Study of NBT–Pluronic F–127 Gels as 1D UV Radiation Dosimeters for Measurement of Artificial Light Sources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Irradiation of Samples
2.3. Reflectance Spectrophotometry Measurements
2.4. Stability of Samples
3. Results and Discussion
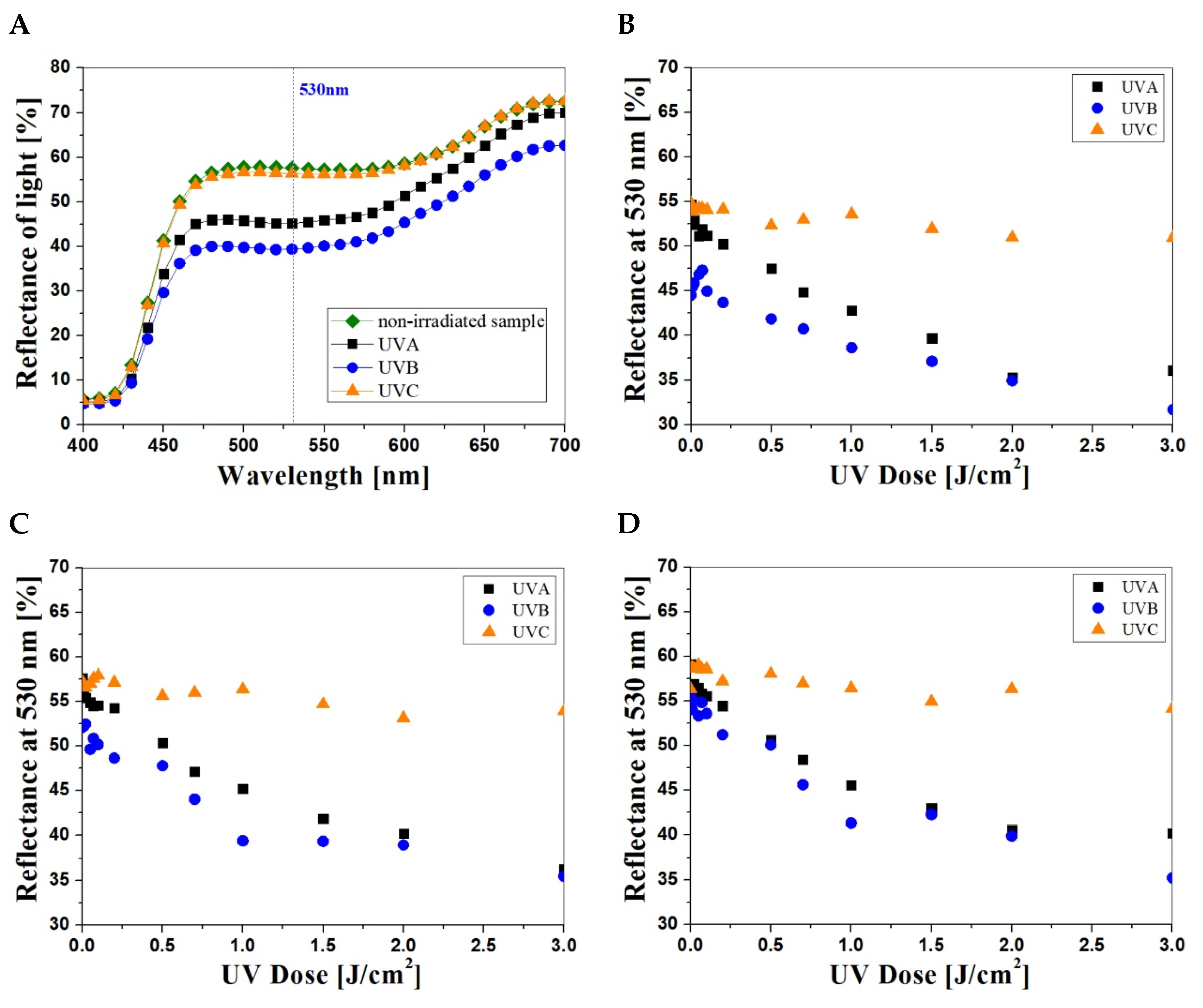
3.1. NBT–Pluronic F–127 UV-Dose Response
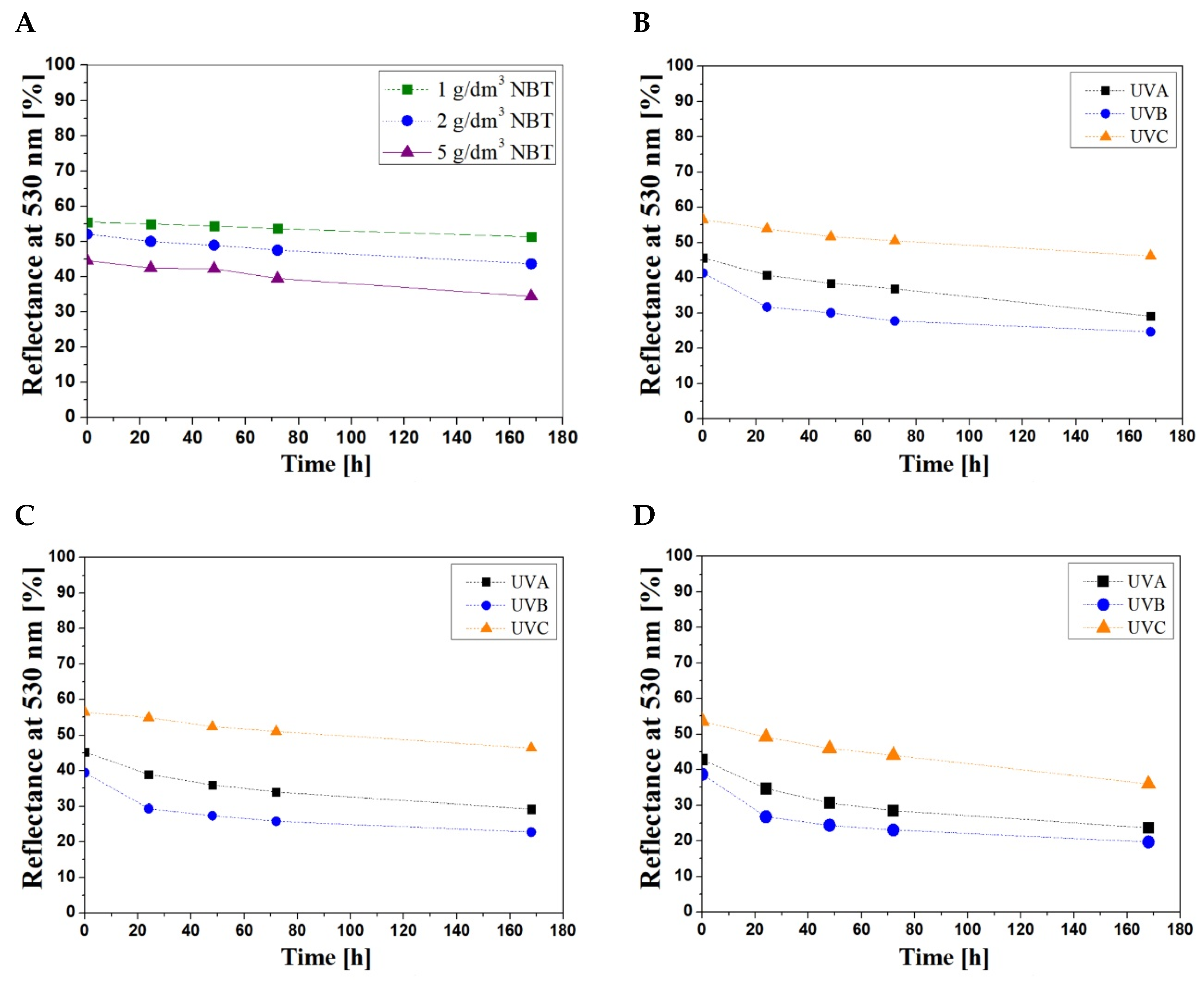
3.2. Stability of Samples
3.3. Proposition of Application
3.4. Pros and Cons of the NBT–Pluronic F–127 Dosimetric System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sachdeva, S. Fitzpatrick skin typing: Applications in dermatology. Indian J. Derm. Venereol. Leprol. 2009, 75, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Roles of solar UV radiation and vitamin D in human health and how to obtain vitamin D. Expert Rev. Dermatol. 2007, 2, 564–566. [Google Scholar] [CrossRef]
- Alfredsson, L.; Armstrong, B.K.; Butterfield, D.A.; Chowdhury, R.; de Gruijl, F.R.; Feelisch, M.; Garland, C.F.; Hart, P.H.; Hoel, D.G.; Jacobsen, R.; et al. Insufficient Sun Exposure Has Become a Real Public Health Problem. Public Health 2020, 17, 5014. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.R. The biological effects of UV-B on the eye. Photochem. Photobiol. 1989, 50, 489–492. [Google Scholar] [CrossRef]
- Zigman, S. Ocular light damage. Photochem. Photobiol. 1993, 57, 1060–1068. [Google Scholar] [CrossRef]
- Urbach, F. Potential effects of altered solar ultraviolet radiation on human skin cancer. Photochem. Photobiol. 1989, 50, 507–513. [Google Scholar] [CrossRef]
- Lee, J.A.H. The relationship between malignant melanoma of skin and exposure to sunlight. Photochem. Photobiol. 1989, 50, 493–496. [Google Scholar] [CrossRef]
- Sayre, R.M.; Dowdy, J.C.; Poh-Fitzpatrick, M. Dermatological Risk of Indoor Ultraviolet Exposure from Contemporary Lighting Sources. Photochem. Photobiol. 2007, 80, 47–51. [Google Scholar] [CrossRef]
- Vecchia, P.; Hietanen, M.; Stuck, B.E.; van Deventer, E.; Niu, S. ICNIRP statement-Protection of workers against ultraviolet radiation. Health Phys. 2010, 99, 66–87. [Google Scholar]
- Shihab, N.; Lim, H.W. Potential cutaneous carcinogenic risk of exposure to UV nail lamp: A review. Photodermatol. Photoimmunol. Photomed. 2018, 34, 362–365. [Google Scholar] [CrossRef]
- Stern, D.K.; Creasey, A.A.; Quijije, J.; Lebwohl, M.G. UV-A and UV-B Penetration of Normal Human Cadaveric Fingernail Plate. Arch. Derm. 2017, 147, 439–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, A.R.; Claveau, J.; Rossi, A.B. Ultraviolet radiation and the skin: Photobiology and sunscreen photoprotection. J. Am. Acad. Derm. 2016, 76, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passeron, T.; Bouillon, R.; Callender, V.; Cestari, T.; Diepgen, T.L.; Green, A.C.; van der Pols, J.C.; Bernard, B.A.; Ly, F.; Bernerd, F.; et al. Sunscreen photoprotection and vitamin D status. Br. J. Dermatol. 2019, 181, 916–927. [Google Scholar] [CrossRef] [Green Version]
- Das, B.R. UV Radiation Protective Clothing. Open Text. J. 2010, 3, 14–21. [Google Scholar]
- Diffey, B.L.; Jansen, C.T.; Urbach, F.; Wulf, H.C. The standard erythema dose: A new photobiological concept. Photodermatol. Photoimmunol. Photomed. 1997, 13, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.M.; Tu, G.C.; Fu, M.N. Simulation Analysis and Experimental Verification of UV-LIGA Process for High-Aspect-Ratio Ni–Fe Micro-Mold Insert. Jpn. J. Appl. Phys. 2003, 42, 6683–6690. [Google Scholar] [CrossRef]
- Smith, G.J. Ultraviolet Radiation Actinometer. U.S. Patent 4,763,011, 23 August 1988. [Google Scholar]
- Beaubien, D.J.; Beaubien, A.F. Reference Grade Solar Ultraviolet Band Pyranometer. U.S. Patent 5,331,168, 19 July 1994. [Google Scholar]
- Chanishvili, A.; Chilaya, G.; Petriashvili, G.; Barberi, R.; Bartolino, R.; De Santo, M.P. Cholesteric liquid crystal mixtures sensitive to different ranges of solar UV irradiation. Mol. Cryst. Liq. Cryst 2005, 434, 353–366. [Google Scholar] [CrossRef]
- Petriashvili, G.; Chanishvili, A.; Chilaya, G.; Matranga, M.A.; De Santo, M.P.; Barberi, R. Novel UV Sensor Based on a Liquid Crystalline Mixture Containing a Photoluminescent Dye. Mol. Cryst. Liq. Cryst. 2009, 500, 82–90. [Google Scholar] [CrossRef]
- Horneck, G. Quantification of biologically effective environmental UV irradiance. Adv. Space Res. 2000, 26, 1983–1994. [Google Scholar] [CrossRef]
- Sąsiadek, E.; Kozicki, M. A Method of 1D UVC Radiation Dose Measurement using a Novel Tablet Dosimeter. Autex Res. J. 2020, 20, 140–147. [Google Scholar] [CrossRef]
- Kozicki, M.; Sasiadek, E.; Kadłubowski, S.; Dudek, M.; Maras, P.; Nosal, A.; Gazicki-Lipman, M. Flat foils as UV and ionising radiation dosimeters. Photochem. Photobiol. 2018, 351, 179–196. [Google Scholar] [CrossRef]
- Kozicki, M.; Sąsiadek, E. Polyamide woven fabrics with 2,3,5-triphenyltetrazoluim chloride or nitro blue tetrazolium chloride as 2D ionizing radiation dosimeters. Radiat. Meas. 2012, 47, 614–621. [Google Scholar] [CrossRef]
- Kozicki, M.; Sąsiadek, E. Textile UV detector with 2,3,5-triphenyltetrazolium chloride as an active compound. Radiat. Meas. 2011, 46, 510–526. [Google Scholar] [CrossRef]
- Kozicki, M.; Sąsiadek, E. UV dosimeter based on polyamide woven fabric and nitro blue tetrazolium chloride as an active compound. Radiat. Meas. 2011, 46, 1123–1137. [Google Scholar] [CrossRef]
- Kozicki, M.; Sąsiadek, E. UV-assisted screen-printing of flat textiles. Coloration Technol. 2012, 128, 251–260. [Google Scholar] [CrossRef]
- Sąsiadek, E.; Jaszczak, M.; Skwarek, J.; Kozicki, M. NBT-Pluronic F–127 Hydrogels Printed on Flat Textiles as UV Radiation Sensors. Materials 2021, 14, 3435. [Google Scholar] [CrossRef]
- Kozicki, M.; Sąsiadek, E.; Kadlubowski, S.; Dudek, M.; Karbownik, I. Radiation sensitive polyacrylonitrile microfibres doped with PDA nanoparticles. Radiat. Phys. Chem. 2020, 169, 107751. [Google Scholar] [CrossRef]
- Sąsiadek, E.; Olejnik, K.; Kozicki, M. Paper Doped with Polyacrylonitrile Fibres Modified with 10,12–Pentacosadiynoic Acid. Materials 2021, 14, 4006. [Google Scholar] [CrossRef]
- Kwiatos, K.; Maras, P.; Kadlubowski, S.; Stempień, Z.; Dudek, M.; Kozicki, M. Tetrazolium salts-Pluronic F–127 gels for 3D radiotherapy dosimetry. Phys. Med. Biol. 2018, 63, 095012. [Google Scholar] [CrossRef]
- Kouvati, K.; Jaszczak, M.; Papagiannis, P.; Kadłubowski, S.; Wach, R.; Maras, P.; Dudek, M. Leuco crystal violet-Pluronic F–127 3D radiochromic gel dosimeter. Phys. Med. Biol. 2019, 64, 175017. [Google Scholar] [CrossRef]
- Kozicki, M.; Bartosiak, M.; Dudek, M.; Kadłubowski, S. LCV-Pluronic F–127 dosimeter for UV light dose distribution measurements. J. Photochem. Photobiol. A Chem. 2021, 405, 112930. [Google Scholar] [CrossRef]
- Kozicki, M.; Kwiatos, K.; Kadłubowski, S.; Dudek, M. TTC-Pluronic 3D radiochromic gel dosimetry of ionizing radiation. Phys. Med. Biol. 2017, 62, 5668–5690. [Google Scholar] [CrossRef] [PubMed]
- Kozicki, M.; Kwiatos, K.; Dudek, M.; Stempien, Z. Radiochromic gels for UV radiation measurements in 3D. J. Photochem. Photobiol. A 2018, 351, 197–207. [Google Scholar] [CrossRef]
- Jaszczak, M.; Wach, R.; Maras, P.; Dudek, M.; Kozicki, M. Substituting gelatine with Pluronic F–127 matrix in 3D polymer gel dosimeters can improve nuclear magnetic resonance, thermal and optical properties. Phys. Med. Biol. 2018, 63, 175010. [Google Scholar] [CrossRef]
- Alexandridis, P.; Hatton, T.A. Poly(ethylene oxide)-poly(propylene oxide)-poly (ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modelling. Colloids Surf. A 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Papadimopoulos, A.N.; Kantartzis, N.V.; Tsitsas, N.L.; Valagiannopoulos, C.A. Wide-angle absorption of visible light from simple bilayers. Appl. Opt. 2017, 56, 9779–9786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valagiannopoulos, C.A.; Tsitsas, N.L. Integral equation analysis of a low-profile receiving planar microstrip antenna with a cloaking superstrate. Radio Sci. 2012, 47, 1–12. [Google Scholar] [CrossRef]
- Wang, H.; Qi, H.; Wang, B.; Cui, Y.; Guo, M.; Zhao, J.; Jin, Y.; Shao, J. Defect analysis of UV high-reflective coatings used in the high power laser system. Opt. Express 2015, 23, 5213–5220. [Google Scholar] [CrossRef]
- ISO/CIE 11664-4:2019; Colorimetry—Part 4: CIE 1976 L*a*b* Colour Space. CIE International Commission on Illumination: Vienna, Austria, 2019.
- Auvinen, A.; Bridges, J.; Dawson, K.; De Jong, W.; Hartemann, P.; Hensten, A.; Hoet, P.; Jung, T.; Mattsson, M.-O.; Norppa, H.; et al. Health Effects of Artificial Light, Scientific Committee on Emerging and Newly Identified Health Risks, SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks); European Union: European Commission DG Health & Consumers: Brussels, Belgium, 2012. [Google Scholar]
- EN 62471:2008; Photobiological Safety of Lamps and Lamp Systems. European Committee for Electrotechnical Standardization: Brussels, Belgium, 2008.
- Xiang, F.; Lucas, R.; de Gruijl, F.; Norval, M. A systematic review of the influence of skin pigmentation on changes in the concentrations of vitamin D and 25-hydroxyvitamin D in plasma/serum following experimental UV irradiation. Photochem. Photobiol. Sci. 2015, 14, 2138–2146. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, T.B. The validity and practicality of sun-reactive skin types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef]
- Hoeppe, P.; Oppenrieder, A.; Erianto, C.; Koepke, P.; Reuder, J.; Seefeldner, M.; Nowak, D. Visualization of UV exposure of the human body based on data from a scanning UV-measuring system. Int. J. Biometeorol. 2004, 49, 18–25. [Google Scholar] [CrossRef] [PubMed]
| NBT [g/dm3] | Dose [J/cm2] | UVA | L | a | b | UVB | L | a | b | UVC | L | a | b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 |  | 79.54 | −3.86 | 14.78 |  | 77.57 | −2.64 | 14.92 |  | 73.79 | −2.06 | 18.84 |
| 0.5 |  | 74.83 | 1.13 | 11.91 |  | 70.60 | 4.71 | 10.55 |  | 74.91 | −1.76 | 13.06 | |
| 1 |  | 68.17 | 6.10 | 9.47 |  | 60.53 | 10.04 | 6.52 |  | 74.62 | −1.25 | 13.00 | |
| 3 |  | 60.37 | 14.57 | 4.91 |  | 50.32 | 20.16 | 3.25 |  | 73.31 | 0.49 | 12.02 | |
| 2 | 0 |  | 78.19 | −4.58 | 20.98 |  | 73.10 | −2.27 | 19.33 |  | 73.79 | −2.06 | 18.84 |
| 0.5 |  | 72.46 | 0.42 | 18.69 |  | 68.54 | 4.59 | 16.05 |  | 72.76 | −1.32 | 18.44 | |
| 1 |  | 69.19 | 4.12 | 15.88 |  | 59.04 | 10.19 | 12.26 |  | 75.14 | −1.28 | 18.79 | |
| 3 |  | 57.39 | 14.38 | 10.93 |  | 48.60 | 20.09 | 6.43 |  | 72.18 | 0.37 | 18.06 | |
| 5 | 0 |  | 75.2 | −4.02 | 31.14 |  | 67.19 | −0.19 | 27.67 |  | 69.00 | −0.22 | 27.77 |
| 0.5 |  | 68.09 | 1.9 | 27.90 |  | 63.24 | 6.50 | 24.09 |  | 67.93 | −0.02 | 25.59 | |
| 1 |  | 65.09 | 5.09 | 25.78 |  | 56.90 | 13.03 | 20.97 |  | 68.56 | 0.39 | 25.29 | |
| 3 |  | 60.55 | 9.01 | 21.46 |  | 43.62 | 20.41 | 11.81 |  | 68.5 | 2.41 | 26.70 |
| NBT (g/dm3) | UV Type | Threshold Dose R0 (J/cm2) | Measuring Range (J/cm2) | Range of Linear Dose–Response (J/cm2) | Sensitivity A (cm2/J) | A0 Intercept | R2 |
|---|---|---|---|---|---|---|---|
| 1 | UVA | 0.2 | 0.2–3.00 | 0.2–2.5 | −8.23 ± 0.35 | 51.56 ± 0.38 | 0.9891 |
| UVB | 0.1 | 0.1–3.00 | 0.1–2.5 | −5.07 ± 0.68 | 45.39 ± 0.41 | 0.9339 | |
| UVC | 0.5 | 0.5–3.00 | 0.5–2.5 | −1.26 ± 0.17 | 54.16 ± 0.19 | 0.8117 | |
| 2 | UVA | 0.2 | 0.2–3.00 | 0.2–2.5 | −7.21 ± 0.57 | 54.97 ± 0.66 | 0.9284 |
| UVB | 0.1 | 0.1–3.00 | 0.1–2.5 | −5.97 ± 0.69 | 50.45 ± 0.79 | 0.9142 | |
| UVC | 0.7 | 0.7–3.00 | 0.7–2.5 | −2.13 ± 0.31 | 57.69 ± 0.31 | 0.8690 | |
| 5 | UVA | 0.1 | 0.1–3.00 | 0.1–2.5 | −11.58 ± 0.32 | 56.81 ± 0.15 | 0.9946 |
| UVB | 0.1 | 0.1–3.00 | 0.1–2.5 | −6.96 ± 0.68 | 53.51 ± 0.78 | 0.8943 | |
| UVC | 0.7 | 0.7–3.00 | 0.7–2.5 | −1.39 ± 0.27 | 58.21 ± 0.31 | 0.7982 |
| UV Radiation Range | Concentration of NBT (g/dm3) | The Percent Changes in the Light Reflectance at 530 nm (%) | ||||
|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 168 h | ||
| UVA | 1 | 0 | 10 | 15 | 19 | 36 |
| UVB | 0 | 23 | 27 | 33 | 40 | |
| UVC | 0 | 4 | 8 | 10 | 18 | |
| UVA | 2 | 0 | 19 | 28 | 33 | 44 |
| UVB | 0 | 30 | 36 | 40 | 49 | |
| UVC | 0 | 8 | 14 | 17 | 32 | |
| UVA | 5 | 0 | 19 | 28 | 33 | 44 |
| UVB | 0 | 31 | 37 | 40 | 49 | |
| UVC | 0 | 8 | 14 | 18 | 33 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sąsiadek-Andrzejczak, E.; Mądrakowska, A.; Kozicki, M. Study of NBT–Pluronic F–127 Gels as 1D UV Radiation Dosimeters for Measurement of Artificial Light Sources. Materials 2022, 15, 2370. https://doi.org/10.3390/ma15072370
Sąsiadek-Andrzejczak E, Mądrakowska A, Kozicki M. Study of NBT–Pluronic F–127 Gels as 1D UV Radiation Dosimeters for Measurement of Artificial Light Sources. Materials. 2022; 15(7):2370. https://doi.org/10.3390/ma15072370
Chicago/Turabian StyleSąsiadek-Andrzejczak, Elżbieta, Agata Mądrakowska, and Marek Kozicki. 2022. "Study of NBT–Pluronic F–127 Gels as 1D UV Radiation Dosimeters for Measurement of Artificial Light Sources" Materials 15, no. 7: 2370. https://doi.org/10.3390/ma15072370
APA StyleSąsiadek-Andrzejczak, E., Mądrakowska, A., & Kozicki, M. (2022). Study of NBT–Pluronic F–127 Gels as 1D UV Radiation Dosimeters for Measurement of Artificial Light Sources. Materials, 15(7), 2370. https://doi.org/10.3390/ma15072370






