Fabrication of MnCuNiFe–CuAlNiFeMn Gradient Alloy by Laser Engineering Net Shaping System
Abstract
:1. Introduction
2. Materials and Methods
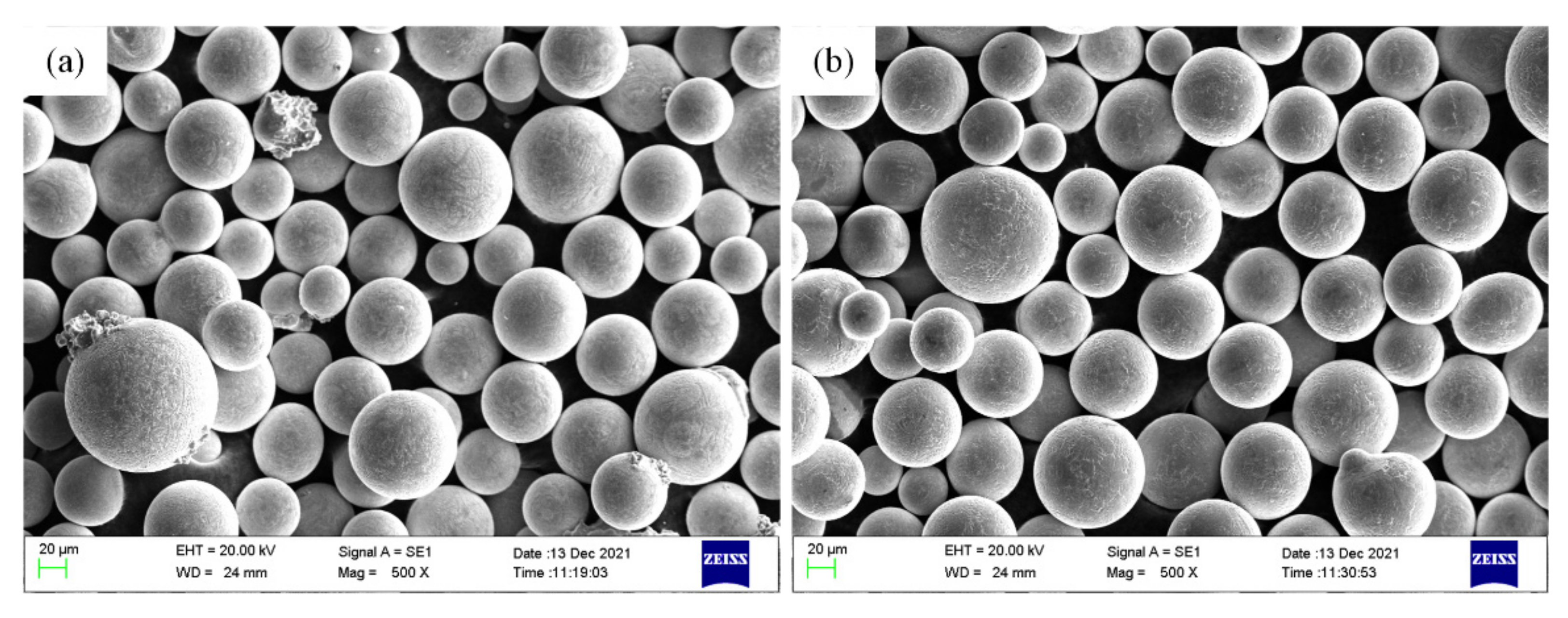
2.1. Materials and Preparation
2.2. Material Characterization Method
3. Results and Discussion
4. Conclusions
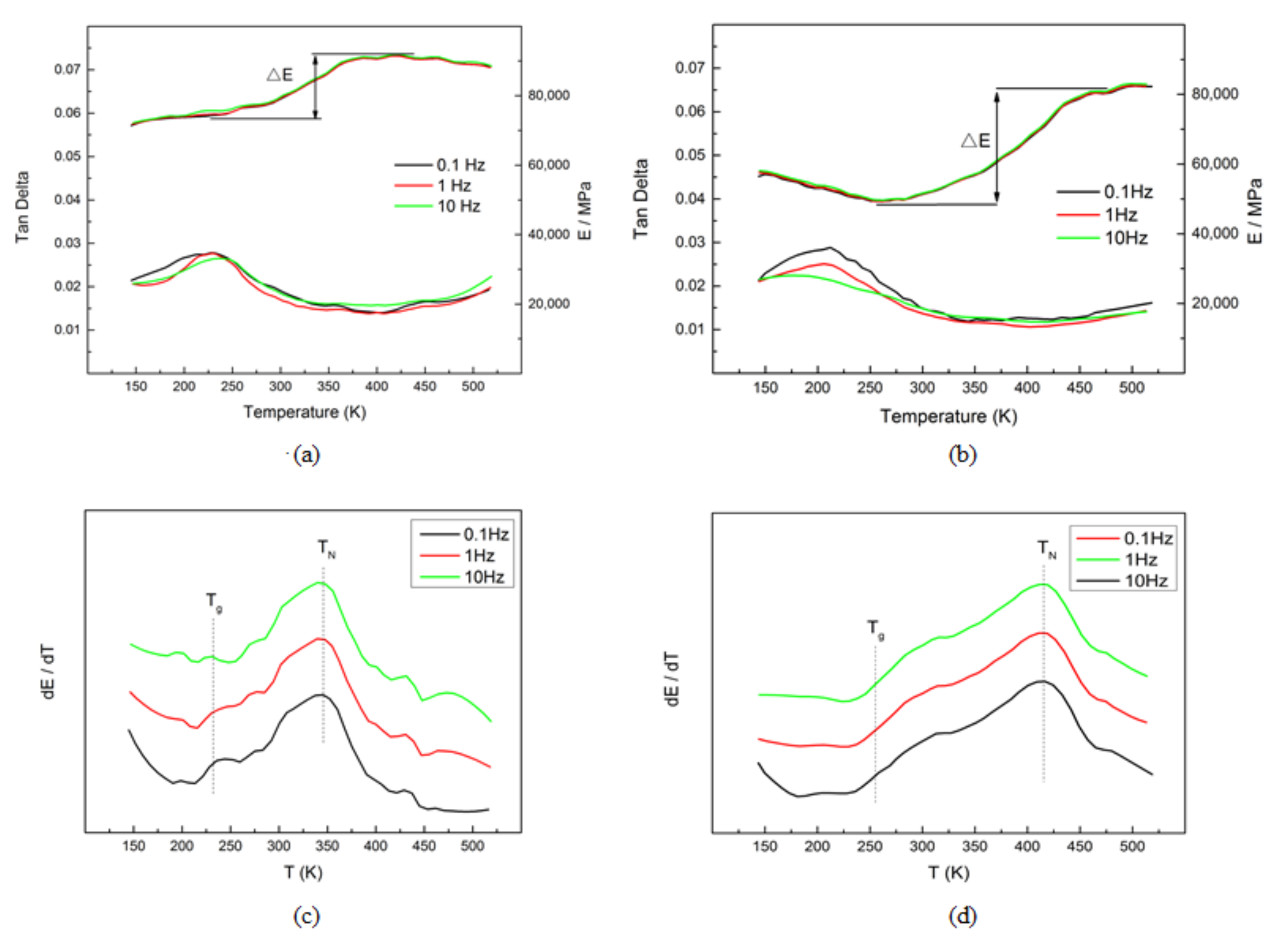
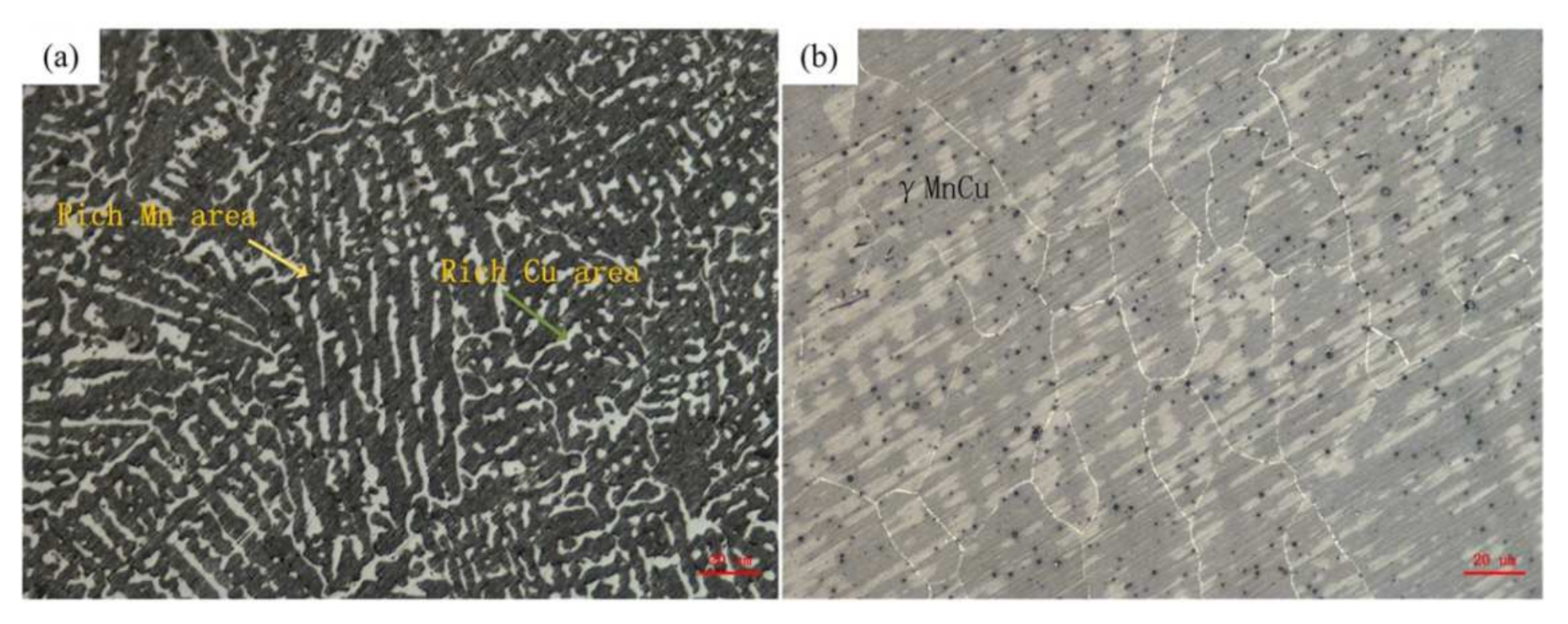
- The microstructure of the as-deposited M2052 alloy was the same as that of the as-cast alloy, with obvious Mn-rich regions and dendrite segregation in the Cu-rich regions. After aging, the dendrite structure in the Mn-rich region disappeared, and the alloy microstructure was equiaxed γ-MnCu. The grain size increased, dislocation density decreased, and apparent modulus softening occurred. These results show that the aged alloy promoted the formation of twins and martensite;
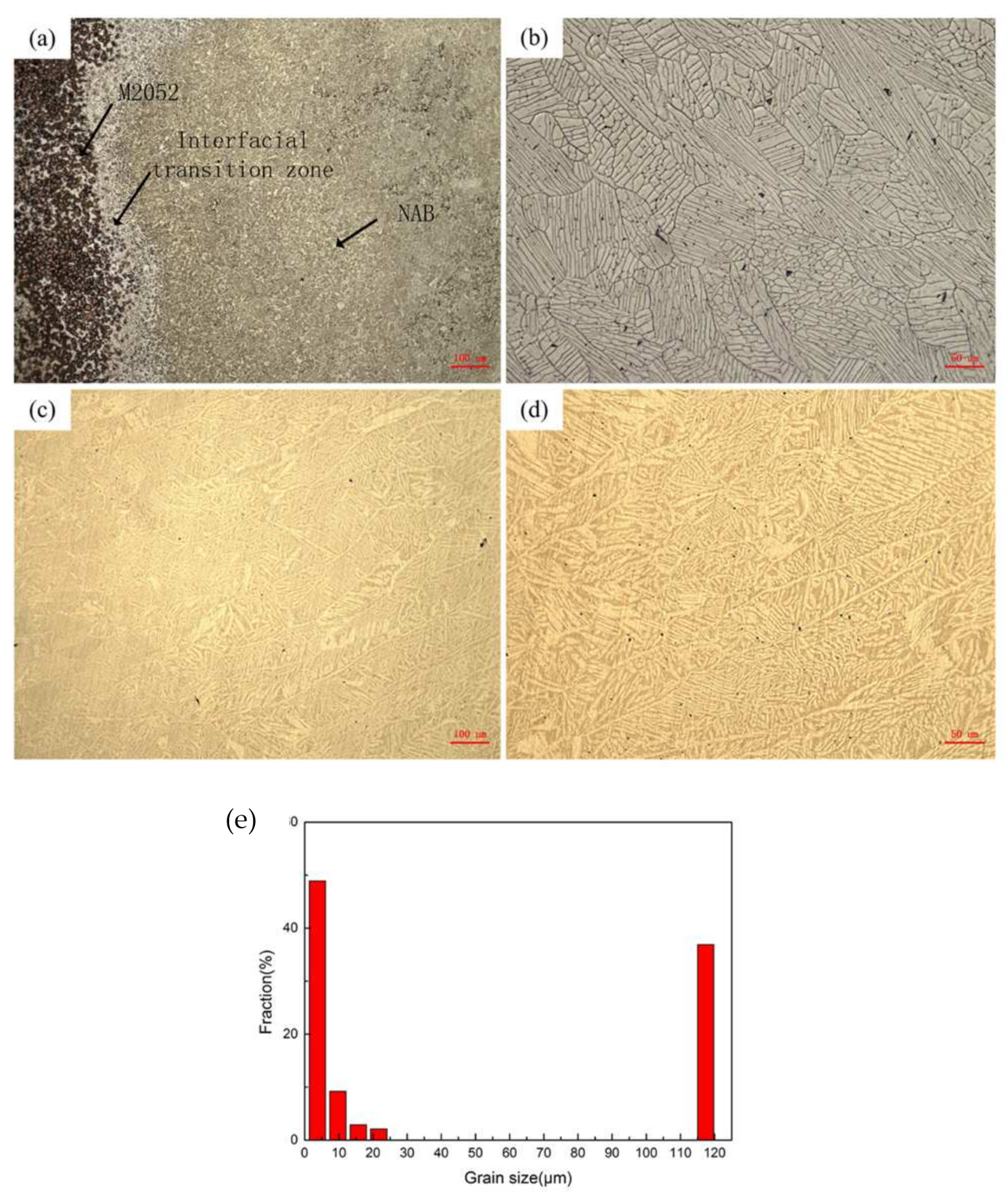
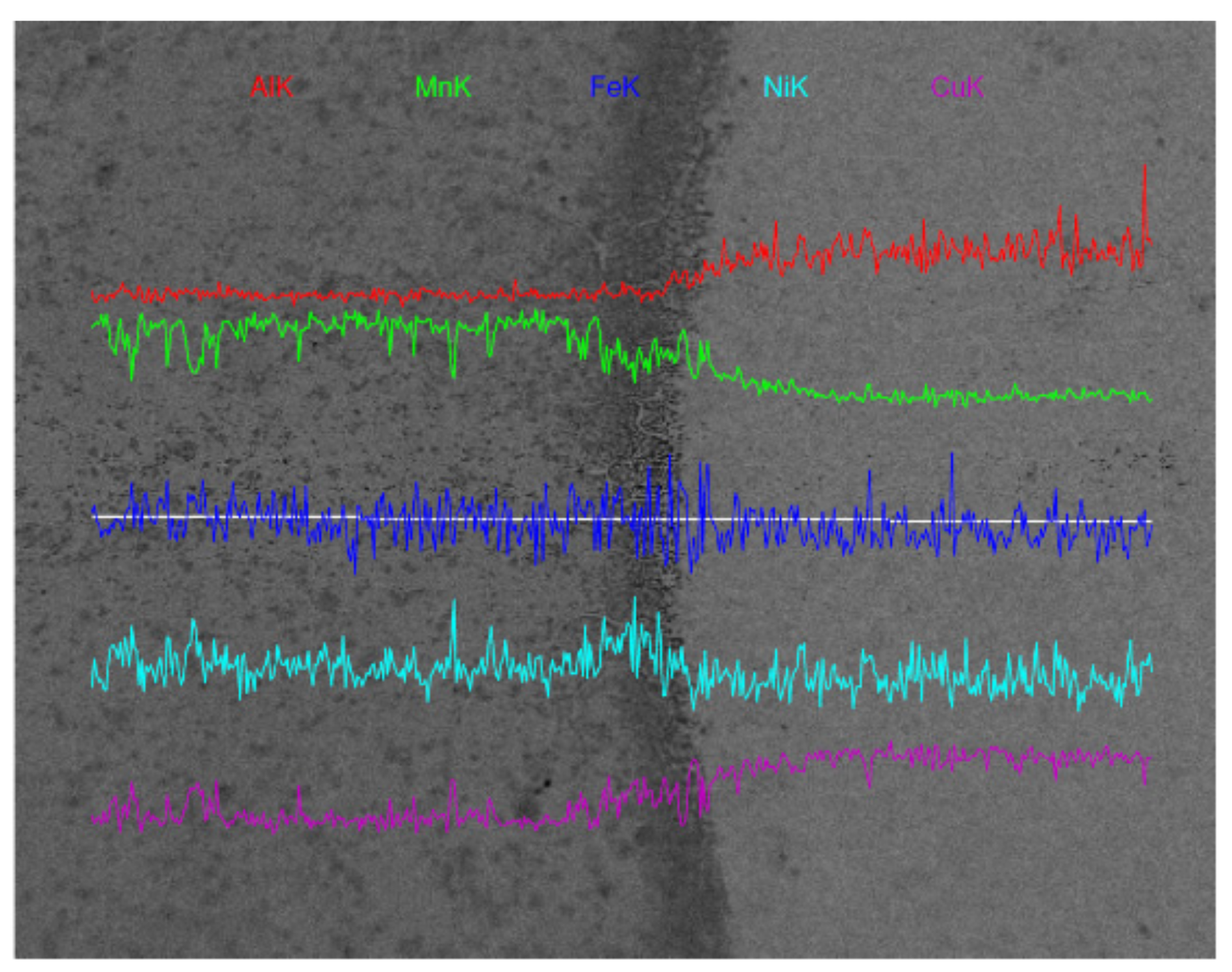
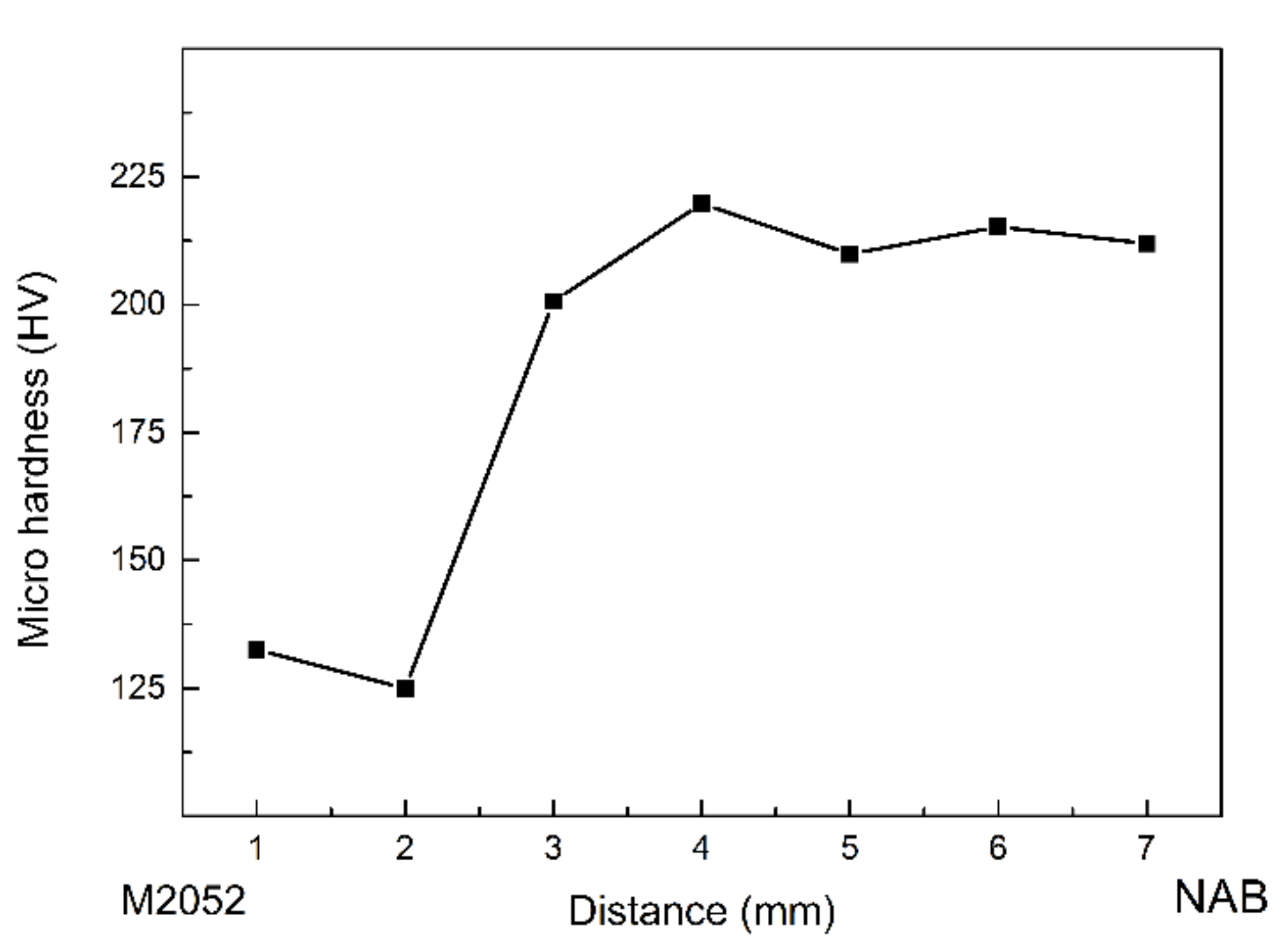
- A transition zone with a width of about 100 μm was formed at the interface of the M2052–NAB gradient alloy, and the alloy had no obvious grain orientation. In XRD analysis, an additional Al–Mn phase appeared in the gradient alloy, indicating that Al in the NAB alloy interacted with Mn in the M2052 matrix to form a new phase in the transition zone. Microhardness testing showed that the hardness of NAB alloy in the as-deposited state was higher than in the as-cast state but lower than that of the alloy after friction stir welding;
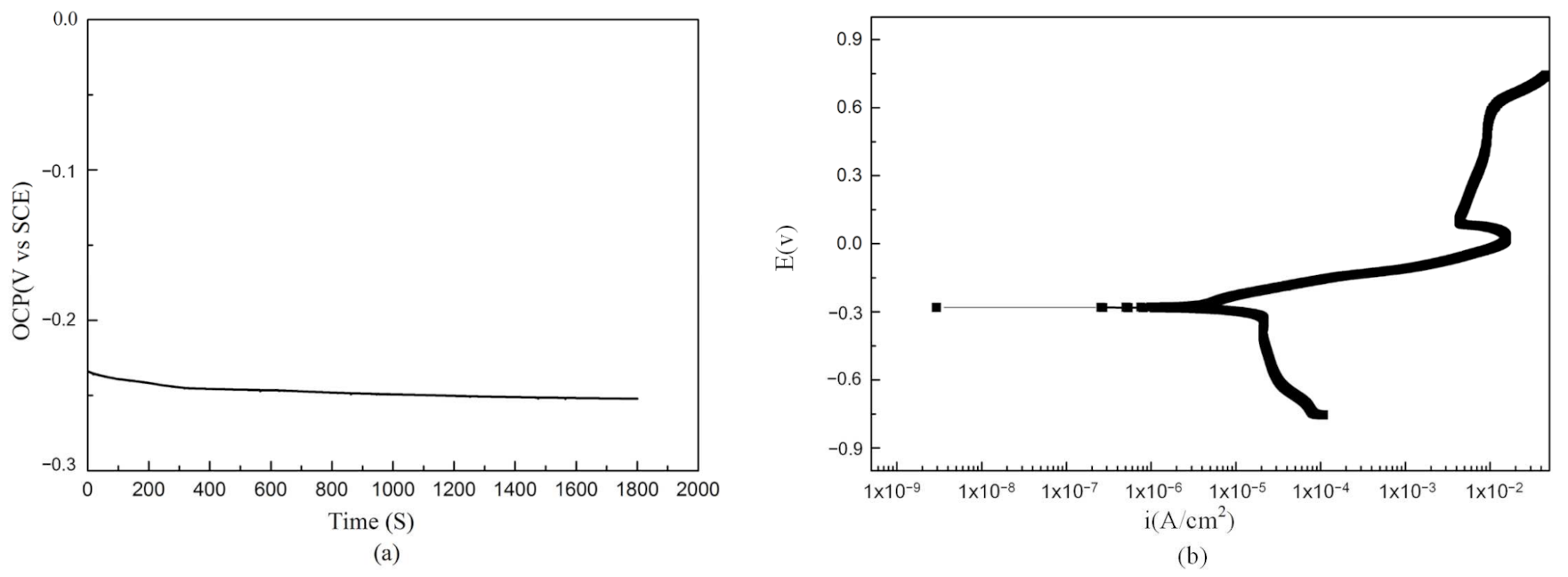
- The NAB alloy deposited by LENS showed good corrosion resistance in 3.5% NaCl. The corrosion potential of −0.28 V was comparable with that of the as-cast and SLM alloys. The self-corrosion current density was 7.24 × 10−6 A/cm2, which is lower than that of the as-cast alloy and SLM deposited alloys;
- The deposited alloy had tiny pores and irregular pore defects caused by molten pool collapse, which affected the material properties. The printing parameters of the alloy have not yet been analyzed and optimized, so there is still potential for improvement in the performance of the gradient alloy;
- Compared with existing studies, the M2052–NAB alloy interface possesses a good metallurgical bond [32], and the corrosion resistance in the 3.5% NaCl solution is higher than that of the as-cast and SLM deposited alloys.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borelli, D.; Gaggero, T.; Rizzuto, E.; Schenone, C. Analysis of noise on board a ship during navigation and manoeuvres. Ocean. Eng. 2015, 105, 256–269. [Google Scholar] [CrossRef]
- Zou, D.; Zhang, J.; Ta, N.; Rao, Z. Study on the axial exciting force characteristics of marine propellers considered the effect of the shaft and blade elasticity. Appl. Ocean. Res. 2019, 89, 141–153. [Google Scholar] [CrossRef]
- Bodger, L.; Helma, S.; Sasaki, N. Vibration control by propeller design. Ocean Eng. 2016, 120, 175–181. [Google Scholar] [CrossRef]
- Raghebi, M.; Keshtan, M.N.; Jafarian, M.M.; Bagheri, M.R. Numerical study and acoustic analysis of propeller and hull surface vessel in self-propulsion mode. Ships Offshore Struct. 2021, 1–10. [Google Scholar] [CrossRef]
- Merz, S.; Kinns, R.; Kessissoglou, N. Structural and acoustic responses of a submarine hull due to propeller forces. J. Sound Vib. 2009, 325, 266–286. [Google Scholar] [CrossRef]
- Razaghian, A.H.; Ebrahimi, A.; Zahedi, F.; Javanmardi, M.R.; Seif, M.S. Investigating the effect of geometric parameters on hydrodynamic and hydro-acoustic performances of submerged propellers. Appl. Ocean. Res. 2021, 114, 102773. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y. Unsteady hydrodynamics of blade forces and acoustic responses of a model scaled submarine excited by propeller’s thrust and side-forces. J. Sound Vib. 2013, 332, 2038–2056. [Google Scholar] [CrossRef]
- Liu, Y.; Song, S.; Xiao, G.; He, Y.; Huang, Y.; Liu, S.; Jiahua, S. The Method and Experiment Research on Down-stroke Abrasive Belt Grinding under Micro Feeding for Noise Reduction Surface. J. Bionic Eng. 2021, 18, 958–973. [Google Scholar] [CrossRef]
- Huang, X.; Su, Z.; Hua, H. Application of a dynamic vibration absorber with negative stiffness for control of a marine shafting system. Ocean. Eng. 2018, 155, 131–143. [Google Scholar] [CrossRef]
- Song, Y.; Wen, J.; Yu, D.; Liu, Y.; Wen, X. Reduction of vibration and noise radiation of an underwater vehicle due to propeller forces using periodically layered isolators. J. Sound Vib. 2014, 333, 3031–3043. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, X.; Tang, Y.; Zhong, S.; Xu, Y. A comparative study on microstructure and damping capacity of Mn-Cu based alloys with dendrite and equiaxial grain. Vacuum 2019, 168, 108814. [Google Scholar] [CrossRef]
- Lynch, S.P.; Edwards, D.P.; Majumdar, A.; Moutsos, S.; Mahoney, M.W. Friction-Stir Processing of a High-Damping Mn-Cu Alloy used for Marine Propellers. Mater. Sci. Forum 2003, 426–432, 2903–2908. [Google Scholar] [CrossRef]
- Jiang, Z.C.; Zhang, S.B.; Tian, Q.C.; Ji, P.G.; Yin, F.X. Phenomenological representation of mechanical spectroscopy of high damping MnCuNiFe alloy. Mater. Sci. Technol. 2020, 36, 743–749. [Google Scholar] [CrossRef]
- Chen, G.; Wang, J.; Xue, L.; Huang, Z. Enhancement of Wear and Corrosion Resistance of M2052 Damping Alloys by Electroless Plating Ni–P Coating. ISIJ Int. 2020, 60, 2276–2284. [Google Scholar] [CrossRef]
- Li, W.S.; Wang, Z.P.; Lu, Y.; Yuan, L.H. Corrosion Wear Behavior of Al-Bronzes in 3.5% NaCl Solution. J. Mater. Eng. Perform. 2006, 15, 102–110. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Z.; Wang, X.; Liang, B.; Zhang, Y. Microstructure and mechanical behavior of Cu–9Al–4Ni-3.5Fe-0.5Mn alloy fabricated by laser melting deposition. Mater. Sci. Eng. A 2021, 826, 142006. [Google Scholar] [CrossRef]
- Amegroud, H.; Guenbour, A.; Bellaouchou, A.; Aoufir, Y.E.; Lgaz, H.; Chung, I.-M.; Alrashdi, A.A. A comprehensive investigation of the electrochemical behavior of nickel-aluminum bronze alloy in alkaline solution: The effect of film formation potential. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126126. [Google Scholar] [CrossRef]
- Lv, Y.; Guo, J.; Zhang, G.; Cao, L.; Sun, X.; Qin, Z.; Xia, D.-H. Insights into the selective phase corrosion of as cast NiAl bronze alloy: Effect of electrical properties of each phase’s protective film. J. Alloys Compd. 2022, 891, 162008. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, Y.; Ni, C.; Yu, L.; Li, C.; Yan, X.; Zhang, X. Research on electromagnetic wave absorption properties of zinc-based acrylate resins for marine antifouling coating. J. Alloys Compd. 2022, 900, 163285. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Cheon, J.; Ji, C. Effect of Filler Metal Type on Microstructure and Mechanical Properties of Fabricated NiAl Bronze Alloy Using Wire Arc Additive Manufacturing System. Metals 2021, 11, 513. [Google Scholar] [CrossRef]
- Basumatary, J.; Wood, R.J.K. Different methods of measuring synergy between cavitation erosion and corrosion for nickel aluminium bronze in 3.5% NaCl solution. Tribol. Int. 2020, 147, 104843. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.-J.; Du, J.; Song, S.-X.; Zhong, Z.-H.; Ge, C.-C. Microstructural characterization of W/Cu functionally graded materials produced by a one-step resistance sintering method. J. Alloys Compd. 2007, 428, 146–150. [Google Scholar] [CrossRef]
- Gao, Y.X.; Wang, X.P.; Jiang, W.B.; Yang, J.F.; Zeng, L.F.; Fang, Q.F. High damping capacity and low density M2052/Al composites fabricated by accumulative roll bonding. J. Alloys Compd. 2018, 757, 415–422. [Google Scholar] [CrossRef]
- Duda, T.R.; Venkat, L. 3D Metal Printing Technology. IFAC-PapersOnLine 2016, 49, 103–110. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components—Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Wei, C.; Sun, Z.; Chen, Q.; Liu, Z.; Li, L. Additive Manufacturing of Horizontal and 3D Functionally Graded 316L/Cu10Sn Components via Multiple Material Selective Laser Melting. J. Manuf. Sci. Eng. 2019, 141, 081014. [Google Scholar] [CrossRef]
- Tan, C.; Zhou, K.; Kuang, T. Selective laser melting of tungsten-copper functionally graded material. Mater. Lett. 2019, 237, 328–331. [Google Scholar] [CrossRef]
- Khaleed, H.M.T.; Badruddin, I.A.; Saquib, A.N.; Addas, M.F.; Kamangar, S.; Yunus Khan, T.M. Novel Approach to Manufacture an AUV Propeller by Additive Manufacturing and Error Analysis. Appl. Sci. 2019, 9, 4413. [Google Scholar] [CrossRef] [Green Version]
- He, T.; Yu, S.; Shi, Y.; Dai, Y. High-accuracy and high-performance WAAM propeller manufacture by cylindrical surface slicing method. Int. J. Adv. Manuf. Technol. 2019, 105, 4773–4782. [Google Scholar] [CrossRef]
- Kumar, S.; Pityana, S. Laser-Based Additive Manufacturing of Metals. Adv. Mater. Res. 2011, 227, 92–95. [Google Scholar] [CrossRef]
- Barwinska, I.; Kopec, M.; Lazinska, M.; Brodecki, A.; Durejko, T.; Kowalewski, Z.L. Suitability of Laser Engineered Net Shaping Technology for Inconel 625 Based Parts Repair Process. Materials 2021, 14, 7302. [Google Scholar] [CrossRef] [PubMed]
- Hindy, A.; Farahmand, F.; Pourdanesh, F.; Torshabi, M.; Al Janabi, A.H.; Rasoulianboroujeni, M.; Tayebi, L.; Tabatabaei, F.S. Synthesis and characterization of 3D-printed functionally graded porous titanium alloy. J. Mater. Sci. 2020, 55, 9082–9094. [Google Scholar] [CrossRef]
- Zhong, Y.; Sakaguchi, T.; Yin, F. Effects of transformation twin on Hall–Petch relationship in MnCu alloy. Mater. Sci. Eng. A 2008, 492, 419–427. [Google Scholar] [CrossRef]
- Fukuhara, M.; Yin, F.; Ohsawa, Y.; Takamori, S. High-damping properties of Mn–Cu sintered alloys. Mater. Sci. Eng. A 2006, 442, 439–443. [Google Scholar] [CrossRef]
- Yin, F.X.; Iwasaki, S.; Sakaguchi, T.; Nagai, K. Susceptibility of Damping Behavior to the Solidification Condition in the As-Cast M2052 High-Damping Alloy. Key Eng. Mater. 2006, 319, 67–72. [Google Scholar] [CrossRef]
- Li, Y.; Lian, Y.; Sun, Y. Comparison of cavitation erosion behaviors between the as-cast and friction stir processed Ni–Al bronze in distilled water and artificial seawater. J. Mater. Res. Technol. 2021, 13, 906–918. [Google Scholar] [CrossRef]
- Murray, T.; Thomas, S.; Wu, Y.; Neil, W.; Hutchinson, C. Selective laser melting of nickel aluminium bronze. Addit. Manuf. 2020, 33, 101122. [Google Scholar] [CrossRef]
- Jiang, Z.; Hou, L.; Tian, Q.; Ren, W.; Ren, Z. Polymorphic microstructure of a MnCu damping alloy solidified under magnetic field. Mater. Res. Express 2019, 6, 0865h2. [Google Scholar] [CrossRef]
- Zhong, Z.; Liu, W.; Li, N.; Yan, J.; Xie, J.; Li, D.; Liu, Y.; Zhao, X.; Shi, S. Mn segregation dependence of damping capacity of as-cast M2052 alloy. Mater. Sci. Eng. A 2016, 660, 97–101. [Google Scholar] [CrossRef]
- Lv, Y.; Ding, Y.; Cui, H.; Liu, G.; Wang, B.; Cao, L.; Li, L.; Qin, Z.; Lu, W. Investigation of microscopic residual stress and its effects on stress corrosion behavior of NiAl bronze alloy using in situ neutron diffraction/EBSD/tensile corrosion experiment. Mater. Charact. 2020, 164, 110351. [Google Scholar] [CrossRef]
- Lv, Y.; Zhao, B.; Zhang, H.; Su, C.; Nie, B.; Wang, R.; Cao, L.; Lyu, F. Improving Corrosion Resistance Properties of Nickel-Aluminum Bronze (NAB) Alloys via Shot Peening Treatment. Mater. Trans. 2019, 60, 1629–1637. [Google Scholar] [CrossRef] [Green Version]

| Mn | Cu | Ni | Fe | Al | O | |
|---|---|---|---|---|---|---|
| NAB powder | 0.6 | 79.0 | 4.3 | 3.6 | 12.5 | 0.0 |
| LENS–NAB alloy | 0.4 | 81.4 | 4.3 | 3.5 | 10.4 | 0.0 |
| M2052 powder | 69.6 | 21.4 | 6.0 | 2.9 | - | 0.0 |
| LENS–M2052 alloy | 68.8 | 21.5 | 5.8 | 3.8 | - | 0.0 |
| Parameters | Value |
|---|---|
| Laser Power (W) | 300 |
| Scanning Speed (cm/min) | 50 |
| Powder Feeder (r/min) | 4 |
| Layer Thickness (μm) | 254 |
| Powder Carrier Gas (MPa) | 0.04 |
| Shielding Gas (MPa) | 0.19 |
| Atmospheric oxygen content (PPM) | <30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, K.; Lin, Z.; Chen, M.; Wang, Y.; Wang, J.; Jiang, H. Fabrication of MnCuNiFe–CuAlNiFeMn Gradient Alloy by Laser Engineering Net Shaping System. Materials 2022, 15, 2336. https://doi.org/10.3390/ma15062336
Yan K, Lin Z, Chen M, Wang Y, Wang J, Jiang H. Fabrication of MnCuNiFe–CuAlNiFeMn Gradient Alloy by Laser Engineering Net Shaping System. Materials. 2022; 15(6):2336. https://doi.org/10.3390/ma15062336
Chicago/Turabian StyleYan, Kuo, Zaiwen Lin, Meng Chen, Yuren Wang, Jun Wang, and Heng Jiang. 2022. "Fabrication of MnCuNiFe–CuAlNiFeMn Gradient Alloy by Laser Engineering Net Shaping System" Materials 15, no. 6: 2336. https://doi.org/10.3390/ma15062336
APA StyleYan, K., Lin, Z., Chen, M., Wang, Y., Wang, J., & Jiang, H. (2022). Fabrication of MnCuNiFe–CuAlNiFeMn Gradient Alloy by Laser Engineering Net Shaping System. Materials, 15(6), 2336. https://doi.org/10.3390/ma15062336







