Eu-Doped Pyrochlore Crystal Nano-Powders as Fluorescent Solid for Fingerprint Visualization and for Anti-Counterfeiting Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Phosphor Samples
2.2. Ball Milling
2.3. Characterization
3. Results
3.1. Structure of the Prepared Samples
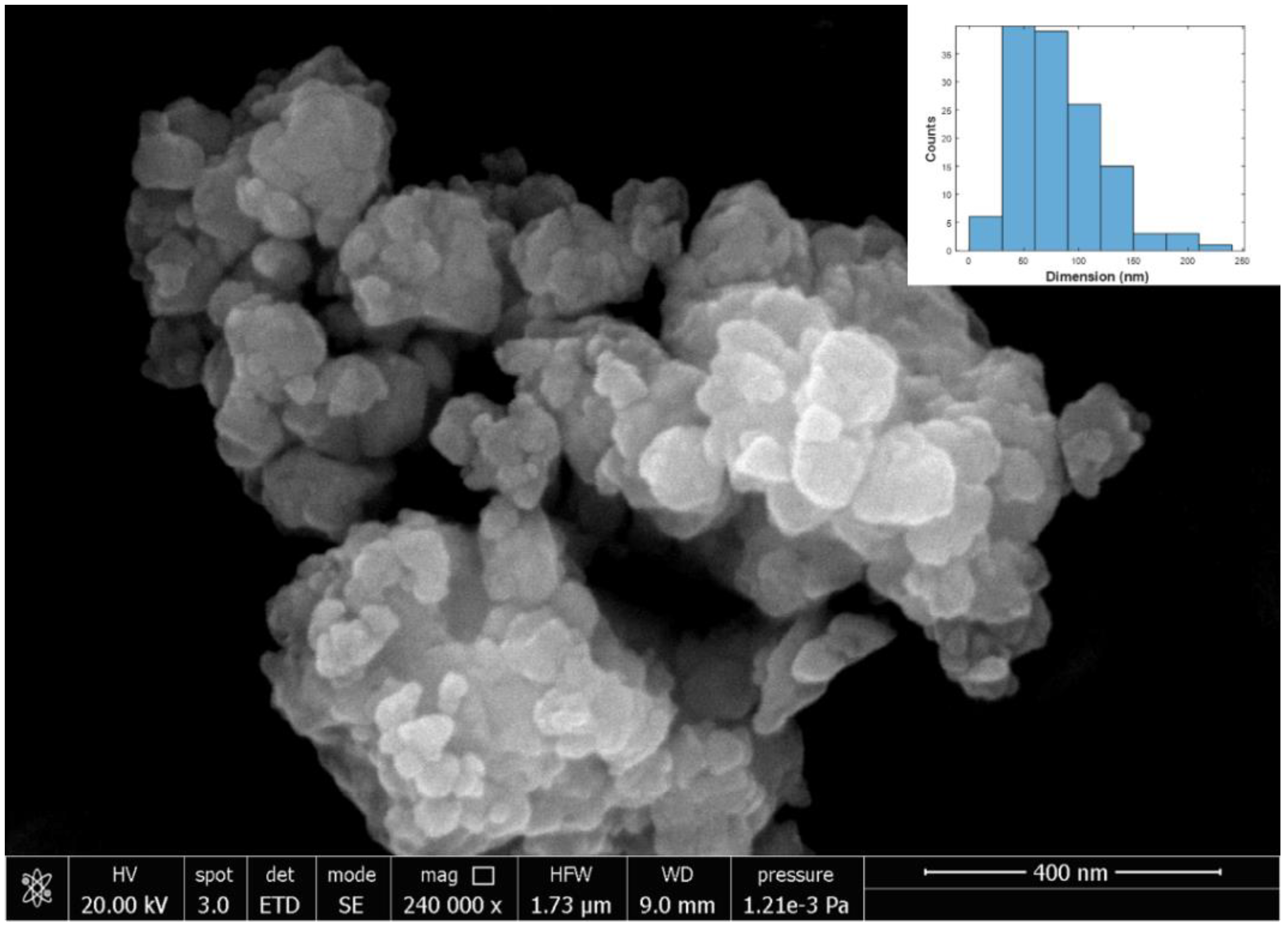
3.2. SEM Characterization
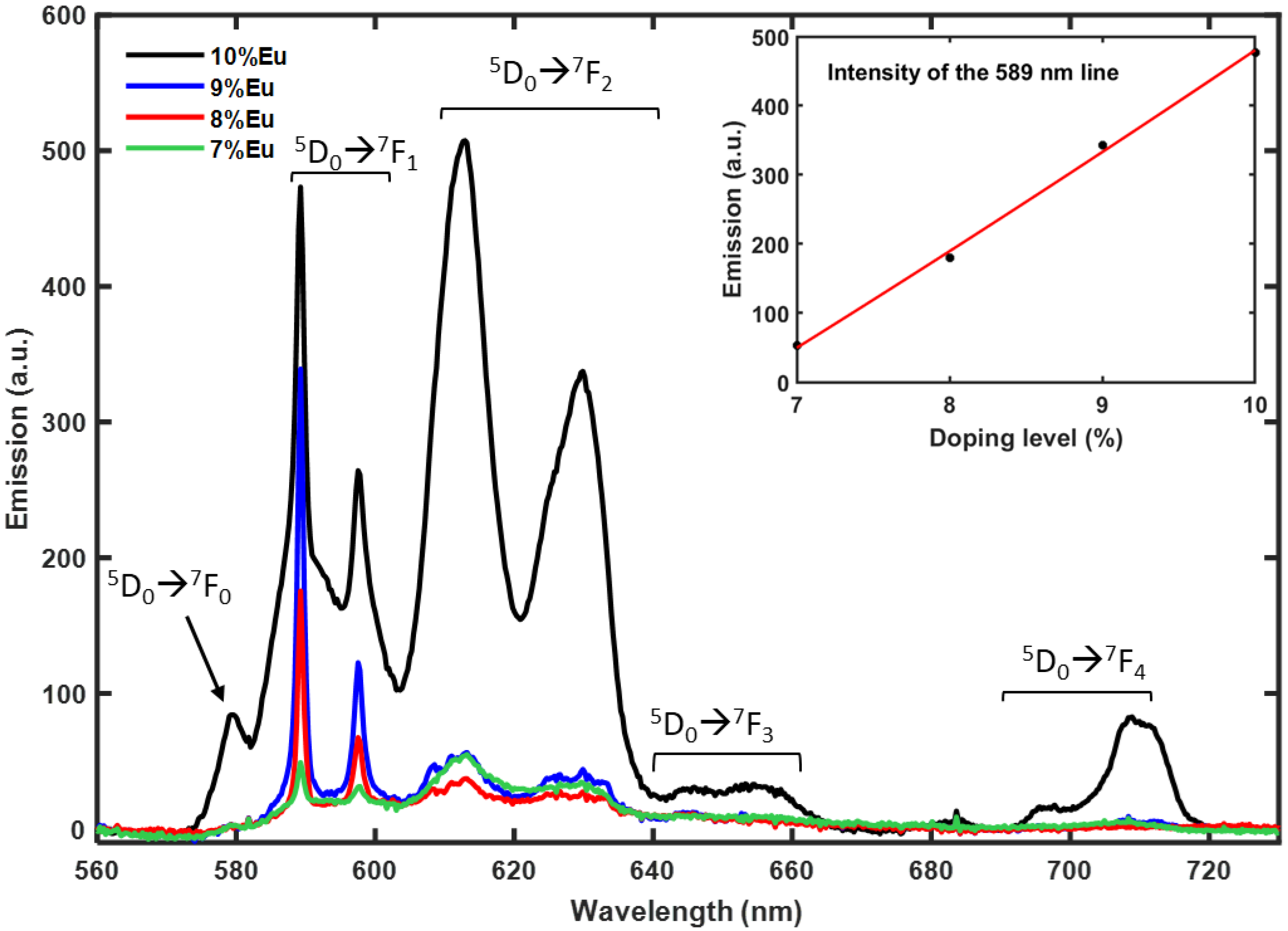
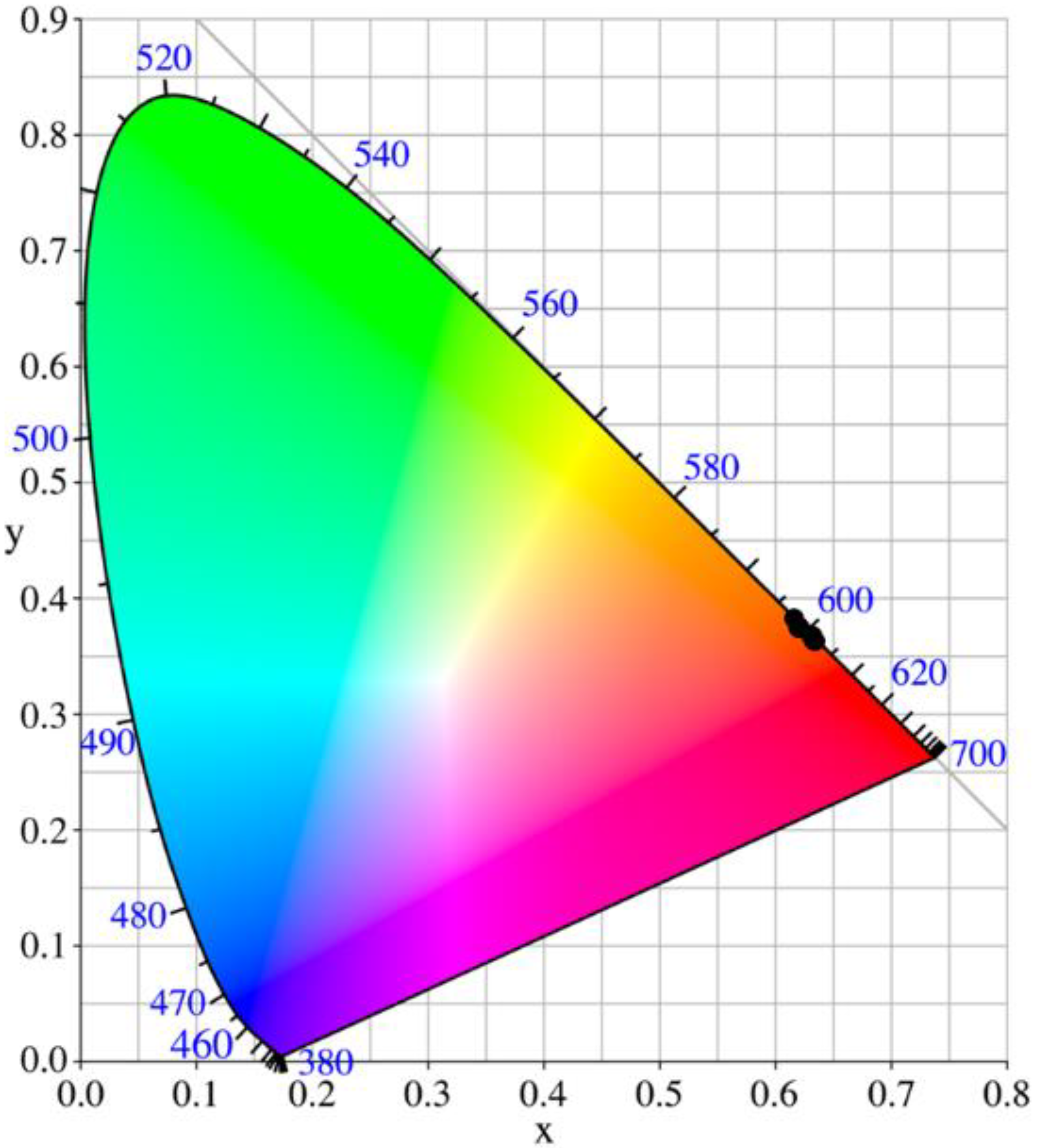
3.3. Optical Characterization
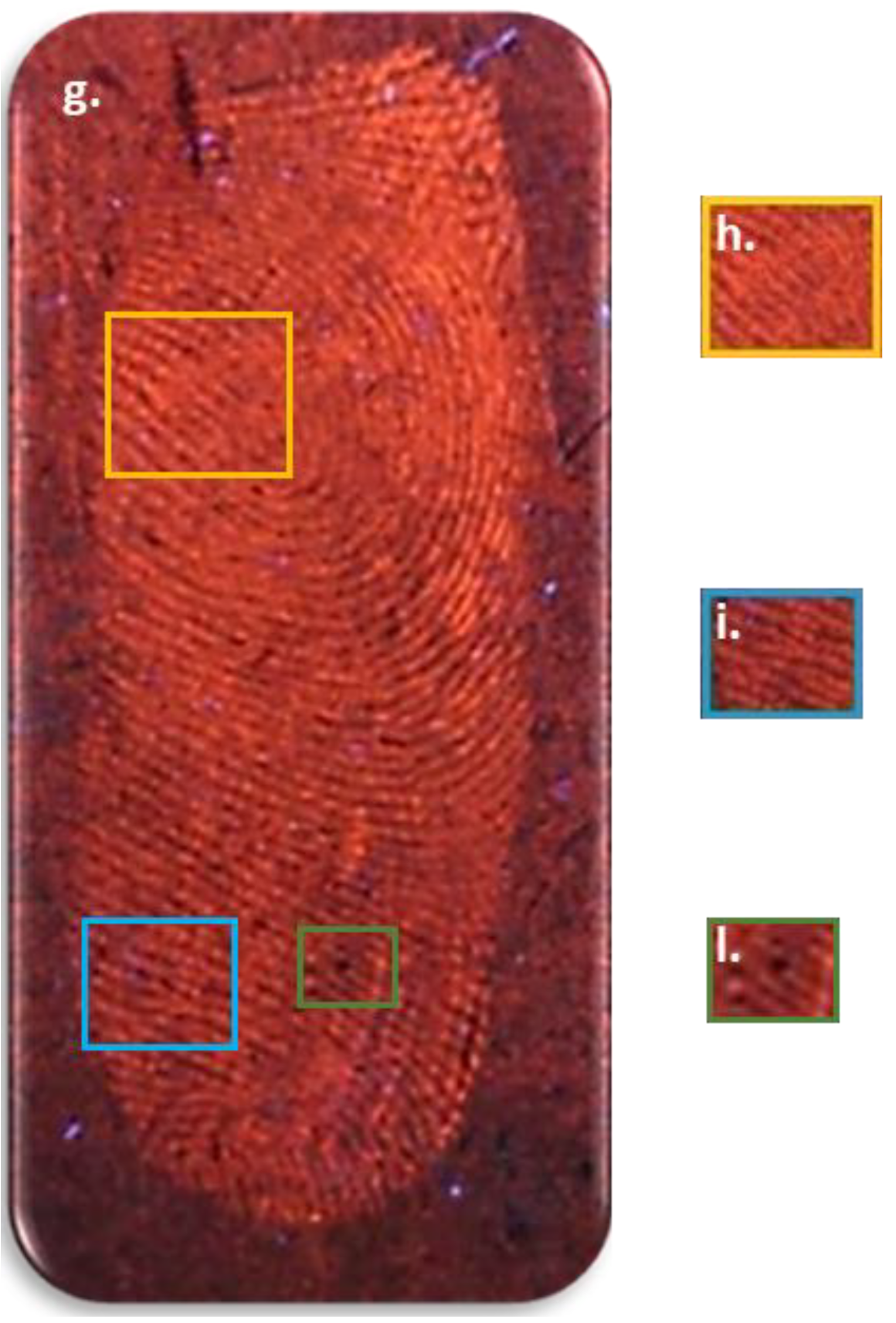
3.4. Visualization of Latent Fingerprints
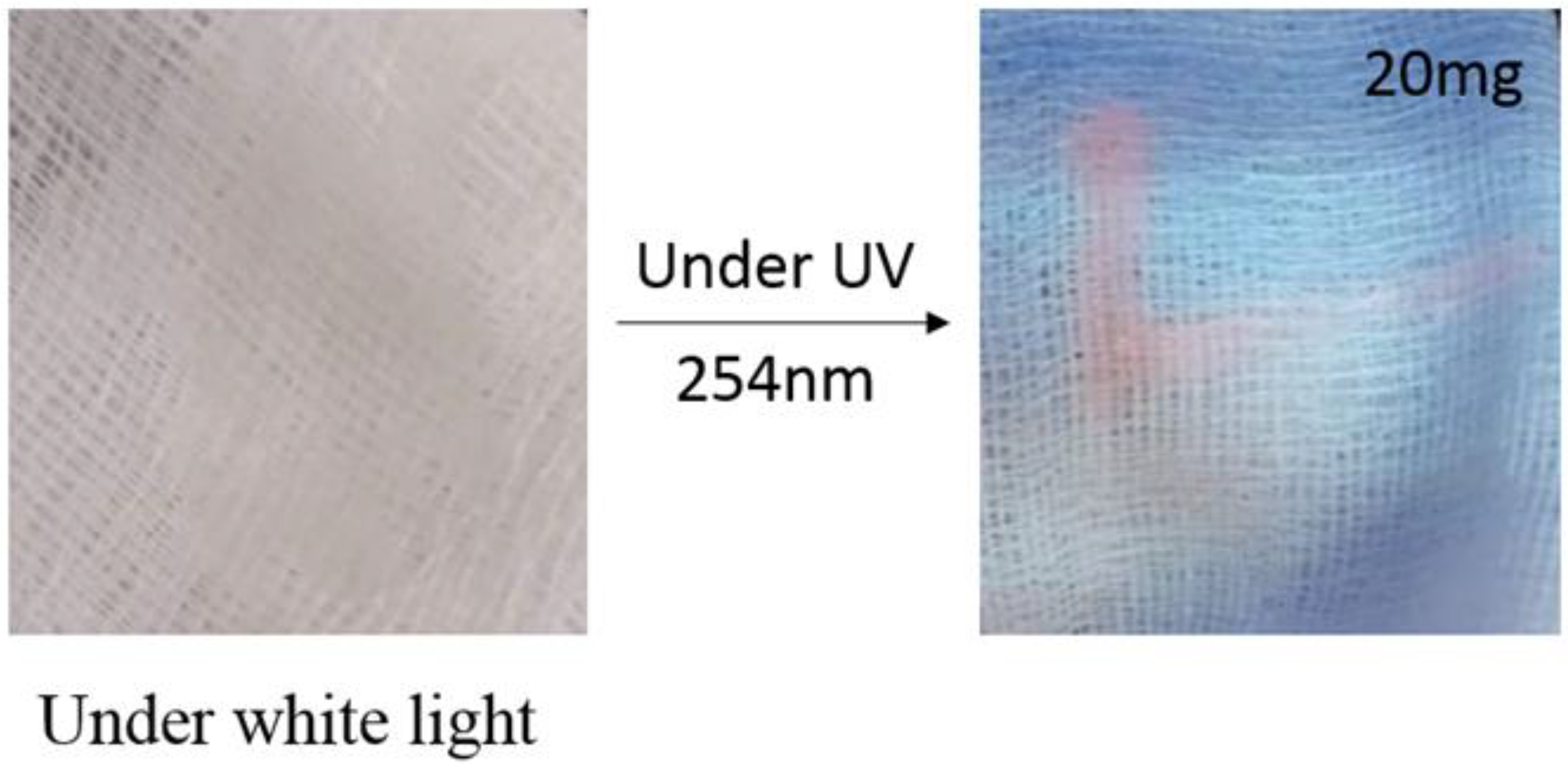
3.5. Application of Luminescent Ink for Detection of Counterfeiting
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prasad, V.; Lukose, S.; Agarwal, P.; Prasad, L. Role of Nanomaterials for Forensic Investigation and Latent Fingerprinting—A Review. J. Forensic Sci. 2019, 65, 26–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singla, N.; Kaur, M.; Sofat, S. Automated Latent Fingerprint Identification System: A Review. Forensic Sci. Int. 2020, 309, 110187. [Google Scholar] [CrossRef] [PubMed]
- Kolhatkar, G.; Parisien, C.; Ruediger, A.; Muehlethaler, C. Latent Fingermark Imaging by Single-Metal Deposition of Gold Nanoparticles and Surface Enhanced Raman Spectroscopy. Front. Chem. 2019, 7, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouiaa, M.; Bennour, I.; Rzouga Haddada, L.; Toncelli, A.; Xu, J.; Mbarek, A.; Moscardini, A.; Essoukri Ben Amara, N.; Maalej, R. Spectroscopic Characterization of Er,Yb:Y2Ti2O7 Phosphor for Latent Fingerprint Detection. Phys. B Condens. Matter 2020, 582, 412009. [Google Scholar] [CrossRef]
- Prabakaran, E.; Pillay, K. Synthesis and Characterization of Fluorescent N-CDs/ZnONPs Nanocomposite for Latent Fingerprint Detection by Using Powder Brushing Method. Arab. J. Chem. 2020, 13, 3817–3835. [Google Scholar] [CrossRef]
- Marappa, B.; Rudresha, M.S.; Basavaraj, R.B.; Darshan, G.P.; Prasad, B.D.; Sharma, S.C.; Sivakumari, S.; Amudha, P.; Nagabhushana, H. EGCG Assisted Y2O3:Eu3+ Nanopowders with 3D Micro-Architecture Assemblies Useful for Latent Finger Print Recognition and Anti-Counterfeiting Applications. Sens. Actuators B Chem. 2018, 264, 426–439. [Google Scholar] [CrossRef]
- Park, J.Y.; Chung, J.W.; Yang, H.K. Versatile Fluorescent Gd2MoO6:Eu3+ Nanophosphor for Latent Fingerprints and Anti-Counterfeiting Applications. Ceram. Int. 2019, 45, 11591–11599. [Google Scholar] [CrossRef]
- Venkatachalaiah, K.N.; Nagabhushana, H.; Darshan, G.P.; Basavaraj, R.B.; Prasad, B.D. Novel and Highly Efficient Red Luminescent Sensor Based SiO2@Y2O3:Eu3+, M+ (M+ = Li, Na, K) Composite Core–Shell Fluorescent Markers for Latent Fingerprint Recognition, Security Ink and Solid State Lightning Applications. Sens. Actuators B Chem. 2017, 251, 310–325. [Google Scholar] [CrossRef]
- Darshan, G.P.; Premkumar, H.B.; Nagabhushana, H.; Sharma, S.C.; Daruka Prasad, B.; Prashantha, S.C.; Basavaraj, R.B. Superstructures of Doped Yttrium Aluminates for Luminescent and Advanced Forensic Investigations. J. Alloys Compd. 2016, 686, 577–587. [Google Scholar] [CrossRef]
- Darshan, G.P.; Premkumar, H.B.; Nagabhushana, H.; Sharma, S.C.; Prashanth, S.C.; Prasad, B.D. Effective Fingerprint Recognition Technique Using Doped Yttrium Aluminate Nano Phosphor Material. J. Colloid Interface Sci. 2016, 464, 206–218. [Google Scholar] [CrossRef]
- Sodhi, G.S.; Kaur, J. Powder Method for Detecting Latent Fingerprints: A Review. Forensic Sci. Int. 2001, 120, 172–176. [Google Scholar] [CrossRef]
- Shahbazi, S.; Boseley, R.; Grant, B.; Chen, D.; Becker, T.; Adegoke, O.; Nic Daéid, N.; Jia, G.; Lewis, S.W. Luminescence Detection of Latent Fingermarks on Non-Porous Surfaces with Heavy-Metal-Free Quantum Dots. Forensic Chem. 2020, 18, 100222. [Google Scholar] [CrossRef]
- Sodhi, G.S.; Kaur, J.; Garg, R.K. Fingerprint Powder Formulations Based on Organic, Fluorescent Dyes. J. Forensic Identif. 2004, 54, 4–8. [Google Scholar]
- Subramanian, M.A.; Aravamudan, G.; Subba Rao, G.V. Oxide Pyrochlores—A Review. Prog. Solid State Chem. 1983, 15, 55–143. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, G.; Jiang, D.; Wang, S. Recent Development of A2B2O7 System Transparent Ceramics. J. Adv. Ceram. 2018, 7, 289–306. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Wang, L.; Lu, Z. A General Aqueous Sol–Gel Route to Ln2Sn2O7 Nanocrystals. Nanotechnology 2008, 19, 025706. [Google Scholar] [CrossRef]
- Sharma, V.; Nigam, S.; Sudarsan, V.; Vatsa, R.K. High Temperature Stabilization of Y2Sn2O7:Eu Luminescent Nanoparticles—A Facile Synthesis. J. Lumin. 2016, 179, 248–253. [Google Scholar] [CrossRef]
- Trujillano, R.; Rives, V.; Douma, M.; Chtoun, E.H. Microwave Hydrothermal Synthesis of A2Sn2O7 (A=Eu or Y). Ceram. Int. 2015, 41, 2266–2270. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Tang, Y.; Li, Y. Synthesis and Photoluminescence of Eu3+-Doped Y2Sn2O7 Nanocrystals. J. Solid State Chem. 2004, 177, 3075–3079. [Google Scholar] [CrossRef]
- Dai, P.; Zhang, X.; Zhou, M.; Li, X.; Yang, J.; Sun, P.; Xu, C.; Liu, Y. Thermally Stable Pyrochlore Y2Ti2O7: Eu3+ Orange-Red Emitting Phosphors. J. Am. Ceram. Soc. 2012, 95, 658–662. [Google Scholar] [CrossRef]
- Nigam, S.; Sudarsan, V.; Vatsa, R.K. Effect of Annealing Temperature on the Structural and Photoluminescence Properties of Y2Sn2O7:Eu Nanoparticles. Eur. J. Inorg. Chem. 2013, 2013, 357–363. [Google Scholar] [CrossRef]
- Ege, A.; Ayvacikli, M.; Dinçer, O.; Satılmış, S.U. Spectral Emission of Rare Earth (Tb, Eu, Dy) Doped Y2Sn2O7 Phosphors. J. Lumin. 2013, 143, 653–656. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent Advances in Magnetic Structure Determination by Neutron Powder Diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Papan, J.; Jovanović, D.; Sekulić, M.; Glais, E.; Dramićanin, M.D. Photoluminescence Properties and Thermal Stability of RE2-XEuxSn2O7 (RE = Y3+, Gd3+, Lu3+) Red Nanophosphors: An Experimental and Theoretical Study. Powder Technol. 2019, 346, 150–159. [Google Scholar] [CrossRef]
- Fu, Z.; Gong, W.; Li, H.; Wu, Q.; Li, W.; Yang, H.K.; Jeong, J.H. Synthesis and Spectral Properties of Nanocrystalline Eu3+-Doped Pyrochlore Oxide M2Sn2O7 (M = Gd and Y). Curr. Appl. Phys. 2011, 11, 933–938. [Google Scholar] [CrossRef]
- Peng, H.; Xu, J.; Tian, J.; Liu, Y.; He, Y.; Tan, J.; Xu, X.; Liu, W.; Zhang, N.; Wang, X. Mesoporous Y2Sn2O7 Pyrochlore with Exposed (111) Facets: An Active and Stable Catalyst for CO Oxidation. RSC Adv. 2016, 6, 71791–71799. [Google Scholar] [CrossRef]
- Nigam, S.; Sudarsan, V.; Majumder, C.; Vatsa, R.K. Structural Differences Existing in Bulk and Nanoparticles of Y2Sn2O7: Investigated by Experimental and Theoretical Methods. J. Solid State Chem. 2013, 200, 202–208. [Google Scholar] [CrossRef]
- Fujihara, S.; Tokumo, K. Multiband Orange-Red Luminescence of Eu3+ Ions Based on the Pyrochlore-Structured Host Crystal. Chem. Mater. 2005, 17, 5587–5593. [Google Scholar] [CrossRef]
- Mrázek, J.; Surýnek, M.; Bakardjieva, S.; Buršík, J.; Proboštová, J.; Kašík, I. Luminescence Properties of Nanocrystalline Europium Titanate Eu2Ti2O7. J. Alloys Compd. 2015, 645, 57–63. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of Europium(III) Spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Andres, J.; Chauvin, A.-S. Colorimetry of Luminescent Lanthanide Complexes. Molecules 2020, 25, 4022. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.; Dalal, M.; Bala, M.; Khatkar, S.P.; Taxak, V.B.; Boora, P. Synthesis, Photoluminescence Features with Intramolecular Energy Transfer and Judd–Ofelt Analysis of Highly Efficient Europium(III) Complexes. J. Mater. Sci. Mater. Electron. 2016, 27, 12506–12516. [Google Scholar] [CrossRef]
- Azman, A.R.; Mahat, N.A.; Wahab, R.A.; Ahmad, W.A.; Huri, M.A.M.; Hamzah, H.H. Relevant Visualization Technologies for Latent Fingerprints on Wet Objects and Its Challenges: A Review. Egypt. J. Forensic Sci. 2019, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.K.; Haranath, D.; Saini, S.; Singh, V.; Shanker, V. Synthesis and characterization of ultra-fine Y2O3:Eu3+ nanophosphors for luminescent security ink applications. Nanotechnology 2010, 21, 055607. [Google Scholar] [CrossRef] [PubMed]
- Trinh, C.D.; Hau, P.T.P.; Dang, T.M.D.; Dang, C.M. Sonochemical Synthesis and Properties of YVO4:Eu3+ Nanocrystals for Luminescent Security Ink Applications. J. Chem. 2019, 2019, 5749702. [Google Scholar] [CrossRef] [Green Version]
- Attia, M.; Elsaadany, S.A.; Ahmed, K.A.; El-Molla, M.M.; Abdel-Mottaleb, M.S.A. Inkjet Printable Luminescent Eu3+-TiO2 Doped in Sol Gel Matrix for Paper Tagging. J. Fluoresc. 2015, 25, 119–125. [Google Scholar] [CrossRef]
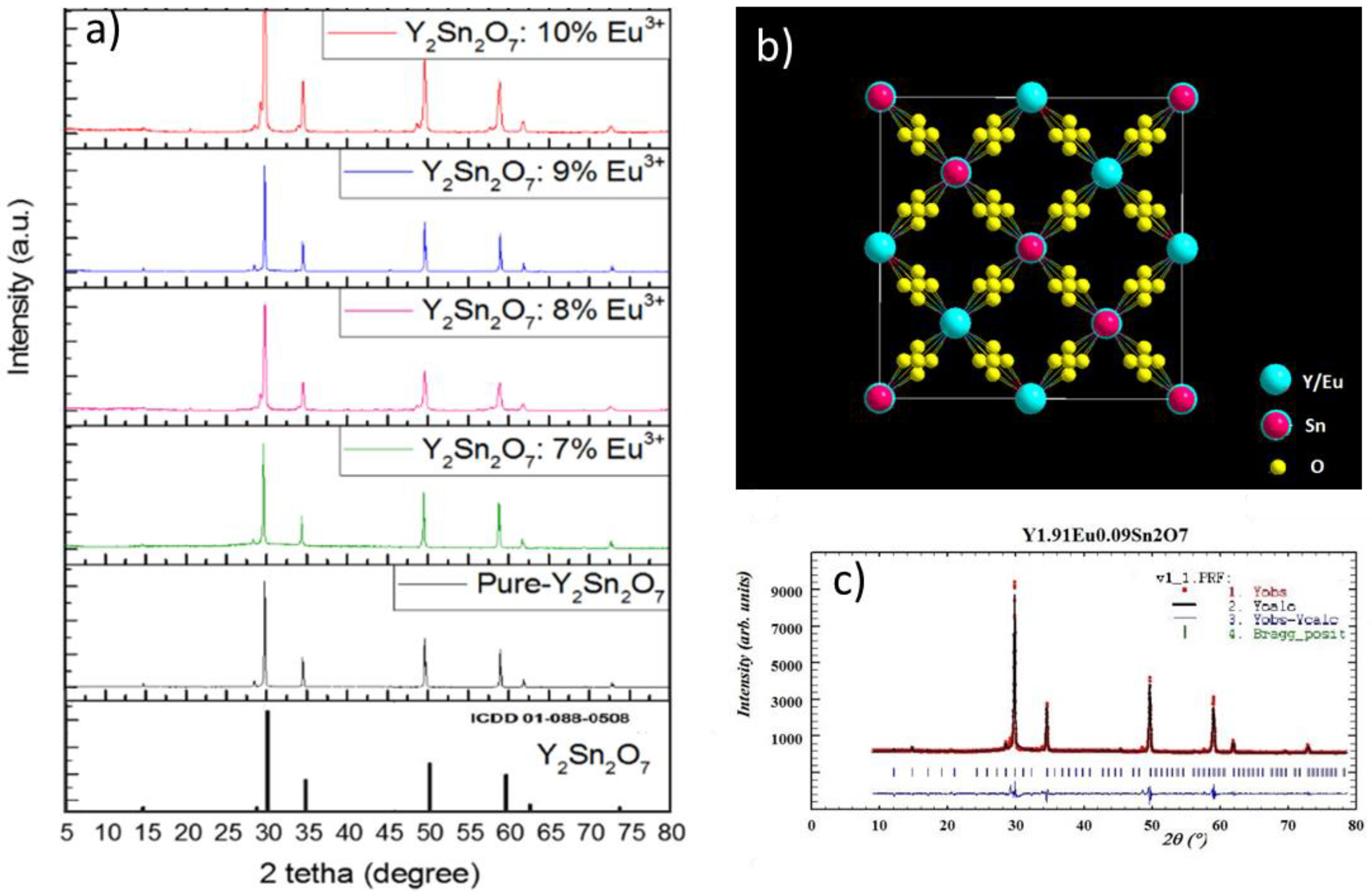

| Atom | Site | Symmetry | X | Y | Z |
|---|---|---|---|---|---|
| Y/Eu | 16d | D3d | 0.5 | 0.5 | 0.5 |
| Sn | 16c | D3d | 0 | 0 | 0 |
| O | 48f | C2v | 0.337 | 0.125 | 0.125 |
| O’ | 8b | Td | 0.375 | 0.375 | 0.375 |
| Composition | Crystallite Size from XRD (nm) | Crystallite Size from DLS (nm) |
|---|---|---|
| Y2Sn2O7:7%Eu | 384.5 | 405 |
| Y2Sn2O7:8%Eu | 311.6 | 487 |
| Y2Sn2O7:9%Eu | 360.5 | 503 |
| Y2Sn2O7:10%Eu | 326.5 | 387 |
| Sample | Size (nm) | Standard Deviation (nm) |
| Y2Sn2O7 | 56 | 31 |
| Y2Sn2O7:7%Eu | 99 | 57 |
| Y2Sn2O7:8%Eu | 57 | 34 |
| Y2Sn2O7:9%Eu | 70 | 50 |
| Y2Sn2O7:10%Eu | 82 | 39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brini, L.; Bennour, I.; Toncelli, A.; Maalej, R.; Abdelhedi, M. Eu-Doped Pyrochlore Crystal Nano-Powders as Fluorescent Solid for Fingerprint Visualization and for Anti-Counterfeiting Applications. Materials 2022, 15, 2423. https://doi.org/10.3390/ma15072423
Brini L, Bennour I, Toncelli A, Maalej R, Abdelhedi M. Eu-Doped Pyrochlore Crystal Nano-Powders as Fluorescent Solid for Fingerprint Visualization and for Anti-Counterfeiting Applications. Materials. 2022; 15(7):2423. https://doi.org/10.3390/ma15072423
Chicago/Turabian StyleBrini, Layla, Ines Bennour, Alessandra Toncelli, Ramzi Maalej, and Mohamed Abdelhedi. 2022. "Eu-Doped Pyrochlore Crystal Nano-Powders as Fluorescent Solid for Fingerprint Visualization and for Anti-Counterfeiting Applications" Materials 15, no. 7: 2423. https://doi.org/10.3390/ma15072423
APA StyleBrini, L., Bennour, I., Toncelli, A., Maalej, R., & Abdelhedi, M. (2022). Eu-Doped Pyrochlore Crystal Nano-Powders as Fluorescent Solid for Fingerprint Visualization and for Anti-Counterfeiting Applications. Materials, 15(7), 2423. https://doi.org/10.3390/ma15072423










