1. Introduction
The conversion of methanol-to-hydrocarbons (MTH), including gasoline, aromatic or olefin, on acidic zeolites is a very important way for fossil fuels and biomass to produce bulk chemicals from syngas; it has become one of the hot topics in the field of catalysis in the past twenty years [
1,
2]. The present situation of energy in China is that it is rich in coal resources and poor in the resources of oil and natural gas. The technology of methanol production from syngas has been matured, which could provide a good raw material foundation for MTH. ZSM-5 is the most commonly used zeolite catalyst in the MTH process, with a three-dimensional crosspore structure and a high Si/Al ratio; as a result, it has unique shape selectivity, good hydrothermal stability and strong resistance to coke deposition ability [
3,
4]. ZSM-5 zeolites are usually synthesized by the hydrothermal method in an alkaline sol–gel system, and the product of ZSM-5 zeolites is an alkaline crystal powder containing template and alkali metal cations. The template needs to be removed by drying and calcining, and then the zeolite powder is exchanged with an acid solution or an ammonium salt solution to form hydrogen-type ZSM-5 (HZSM-5) raw powder. In industrial applications HZSM-5 raw powder is molded with a certain amount of binder by wet extrusion, dried and calcined to achieve the mechanical strength required for industrial applications and then the finished MTH catalyst is prepared. The molding of HZSM-5 zeolite raw powder and binders is an important part in the industrial preparation process of an MTH catalyst. This process can not only enhance the strength of the catalyst but also affect the acid active centers and textural properties of the catalyst by adding a binder, thus affecting the reaction performance of the catalyst [
5,
6,
7]. In addition, coke deposition during the MTH reaction is the main reason for the one-way deactivation of the catalyst [
8,
9,
10]. The preparation methods affect the acidity and pore distribution of the catalyst, thus affecting the deactivation behavior of coke deposition. Therefore, it is also of great significance to study the effect of the preparation method on the catalytic performance and coke behaviors of HZSM-5 zeolites for the development of an MTH catalyst in industrial applications.
The typically used binders include silica, alumina, kaolin and so on, which play different roles in the molding process. Pseudoboehmite is a kind of alumina binder used often and commonly in the extrusion molding process of a ZSM-5 zeolite catalyst [
11,
12,
13,
14]. In order to investigate the influence of the binder content and molding method on the MTH catalytic performance of ZSM-5 zeolite catalysts, pseudoboehmite was used as the binder to prepare different kinds of catalysts (wet extrusion molding with dilute nitric acid, mechanical mixing pressing molding and wet extrusion molding with water). In our study the effects of the molding method and binder content on the catalytic performance of MTH were discussed, and a catalyst with industrial application value was prepared. The content and C/H ratio of coke deposition on the catalyst were compared, and the influence of the molding method on coke behavior was analyzed.
2. Materials and Methods
2.1. Catalyst Preparation
ZSM-5 zeolites were synthesized by the hydrothermal method, using sodium silicate (modulus 3.3, Qingdao Dongyue sodium silicate Co., Ltd., Qingdao, China) as a silicon source, aluminum sulfate octadecahydrate (Al2(SO4)3·18H2O, analytical purity, Tianjin Beichen Fangzheng Reagent Factory, Tianjin, China) as an aluminum source, tetrapropylammonium bromide as a template (TPABr, chemical purity, Zhejiang Kente Chemical Co., Ltd., Xianju, China) and concentrated sulfuric acid (H2SO4, chemical purity, Sinopharm Chemical Reagent Co., Ltd., Beijing, China) as a pH regulator, to prepare a certain proportion of gel. The obtained gel was put into a 2 L stainless steel autoclave, and the stirring speed was 400 rev/min. ZSM-5 zeolite crystals were obtained through aging at low temperature and crystallization at high temperature, controlling the synthetic temperature and crystallization time. The obtained crystals were washed to neutral with deionized water, dried at 393 K for 12 h and calcinated at 813 K for 4 h to remove the organic template. According to 50 mL of a 0.5 mol/L ammonium nitrate (NH4NO3, chemical purity, Sinopharm Chemical Reagent Co., Ltd., Beijing, China) solution of each gram of NaZSM-5 zeolites, the hydrogen-type ZSM-5 zeolite (HZSM-5) raw powder was obtained by exchanging at 353 K for three times. The HZSM-5 raw powder was pressed into tablets and broken into a 20–40 mesh for standby, which was recorded as CZ.
Weigh 8 g, 7 g, 6 g and 5 g of HZSM-5 zeolite powder, then weigh 2 g, 3 g, 4 g and 5 g of pseudoboehmite (Zibo Baida Chemical Co., Ltd., Zibo, China), respectively. Mix them evenly, add dilute nitric acid solution, knead evenly and extrude them. The formed HZSM-5 catalysts with pseudoboehmite contents of 20%, 30%, 40% and 50% were prepared, which were recorded as CN-20, CN-30, CN-40 and CN-50 successively. Weigh 7 g of HZSM-5 zeolite raw powder and 3 g of pseudoboehmite, mix them mechanically, grind them evenly in an agate mortar and press them into tablets, recorded as CP-30. Weigh 7 g of HZSM-5 zeolite raw powder and 3 g of pseudoboehmite, mix them evenly, add deionized water, knead them evenly and extrude them into shape, recorded as CW-30. All the formed catalysts were dried at 393 K for 12 h, calcinated at 813 K for 4 h and broken into a 20–40 mesh for subsequent catalyst evaluation.
2.2. Catalyst Characterization
X-ray diffraction (XRD) phase analysis was carried out on a D8 ADVANCE X-ray powder diffractometer (Bruker, Germany). Cu target, Kα ray (λ = 0.15 nm), tube voltage of 40 kV, tube current of 30 mA, 5–50° scanning and step size of 0.02°.
Fourier transform infrared (FT-IR) spectra characterization was carried out on a Bruker Vector 22 infrared spectrometer (Bruker, Germany). The samples and potassium bromide powder were mixed and ground as the mass ratio of 1:(200–400), and then the spectra were scanning at room temperature after forming by pressing.
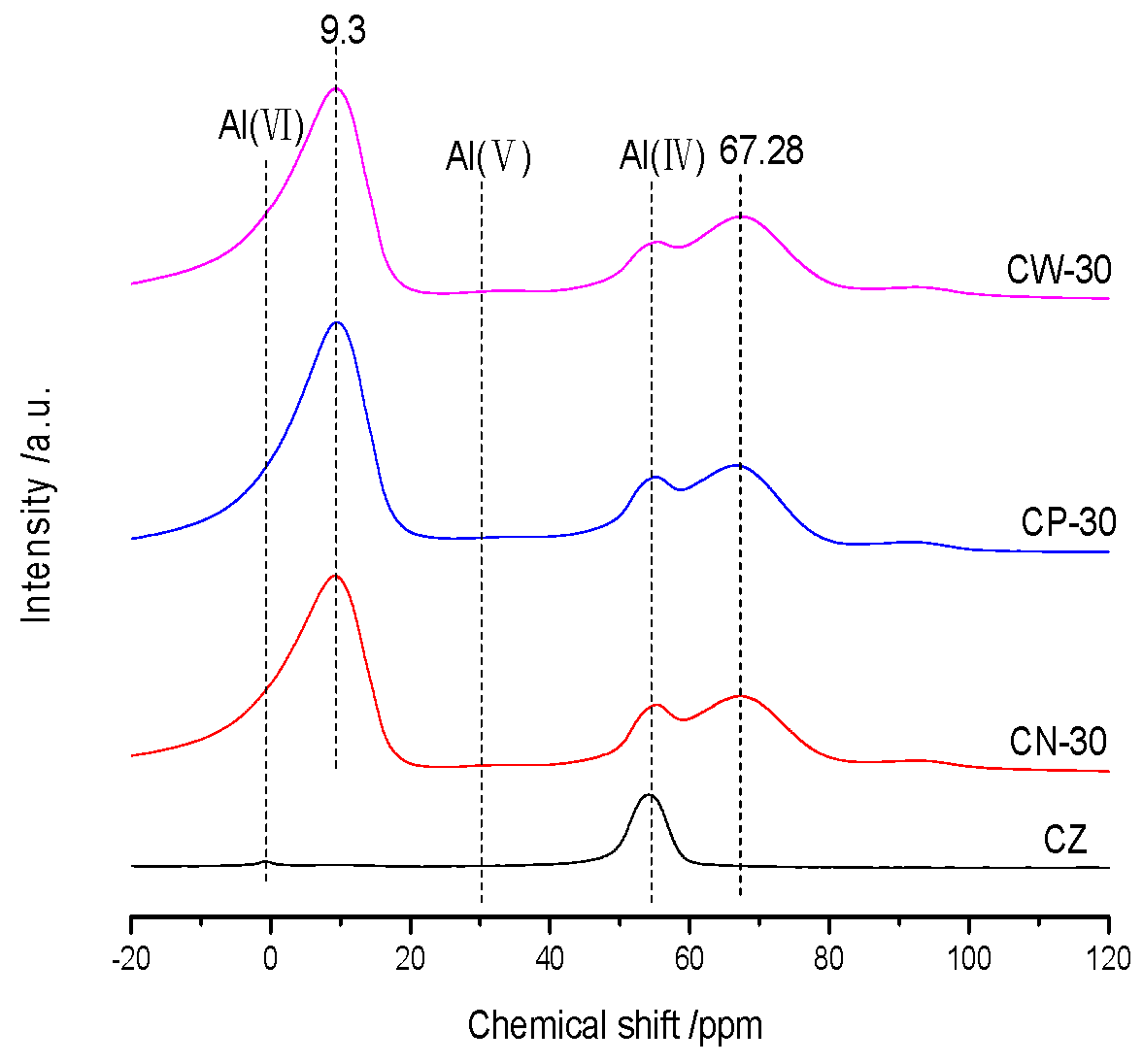
An 27Al NMR test was carried out on an AVANCE IIITM 600 MHz superconducting NMR spectrometer (Bruker, Switzerland). 27Al NMR spectra were obtained at 10.0 kHz using a 1 s delay, for a total of 10000 pulses.
The catalysts’ morphology was determined by a JSM-7001F thermal field emission scanning electron microscope (SEM) with a voltage of 10 kV (JEOL, Akishima, Tokyo, Japan). Elemental distributions were acquired with an X-ray energy-dispersive spectrometer (EDS, X-Flash 5010) detector at 15 kV.
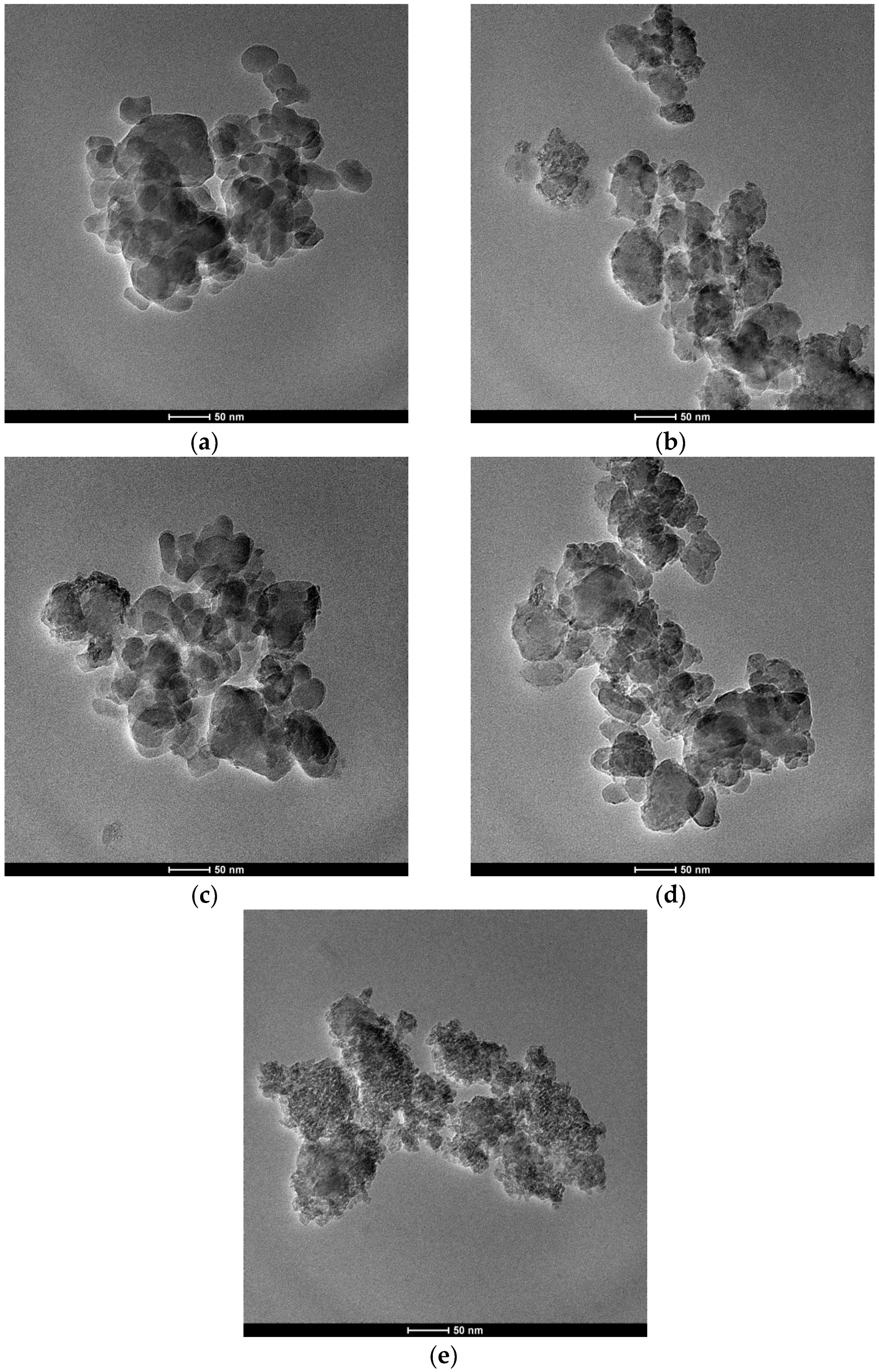
Transmission electron microscopy (TEM) images were recorded on a Tecnai G2 F20 S-Twin microscope operated at 200 kV (FEI, Hillsboro, America).
The composition of HZSM-5 zeolites were determined by a Thermo iCAP 6300 inductively coupled plasma (ICP) atomic emission spectrometer (Thermo Fisher, Waltham, America).
Low-temperature N2 adsorption and desorption isotherms at 77 K were recorded using a Micromeritics ASAP 2010 instrument (Micromeritics, America). Before the measurements the samples were heated to 570 K in a vacuum for at least 12 h. The specific surface area, mesopore size pore distribution and micropore volume were calculated by the Brunauer–Emmett–Teller (BET) method, the Barret–Joyner–Halenda (BJH) method and the t-plot method by Harkins and Jura (DeBoer) thickness equation, with a thickness range of 3.5 to 5 Å, respectively.
Ammonia temperature-programmed desorption (NH3-TPD) characterization was carried out on a micro automatic multipurpose adsorption instrument, TP-5080 (Tianjin Xianquan, China). The catalyst was first purged with N2 at 773 K for 60 min, with a N2 flow rate of 30 mL/min, and then reduced to 373 K for the adsorption of NH3. After the baseline was stable the ammonia desorption experiment was conducted at 10 K/min to 973 K.
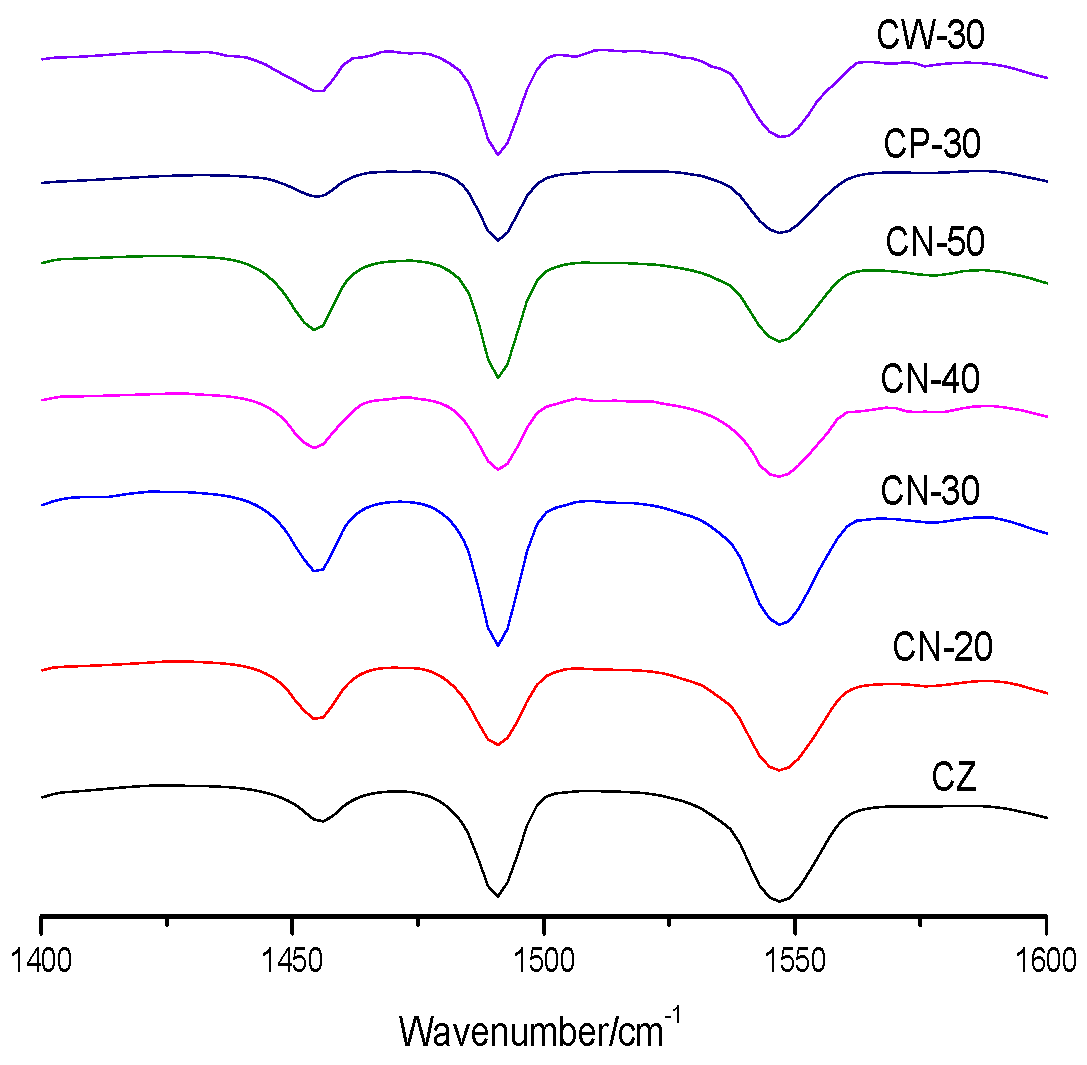
Pyridine Fourier transform infrared (Py-FTIR) was characterized on a Bruker Vector 22 infrared spectrometer (Bruker, Karlsruhe, Germany). The catalyst was first treated at 673 K and 0.05 Pa for 30 min to remove the adsorbed impurities. Pyridine was adsorbed at room temperature. After desorption at 573 K and 0.05 Pa, the characteristic peaks of Brönsted (B) acid and Lewis (L) acid were integrated. The distribution of B acid and L acid was represented by the ratio of the peak area of the two kinds of acids.
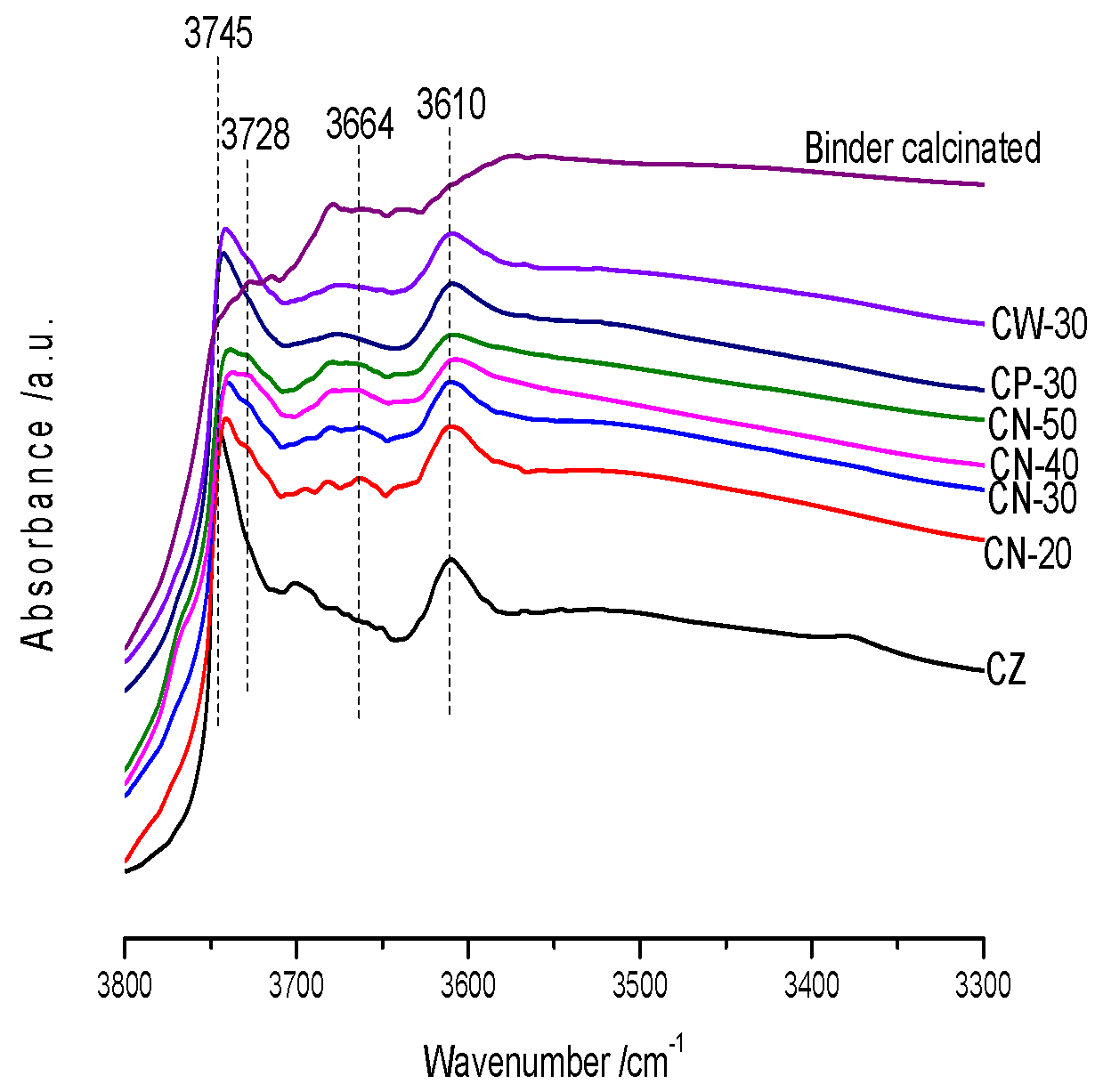
Diffuse reflectance Fourier transform infrared (FT-IR) spectra were measured on a Bruker Tensor 27 FT-IR spectrometer (Bruker, Germany). The catalyst samples were first calcinated at 813 K for 4 h in the muffle furnace. Prior to the measurement the sample was heated at 723 K for 2 h with a N2 flow of 15 mL/min in situ cell. The IR spectra were then recorded at room temperature.
C and H elements were analyzed by a Vario EL CUBE element analyzer (Elementar, Frankfurt, Germany).
The thermogravimetry (TG) analysis of deposited coke catalysts was carried out on a Setsys Evolution thermogravimetric analyzer (Setaram, Lyon, France). O2 atmosphere, from 303 K to 1173 K, with a heating rate of 10 K/min and a gas flow rate of 20 mL/min.
2.3. Catalyst Evaluation
The catalyst was evaluated in a continuous-flow fixed-bed reactor with stainless steel tubes of 100 cm in length and 1 cm in inner diameter. Three grams of catalyst was loaded into the constant temperature section of the reactor, and quartz sand was filled at both ends of the bed. MTH reaction performance was evaluated at atmospheric pressure, 653 K and a methanol weight space time velocity (WHSV) of 4 h
−1. The products were separated by cold trap gas–liquid separation. The gas- and water-phase products were analyzed by an SP-2000 gas chromatograph of a Beijing Beifen Ruili analytical instrument (Group) Co., Ltd. (Beijing, China). The gas-phase products were analyzed by a TDX-102 packed column, a thermal conductivity detector (TCD), an aluminum oxide (Al
2O
3) capillary column and a hydrogen flame (FID) detector. A Porapak-Q packed column and TCD detector were used for the analysis of aqueous products. Oil-phase products in the liquid-phase products were analyzed by an SP-3420 gas chromatograph of a Beijing Beifen Ruili analytical instrument (Group) Co., Ltd. The chromatographic columns for oil-phase products were an HP INNOWAX capillary column and an FID detector. The methanol conversion, product distribution and hydrogen transfer index (HTI) are defined as follows:
4. Discussion
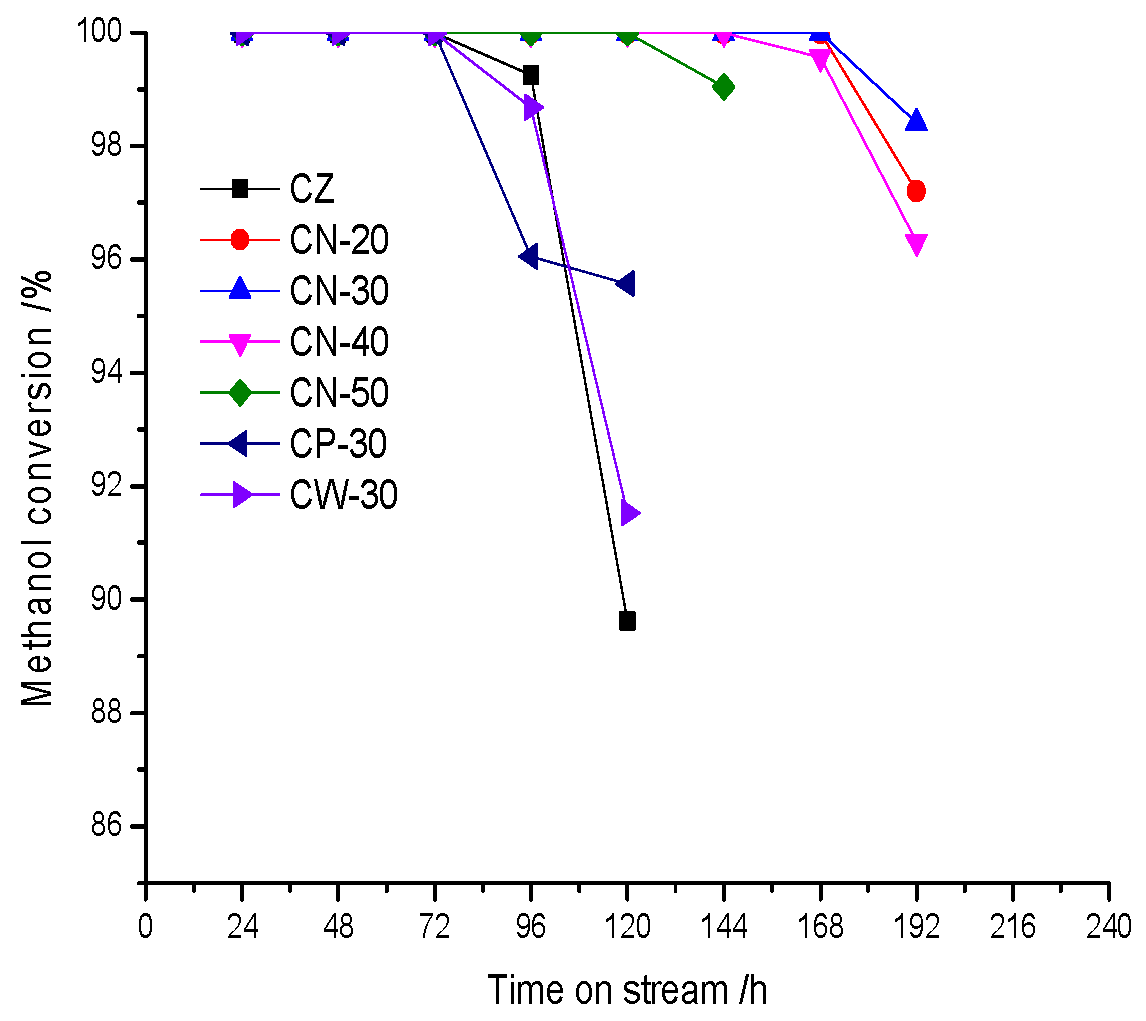
CN-30 has the best catalytic performance. It suggested that adding a binder and wet extrusion molding with dilute nitric acid can effectively reduce the deactivation rate and improve activity stability. As we all know, coke deposition is the main reason of one-way deactivation in the MTH reaction and the catalyst activity can be restored by burning coke deposition. Strong B acid sites are conducive to the reactions of polymerization, cracking, cyclization and hydrogen transfer, which eventually generate macromolecular polycyclic aromatics, and they are the active centers of deep coke deposition. The addition of the binder not only increases the mechanical strength of the catalyst but also disperses and covers strong B acid sites [
29]. A strong acid amount and strong acid strength decreased significantly with the addition of the binder, and strong acid strength decreased gradually with an increase in binder content. On the one hand, although the acid amount of CN-30 increases and its acid density is the highest, the acid strength of CN-30 decreases significantly and its proportion of B acid is moderate. On the other hand, there are two kinds of concentrated pore distribution in CN-30, forming a hierarchical pore structure that greatly improves diffusion performance. CN-30 therefore has the best activity stability due to the combination effect of acidity and pore distribution. The B acid ratio of CP-30 and CW-30 obtained by the mechanical mixing method and water extrusion method is higher, after which a deep coke deposition reaction is more likely to occur; therefore, the deactivation is faster.
NH3-TPD and Py-IR characterizations show that CZ has strong acidity, high acid density and the highest proportion of B acid, which makes it easier to deactivate via carbon deposition. However, the activity stability of CZ is the same as that of CP-30 and CW-30, which is 72 h, methanol conversion of CZ decreases more rapidly at 96 h than that of CP-30 and CW-30, and begins to decrease sharply at 120 h. This indicates that pore structure also has some certain influence on activity stability. CZ molded by the pressing of HZSM-5 zeolites into tablet has formed a large number of mesopores and macropores via nano-HZSM-5 crystals, which improves diffusion performance and is conducive to the diffusion of precursors of macromolecular coke deposition; therefore, the deactivation rate of CZ is reduced to a certain extent. The proportion of B acid decreases with an increase in binder content, indicating that the strength and density of B acid decreased significantly, while the proportion of B acid of CP-30 and CW-30 is higher than that of CN series catalysts, which aggravated the deep coke reaction. Pore structure data show that the micropore volume and micropore surface area of CP-30 and CW-30 decreased, which indicates that the binder covered the surface and pore entrance of HZSM-5 zeolites in addition to blocking the micropore. The pore distribution also confirms the above statement. Consequently, the catalysts prepared by the mechanical mixing method and water wet extrusion method are detrimental to the diffusion of macromolecular products. The activity stability of CP-30 and CW-30 is not improved, and the activity stability is close to that of CZ with the effect of the acid distribution and pore structure.
According to the “hydrocarbon pool” reaction mechanism, methanol first forms “hydrocarbon pool” active species, after which methanol and “hydrocarbon pool” active species continue to react to generate various hydrocarbon products. The “hydrocarbon pool” species on HZSM-5 are mainly long-chain olefins, polymethylbenzene and cyclopentene carbon cations [
30,
31]. These species are also important deactivate intermediates, and they can also be called coke deposition precursors. With the progress of the reaction the coke deposition on the catalyst gradually increases, covering the surface of the acid center, reducing the acid strength and acid density; therefore, the capacity of polymerization, cracking, cyclization and hydrogen transfer gradually decreases, the olefin content gradually increases, the alkane content gradually decreases and C
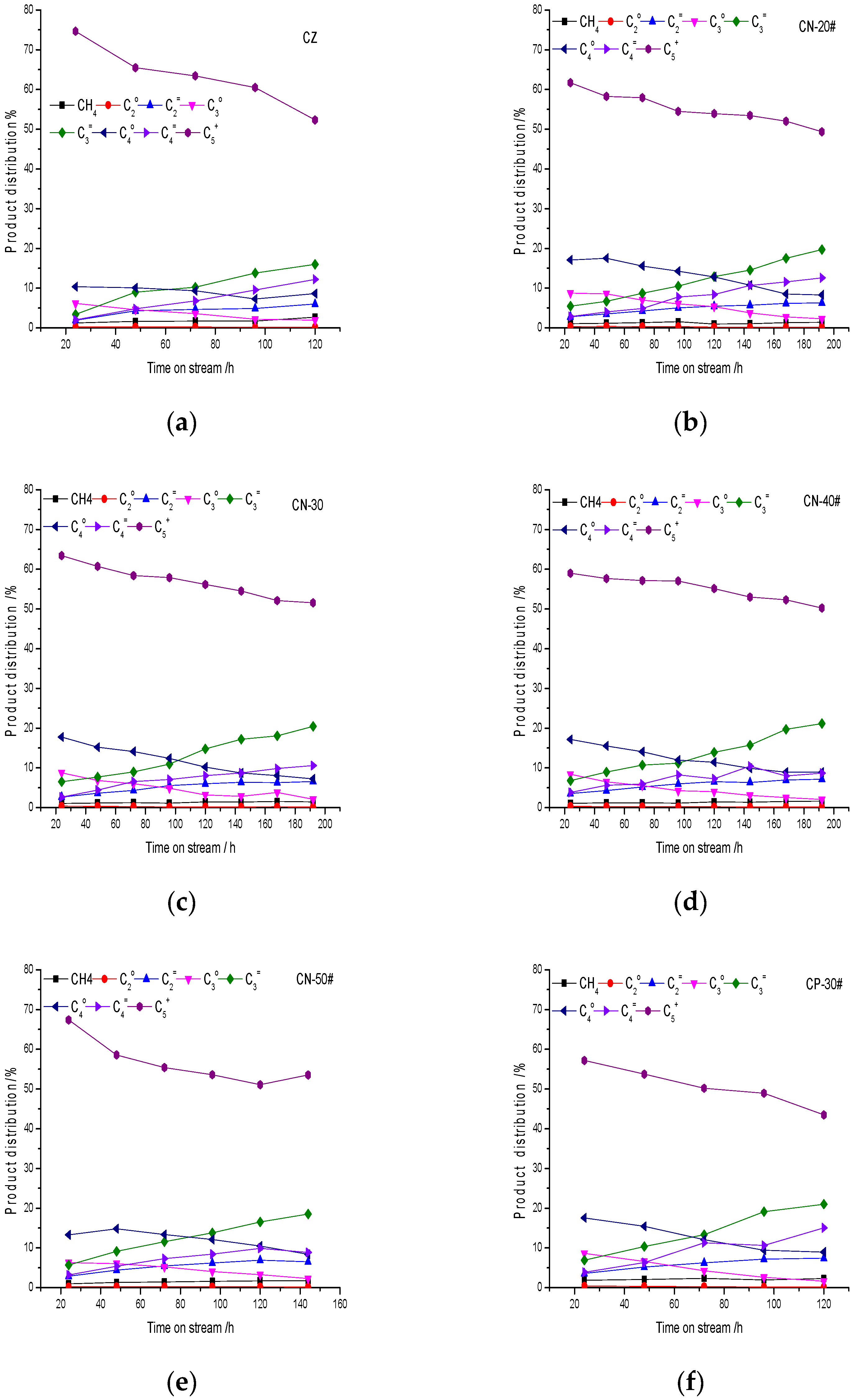
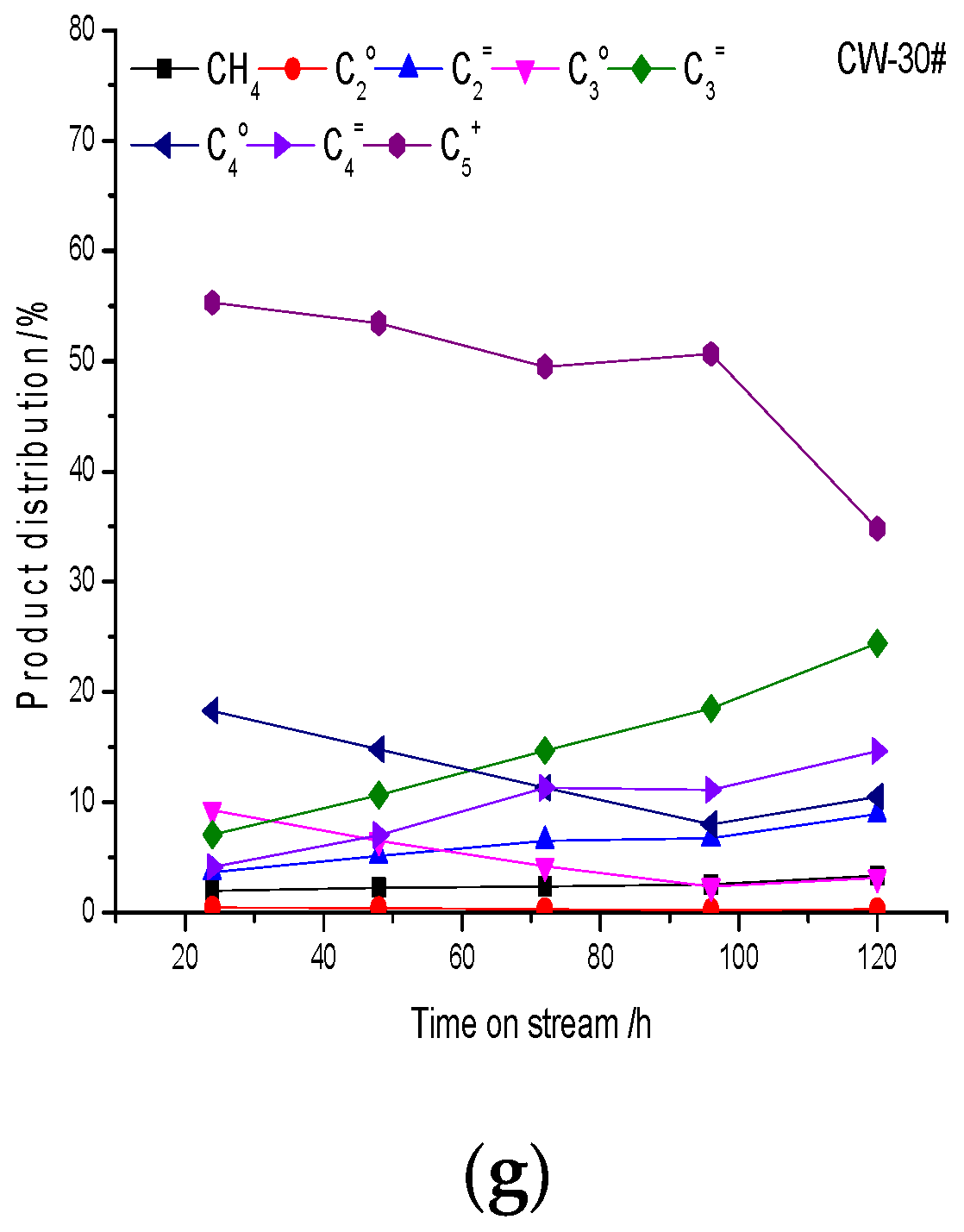
5+ hydrocarbon products decrease gradually. In the same way, the catalysts with strong acidity, high proportions of B acid centers and large acid amounts have strong abilities of polymerization, cracking, cyclization and hydrogen transfer, so olefins are lower and alkanes are higher. The acid center is the active center of the reaction, especially the strong B acid center, which is the key factor of the reaction performance. If acidity is strong and the acid density is high the combination of “hydrocarbon pool” species and acid sites is strong, and products will not be easy to desorb from catalysts, resulting in deep coke deposition. If the acidity is moderate methanol can continuously react with “hydrocarbon pool” species, and the catalyst will have a long lifetime. Gas-phase hydrocarbon products of the catalysts with the addition of the binder increased, while high-carbon-number C
5+ hydrocarbon products increase gradually, which is mainly due to the decrease in strong acid strength and acid density.
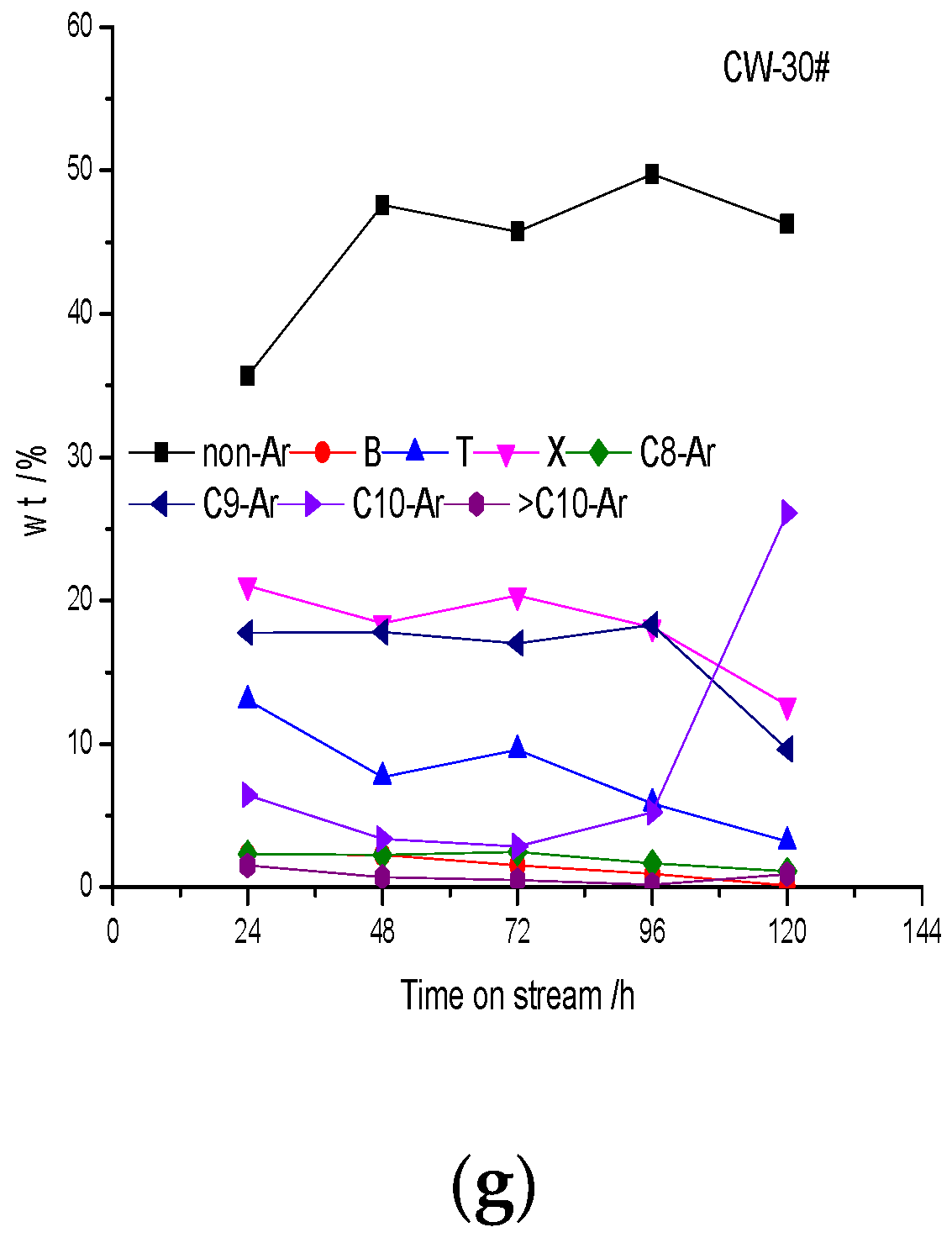
The oil composition change is also mainly due to the effect of coke deposition on the acidity during the reaction. As mentioned above, with an increase in coke deposition the acid center is covered by coke deposition, acid strength decreases, acid density decreases and the ability of polymerization and cyclization decrease. Therefore, the content of nonaromatic hydrocarbons with a low carbon number increases and the content of aromatics decreases. Polymethylbenzenes (toluene, xylene, trimethylbenzene and tetramethylbenzene) are the main aromatics. The content of aromatics with carbon number larger than C10 is less; the trend is not obvious, which is due to the shape selectivity of HZSM-5 crystal micropores. Benzene, toluene and C8 aromatics are mainly obtained from cracking reactions; the cracking ability is stronger at the initial stage of the reaction, so the content of benzene, toluene and C8 aromatics is higher at first and then decreases gradually, which is due to the influence of coke deposition on acidity. The change in C9 aromatics is not obvious, and the main C9 aromatics component is the thermodynamic equilibrium product meta-trimethylbenzene. C10 aromatics are mainly tetramethylbenzenes, in which the content of durene is the largest, and its content increases with time on stream. As the reaction proceeds the cracking activity is insufficient, so durene increases. The results suggest that the relationship of aromatics distribution and pore distribution is not obvious, which is mainly related to acidity. The acid distribution affects product distribution directly. A catalyst with strong acidity and a high density of acid centers has strong cracking ability, and the content of low-carbon-number aromatics is high. On the contrary, the content of polymethyl aromatics is high.
The change in HTI is also due to carbon deposits formed in the reaction process, which modifies the acidity, weakens strong acid strength and decreases acid density. Therefore, the HTI decreases continuously with the reaction. The trend in the HTI showed that the coke deposition rate of a catalyst with a high B/L ratio was very fast. The addition of the binder can reduce the acid strength, make the distribution of acid centers more dispersed and reduce the density of acid centers, which can delay coke deposition; therefore, the HTI of the catalysts with a high binder content decreased slowly. The HTI corresponds to the difference in catalysts’ acidity, which is related to the B/L ratio and acid density directly. The essential reason is that the molding methods and binder content have different effects on acidity, which was mentioned earlier.
Acidity is the main factor that affects the properties of coke deposition; aromatics are prone to deep coking reactions over catalysts with a high acid density and strong acidity. Therefore, the amount of coke deposition of CZ is the largest and the C/H ratio of coke deposition is the highest. The interaction between the binder and strong acid sites prepared by the mechanical mixing method is weak, its strong acid sites are retained more and the B acid ratio is higher; therefore, its C/H ratio is higher, followed by CW-30. Adding dilute acid to the molding process of CN-30, which enhances the interaction between the binder and HZSM-5 zeolites, covers strong acid centers, reduces acid strength, and inhibits the deep coking reaction effectively. The trend in the TG and C/H ratio of coke deposition corresponds to acidity changing, which verifies the influence of acidity difference on the reaction performance.
5. Conclusions
Molding by adding a binder of pseudoboehmite changes the acid distribution of the catalysts, the amount of medium strength acid increases, the amount of strong acid decreases, the strength of strong acid decreases and the B/L ratio decreases. The medium-strength acid amount of the catalyst molded by wet extrusion with dilute nitric acid is increased and the B acid proportion is moderate, while the mechanical mixing and water wet extrusion methods retained more B acid sites. With the increase in binder content the acid amount first increases and then decreases, and the B/L ratio decreases gradually.
For nano-HZSM-5 zeolite crystals a large number of intercrystal mesopores can be formed by nanocrystals. The addition of the binder can form an obvious hierarchical pore distribution, but the total pore volume decreases. The mechanical mixing method and water wet extrusion molding method cause micropore blockage; the molding method of wet extrusion with dilute nitric acid is conducive to the formation of a more obvious hierarchical pore distribution. Hierarchical pore distribution is beneficial to product diffusion, reducing the deactivation rate of carbon deposition and improving the activity stability of the catalyst.
The addition of dilute nitric acid in the molding process strengthens the interaction of HZSM-5 zeolites and binders, increases the medium-strength acid amount and forms a more obvious hierarchical pore distribution. However, the hierarchical pore structure of the mechanical mixing method and water wet extrusion molding method is not obvious. The acidity of the 30% binder content catalyst prepared by wet extrusion molding with a dilute nitric acid solution is moderate, especially its strong acid strength and B/L ratio, whose hierarchical pore structure is the most obvious; therefore, the activity stability of CN-30 is the highest.
Acid distribution is the main reason for product distribution of the catalysts, which is related to the ability of the hydrogen transfer reaction, the amount of coke deposition, the rate of coke deposition and the C/H ratio of coke deposition. The catalyst with strong acidity and a large strong acid amount has high hydrogen transfer ability, large coke deposition, a fast coke deposition rate and a high carbon C/H ratio. A strong B acid center is conducive to the formation of high-carbon hydrocarbons and aromatics, while pore distribution has little influence on product distribution.