Effect of the P/Al Molar Ratio and Heating Rate on the Composi-Tion of Alumino-Phosphate Binders
Abstract
:1. Introduction
2. Experimental
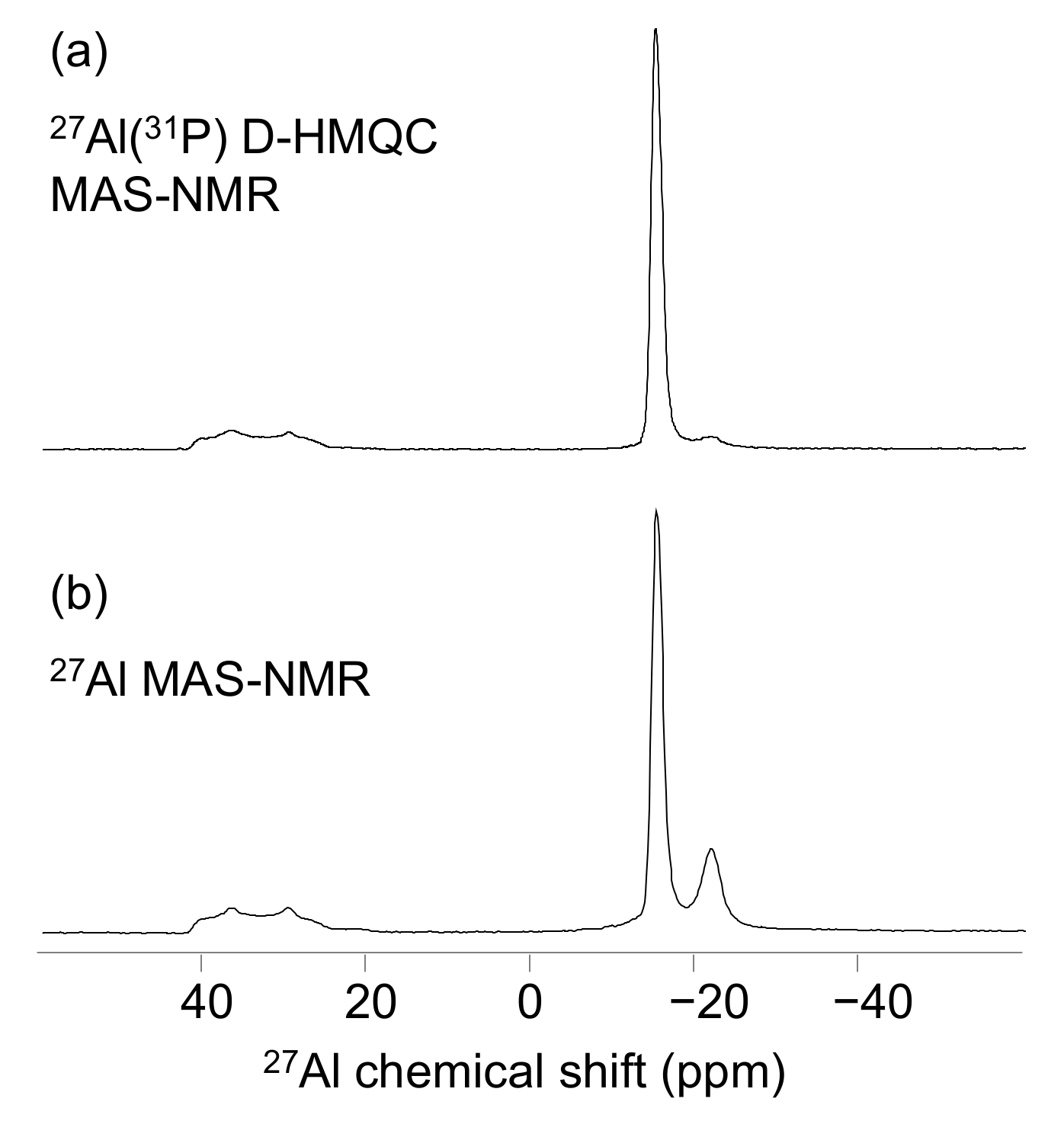
3. Results
4. Discussion
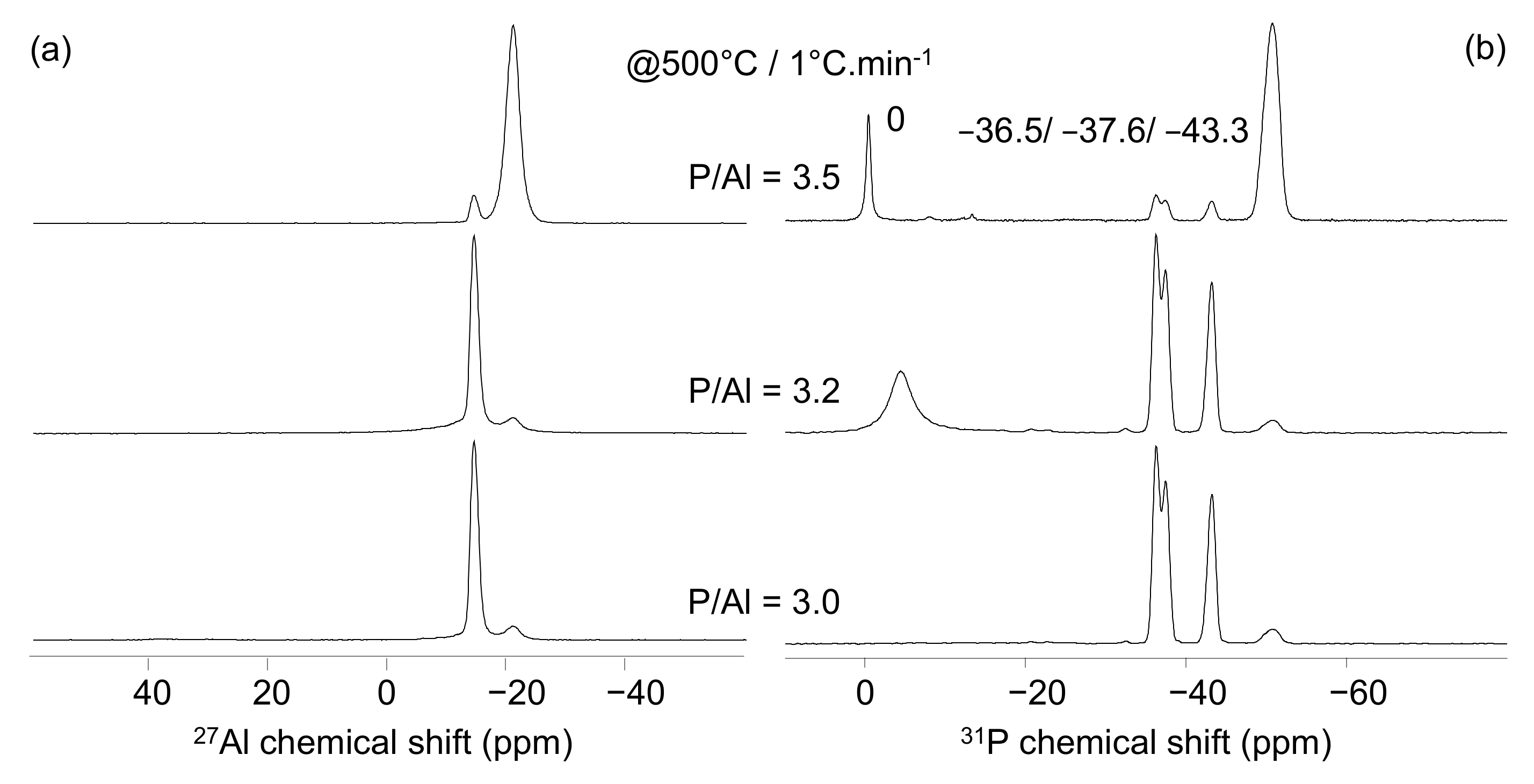
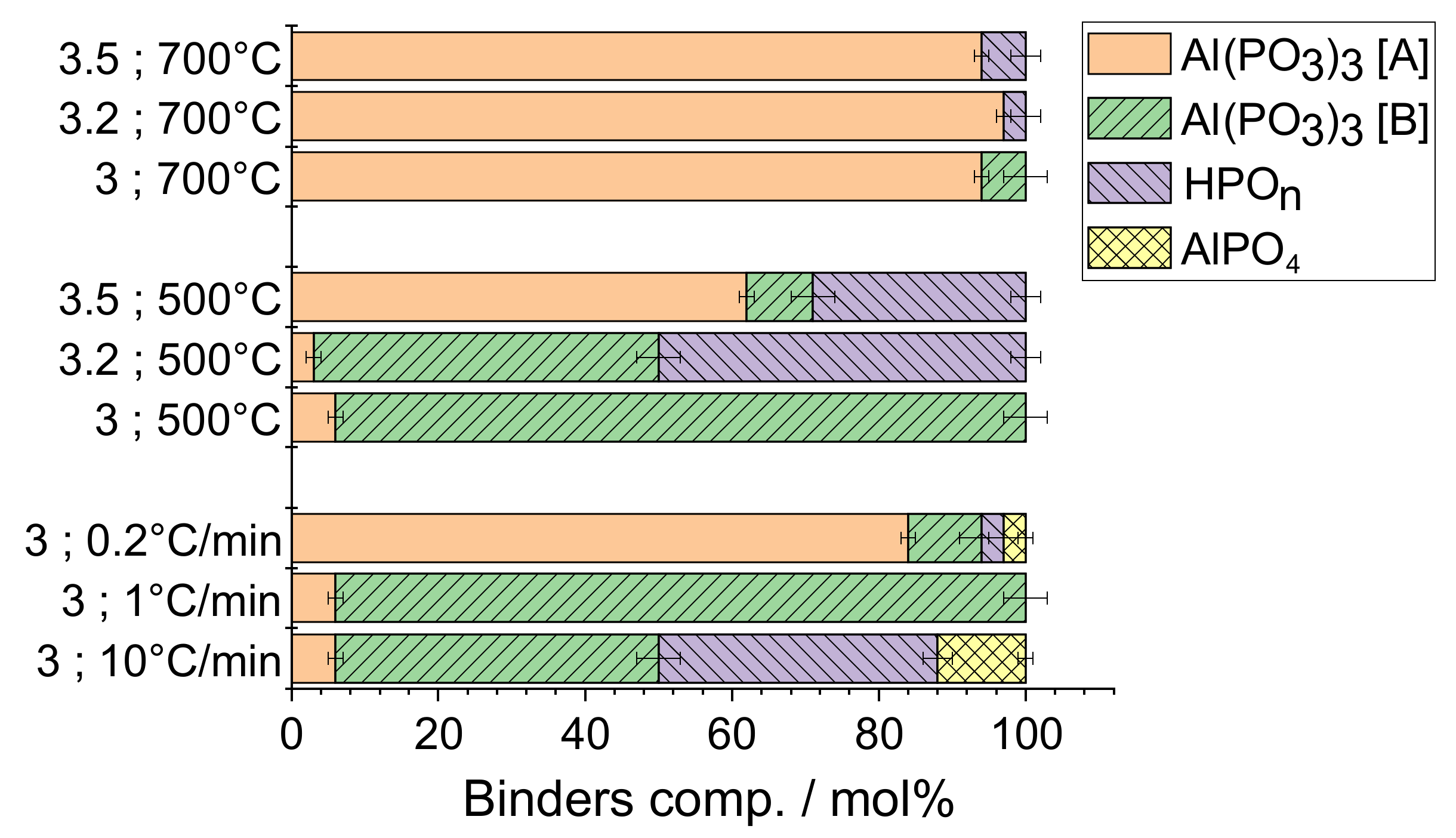
4.1. Effect of the P/Al Molar Ratio
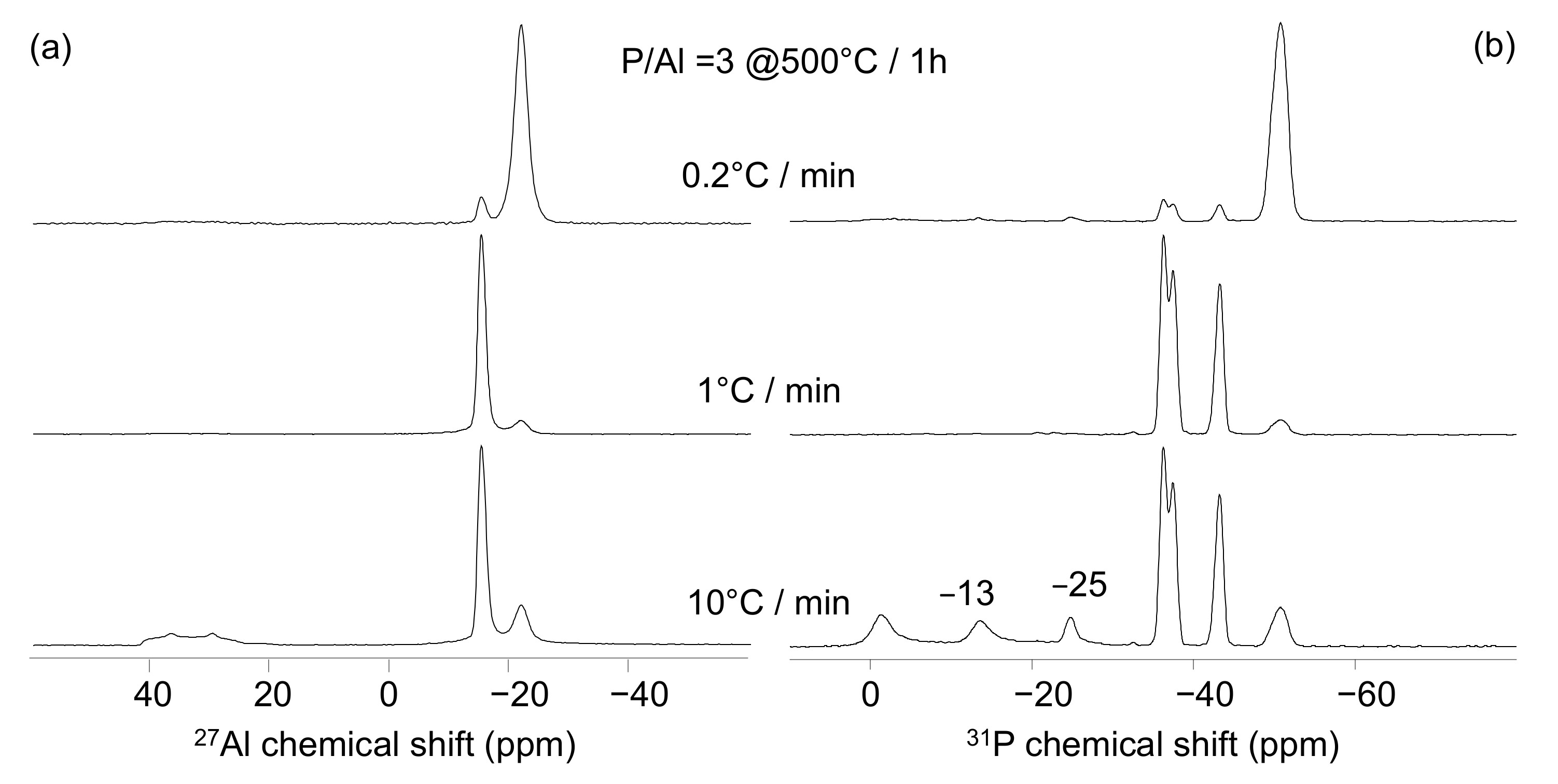
4.2. Effect of The Heating Rate
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vippola, M.; Keränen, J.; Zou, X.; Hovmöller, S.; Lepistö, T.; Mäntylä, T. Structural characterization of aluminum phosphate binder. J. Am. Ceram. Soc. 2000, 83, 1834–1836. [Google Scholar] [CrossRef]
- Vippola, M.; Vuorinen, J.; Vuoristo, P.; Lepistö, T.; Mäntylä, T. Thermal analysis of plasma sprayed oxide coatings sealed with aluminium phosphate. J. Eur. Ceram. Soc. 2002, 22, 1937–1946. [Google Scholar] [CrossRef]
- Chen, D.; He, L.; Shang, S. Study on aluminum phosphate binder and related Al2O3–SiC ceramic coating. Mater. Sci. Eng. A. 2003, 348, 29–35. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Tong, W.; Zhou, Q.; Li, Z. Effect of Al:P ratio on bonding performance of high-temperature resistant aluminum phosphate adhesive. Int. J. Adhes. Adhes. 2020, 100, 102627. [Google Scholar] [CrossRef]
- Ernst, A. Tomic, Phosphate Cement and Mortar. U.S. Patent 4,394,174A, 1 June 1982. [Google Scholar]
- Balhadere, A.; Thebault, J.; Bernard, B. Method of Protecting a Part Made of a Carbon Containing Composite Material against Oxidation. U.S. Patent 5,853,821, 29 December 1998. [Google Scholar]
- Tricot, G.; Nicolaus, N.; Diss, P.; Montagne, L. Inhibition of the catalytic oxidation of carbon/carbon composite materials by an aluminophosphate coating. Carbon 2012, 50, 3440–3445. [Google Scholar] [CrossRef]
- Joly, A.; Brun, P.; Lacombe, J.; Tricot, G.; Denoirjean, A. Structural characterization of an electrically insulating diffusion barrier on a plasma-sprayed ceramic for severe environment applications. Surf. Coat. Technol. 2013, 220, 204–208. [Google Scholar] [CrossRef]
- D’Yvoire, F. Sur les polyphosphates à longues chaînes et les metaphosphates d’aluminium et de fer trivalent. C. R. Seances Acad. Sci. 1960, 251, 2182–2184. [Google Scholar]
- Tricot, G.; Coillot, D.; Creton, E.; Montagne, L. New insights into the thermal evolution of aluminophosphate solutions: A complementary XRD and solid state NMR study. J. Eur. Ceram. Soc. 2008, 28, 1135–1141. [Google Scholar] [CrossRef]
- Hahn, D.; Masoudi Alavi, A.; Hopp, V.; Quirmbach, P. Phase development of phosphate-bonded Al2O3-MgAl2O4 high-temperature ceramics: XRD and solid-state NMR investigations. J. Am. Ceram. Soc. 2021, 104, 6625–6642. [Google Scholar] [CrossRef]
- Li, Y.; Chen, G.; Zhu, S.; Li, H.; Ma, Z.; Liu, Y.; Liu, L. Preparation of an aluminium phosphate binder and its influence on the bonding strength of coating. Bull. Mater. Sci. 2019, 42, 200. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.-Y.; Han, H.-J.; Ha, H.; Lee, J.-Y.; Kim, D.-P. Development of Cr-free aluminum phosphate binders and their composite applications. Compos. Sci. Technol. 2007, 67, 1195–1201. [Google Scholar] [CrossRef]
- Bemmer, V.; Bowker, M.; Carter, J.H.; Davies, P.R.; Edwards, L.E.; Harris, K.D.M.; Hughes, C.E.; Robinson, F.; Morgan, D.J.; Thomas, M.G. Rationalization of the X-ray photoelectron spectroscopy of aluminium phosphates synthesized from different precursors. RSC Adv. 2020, 10, 8444–8452. [Google Scholar] [CrossRef]
- Tricot, G.; Delevoye, L.; Palavit, G.; Montagne, L. Phase identification and quantification in a devitrified glass using homo-and heteronuclear solid-state NMR. Chem. Commun. 2005, 42, 5289–5291. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.M.; Douglas, D.C. On the 31P chemical shift anisotropy in condensed phosphates. Chem. Phys. 1984, 87, 339–349. [Google Scholar] [CrossRef]
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; Le Calvé, S.; Alonso, B.; Durand, J.-O.; Bujoli, B.; Gan, Z.; Hoatson, G. Modelling one- and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]
- Lesage, A.; Sakellariou, D.; Steuernagel, S.; Emsley, L. Carbon–Proton Chemical Shift Correlation in Solid-State NMR by Through-Bond Multiple-Quantum Spectroscopy. J. Am. Chem. Soc. 1998, 120, 13194–13201. [Google Scholar] [CrossRef]
- Trebosc, J.; Hu, B.; Amoureux, J.P.; Gan, Z. Through-space R3-HETCOR experiments between spin-1/2 and half-integer quadrupolar nuclei in solid-state NMR. J. Magn. Reson. 2007, 186, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Tricot, G.; Trébosc, J.; Pourpoint, F.; Gauvin, R.; Delevoye, L. The D-HMQC MAS-NMR Technique: An Efficient Tool for the Editing of Through-Space Correlation Spectra Between Quadrupolar and Spin-1/2 (31P, 29Si, 1H, 13C) Nuclei. Annu. Rep. NMR Spectrosc. 2014, 81, 145–184. [Google Scholar] [CrossRef]
- Tricot, G. Mixed Network Phosphate Glasses: Seeing Beyond the 1D 31P MAS-NMR Spectra With 2D X/31P NMR Correlation Maps. Annu. Rep. NMR Spectrosc. 2019, 96, 35–75. [Google Scholar]
- Müller, D.; Jahn, E.; Ladwig, G.; Haubenreisser, U. High-resolution solid-state 27Al and 31P NMR: Correlation between chemical shift and mean Al-O-P angle in AlPO4 polymorphs. Chem. Phys. Lett. 1984, 109, 332–336. [Google Scholar] [CrossRef]

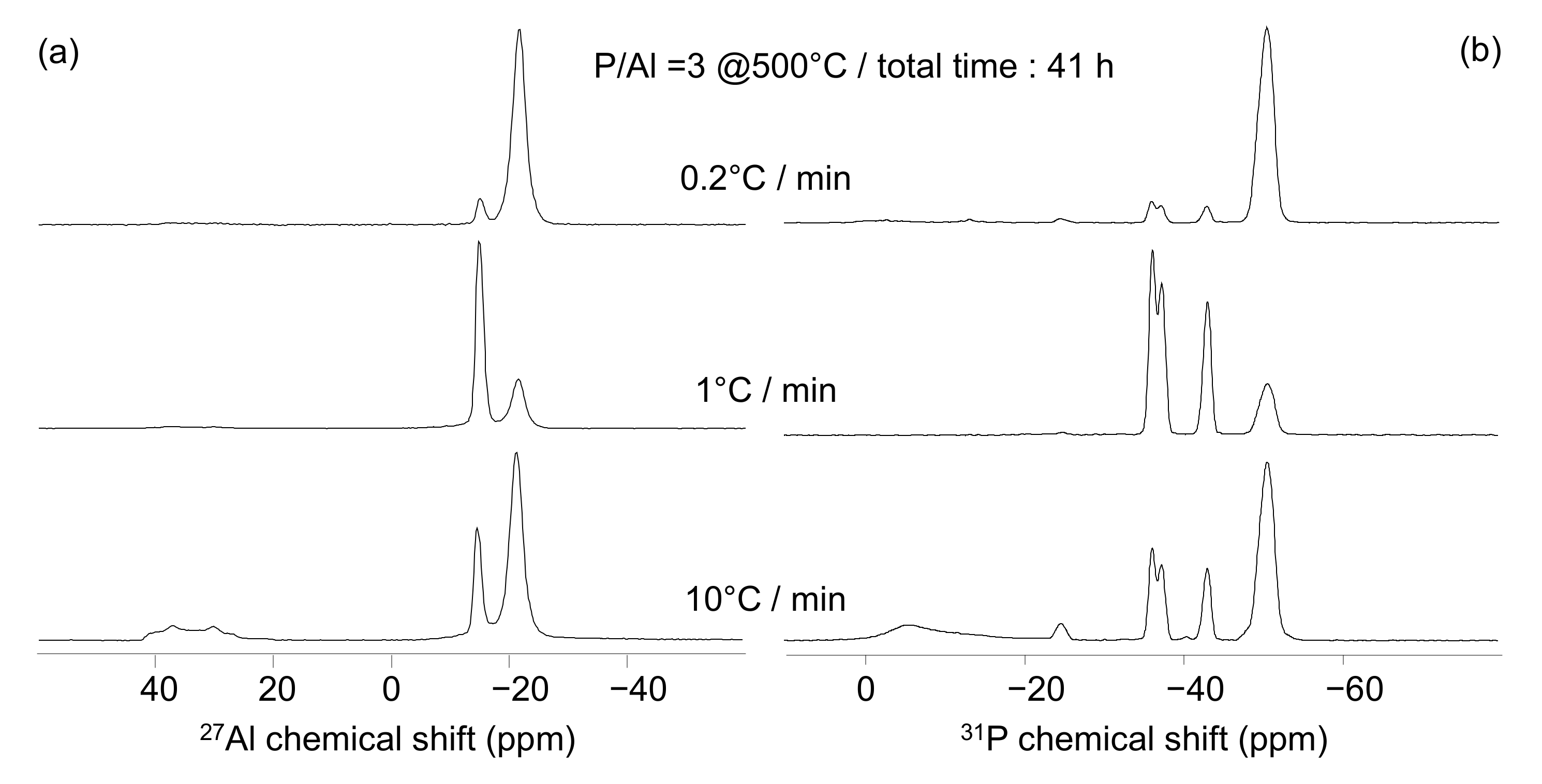
| T/°C | Compounds |
|---|---|
| 150–250 °C | Al(H2PO4)3 |
| 350 °C | AlH2P3O10, × H2O |
| 500 °C | Al(PO3)3 [B] + [A] |
| 1000 °C | Al(PO3)3 [A] |
| δ(31P) (ppm) | δ(27Al) (ppm) | |
|---|---|---|
| Al(PO3)3 [A] | −50.8 | −21.4 |
| Al(PO3)3 [B] | −36.5/−37.6/−43.3 | −15 |
| AlH2P3O10, × H2O | −20.9/−22.9/−32.5 | −13.2/−15.2 |
| Al(H2PO4)3 | −15.8 | −16.5 |
| Phosphoric Acids HPOn | 0, −13 | - |
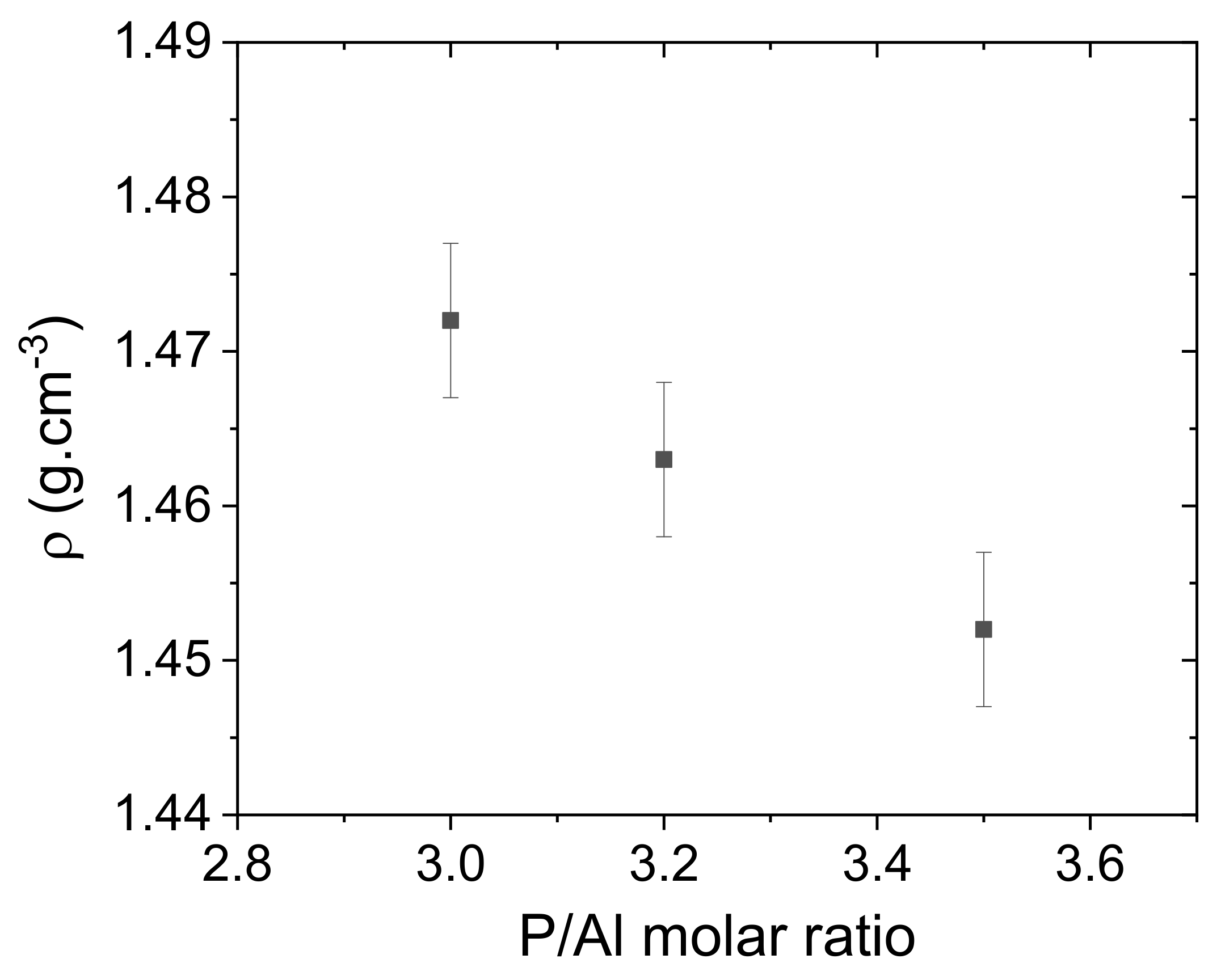
| P/Al | mAl(OH)3 (g) | vol H3PO4/H2O (mL/ mL) | [P] (g.mol−1) | ρ (g.cm−3) |
|---|---|---|---|---|
| 3 | 18.936 | 50/50 | 7.41 | 1.472 |
| 3.2 | 17.753 | 50/50 | 7.41 | 1.463 |
| 3.5 | 16.458 | 50/50 | 7.41 | 1.452 |
| P/Al; Final T; Heating Rate | Major Compound | Minor Compound |
|---|---|---|
| 3; 700 °C; 1 °C/min | Al(PO3)3 [A] | Al(PO3)3 [B] |
| 3.2; 700 °C; 1 °C/min | Al(PO3)3 [A] | - |
| 3.5; 700 °C; 1 °C/min | Al(PO3)3 [A] | - |
| 3; 500 °C; 1 °C/min | Al(PO3)3 [B] | Al(PO3)3 [A] |
| 3.2; 500 °C; 1 °C/min | Al(PO3)3 [B] | Al(PO3)3 [A] |
| 3.5; 500 °C; 1 °C/min | Al(PO3)3 [A] | Al(PO3)3 [B] |
| 3; 500 °C; 0.2 °C/min | Al(PO3)3 [A] | Al(PO3)3 [B] |
| 3; 500 °C; 10 °C/min | Al(PO3)3 [B] | Al(PO3)3 [A], AlPO4 (berlinite) |
| % of total P (+/−1) involved in | ||||
|---|---|---|---|---|
| P/Al; Final T; Heating Rate | Al(PO3)3 [A] | Al(PO3)3 [B] | HPOn | AlPO4 |
| 3; 700 °C; 1 °C/min | 94 | 6 | - | - |
| 3.2; 700 °C; 1 °C/min | 99 | - | 1 | - |
| 3.5; 700 °C; 1 °C/min | 98 | - | 2 | - |
| 3; 500 °C; 1 °C/min | 6 | 94 | - | - |
| 3.2; 500 °C; 1 °C/min | 4 | 71 | 25 | |
| 3.5; 500 °C; 1 °C/min | 77 | 11 | 12 | - |
| 3; 500 °C; 0.2 °C/min | 84 | 10 | 3 | 3 |
| 3; 500 °C; 10 °C/min | 9 | 66 | 19 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tricot, G.; Hu, H.; Beaussart, A.; Fernandes, I.; Perrot, C. Effect of the P/Al Molar Ratio and Heating Rate on the Composi-Tion of Alumino-Phosphate Binders. Materials 2022, 15, 2337. https://doi.org/10.3390/ma15062337
Tricot G, Hu H, Beaussart A, Fernandes I, Perrot C. Effect of the P/Al Molar Ratio and Heating Rate on the Composi-Tion of Alumino-Phosphate Binders. Materials. 2022; 15(6):2337. https://doi.org/10.3390/ma15062337
Chicago/Turabian StyleTricot, Grégory, Hanyu Hu, Amélie Beaussart, Ismérie Fernandes, and Clément Perrot. 2022. "Effect of the P/Al Molar Ratio and Heating Rate on the Composi-Tion of Alumino-Phosphate Binders" Materials 15, no. 6: 2337. https://doi.org/10.3390/ma15062337
APA StyleTricot, G., Hu, H., Beaussart, A., Fernandes, I., & Perrot, C. (2022). Effect of the P/Al Molar Ratio and Heating Rate on the Composi-Tion of Alumino-Phosphate Binders. Materials, 15(6), 2337. https://doi.org/10.3390/ma15062337






