An Inverse Analysis for Establishing the Temperature-Dependent Thermal Conductivity of a Melt-Cast Explosive across the Whole Solidification Process
Abstract
:1. Introduction
2. Experiments and Inverse Analysis Method
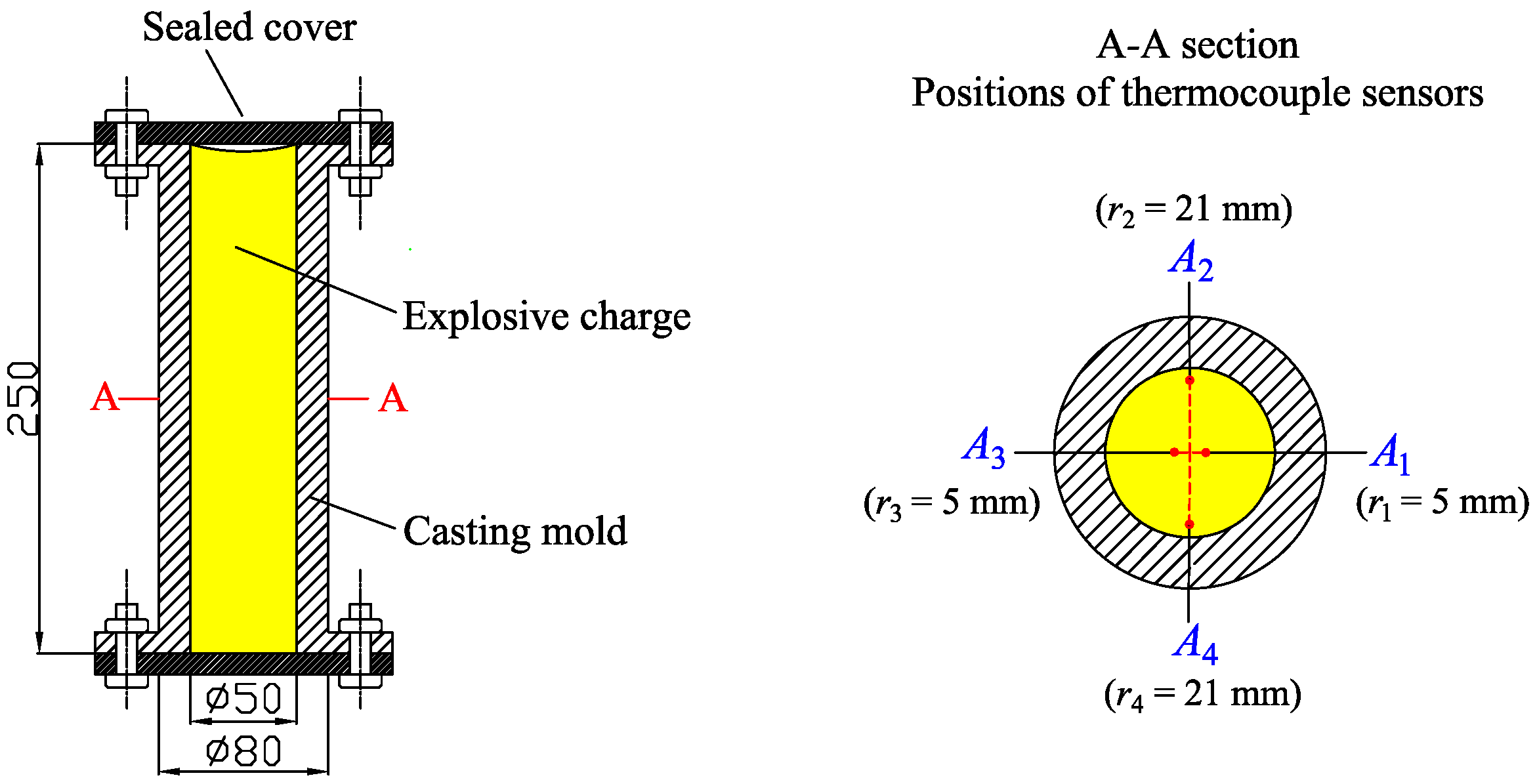
2.1. Experimental Design
- The molten DNAN/HMX explosive was prepared with an initial temperature of 100.1 .
- The molten explosive was poured into the mold and the sealing cover was placed immediately on the top of the mold and held securely in place using tightening bolts.
- The molten explosive began to cool, and the four thermocouples recorded the temperature time history until the temperature had decreased to reach that of the ambient environment.
- The molten explosive was poured into a steel mold with an inner diameter of 25 mm and a height of 100 mm, ensuring that the mold was completely filled so the volume (V) of the molten explosive was roughly equal to that of the mold.
- The mass (M) of the molten explosive was measured with a balance after it had solidified and cooled down to normal temperature.
- The density () of the molten explosive was evaluated according to .
2.2. Inverse Analysis of Thermal Conductivity
2.2.1. Direct Problem
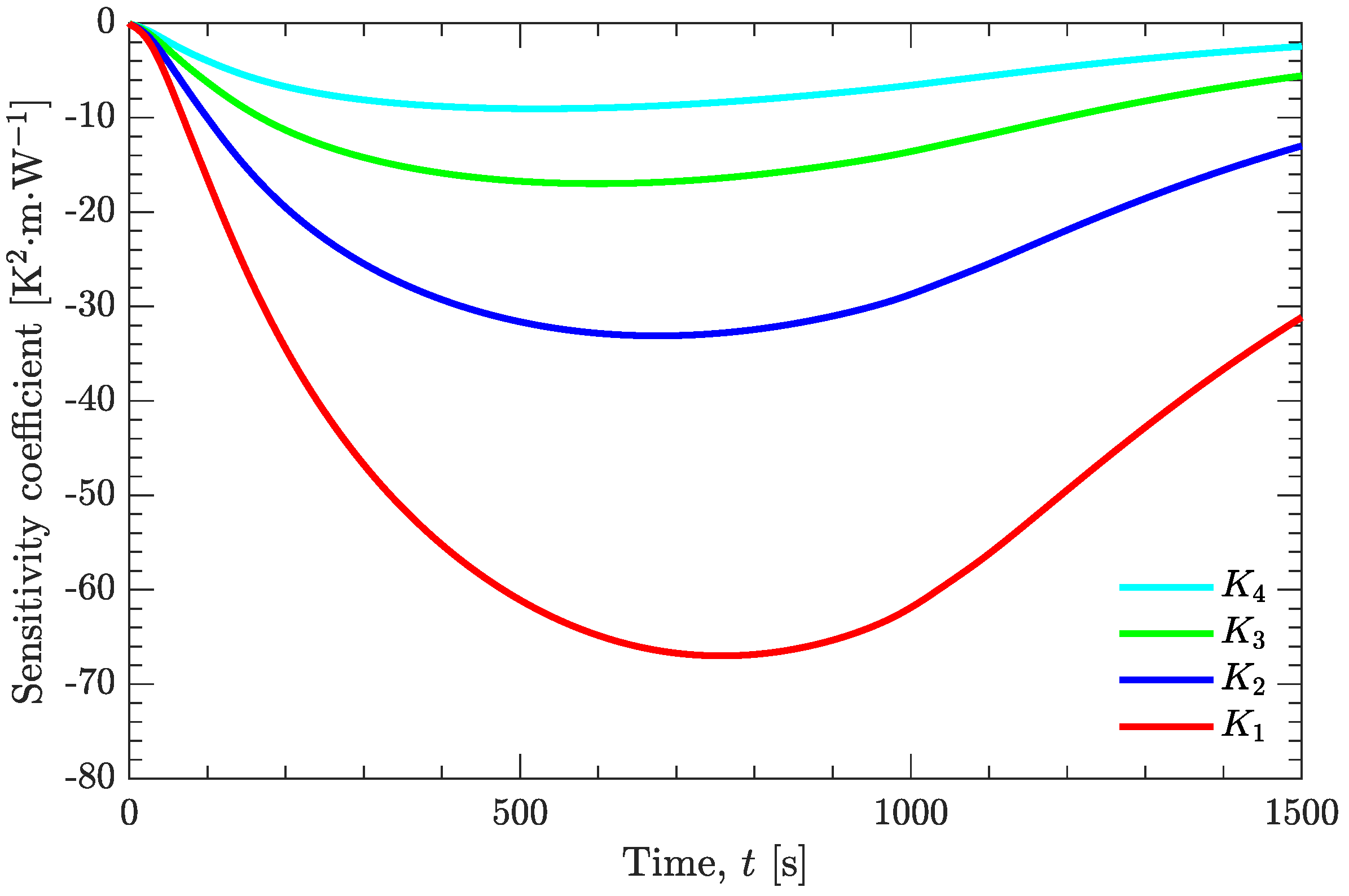
2.2.2. Sensitivity Problem
2.2.3. Gauss–Newton Algorithm for Minimization
2.2.4. Stopping Criterion
2.2.5. Computational Procedure
3. Results and Discussion
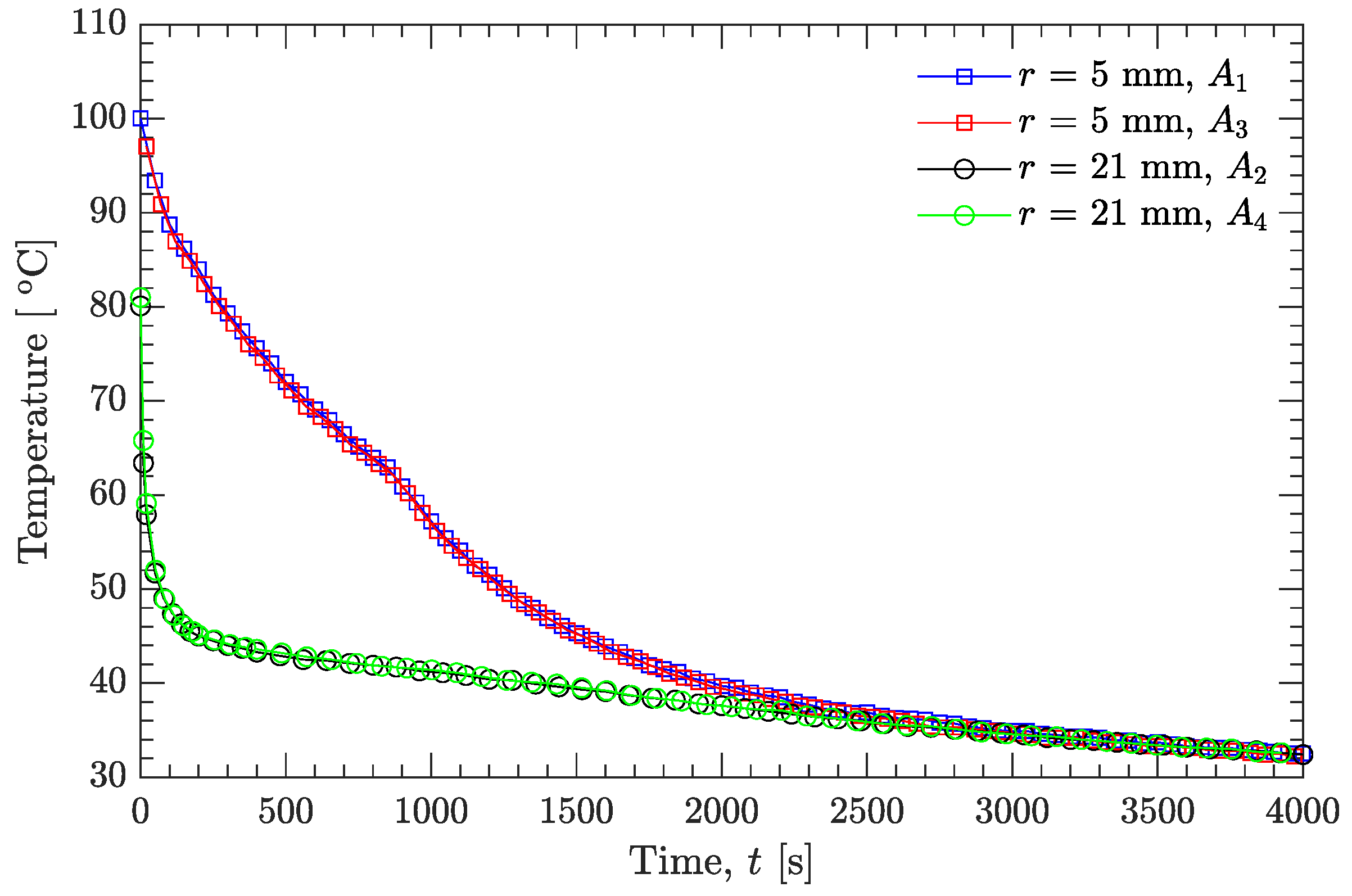
3.1. Experimental Temperatures and Thermophysical Properties
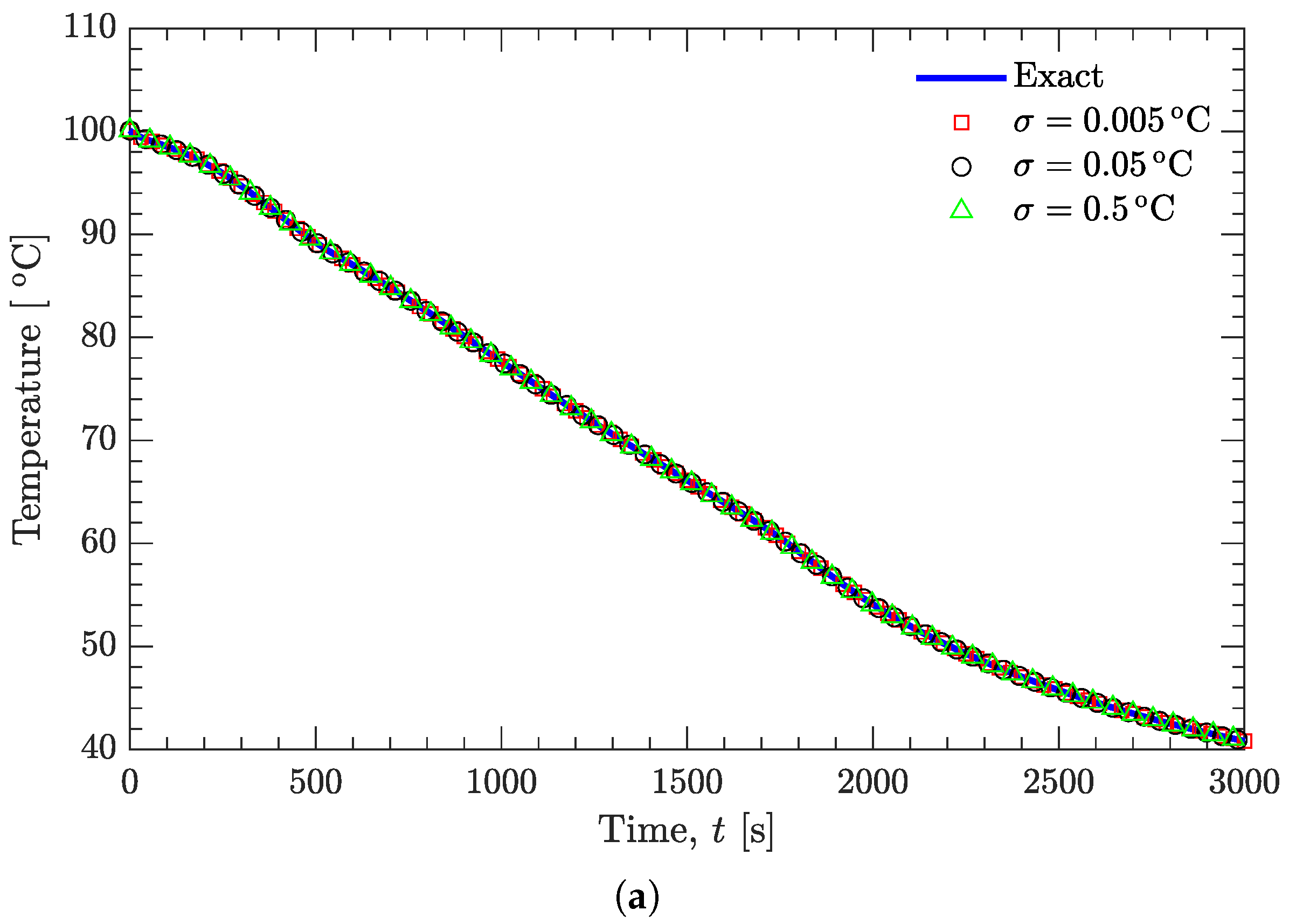
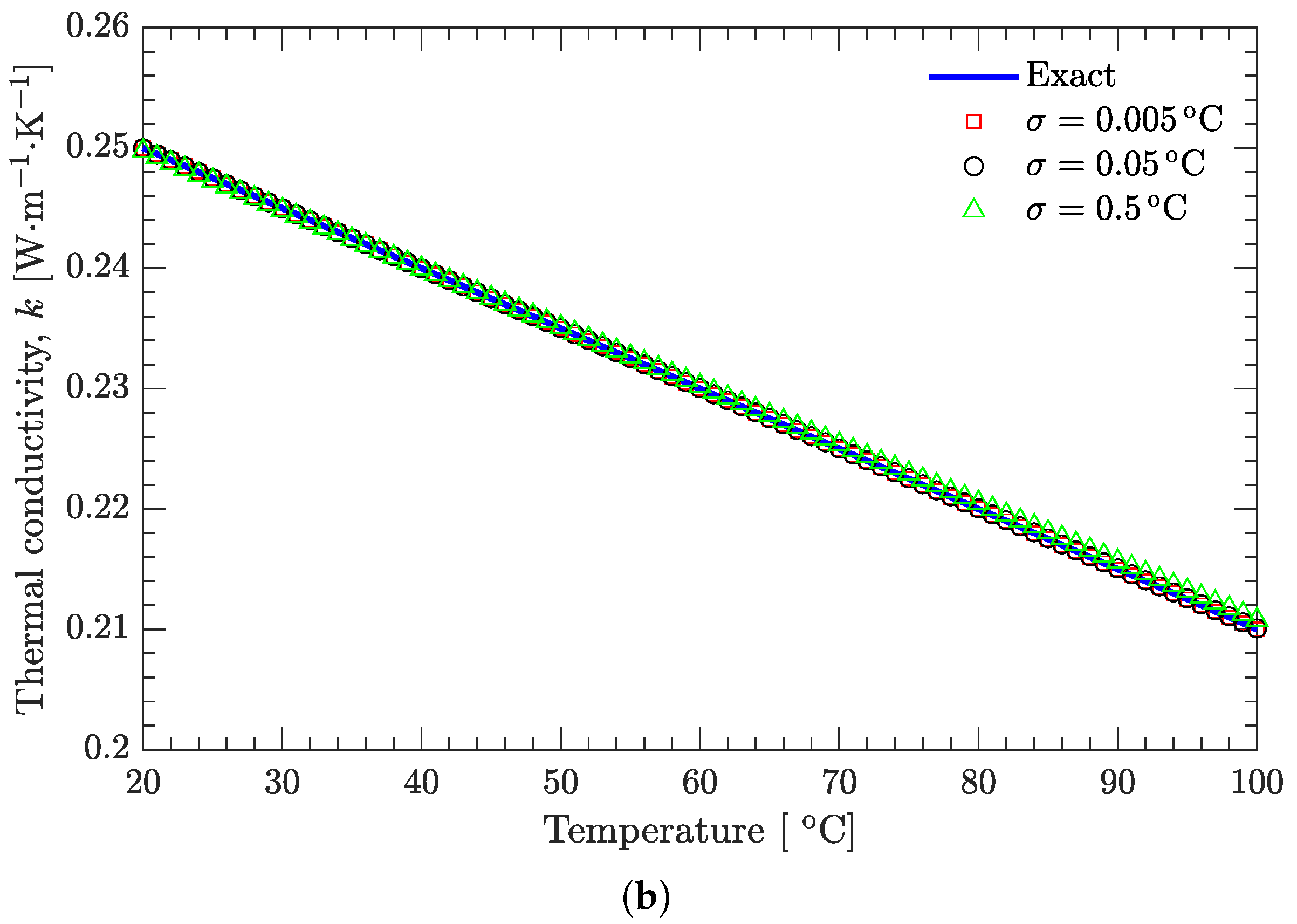
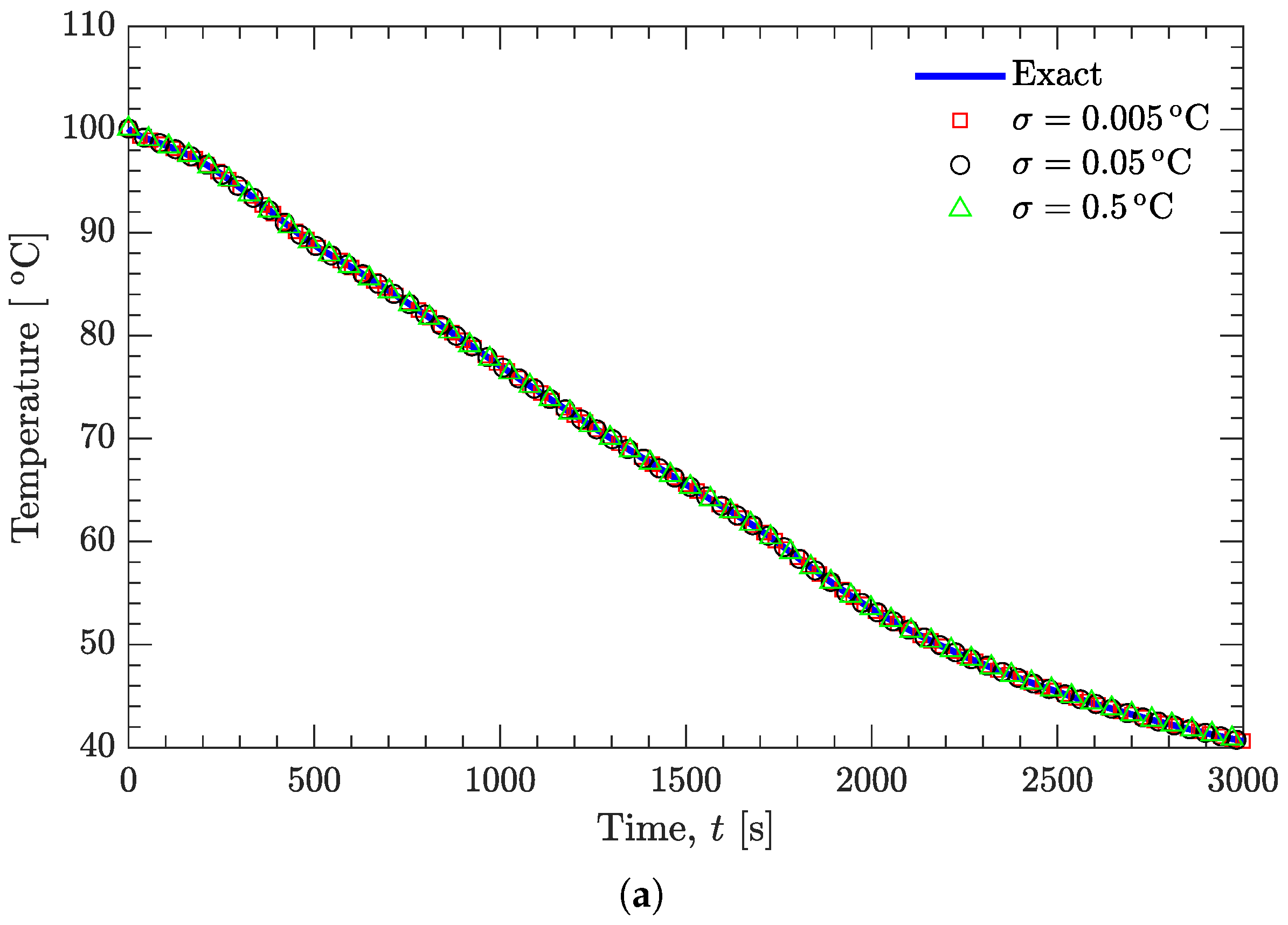
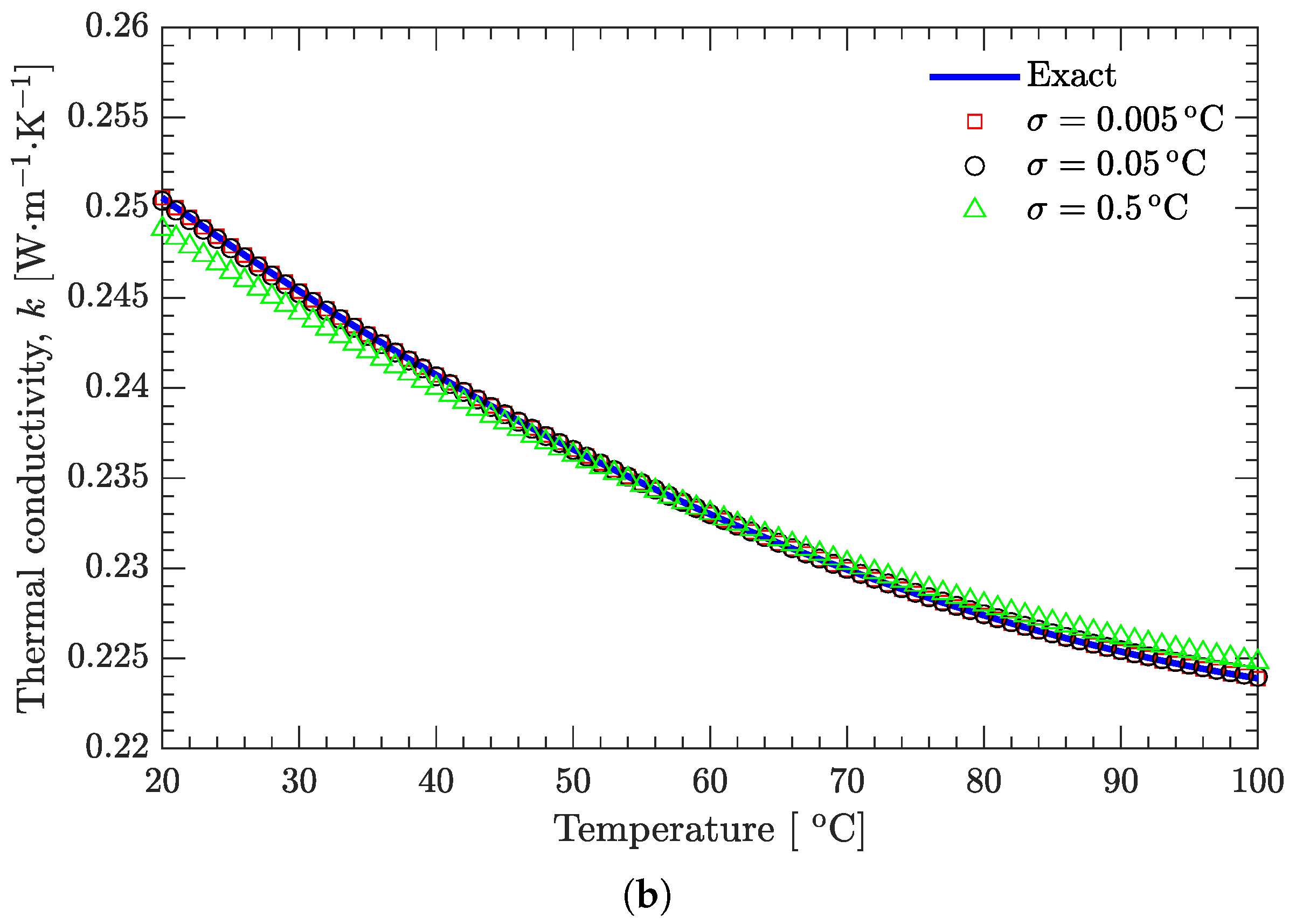
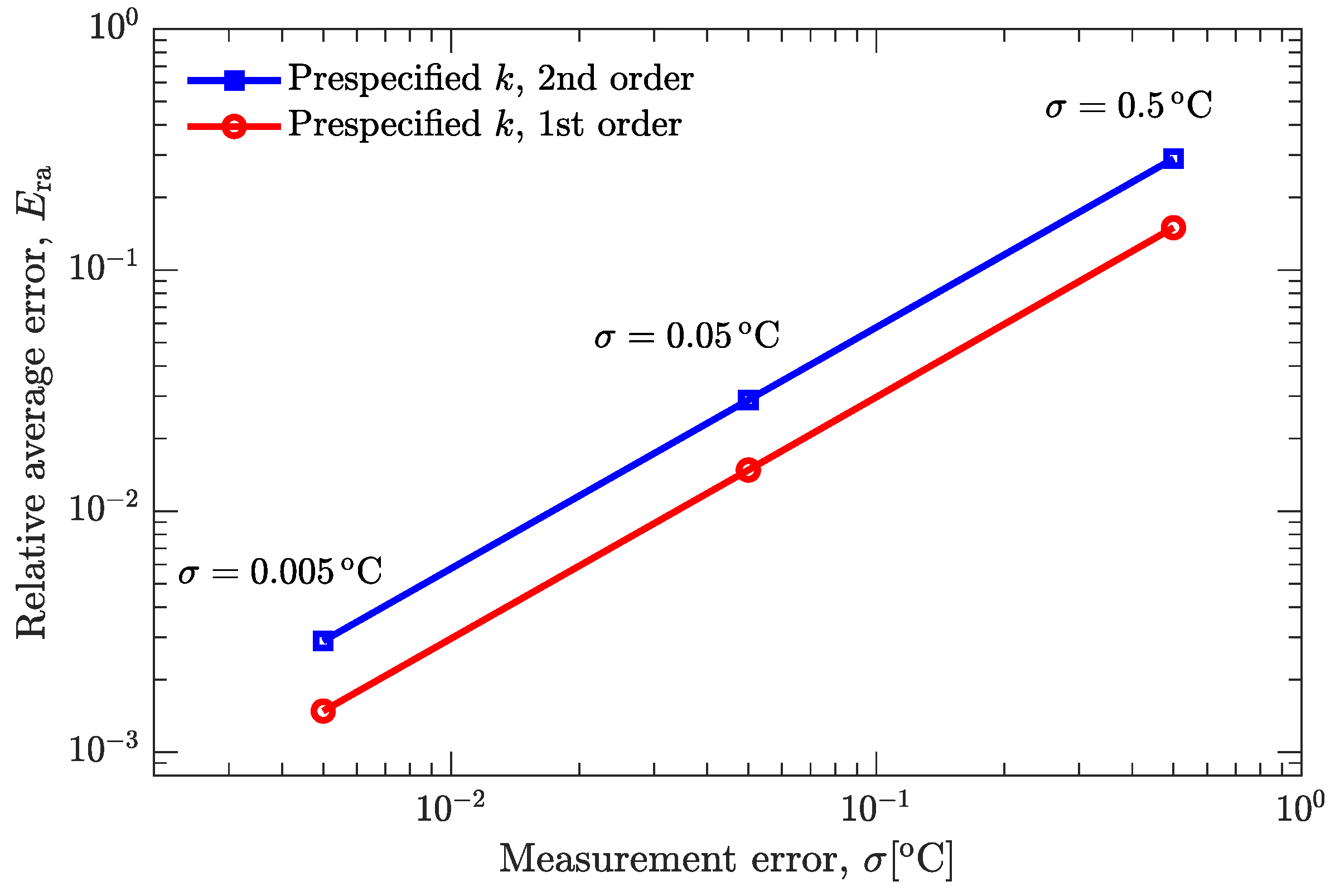
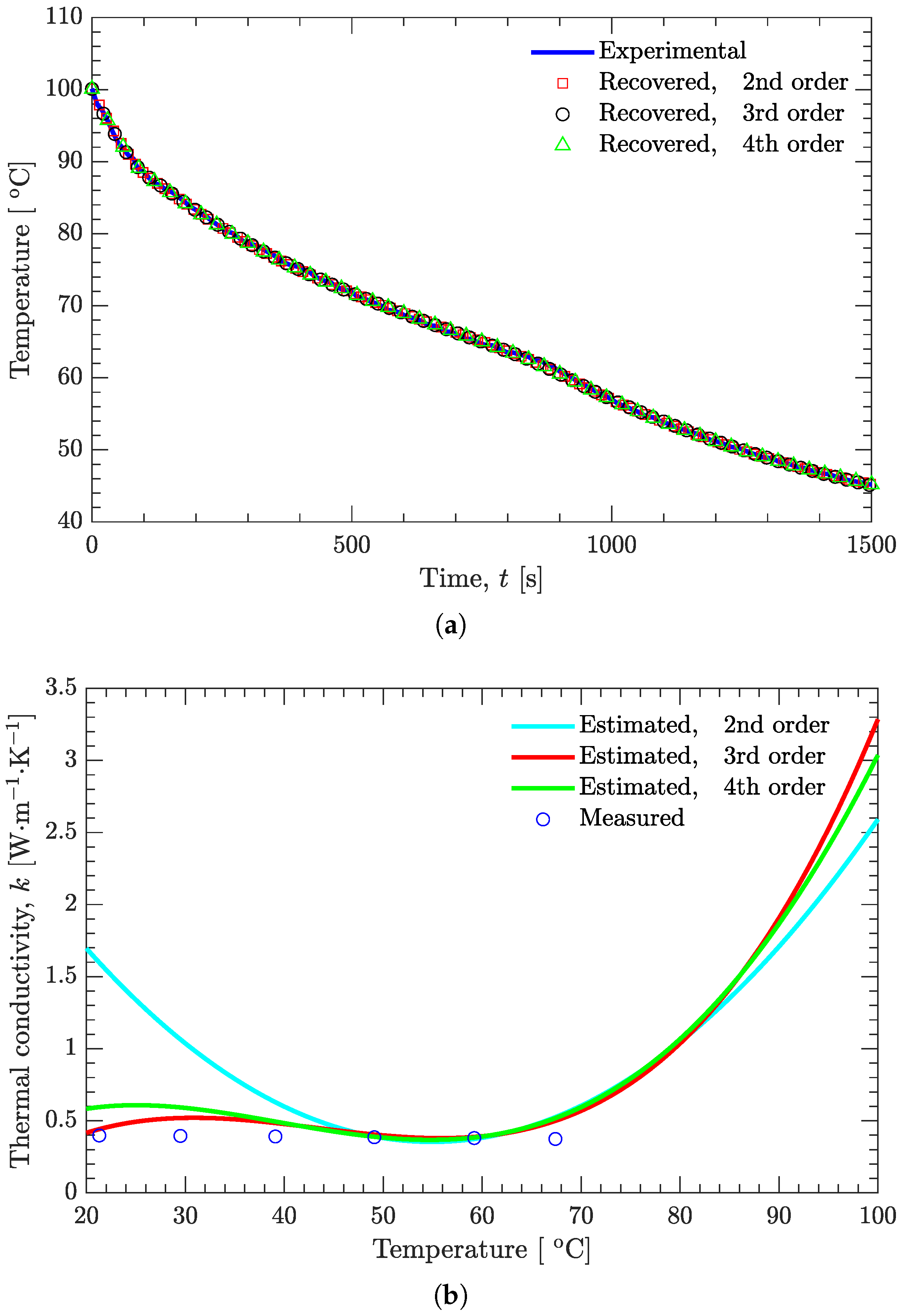
3.2. Verification of Inverse Analysis Method
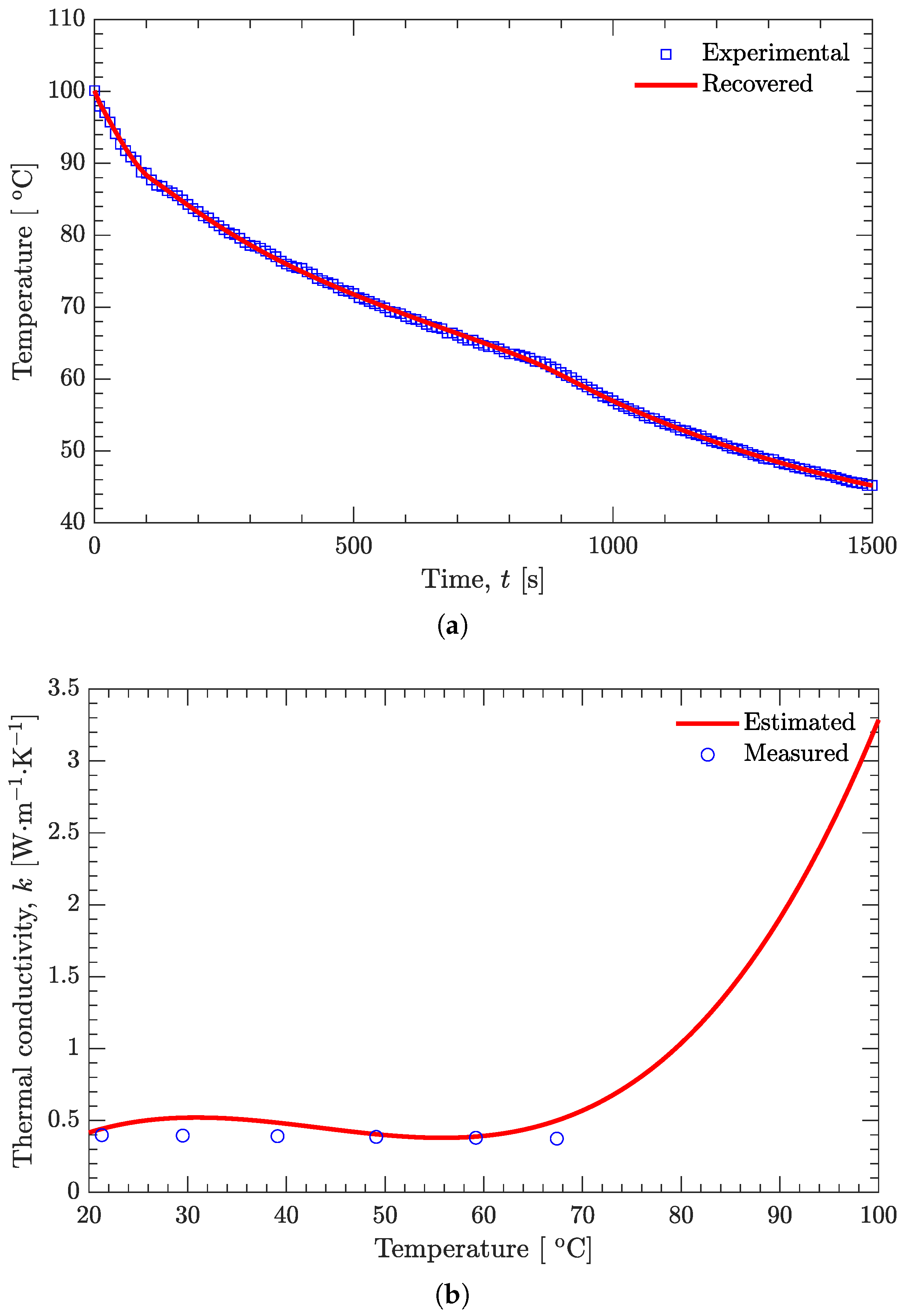
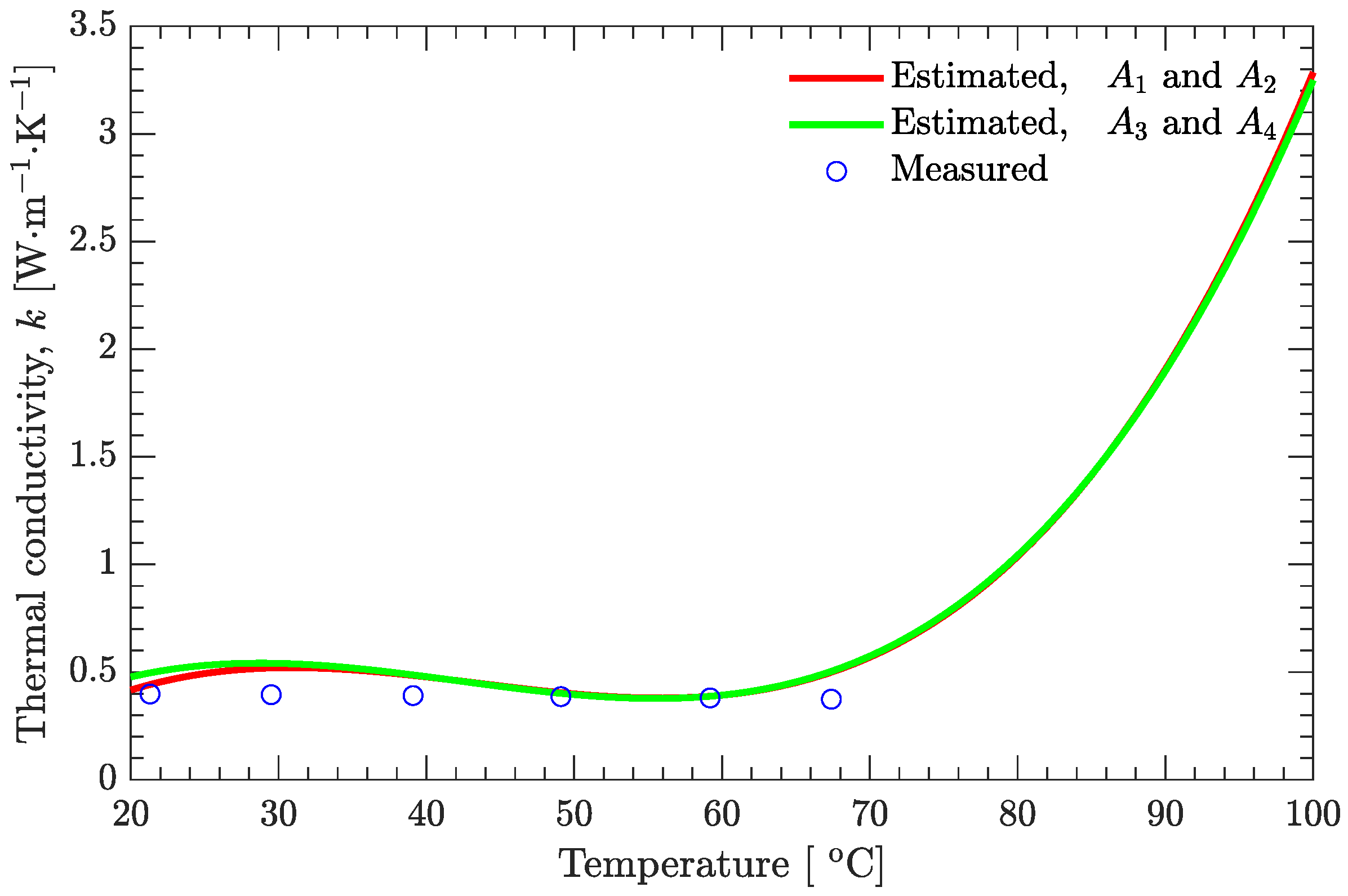
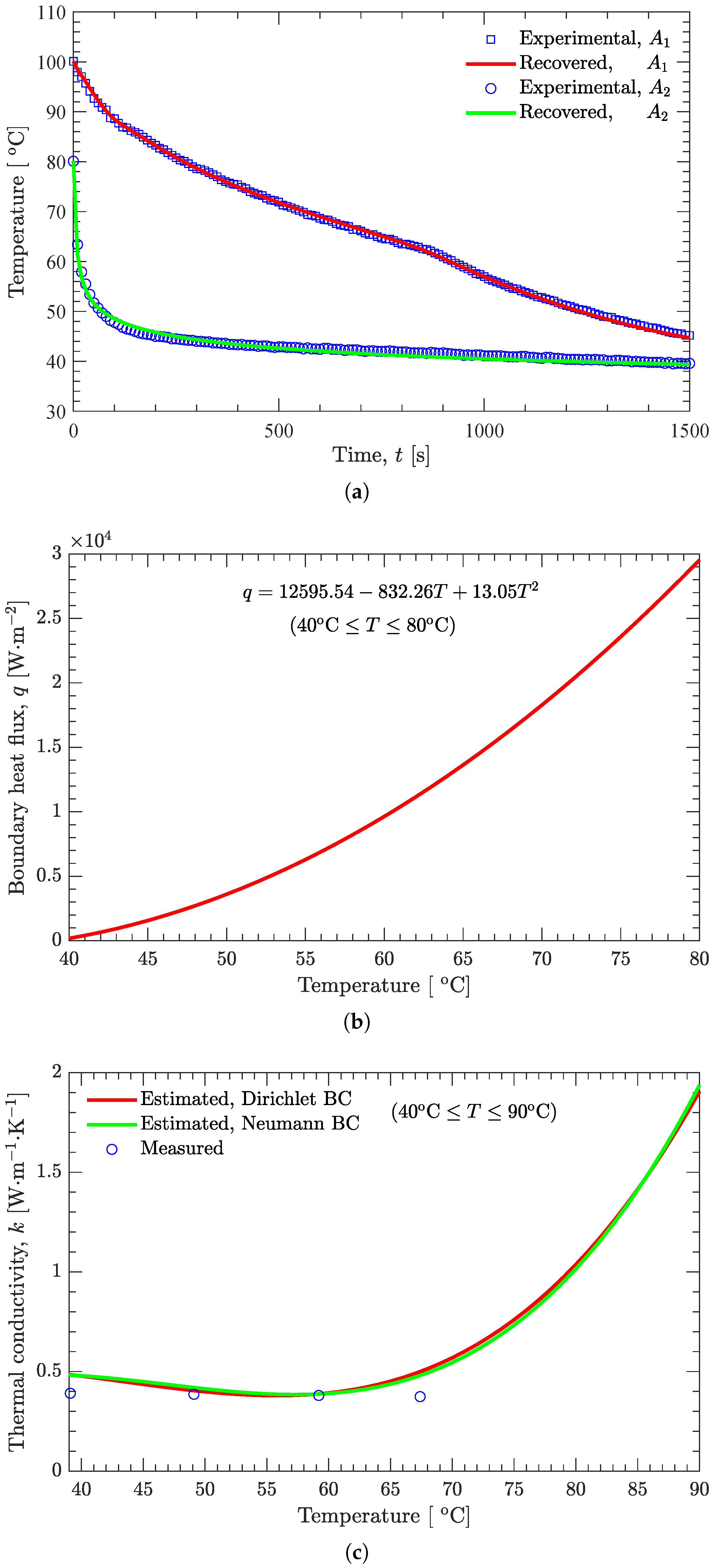
3.3. Estimation of Temperature-Dependent Thermal Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanhye, W.; Dubois, C.; Laroche, I.; Pelletier, P. Simulation of the Cooling and Phase Change of a Melt-Cast Explosive. In Proceedings of the COMSOL Conference, Boston, MA, USA, 7–9 October 2015. [Google Scholar]
- Liu, Z.W.; Xie, H.M.; Li, K.X.; Chen, P.W.; Huang, F.L. Fracture Behavior of PBX Simulation Subject to Combined Thermal and Mechanical Loads. Polym. Test. 2009, 28, 627–635. [Google Scholar] [CrossRef]
- Huang, X.; Huang, Z.; Lai, J.C.; Li, L.; Yang, G.C.; Li, C.H. Self-Healing Improves the Stability and Safety of Polymer Bonded Explosives. Compos. Sci. Technol. 2018, 167, 346–354. [Google Scholar] [CrossRef]
- He, G.; Yang, Z.; Zhou, X.; Zhang, J.; Pan, L.; Liu, S. Polymer Bonded Explosives (PBXs) with Reduced Thermal Stress and Sensitivity by Thermal Conductivity Enhancement with Graphene Nanoplatelets. Compos. Sci. Technol. 2016, 131, 22–31. [Google Scholar] [CrossRef]
- He, G.; Zhou, X.; Liu, J.; Zhang, J.; Pan, L.; Liu, S. Synergetic Enhancement of Thermal Conductivity for Highly Explosive-Filled Polymer Composites through Hybrid Carbon Nanomaterials. Polym. Compos. 2018, 39, E1452–E1462. [Google Scholar] [CrossRef]
- Wang, S.; An, C.; Wang, J.; Ye, B. Reduce the Sensitivity of CL-20 by Improving Thermal Conductivity Through Carbon Nanomaterials. Nanoscale Res. Lett. 2018, 13, 85. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Nie, S.; He, G.; Ding, L.; Wen, Y.; Zhang, J.; Yang, Z.; Liu, J.; Liu, S.; Li, J.; et al. Construction of Efficient Thermally Conductive Networks with Macroscopic Separated Architectures for Polymer Based Energetic Composites. Compos. Part Eng. 2020, 203, 108447. [Google Scholar] [CrossRef]
- Hapenciuc, C.L.; Negut, I.; Borca-Tasciuc, T.; Mihailescu, I.N. A Steady-State Hot-Wire Method for Thermal Conductivity Measurements of Fluids. Int. J. Heat Mass Transf. 2019, 134, 993–1002. [Google Scholar] [CrossRef]
- Adamczyk, W.P.; Pawlak, S.; Ostrowski, Z. Determination of Thermal Conductivity of CFRP Composite Materials Using Unconventional Laser Flash Technique. Measurement 2018, 124, 147–155. [Google Scholar] [CrossRef]
- Al Ghossein, R.M.; Hossain, M.S.; Khodadadi, J.M. Experimental Determination of Temperature-Dependent Thermal Conductivity of Solid Eicosane-Based Silver Nanostructure-Enhanced Phase Change Materials for Thermal Energy Storage. Int. J. Heat Mass Transf. 2017, 107, 697–711. [Google Scholar] [CrossRef] [Green Version]
- Lawless, Z.D.; Hobbs, M.L.; Kaneshige, M.J. Thermal Conductivity of Energetic Materials. J. Energetic Mater. 2020, 38, 214–239. [Google Scholar] [CrossRef]
- Beck, J.V.; Blackwell, B.; Clair, C.R.S., Jr. Inverse Heat Conduction: Ill-Posed Problems; Wiley Interscience: New York, NY, USA, 1985. [Google Scholar]
- Alifanov, O.M. Inverse Heat Transfer Problems; Springer: New York, NY, USA, 1994. [Google Scholar]
- Özisik, M.N.; Orlande, H.R.B. Inverse Heat Transfer: Fundamentals and Applications, 1st ed.; Taylor & Francis Inc.: New York, NY, USA, 2000. [Google Scholar]
- Bokar, J.C.; Özisik, M.N. An Inverse Analysis for Estimating the Time-Varying Inlet Temperature in Laminar Flow inside a Parallel Plate Duct. Int. J. Heat Mass Transf. 1995, 38, 39–45. [Google Scholar] [CrossRef]
- Huang, C.H.; Chao, B.H. An Inverse Geometry Problem in Identifying Irregular Boundary Configurations. Int. J. Heat Mass Transf. 1997, 40, 2045–2053. [Google Scholar] [CrossRef]
- Huang, C.H.; Chaing, M.T. A Three-Dimensional Inverse Geometry Problem in Identifying Irregular Boundary Configurations. Int. J. Therm. Sci. 2009, 48, 502–513. [Google Scholar] [CrossRef]
- Chen, W.L.; Yang, Y.C. Inverse Problem of Estimating the Heat Flux at the Roller/Workpiece Interface during a Rolling Process. Appl. Therm. Eng. 2010, 30, 1247–1254. [Google Scholar] [CrossRef]
- Ranut, P. Optimization and Inverse Problems in Heat Transfer. Ph.D Thesis, University of Udine, Udine, Italy, 2012. [Google Scholar]
- Sun, S.C.; Wang, G.J.; Chen, H. An Efficient Inverse Approach for Reconstructing Time- and Space-Dependent Heat Flux of Participating Medium. Chin. Phys. B 2020, 29, 110202. [Google Scholar] [CrossRef]
- Sun, S. Simultaneous Reconstruction of Thermal Boundary Condition and Physical Properties of Participating Medium. Int. J. Therm. Sci. 2021, 163, 106853. [Google Scholar] [CrossRef]
- Huang, C.H.; Yan, J.Y. An Inverse Problem in Simultaneously Measuring Temperature-Dependent Thermal Conductivity and Heat Capacity. Int. J. Heat Mass Transf. 1995, 38, 3433–3441. [Google Scholar] [CrossRef]
- Czél, B.; Woodbury, K.A.; Gróf, G. Simultaneous Estimation of Temperature-Dependent Volumetric Heat Capacity and Thermal Conductivity Functions via Neural Networks. Int. J. Heat Mass Transf. 2014, 68, 1–13. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, L.; Zhang, X.; Zhao, J. Simultaneous Determination of Multiple Mechanical Parameters for a DNAN/HMX Melt-Cast Explosive by Brazilian Disc Test Combined with Digital Image Correlation Method. Propellants Explos. Pyrotech. 2017, 42, 864–872. [Google Scholar] [CrossRef]
- Jaffe, I.; Price, D. Determination of the Critical Diameter of Explosive Materials. ARS J. 1962, 32, 1060–1065. [Google Scholar] [CrossRef]
- Yang, C. Estimation of the Temperature-Dependent Thermal Conductivity in Inverse Heat Conduction Problems. Appl. Math. Model. 1999, 23, 469–478. [Google Scholar] [CrossRef]
- Yang, C. Determination of the Temperature Dependent Thermophysical Properties from Temperature Responses Measured at Medium’s Boundaries. Int. J. Heat Mass Transf. 2000, 43, 1261–1270. [Google Scholar] [CrossRef]
- Ni, L.; Zhang, X.; Zhou, L.; Zhu, D. An Inverse Analysis for Contact Conductance at the Charge/Mold Interface during Pressurized Loading of Melt-Cast Explosives. J. Appl. Phys. 2019, 126, 094902. [Google Scholar] [CrossRef]
- Voller, V.; Cross, M. Accurate Solutions of Moving Boundary Problems Using the Enthalpy Method. Int. J. Heat Mass Transf. 1981, 24, 545–556. [Google Scholar] [CrossRef]
- Hahn, D.W.; Özisik, M.N. Heat Conduction; John Wiley & Sons: New York, NY, USA, 2012. [Google Scholar]
- Özişik, M.N.; Orlande, H.R.; Colaco, M.J.; Cotta, R.M. Finite Difference Methods in Heat Transfer; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Nocedal, J.; Wright, S. Numerical Optimization; Springer Science & Business Media: New York, NY, USA, 2006. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, L.; Zhang, X.; Zhou, L.; Yang, X.; Yan, B. An Inverse Analysis for Establishing the Temperature-Dependent Thermal Conductivity of a Melt-Cast Explosive across the Whole Solidification Process. Materials 2022, 15, 2077. https://doi.org/10.3390/ma15062077
Ni L, Zhang X, Zhou L, Yang X, Yan B. An Inverse Analysis for Establishing the Temperature-Dependent Thermal Conductivity of a Melt-Cast Explosive across the Whole Solidification Process. Materials. 2022; 15(6):2077. https://doi.org/10.3390/ma15062077
Chicago/Turabian StyleNi, Lei, Xiangrong Zhang, Lin Zhou, Xiufen Yang, and Bo Yan. 2022. "An Inverse Analysis for Establishing the Temperature-Dependent Thermal Conductivity of a Melt-Cast Explosive across the Whole Solidification Process" Materials 15, no. 6: 2077. https://doi.org/10.3390/ma15062077
APA StyleNi, L., Zhang, X., Zhou, L., Yang, X., & Yan, B. (2022). An Inverse Analysis for Establishing the Temperature-Dependent Thermal Conductivity of a Melt-Cast Explosive across the Whole Solidification Process. Materials, 15(6), 2077. https://doi.org/10.3390/ma15062077





