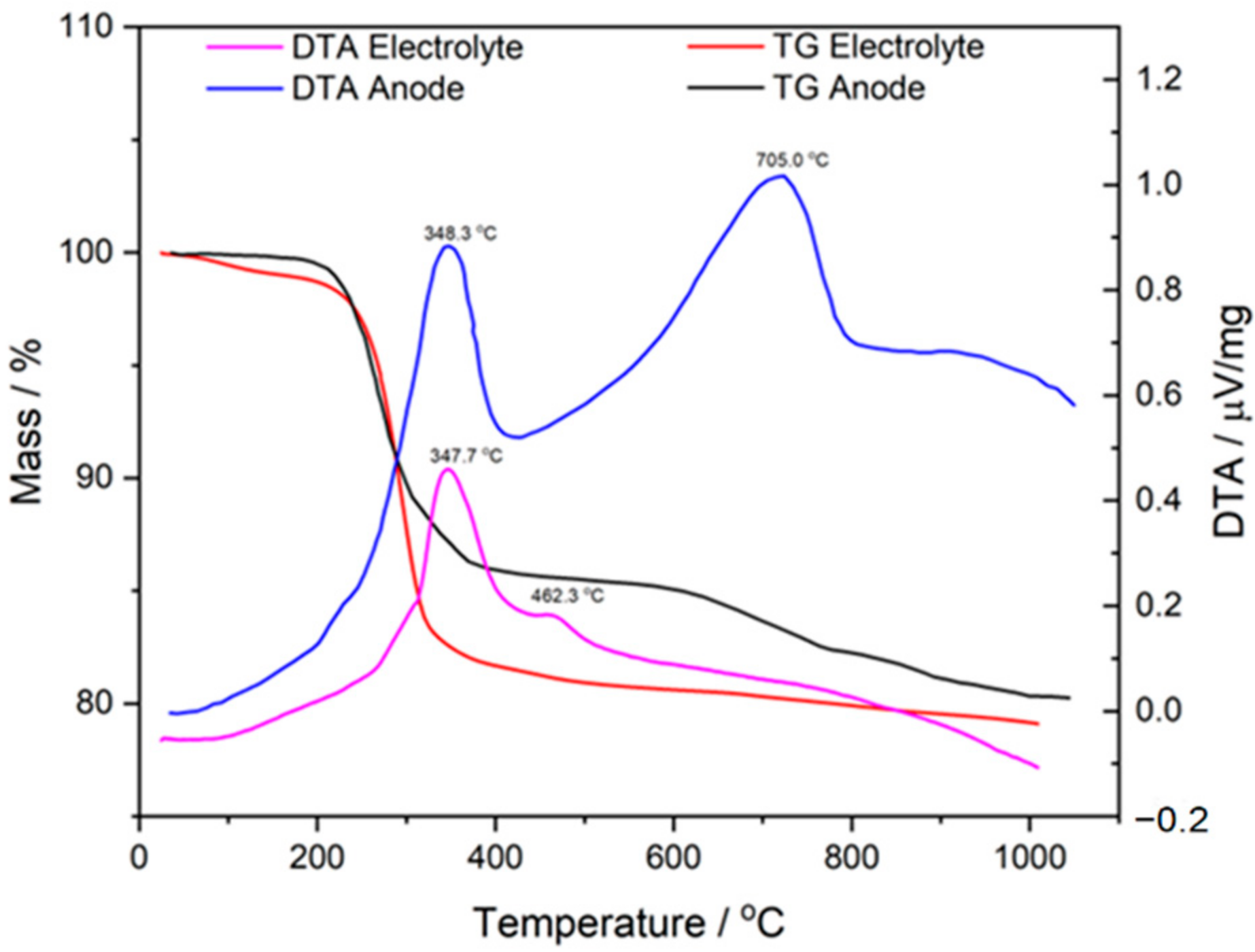
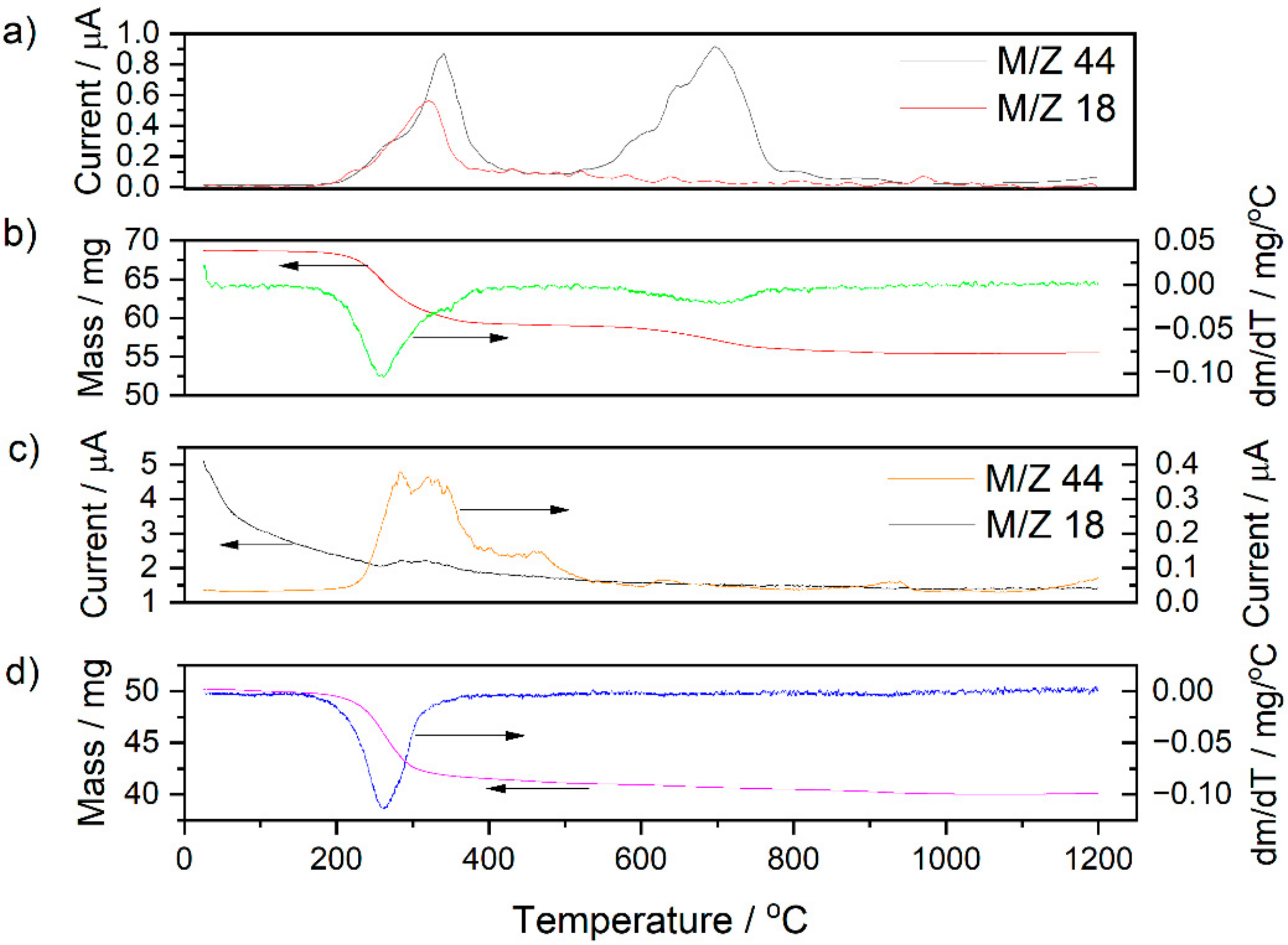
3.2. Analysis of NiO–5BCY Substrate during Thermal Treatment Using Thermal Analysis Methods
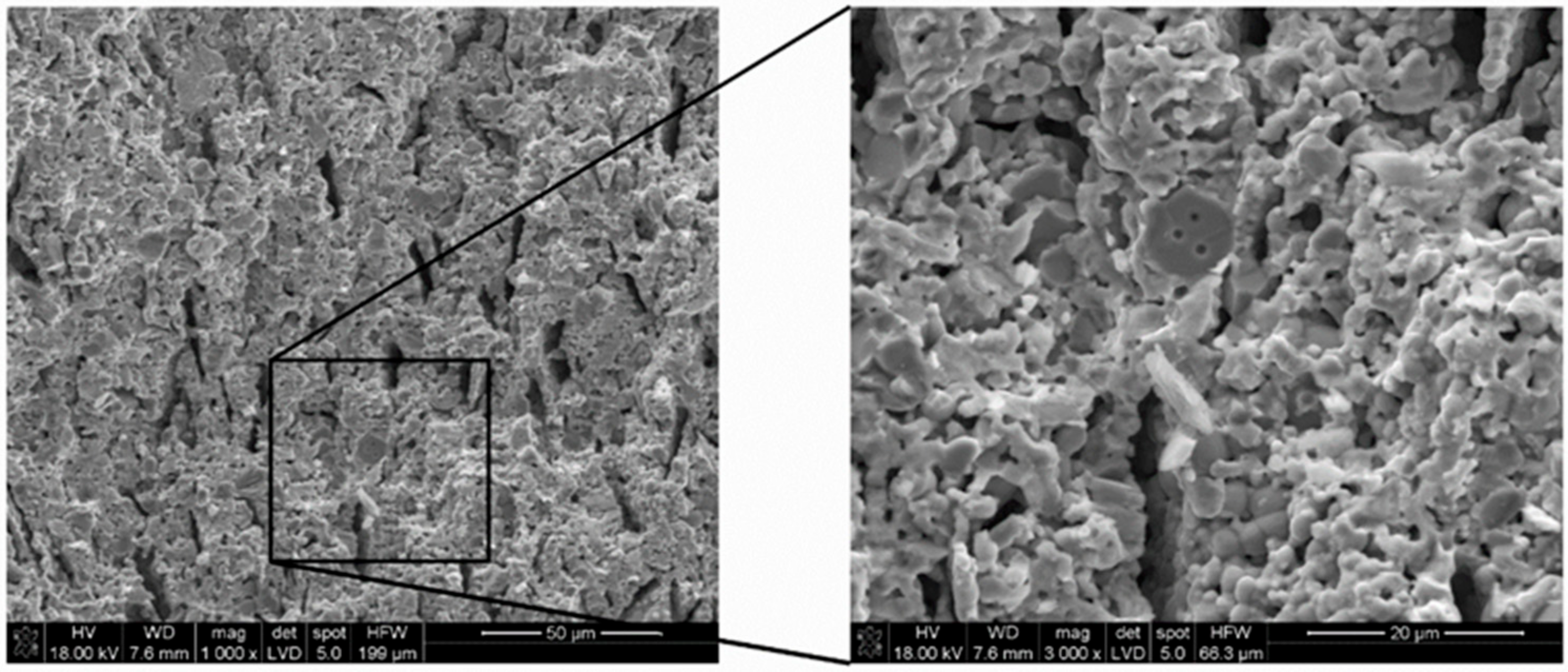
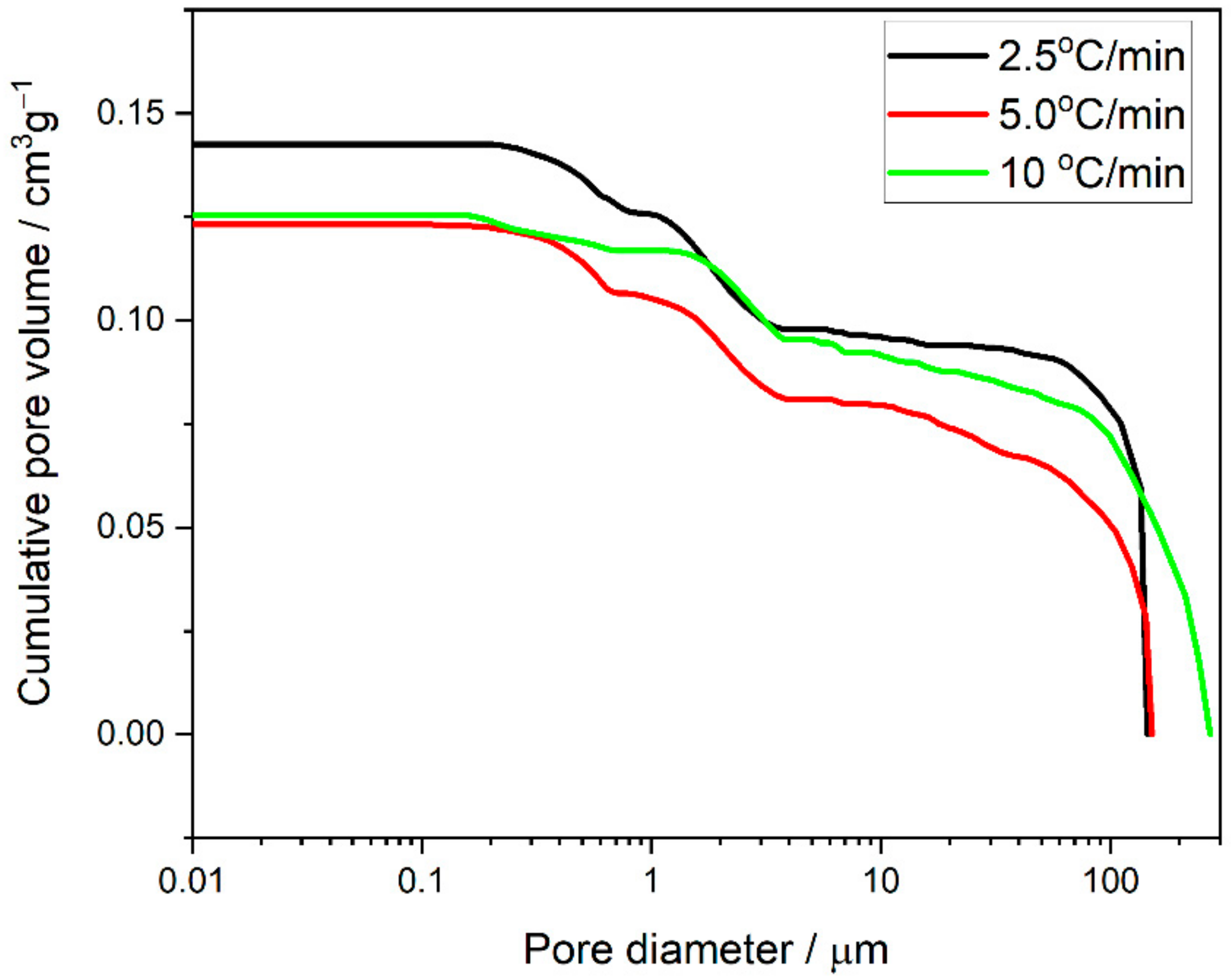
3.3. Scanning Electron Microscopy with Energy Dispersive X-ray Spectroscopy of the Ni–5CBCY Sample Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCFCs | ceramic proton-conducting fuel cells |
| SOFCs | solid oxide fuel cells |
| A-SOFCs | anode-supported solid oxide fuel cells |
| BCY | BaCe0.9Y0.1O3−δ, barium cerate-doped yttria oxide, electrolyte |
| 5CBCY | Ba0.95Ca0.05Ce0.9Y0.1O3−δ, co-doped calcia and yttria barium cerate |
| NiO–5CBCY | NiO-Ba0.95Ca0.05Ce0.9Y0.1O3−δ: sintered anode samples |
| Ni-5CBCY | Ni-Ba0.95Ca0.05Ce0.95Y0.1O3−δ: cermet obtained by thermally treating |
| NiO–5CBCY | in H2-Ar gas atmosphere at 700 °C for 2 h |
| DTA | differential thermal analysis |
| TG | thermogravimetry |
| QMS | quadrupole mass spectrometry |
| SEM | scanning electron microscopy |
| EPD | electrophoretic deposition layers |
| LSCF | La0.8Sr0.2Co0.8Fe0.2O3, cathode |
| YbBC | BaCe0.9Yb0.1O3, barium cerate-doped ytterbium oxide |
| σ | total electrical conductivity |
| RT | room temperature |
| HF | high-frequency |
| LF | low-frequency |
| EEC | electrical equivalent circuit |
References
- Rasaki, S.A.; Liu, C.; Lao, C.; Chen, Z. A review of current performance of rare earth metal-doped barium zirconate perovskite: The promising electrode and electrolyte material for the protonic ceramic fuel cells. Prog. Solid State Chem. 2021, 63, 100325. [Google Scholar] [CrossRef]
- Le, L.Q.; Hernandez, C.H.; Rodriguez, M.H.; Zhu, L.; Duan, C.; Ding, H.; O’Hayre, R.P.; Sullivan, N.P. Proton-conducting ceramic fuel cells: Scale up and stack integration. J. Power Source 2021, 482, 228868. [Google Scholar] [CrossRef]
- Zakowsky, N.; Williamson, S.; Irvine, J.T.S. Elaboration of CO2 tolerance limits of BaCe0.9Y0.1O3–δ electrolytes for fuel cells and other applications. Solid State Ion. 2005, 176, 3019–3026. [Google Scholar] [CrossRef]
- Ma, X.; Dai, J.; Zhang, H.; Reisner, D.E. Protonic conductivity nanostructured ceramic film with improved resistance to carbon dioxide at elevated temperatures. Surf. Coat. Technol. 2005, 200, 1252–1258. [Google Scholar] [CrossRef]
- Radojković, A.; Žunić, M.; Savić, S.M.; Branković, G.; Branković, Z. Enhanced stability in CO2 of Ta doped BaCe0.9Y0.1O3−δ electrolyte for intermediate temperature SOFCs. Ceram. Int. 2013, 39, 2631–2637. [Google Scholar] [CrossRef]
- Radojković, A.; Žunić, M.; Savić, S.M.; Perać, S.; Golić, D.L.; Branković, Z.; Branković, G. Co-doping as a strategy for tailoring the electrolyte properties of BaCe0.9Y0.1O3–δ. Ceram. Int. 2019, 45, 8279–8285. [Google Scholar] [CrossRef]
- Melnik, J.; Luo, J.J.; Chuang, K.T.; Sanger, A.R. Stability and electric conductivity of barium cerate perovskites co-doped with praseodymium. Open Fuels Energy Sci. J. 2008, 1, 7–10. [Google Scholar] [CrossRef]
- Dudek, M.; Lis, B.; Lach, R.; Daugėla, S.; Šalkus, T.; Kežionis, A.; Mosiałek, M.; Socha, R.P.; Morgiel, J.; Gajek, M.; et al. Ba0.95Ca0.05Ce0.9Y0.1O3 as an electrolyte for proton-conducting ceramic fuel cells. Electrochim. Acta 2019, 304, 70–79. [Google Scholar] [CrossRef]
- Dudek, M.; Lis, B.; Lach, R.; Daugėla, S.; Šalkus, T.; Kežionis, A.; Mosiałek, M.; Sitarz, M.; Rapacz-Kmita, A.; Grzywacz, P. Samples of Ba1−xSrxCe0.9Y0.1O3−δ, 0 < x < 0.1, with improved chemical stability in CO2-H2 gas-involving atmospheres as potential electrolytes for a proton ceramic fuel cell. Materials 2020, 13, 1874. [Google Scholar] [CrossRef] [Green Version]
- Bello, I.T.; Zhai, S.; Zhao, S.; Li, Z.; Yu, N.; Ni, M. Scientometric review of proton-conducting solid oxide fuel cells. Int. J. Hydrogen Energy 2021, 46, 37406–37428. [Google Scholar] [CrossRef]
- Ferguson, K.; Dubois, A.; Albrecht, K.; Braun, R.J. High performance protonic ceramic fuel cell systems for distributed power generation. Energy Convers. Manag. 2021, 248, 114763. [Google Scholar] [CrossRef]
- Majhi, S.M.; Behura, S.K.; Bhattacharjee, S.; Singh, B.P.; Chongdar, T.K.; Gokhale, N.M.; Besra, L. Anode supported solid oxide fuel cells (SOFC) by electrophoretic deposition. Int. J. Hydrogen Energy 2011, 22, 14930–14935. [Google Scholar] [CrossRef]
- Marrony, M. Proton-Conducting Ceramics: From Fundamentals to Applied Research, 2nd ed.; Pan Stanford Publishing Pte. Ltd.: Singapore, 2016. [Google Scholar]
- Taillades, G.; Batocchi, P.; Essoumhi, A.; Taillades, M.; Jones, D.J.; Rozière, J. Engineering of porosity, microstructure and electrical properties of Ni–BaCe0.9Y0.1O2.95 cermet fuel cell electrodes by gelled starch porogen processing. Microporous Mesoporous Mater. 2011, 145, 26–31. [Google Scholar] [CrossRef]
- Agarwal, V.; Liu, M. Electrochemical properties of BaCe0.8Gd0.2O3 electrolyte films deposited on Ni-BaCe0.8Gd0.2O3 substrates. J. Electrochem. Soc. 1997, 144, 1035–1040. [Google Scholar] [CrossRef]
- Essoumhi, A.; Taillades, G.; Taillades-Jacquin, M.; Jones, D.J.; Rozière, J. Synthesis and characterization of Ni-Cermet/Proton conducting thin film electrolyte symmetrical assemblies. Solid State Ion. 2008, 179, 2155–2159. [Google Scholar] [CrossRef]
- Chevallier, L.; Zunic, M.; Esposito, V.; Di Bartolomeo, E.; Traversa, E. A wet-chemical route for the preparation of Ni–BaCe0.9Y0.1O3−δ Cermet anodes for IT-SOFCs. Solid State Ion. 2009, 180, 715–720. [Google Scholar] [CrossRef]
- Zunic, M.; Chevallier, L.; Deganello, F.; D’Epifanio, A.; Licoccia, S.; Di Bartolomeo, E.; Traversa, E. Electrophoretic deposition of Dense BaCe0.9Y0.1O3−x electrolyte thick-films on Ni-based anodes for intermediate temperature solid oxide fuel cells. J. Power Source 2009, 190, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Cao, D.; Zhou, M.; Yan, X.; Liu, Z.; Liu, J. High performance low-temperature tubular protonic ceramic fuel cells based on barium cerate-zirconate electrolyte. Electrochem. Commun. 2021, 125, 106986. [Google Scholar] [CrossRef]
- Taillades, G.; Pers, P.; Mao, V.; Taillades, M. High performance anode-supported proton ceramic fuel cell elaborated by wet powder spraying. Int. J. Hydrogen Energy 2016, 41, 12330–12336. [Google Scholar] [CrossRef]
- Anggia, E.; Shin, E.-K.; Nam, J.-T.; Park, J.-S. Fabrication of ceramic composite anode at low temperature for high performance protonic ceramic fuel cells. Ceram. Int. 2020, 46, 236–242. [Google Scholar] [CrossRef]
- Bae, H.; Choi, G.M. Novel modification of anode microstructure for proton-conducting solid oxide fuel cells with BaZr0.8Y0.2O3−δ Electrolytes. J. Power Source 2015, 285, 431–438. [Google Scholar] [CrossRef]
- Xiao, J.; Sun, W.; Zhu, Z.; Tao, Z.; Liu, W. Fabrication and characterization of anode-supported dense BaZr0.8Y0.2O3−δ Electrolyte membranes by a dip-coating process. Mater. Lett. 2012, 73, 198–201. [Google Scholar] [CrossRef]
- Lis, B.; Dudek, M.; Kluczowski, R.; Krauz, M.; Kawalec, M.; Mosiałek, M.; Lach, R. Physicochemical properties of ceramic tape involving Ca0.05 Ba0.95Ce0.9Y0.1O3 as an electrolyte designed for electrolyte-supported solid oxide fuel cells (IT-SOFCs). J. Therm. Anal. Calorim. 2018, 133, 95–105. [Google Scholar] [CrossRef]
- Dudek, M.; Lis, B.; Kocyło, E.; Rapacz-Kmita, A.; Mosiałek, M.; Gajek, M.; Lach, R.; Presto, S.; Viviani, M.; Carpanese, M.P.; et al. Utilisation of methylcellulose as a shaping agent in the fabrication of Ba0.95Ca0.05Ce0.9Y0.1O3 proton-conducting ceramic membranes via the gelcasting method. J. Therm. Anal. Calorim. 2019, 138, 2077–2090. [Google Scholar] [CrossRef] [Green Version]
- James, F.; Roos, M. MINUIT: A system for function minimization and analysis of the parameter errors and corrections. Comput. Phys. Commun. 1975, 10, 343–367. [Google Scholar] [CrossRef]
- Mosiałek, M.; Nowak, P.; Dudek, M.; Mordarski, G. Oxygen reduction at the Ag|Gd0.2Ce0.8O1.9 interface studied by electrochemical impedance spectroscopy and cyclic voltammetry at the silver point electrode. Electrochim. Acta 2014, 120, 248–257. [Google Scholar] [CrossRef]
- Kim, H.; Kim, B.; Lee, J.; Ahn, K.; Kim, H.R.; Yoon, K.J.; Kim, B.K.; Cho, Y.W.; Lee, H.W.; Lee, J.H. Microstructural adjustment of Ni–BaCe0.9Y0.1O3−δ cermet membrane for improved hydrogen permeation. Ceram. Int. 2014, 40, 4117–4126. [Google Scholar] [CrossRef]
- Hao, X.; Han, D.; Wang, J.; Liu, Y.; Rooney, D.; Sun, W.; Qiao, J.; Wang, Z.; Sun, K. Co-tape casting fabrication, field assistant sintering and evaluation of a coke resistant La0.2Sr0.7TiO3–Ni/YSZ functional gradient anode supported solid oxide fuel cell. Int. J. Hydrogen Energy 2015, 40, 12790–12797. [Google Scholar] [CrossRef]
- Myung, J.H.; Ho Shin, T.; Kim, S.D.; Park, H.G.; Moon, J.; Hyun, S.H. Optimization of Ni–Zirconia based anode support for robust and high-performance 5 × 5 cm2 Sized SOFC via tape-casting/co-firing technique and nano-structured anode. Int. J. Hydrogen Energy 2015, 40, 2792–2799. [Google Scholar] [CrossRef]
- Agarkova, E.A.; Zadorozhnaya, O.Y.; Burmistrov, I.N.; Yalovenko, D.V.; Agarkov, D.A.; Rabotkin, S.V.; Solovyev, A.A.; Nepochatov, Y.K.; Levin, M.N.; Bredikhin, S.I. Relationships between mechanical stability of the anode supports and electrochemical performance of intermediate-temperature SOFCs. Mater. Lett. 2021, 303, 130516. [Google Scholar] [CrossRef]
- Fabbri, E.; Pergolesi, D.; Traversa, E. Materials challenges toward proton-conducting oxide fuel cells: A critical review. Chem. Soc. Rev. 2010, 39, 4355–4369. [Google Scholar] [CrossRef] [PubMed]
- Rainwater, B.H.; Liu, M.; Liu, M. A more efficient anode microstructure for SOFCs based on proton conductors. Int. J. Hydrogen Energy 2012, 37, 18342–18348. [Google Scholar] [CrossRef]
- Nasani, N.; Ramasamy, D.; Antunes, I.; Perez, J. Electrochemical behaviour of Ni-BZO and Ni-BZY cermet anodes for Protonic Ceramic Fuel Cells (PCFCs)–A comparative study. Electrochim. Acta 2015, 154, 387–396. [Google Scholar] [CrossRef]
- Drożdż-Cieśla, E.; Wyrwa, J.; Broś, J.; Rękas, M. Structural, microstructural, thermal, and electrical properties of Ni/YSZ Cermet materials. J. Therm. Anal. Calorim. 2012, 108, 1051–1057. [Google Scholar] [CrossRef] [Green Version]
- Prakash, B.S.; Kumar, S.S.; Aruna, S.T. Properties and development of Ni/YSZ as an anode material in solid oxide fuel cell: A review. Renew. Sust. Energy Rev. 2014, 36, 149–179. [Google Scholar] [CrossRef]
- Marinšek, M.; Pejovnik, S.; Maček, J. Modelling of electrical properties of Ni-YSZ composites. J. Eur. Ceram. 2007, 7, 149–179. [Google Scholar] [CrossRef]
- Dailly, J.; Marrony, M.; Taillades, G.; Taillades-Jacquin, M.; Grimaud, A.; Mauvy, F.; Louradour, E.; Salmi, J. Evaluation of proton conducting BCY10-based anode supported cells by co-pressing method: Up-scaling, performances and durability. J. Power Source 2014, 255, 302–307. [Google Scholar] [CrossRef]
- Baquero, T.; Escobar, J.; Frade, J.; Hotza, D. Aqueous tape casting of micro and nano YSZ for SOFC electrolytes. Ceram. Int. 2013, 39, 8279–8285. [Google Scholar] [CrossRef]
- Narendar, N.; Mather, G.M.; Dias, P.A.N.; Duncan, P.F. The importance of phase purity in Ni–BaZr0.85Y0.15O3−δ cermet anodes—Novel nitrate-free combustion route and electrochemical study. RSC Adv. 2013, 3, 859–869. [Google Scholar] [CrossRef]
- Zunic, M.; Chevallier, L.; Radojkovic, A.; Brankovic, G.; Brankovic, Z.; Bartolomeo, E.D. Influence of the ratio between Ni and BaCe0.9Y0.1O3−δ on microstructural and electrical properties of proton conducting Ni–BaCe0.9Y0.1O3−δ anodes. J. Alloys Compd. 2011, 509, 1157–1162. [Google Scholar] [CrossRef]
- Tomczyk, P.; Zurek, S.; Mosiałek, M. Effect of time and polarization on kinetics of the oxygen electrode reaction at an Au Ι YSZ interface. J. Electroceram. 2009, 23, 25–36. [Google Scholar] [CrossRef]
- Drożdż-Cieśla, E.; Wrwa, J.; Pyda, W.; Rękas, M. A new method of preparing Ni / YSZ cermet materials. J. Mater. Sci. 2012, 47, 2807–2817. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance Spectroscopy; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Mosiałek, M.; Dudek, M.; Michna, A.; Tatko, M.; Kędra, A.; Zimowska, M. Composite cathode materials Ag-Ba0.5Sr0.5Co0.8Fe0.2O3 for solid oxide fuel cells. J. Solid State Electrochem. 2014, 18, 3011–3021. [Google Scholar] [CrossRef] [Green Version]
- Kubota, M.; Okanishi, T.; Muroyama, H.; Matsui, T.; Eguchi, K. Microstructural evolution of Ni–YSZ cermet anode under thermal cycles with redox treatments. J. Electrochem. Soc. 2015, 162, F380–F386. [Google Scholar] [CrossRef]
- Nasani, N.; Wang, Z.J.; Willinger, M.G.; Yaremchenko, A.A.; Fagg, D.P. In-Situ redox cycling behaviour of Ni–BaZr0.85Y0.15O3−δ cermet anodes for protonic ceramic fuel cells. Int. J. Hydrogen Energy 2014, 39, 19780–19788. [Google Scholar] [CrossRef]
- Shen, C.T.; Lee, Y.H.; Xie, K.; Yen, C.P.; Jhuang, J.W.; Lee, K.R.; Lee, S.W.; Tseng, C.J. Correlation between microstructure and catalytic and mechanical properties during redox cycling for Ni-BCY and Ni-BCZY composites. Ceram. Int. 2017, 43, S671–S674. [Google Scholar] [CrossRef]
- Matsumoto, H.; Nomura, I.; Okada, S.; Ishihara, T. Intermediate-temperature solid oxide fuel cells using perovskite-type oxide based on barium cerate. Solid State Ion. 2008, 179, 1486–1489. [Google Scholar] [CrossRef]
- Rashid, N.L.R.M.; Samat, A.A.; Jais, A.A.; Somalu, M.R.; Muchtar, A.; Baharuddin, N.A.; Wan Isahak, W.N.R. Review on zirconate-cerate-based electrolytes for proton-conducting solid oxide fuel cell. Ceram. Int. 2019, 45, 6605–6615. [Google Scholar] [CrossRef]


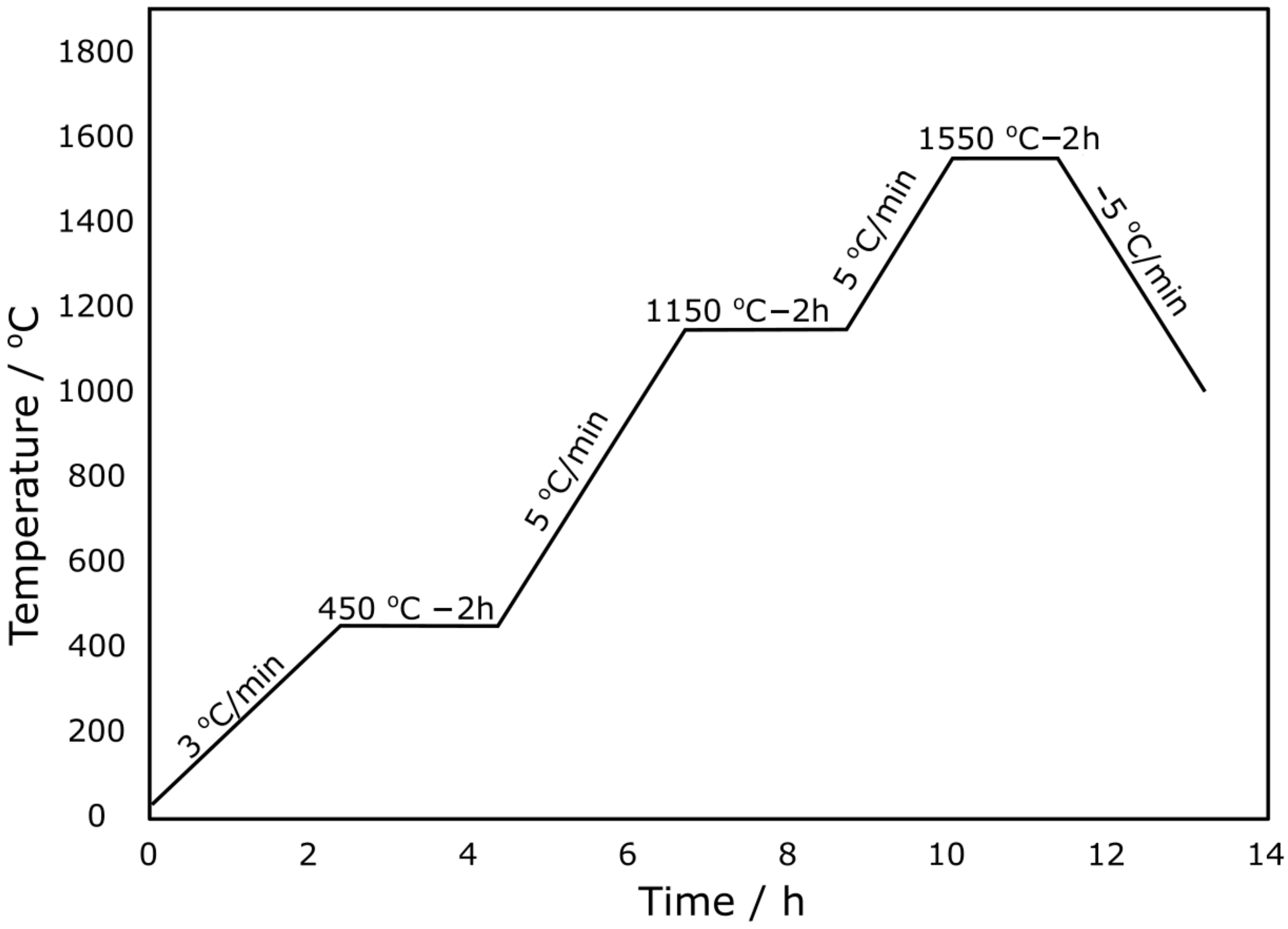
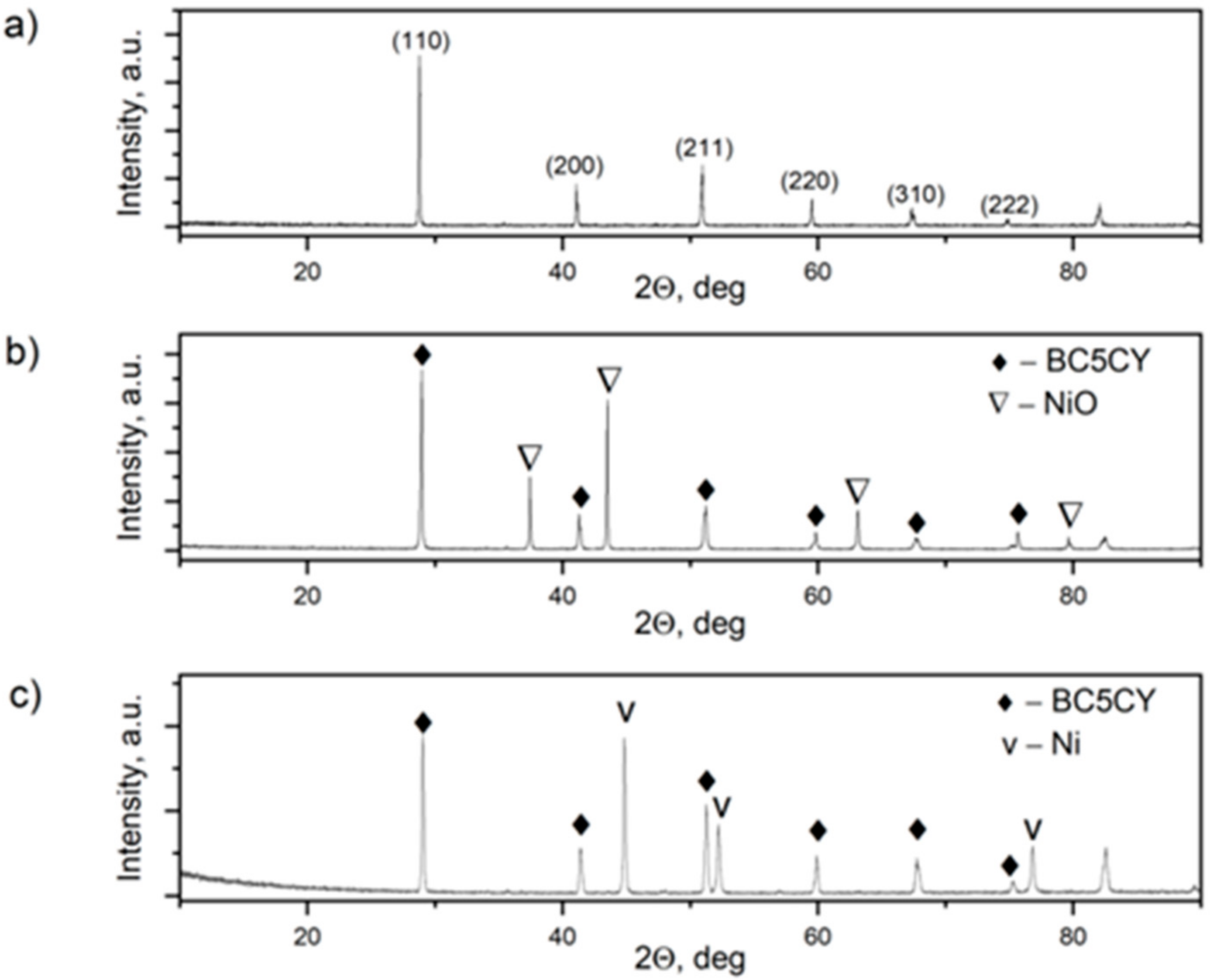
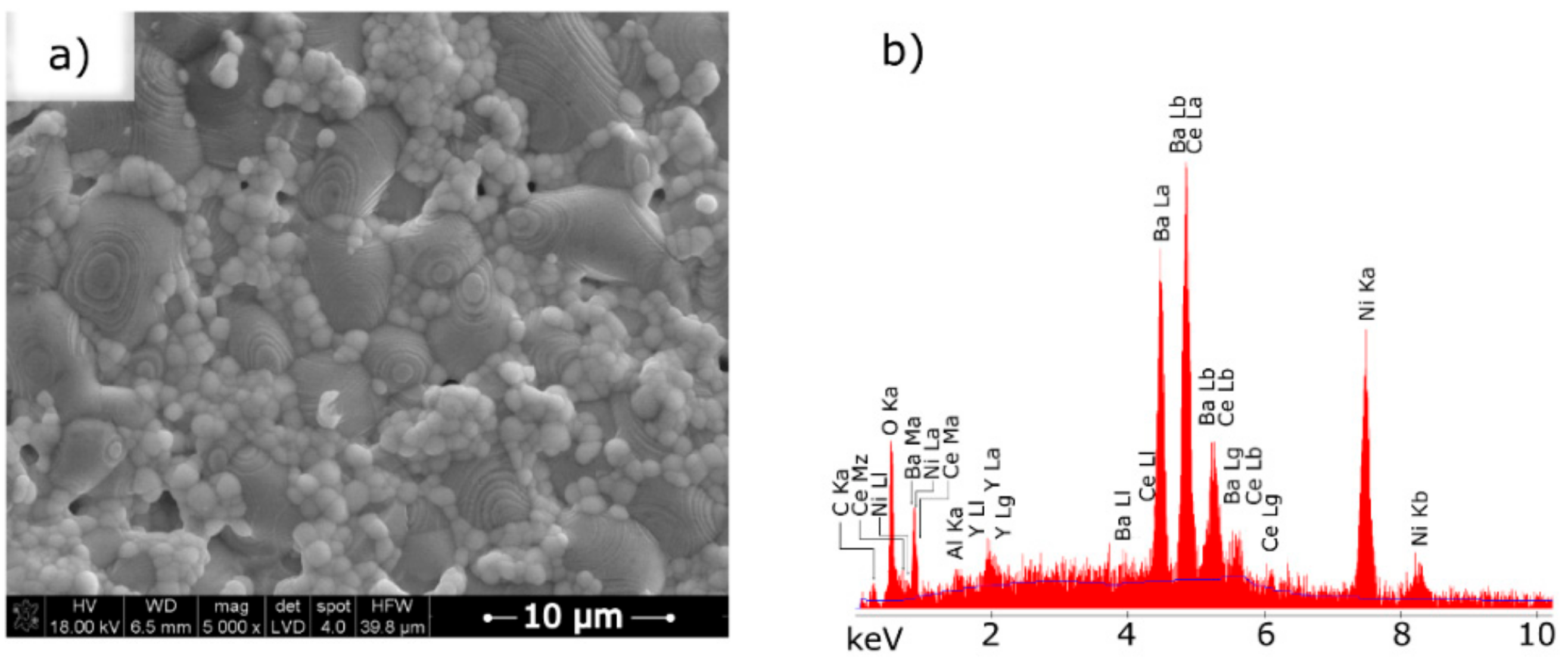
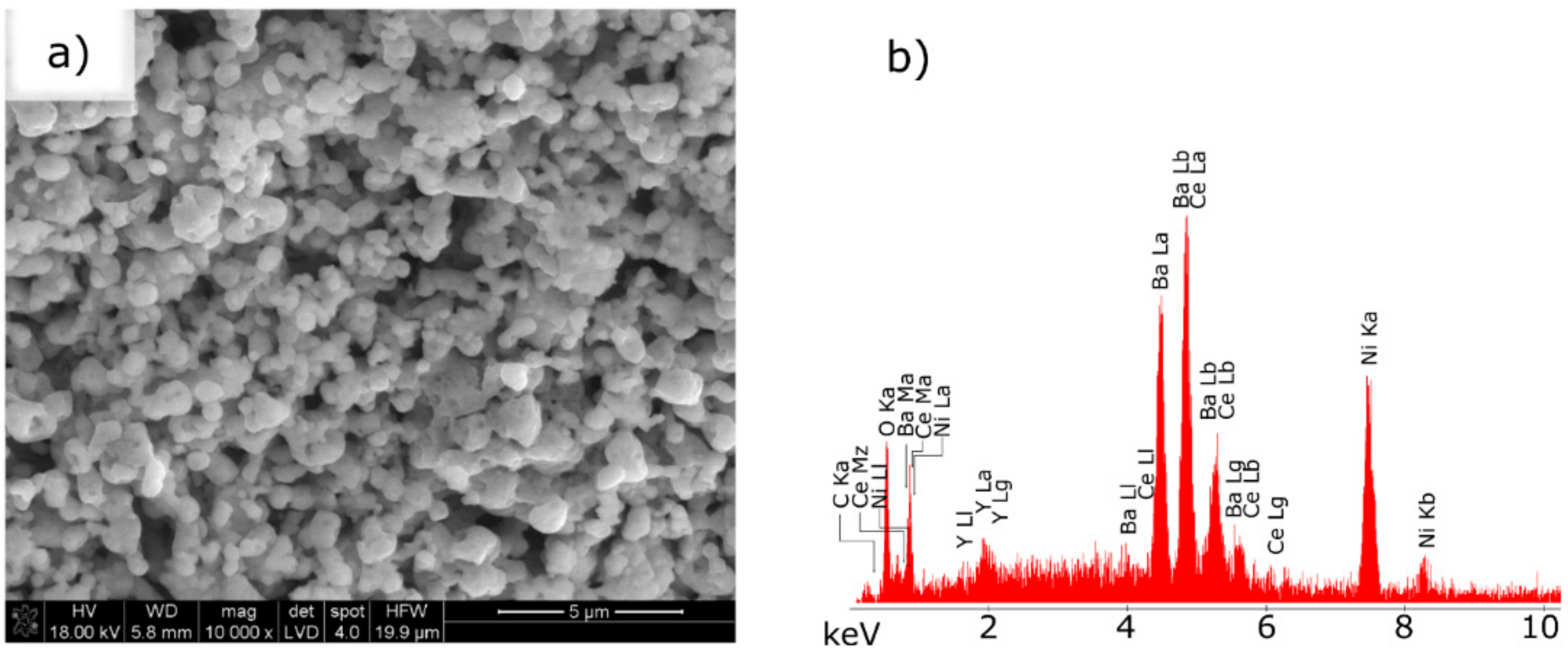

| Component | Mass/g |
|---|---|
| BC5CY electrolyte powder | 38 |
| Nickel (II) oxide | 57 |
| Graphite | 5 |
| Organic binder PVB, plasticiser, solvent | 85 |
| No | σ/S cm−1 | Anode Material/Conditions | Ref. |
|---|---|---|---|
| 1 | 1.1 | Ni–5CBCY (57 wt% NiO) Humidified 10 vol.% H2 in Ar | This work |
| 2 | ~3.1 | Humidified 10 vol.% H2 Ni–BZY—50 vol.% Ni BZY = BaZr0.85Y0.15O3−d | [40] |
| 3 | ~40 | Ni–BCY (40–60 wt% NiO) Humidified H2 | [41] |
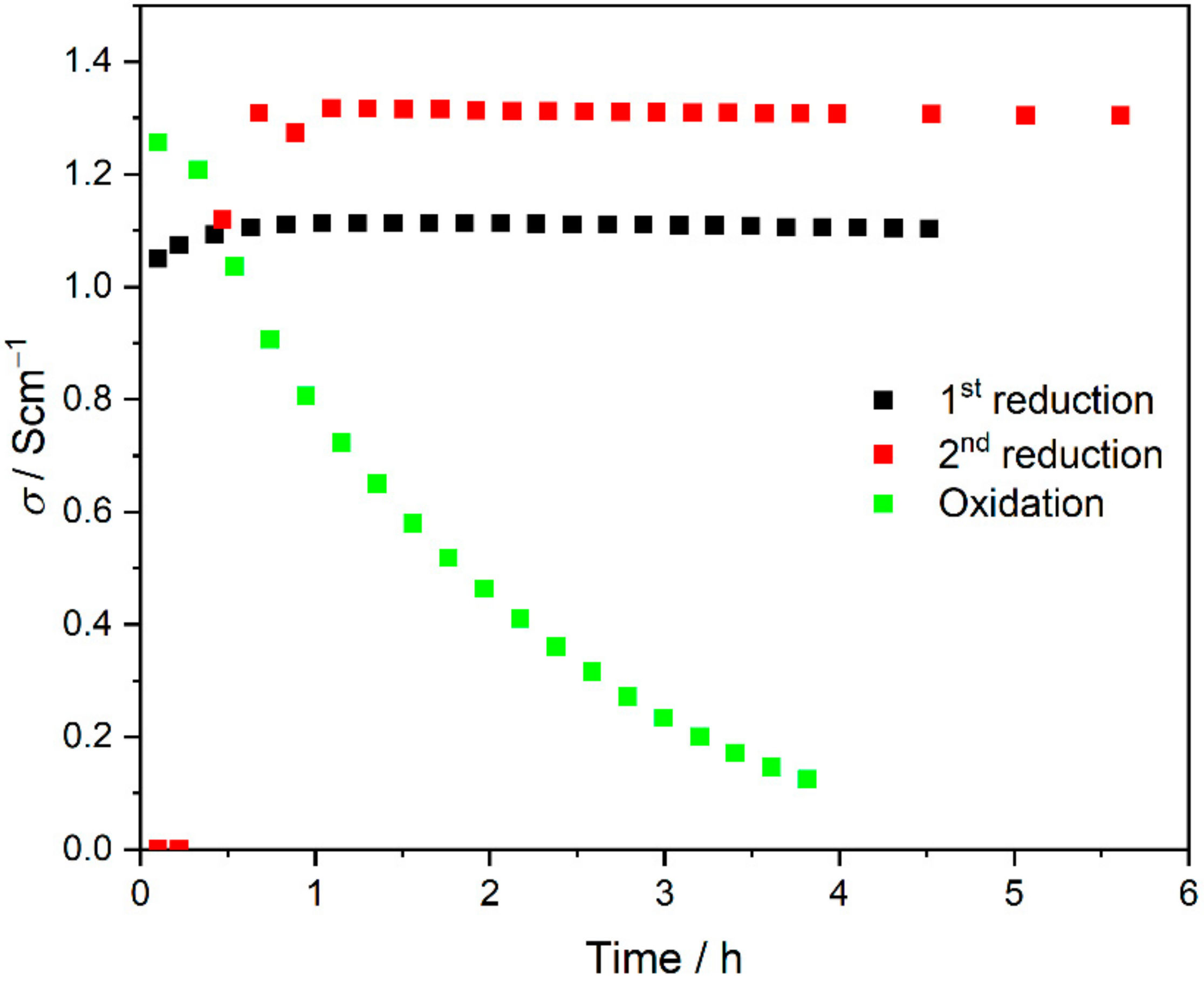
| t/h | s/% | L/mH | RL/W cm | σ/S cm−1 |
|---|---|---|---|---|
| 0.00 | 1.06 | 0.410 | 0.95 | 1.05 |
| 0.12 | 0.88 | 0.412 | 0.93 | 1.07 |
| 0.33 | 0.88 | 0.410 | 0.91 | 1.09 |
| 0.53 | 0.90 | 0.409 | 0.90 | 1.11 |
| 0.73 | 0.91 | 0.408 | 0.90 | 1.11 |
| 0.94 | 0.91 | 0.407 | 0.90 | 1.11 |
| 1.14 | 0.90 | 0.407 | 0.90 | 1.11 |
| 1.35 | 0.91 | 0.406 | 0.90 | 1.11 |
| 1.55 | 0.90 | 0.405 | 0.90 | 1.11 |
| 1.76 | 0.91 | 0.405 | 0.90 | 1.11 |
| 1.96 | 0.92 | 0.405 | 0.90 | 1.11 |
| 2.17 | 0.89 | 0.405 | 0.90 | 1.11 |
| 2.37 | 1.08 | 0.406 | 0.90 | 1.11 |
| 2.57 | 0.91 | 0.405 | 0.90 | 1.11 |
| 2.78 | 0.68 | 0.409 | 0.90 | 1.11 |
| 2.98 | 0.89 | 0.405 | 0.90 | 1.11 |
| 3.19 | 0.81 | 0.407 | 0.90 | 1.11 |
| 3.39 | 0.90 | 0.405 | 0.90 | 1.11 |
| 3.60 | 0.91 | 0.405 | 0.90 | 1.11 |
| 3.80 | 0.91 | 0.405 | 0.90 | 1.11 |
| 4.01 | 0.89 | 0.405 | 0.90 | 1.11 |
| 4.21 | 0.91 | 0.404 | 0.91 | 1.10 |
| 4.41 | 0.91 | 0.405 | 0.91 | 1.10 |
| t/h | s/% | R1/W cm | L/nH | RL/W cm | σ/S cm−1 |
|---|---|---|---|---|---|
| 0.37 | 2.88 | 1.55 | 0.011 | 2.10 | 1.12 |
| 0.58 | 0.38 | 8.56 | 0.575 | 0.84 | 1.31 |
| 0.99 | 1.75 | 1.58 | 9.21 | 1.46 | 1.32 |
| 1.20 | 1.41 | 3.73 | 51.63 | 0.96 | 1.31 |
| 1.41 | 3.06 | 1.39 | 0.91 | 1.68 | 1.31 |
| 1.62 | 0.79 | 1.73 | 0.228 | 1.36 | 1.31 |
| 1.82 | 2.37 | 3.09 | 1.00 | 1.01 | 1.31 |
| 2.03 | 1.50 | 1.72 | 0.99 | 1.37 | 1.31 |
| 2.23 | 1.53 | 1.12 | 0.58 | 2.39 | 1.31 |
| 2.23 | 2.86 | 3.04 | 0.70 | 1.03 | 1.30 |
| 2.44 | 0.60 | 1.98 | 0.011 | 1.24 | 1.31 |
| 2.65 | 1.22 | 2.15 | 0.060 | 1.18 | 1.31 |
| 2.86 | 0.84 | 3.73 | 0.012 | 0.96 | 1.31 |
| 3.06 | 0.97 | 3.62 | 0.68 | 0.97 | 1.31 |
| 3.26 | 5.03 | 2.86 | 0.90 | 1.04 | 1.31 |
| 3.47 | 4.93 | 2.38 | 1.06 | 1.13 | 1.31 |
| 3.68 | 2.92 | 1.52 | 1.60 | 1.55 | 1.30 |
| 3.89 | 2.03 | 3.21 | 0.76 | 1.00 | 1.31 |
| 4.43 | 0.79 | 3.06 | 0.710 | 1.02 | 1.31 |
| 4.97 | 0.53 | 5.36 | 0.542 | 0.89 | 1.31 |
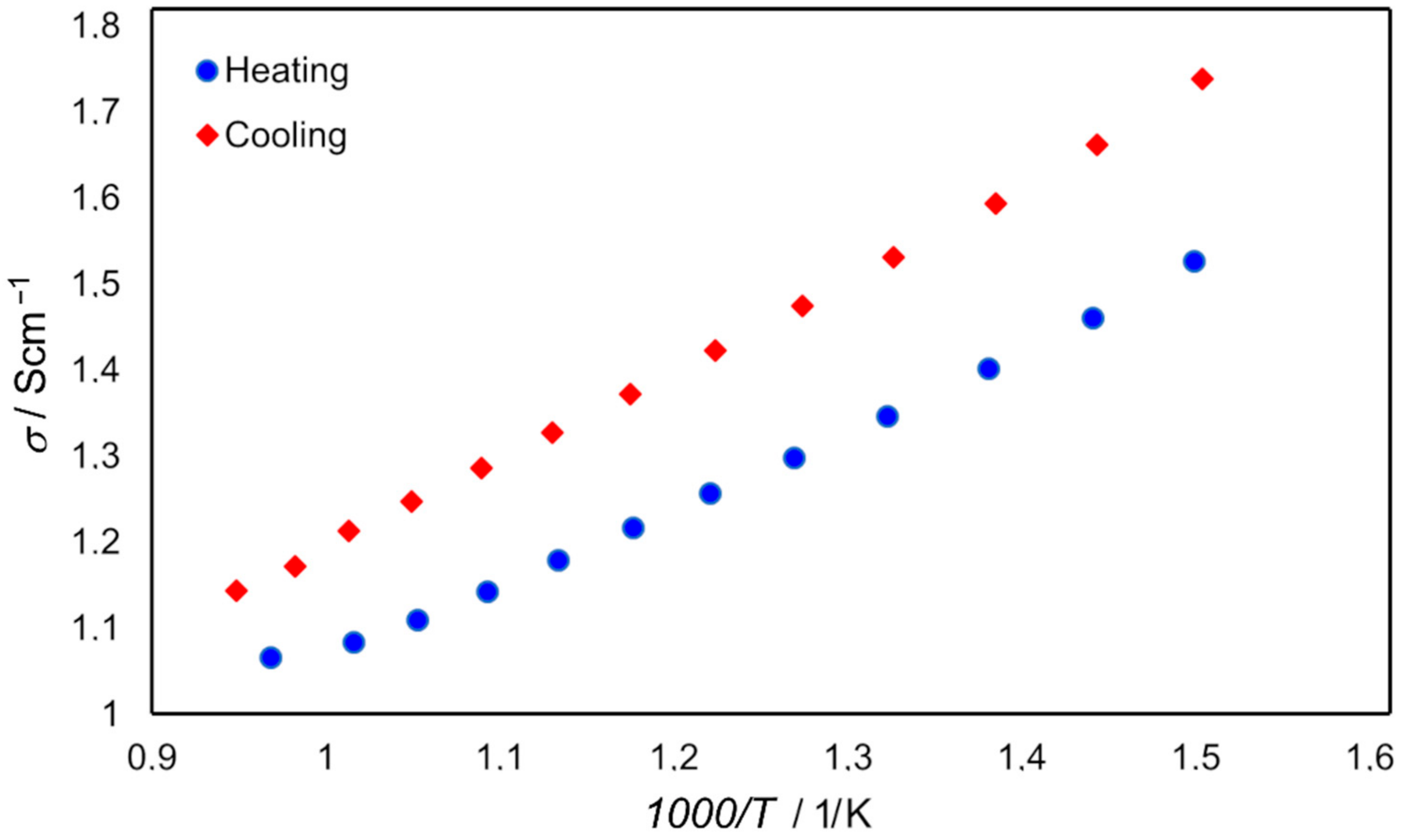
| T/°C | s/% | R1/W cm | L/mH | RL/W cm | σ/S cm−1 |
|---|---|---|---|---|---|
| 392 | 2.96 | 4.49 | 0.53 | 0.66 | 1.73 |
| 420 | 1.81 | 3.56 | 0.59 | 0.72 | 1.67 |
| 449 | 1.62 | 3.86 | 0.60 | 0.75 | 1.60 |
| 481 | 2.09 | 2.02 | 0.93 | 0.96 | 1.53 |
| 512 | 2.70 | 3.39 | 0.65 | 0.85 | 1.47 |
| 544 | 2.10 | 24.18 | 0.41 | 0.73 | 1.42 |
| 578 | 1.59 | 11.28 | 0.45 | 0.78 | 1.37 |
| 612 | 0.24 | 14.76 | 0.42 | 0.79 | 1.33 |
| 645 | 2.08 | 5.60 | 0.54 | 0.90 | 1.28 |
| 680 | 1.76 | 18.54 | 0.41 | 0.84 | 1.25 |
| 714 | 1.47 | 18.37 | 0.41 | 0.86 | 1.21 |
| 745 | 1.45 | 3.15 | 0.77 | 1.17 | 1.17 |
| 748 | 1.71 | 4.60 | 0.59 | 1.05 | 1.17 |
| 781 | 1.73 | 5.00 | 0.58 | 1.06 | 1.15 |
| T/°C | s/% | R1/W cm | L/mH | RL/W cm | σ/S cm−1 |
|---|---|---|---|---|---|
| 394 | 0.73 | 3.77 | 0.64 | 0.79 | 1.53 |
| 421 | 0.61 | 4.00 | 0.62 | 0.83 | 1.46 |
| 451 | 1.88 | 2.96 | 0.79 | 0.94 | 1.40 |
| 483 | 1.24 | 8.22 | 0.49 | 0.82 | 1.35 |
| 515 | 0.31 | 4.93 | 0.56 | 0.91 | 1.30 |
| 546 | 0.43 | 10.24 | 0.49 | 0.86 | 1.26 |
| 577 | 1.17 | 12.71 | 0.45 | 0.88 | 1.22 |
| 609 | 1.13 | 10.35 | 0.47 | 0.92 | 1.18 |
| 642 | 0.40 | 4.96 | 0.54 | 1.06 | 1.14 |
| 677 | 0.58 | 3.67 | 0.71 | 1.19 | 1.11 |
| 711 | 3.45 | 14.64 | 0.39 | 0.99 | 1.08 |
| 760 | 2.41 | 6.15 | 0.53 | 1.11 | 1.07 |
| t/h | s/% | L/mH | RL/W cm | σ/S cm−1 |
|---|---|---|---|---|
| 0.00 | 0.88 | 0.406 | 0.797 | 1.25 |
| 0.23 | 1.33 | 0.397 | 0.83 | 1.20 |
| 0.44 | 1.33 | 0.379 | 0.97 | 1.03 |
| 0.64 | 1.36 | 0.371 | 1.10 | 0.91 |
| 0.85 | 0.89 | 0.361 | 1.24 | 0.806 |
| 1.05 | 0.86 | 0.356 | 1.38 | 0.724 |
| 1.25 | 1.00 | 0.336 | 1.54 | 0.651 |
| 1.46 | 0.95 | 0.329 | 1.72 | 0.580 |
| 1.66 | 1.59 | 0.298 | 1.94 | 0.517 |
| 1.85 | 1.81 | 0.340 | 2.17 | 0.460 |
| t/h | s/% | R0/Ω cm | R1/Ω cm | R2/Ω cm | R3/Ω cm | C1/pF cm−1 | C2/μF cm−1 | C3/μF cm−1 | α1 | α2 | α3 | σ/mS cm−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.98 | 3.85 | 0.0 | 97.3 | 6.0 | 1.22 | 393 | 12.7 | 1510 | 1.00 | 0.74 | 0.67 | 10.3 |
| 2.18 | 4.89 | 0.0 | 113.0 | 5.3 | 3.15 | 235 | 4.95 | 107 | 1.20 | 1.00 | 0.87 | 8.85 |
| 2.39 | 0.5 | 0.0 | 123.8 | 4.49 | 10.0 | 153 | 2.70 | 7.55 | 1.08 | 0.61 | 0.59 | 8.08 |
| 2.59 | 0.17 | 0.0 | 139.4 | 5.61 | 11.7 | 135 | 2.04 | 7.25 | 1.10 | 0.74 | 0.58 | 7.17 |
| 2.8 | 0.16 | 0.0 | 154.2 | 7.09 | 12.3 | 119 | 1.40 | 7.32 | 1.13 | 0.78 | 0.61 | 6.49 |
| 3 | 0.19 | 0.0 | 166.4 | 10.3 | 12.4 | 137 | 1.99 | 6.62 | 1.01 | 0.60 | 0.55 | 6.01 |
| 3.21 | 0.57 | 14.8 | 166.9 | 7.70 | 15.4 | 122 | 1.25 | 6.53 | 1.16 | 0.81 | 0.64 | 5.50 |
| 3.42 | 2.38 | 4.78 | 190.4 | 31.0 | 3.25 | 80 | 1.17 | 88 | 1.15 | 0.53 | 0.76 | 5.12 |
| 3.62 | 0.51 | 15.2 | 198.5 | 21.6 | 9.82 | 104 | 1.09 | 13.0 | 1.11 | 0.62 | 0.70 | 4.68 |
| 3.83 | 0.12 | 10.1 | 218.2 | 22.14 | 12.0 | 93.5 | 0.89 | 10.30 | 1.10 | 0.65 | 0.70 | 4.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudek, M.; Lis, B.; Kluczowski, R.; Krauz, M.; Ziąbka, M.; Gajek, M.; Rapacz-Kmita, A.; Mosiałek, M.; Dudek, P.; Majda, D.; et al. NiO–Ba0.95Ca0.05Ce0.9Y0.1O3−δ as a Modified Anode Material Fabricated by the Tape Casting Method. Materials 2022, 15, 2489. https://doi.org/10.3390/ma15072489
Dudek M, Lis B, Kluczowski R, Krauz M, Ziąbka M, Gajek M, Rapacz-Kmita A, Mosiałek M, Dudek P, Majda D, et al. NiO–Ba0.95Ca0.05Ce0.9Y0.1O3−δ as a Modified Anode Material Fabricated by the Tape Casting Method. Materials. 2022; 15(7):2489. https://doi.org/10.3390/ma15072489
Chicago/Turabian StyleDudek, Magdalena, Bartłomiej Lis, Ryszard Kluczowski, Mariusz Krauz, Magdalena Ziąbka, Marcin Gajek, Alicja Rapacz-Kmita, Michał Mosiałek, Piotr Dudek, Dorota Majda, and et al. 2022. "NiO–Ba0.95Ca0.05Ce0.9Y0.1O3−δ as a Modified Anode Material Fabricated by the Tape Casting Method" Materials 15, no. 7: 2489. https://doi.org/10.3390/ma15072489
APA StyleDudek, M., Lis, B., Kluczowski, R., Krauz, M., Ziąbka, M., Gajek, M., Rapacz-Kmita, A., Mosiałek, M., Dudek, P., Majda, D., & Raźniak, A. (2022). NiO–Ba0.95Ca0.05Ce0.9Y0.1O3−δ as a Modified Anode Material Fabricated by the Tape Casting Method. Materials, 15(7), 2489. https://doi.org/10.3390/ma15072489








