Characterization of Bioactive Colored Materials Produced from Bacterial Cellulose and Bacterial Pigments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Resources: Production and Recovery
2.2.1. Bacterial Cellulose
2.2.2. Prodigiosin Pigment
2.2.3. Flexirubin-Type Pigment
2.3. BC Functionalization with Prodigiosin and Flexirubin-Type Pigment
2.4. BC samples Characterization
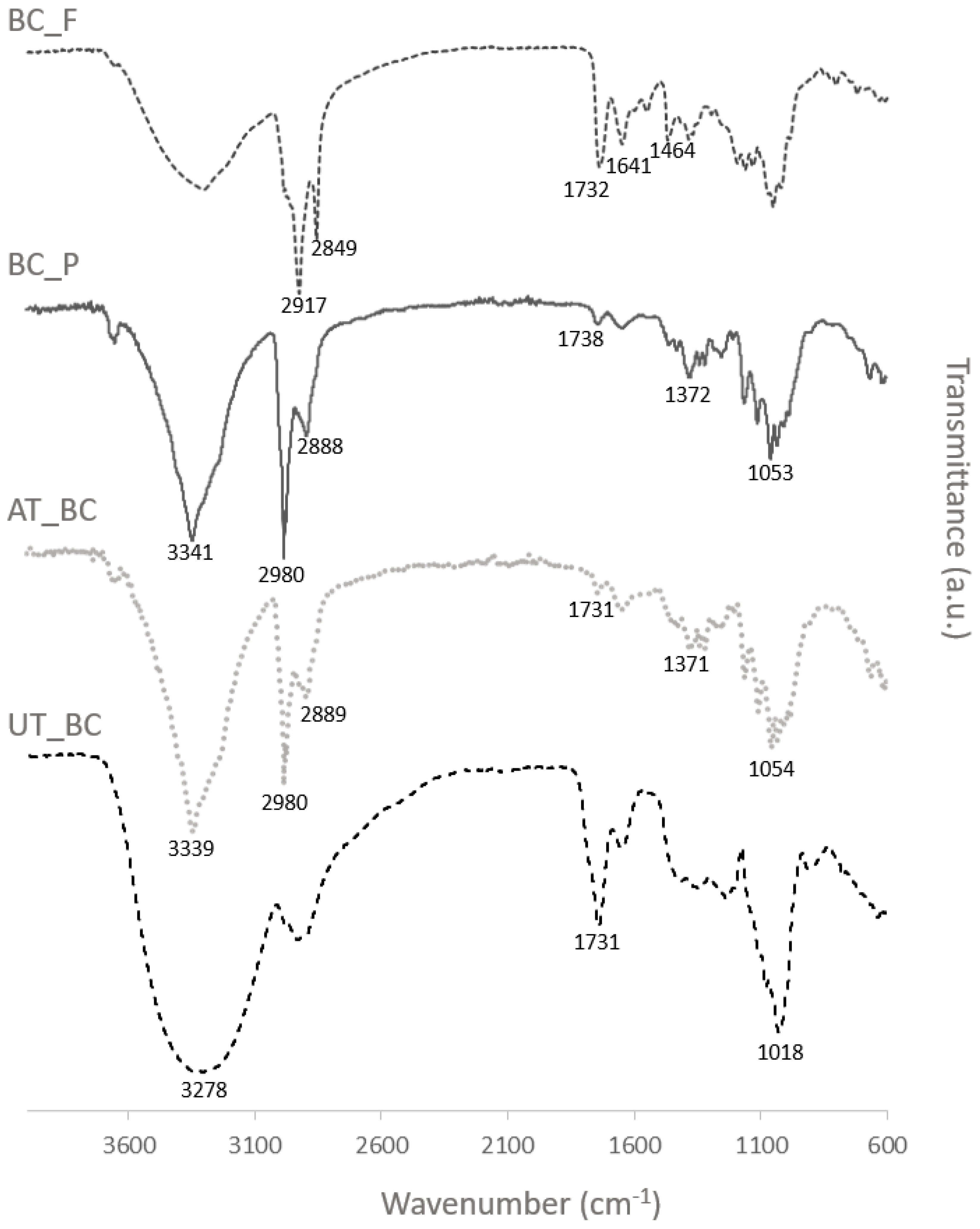
2.4.1. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.4.2. Color Characteristics
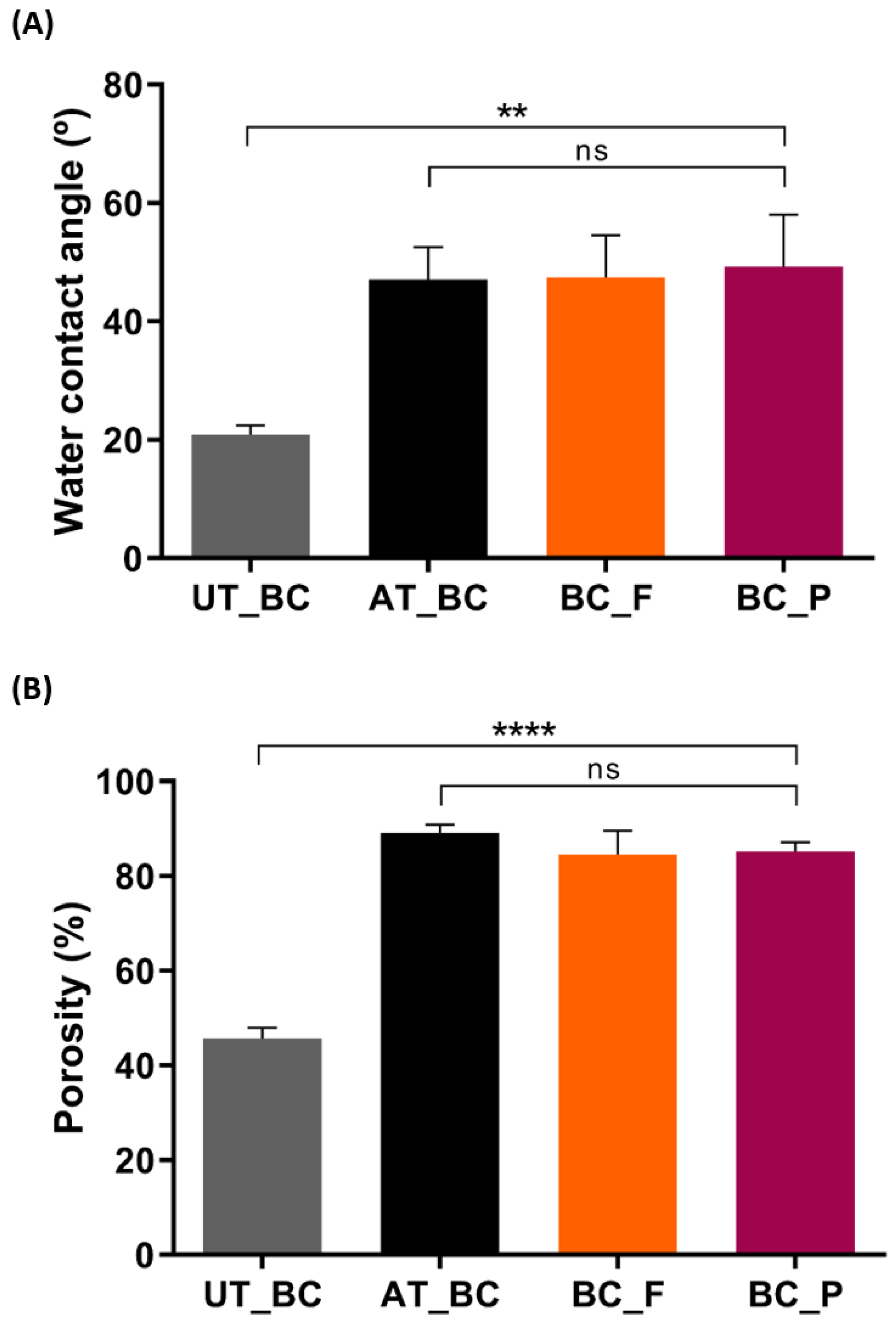
2.4.3. Water Contact Angle Measurements
2.4.4. Porosity
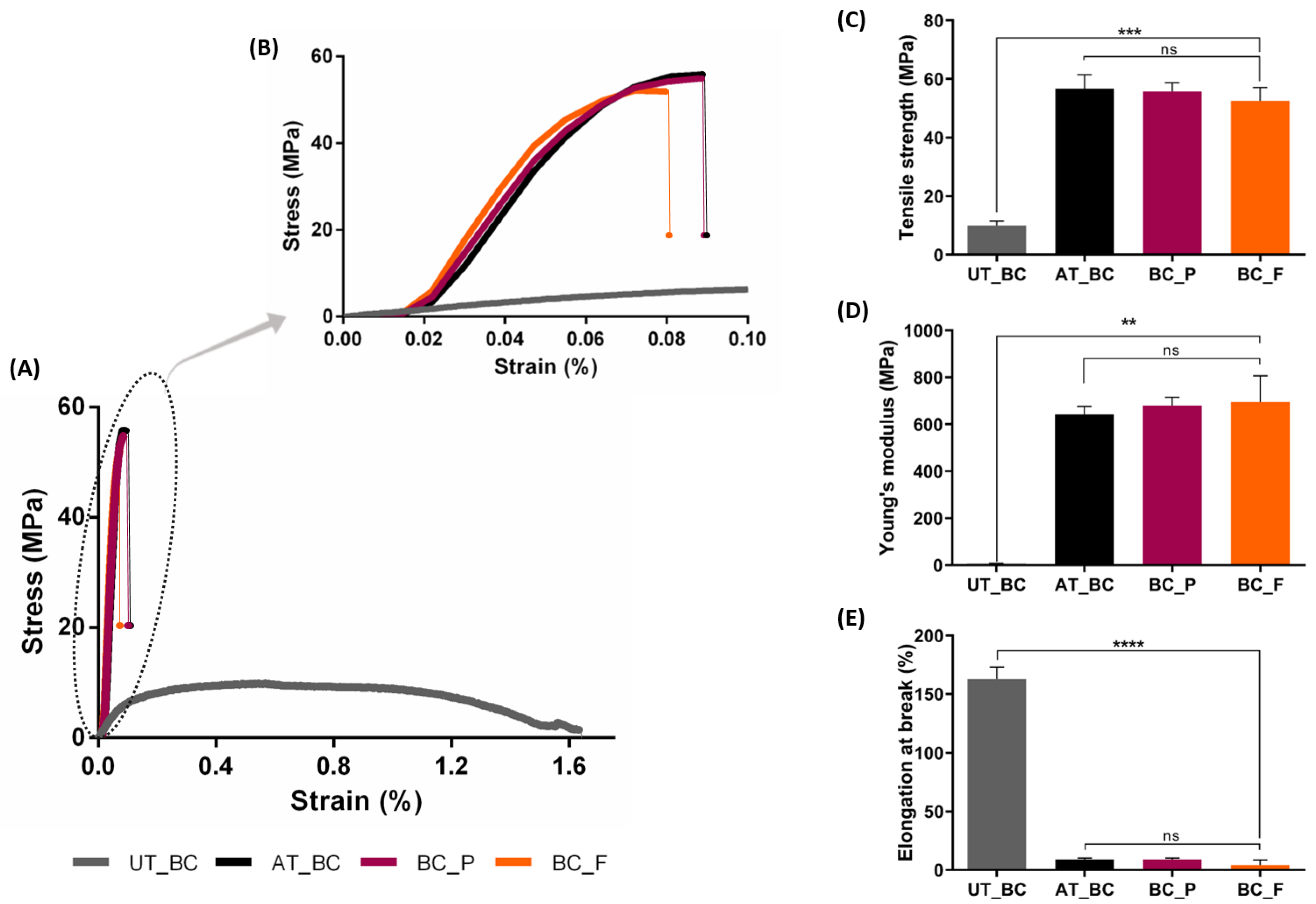
2.4.5. Mechanical Testing
2.5. Antibacterial Activity
2.6. Antioxidant Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. BC films Functionalization with Prodigiosin and Flexirubin-Type Pigment
3.2. BC Film-Based Materials Characterization
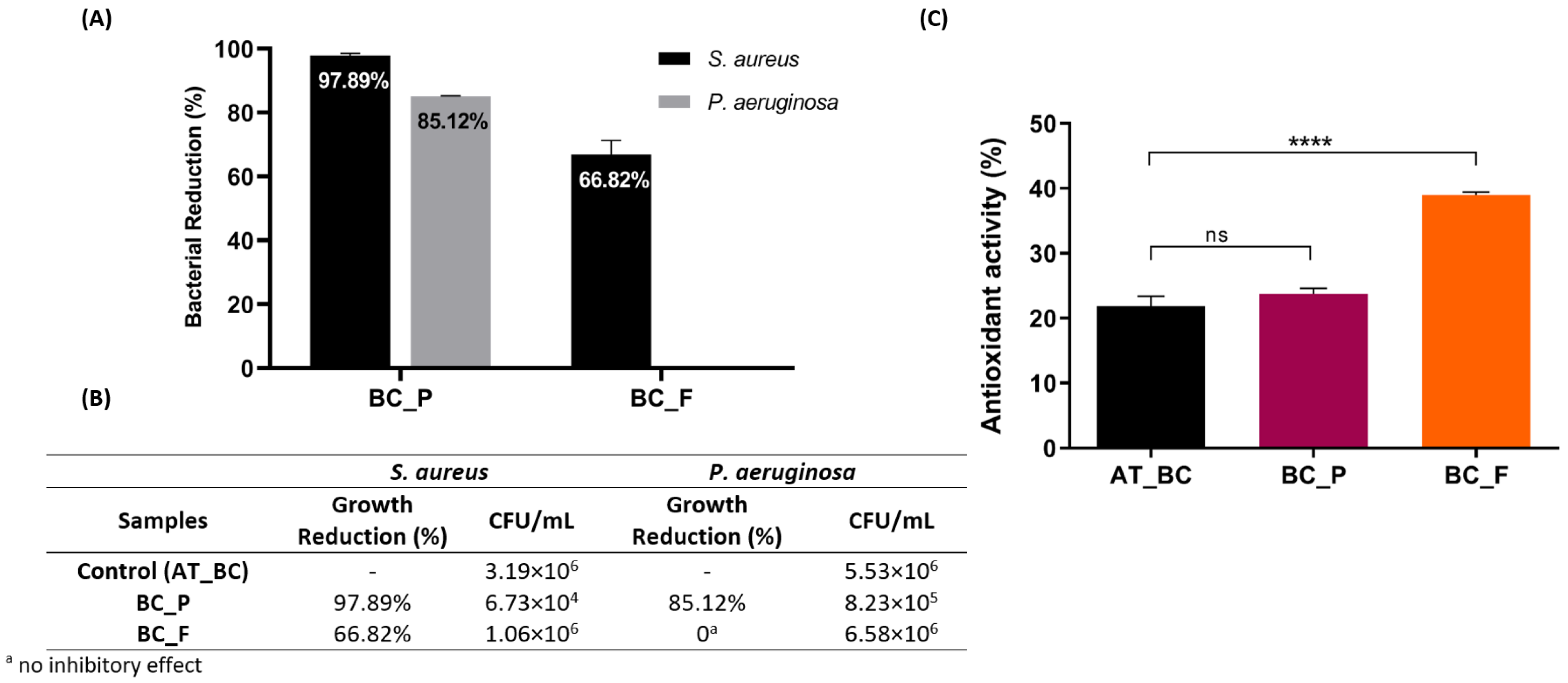
3.3. Bioactivities of the Functionalized BC Materials: Antibacterial and Antioxidant Activity Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mona, S.; Bajar, S.; Deepak, B.; Kiran, B.; Kaushik, A. Microbial Cellulose: Production and Application. In Materials for Biomedical Engineering; Grumezescu, V., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 309–322. ISBN 978-0-12-818415-8. [Google Scholar]
- Heinze, T.; el Seoud, O.A.; Koschella, A. Production and Characteristics of Cellulose from Different Sources. In Cellulose Derivatives; Springer: Cham, Switzerland, 2018; pp. 1–38. ISBN 978-3-31-973168-1. [Google Scholar]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Gregory, D.A.; Tripathi, L.; Fricker, A.T.R.; Asare, E.; Orlando, I.; Raghavendran, V.; Roy, I. Bacterial Cellulose: A Smart Biomaterial with Diverse Applications. Mater. Sci. Eng. R Rep. 2021, 145, 100623. [Google Scholar] [CrossRef]
- Andriani, D.; Apriyana, A.Y.; Karina, M. The Optimization of Bacterial Cellulose Production and Its Applications: A Review. Cellulose 2020, 27, 6747–6766. [Google Scholar] [CrossRef]
- Lupașcu, R.E.; Ghica, M.V.; Dinu-Pîrvu, C.E.; Popa, L.; Velescu, B.Ș.; Arsene, A.L. An Overview Regarding Microbial Aspects of Production and Applications of Bacterial Cellulose. Materials 2022, 15, 676. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Sindhi, V.; Gupta, V.; Sharma, K.; Bhatnagar, S.; Kumari, R.; Dhaka, N. Potential Applications of Antioxidants—A Review. J. Pharm. Res. 2013, 7, 828–835. [Google Scholar] [CrossRef]
- Ren, Y.; Gong, J.; Fu, R.; Li, Z.; Li, Q.; Zhang, J.; Yu, Z.; Cheng, X. Dyeing and Antibacterial Properties of Cotton Dyed with Prodigiosins Nanomicelles Produced by Microbial Fermentation. Dye. Pigment. 2017, 138, 147–153. [Google Scholar] [CrossRef]
- Darshan, N.; Manonmani, H.K. Prodigiosin and Its Potential Applications. J. Food Sci. Technol. 2015, 52, 5393–5407. [Google Scholar] [CrossRef] [Green Version]
- Nisha, N.; Kumar, K.; Kumar, V. Prodigiosin Alkaloids: Recent Advancements in Total Synthesis and Their Biological Potential. RSC Adv. 2015, 5, 10899–10920. [Google Scholar] [CrossRef]
- Stankovic, N.; Senerovic, L.; Ilic-Tomic, T.; Vasiljevic, B.; Nikodinovic-Runic, J. Properties and Applications of Undecylprodigiosin and Other Bacterial Prodigiosins. Appl. Microbiol. Biotechnol. 2014, 98, 3841–3858. [Google Scholar] [CrossRef]
- Gong, J.; Ren, Y.; Fu, R.; Li, Z.; Zhang, J. PH-Mediated Antibacterial Dyeing of Cotton with Prodigiosins Nanomicelles Produced by Microbial Fermentation. Polymers 2017, 9, 468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suryawanshi, R.K.; Patil, C.D.; Borase, H.P.; Salunke, B.K.; Patil, S.V. Studies on Production and Biological Potential of Prodigiosin by Serratia Marcescens. Appl. Biochem. Biotechnol. 2014, 173, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, C.; Vinithkumar, N.V.; Kirubagaran, R.; Venil, C.K.; Dufossé, L. Applications of Prodigiosin Extracted from Marine Red Pigmented Bacteria Zooshikella sp. and Actinomycete Streptomyces sp. Microorganisms 2020, 8, 556. [Google Scholar] [CrossRef] [PubMed]
- Kulandaisamy Venil, C.; Lakshmanaperumalsamy, P. An Insightful Overview on Microbial Pigment, Prodigiosin. Electron. J. Biol. 2009, 5, 49–61. [Google Scholar]
- Alström, S.; Gerhardson, B. Characteristics of ASerratia Plymuthica Isolate from Plant Rhizospheres. Plant Soil 1987, 103, 185–189. [Google Scholar] [CrossRef]
- Giacomelli, F.O. Avaliação Das Condições de Crescimento Da Serratia Plymuthica Para Produção de Prodigiosina. Master’s Thesis, University of Beira Interior, Covilhã, Portugal, 2018. [Google Scholar]
- Mumtaz, R.; Bashir, S.; Numan, M.; Shinwari, Z.K.; Ali, M. Pigments from Soil Bacteria and Their Therapeutic Properties: A Mini Review. Curr. Microbiol. 2019, 76, 783–790. [Google Scholar] [CrossRef]
- Mogadem, A.; Almamary, M.A.; Mahat, N.A.; Jemon, K.; Ahmad, W.A.; Ali, I. Antioxidant Activity Evaluation of FlexirubinType Pigment from Chryseobacterium Artocarpi CECT 8497 and Related Docking Study. Molecules 2021, 26, 979. [Google Scholar] [CrossRef]
- Shimomura, K.; Kaji, S.; Hiraishi, A. Chryseobacterium Shigense Sp. Nov., a Yellow-Pigmented, Aerobic Bacterium Isolated from a Lactic Acid Beverage. Int. J. Syst. Evol. Microbiol. 2005, 55, 1903–1906. [Google Scholar] [CrossRef]
- Venil, C.K.; Zakaria, Z.A.; Usha, R.; Ahmad, W.A. Isolation and Characterization of Flexirubin Type Pigment from Chryseobacterium Sp. UTM-3T. Biocatal. Agric. Biotechnol. 2014, 3, 103–107. [Google Scholar] [CrossRef]
- Venil, C.K.; Khasim, A.R.; Aruldass, C.A.; Ahmad, W.A. Microencapsulation of Flexirubin-Type Pigment by Spray Drying: Characterization and Antioxidant Activity. Int. Biodeterior. Biodegrad. 2016, 113, 350–356. [Google Scholar] [CrossRef]
- Jehlička, J.; Osterrothová, K.; Oren, A.; Edwards, H.G.M. Raman Spectrometric Discrimination of Flexirubin Pigments from Two Genera of Bacteroidetes. FEMS Microbiol. Lett. 2013, 348, 97–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, E.; Kim, H.R. Coloration of Bacterial Cellulose Using in Situ and Ex Situ Methods. Text. Res. J. 2019, 89, 1297–1310. [Google Scholar] [CrossRef]
- Mohammadalinejhad, S.; Almasi, H.; Moradi, M. Immobilization of Echium Amoenum Anthocyanins into Bacterial Cellulose Film: A Novel Colorimetric PH Indicator for Freshness/Spoilage Monitoring of Shrimp. Food Control 2020, 113, 107169. [Google Scholar] [CrossRef]
- Song, J.E.; Su, J.; Noro, J.; Cavaco-Paulo, A.; Silva, C.; Kim, H.R. Bio-Coloration of Bacterial Cellulose Assisted by Immobilized Laccase. AMB Express 2018, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Wonganu, B.; Kongruang, S. Red Bacterial Cellulose Production by Fermentation of Monascus Purpureus. In Proceedings of the ICCCE 2010—2010 International Conference on Chemistry and Chemical Engineering, Proceedings, Kyoto, Japan, 1–3 August 2010; pp. 137–141. [Google Scholar]
- Ochaikul, D.; Chotirittikrai, K.; Chantra, J. Studies on Fermentation of Monascus Purpureus TISTR 3090 with Bacterial Cellulose from Acetobacter Xylinum TISTR 967. KMITL Sci. Tech. J. 2006, 6, 13–17. [Google Scholar]
- Araújo, S.; da Silva, F.M.; Gouveia, I.C. The Role of Technology towards a New Bacterial-Cellulose-Based Material for Fashion Design. J. Ind. Intell. Inf. 2015, 3, 168–172. [Google Scholar] [CrossRef] [Green Version]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on Kombucha Tea-Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Coelho, R.M.D.; de Almeida, A.L.; do Amaral, R.Q.G.; da Mota, R.N.; de Sousa, P.H.M. Kombucha: Review. Int. J. Gastron. Food Sci. 2020, 22, 100272. [Google Scholar] [CrossRef]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current Challenges, Applications and Future Perspectives of SCOBY Cellulose of Kombucha Fermentation. J. Clean. Prod. 2021, 295, 126454. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.-P.; Taillandier, P. Understanding Kombucha Tea Fermentation: A Review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef]
- Ramírez Tapias, Y.A.; di Monte, M.V.; Peltzer, M.A.; Salvay, A.G. Bacterial Cellulose Films Production by Kombucha Symbiotic Community Cultured on Different Herbal Infusions. Food Chem. 2022, 372, 131346. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Yang, Y.K.; Hwang, J.K.; Pyun, Y.R.; Kim, Y.S. Effects of PH and Dissolved Oxygen on Cellulose Production by Acetobacter Xylinum BRC5 in Agitated Culture. J. Biosci. Bioeng. 1999, 88, 183–188. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Catchmark, J.M.; Demirci, A. Effect of Different Additives on Bacterial Cellulose Production by Acetobacter Xylinum and Analysis of Material Property. Cellulose 2009, 16, 1033–1045. [Google Scholar] [CrossRef]
- Elkenawy, N.M.; Yassin, A.S.; Elhifnawy, H.N.; Amin, M.A. Optimization of Prodigiosin Production by Serratia Marcescens Using Crude Glycerol and Enhancing Production Using Gamma Radiation. Biotechnol. Rep. 2017, 14, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Rather, L.J.; Shahid-Ul-Islam; Shabbir, M.; Bukhari, M.N.; Shahid, M.; Khan, M.A.; Mohammad, F. Ecological Dyeing of Woolen Yarn with Adhatoda Vasica Natural Dye in the Presence of Biomordants as an Alternative Copartner to Metal Mordants. J. Environ. Chem. Eng. 2016, 4, 3041–3049. [Google Scholar] [CrossRef]
- Yasukawa, A.; Chida, A.; Kato, Y.; Kasai, M. Dyeing Silk and Cotton Fabrics Using Natural Blackcurrants. Text. Res. J. 2017, 87, 2379–2387. [Google Scholar] [CrossRef]
- Han, J.; Shim, E.; Kim, H.R. Effects of Cultivation, Washing, and Bleaching Conditions on Bacterial Cellulose Fabric Production. Text. Res. J. 2019, 89, 1094–1104. [Google Scholar] [CrossRef]
- Justin Thomas, K.R. GoCIE V2; Organic Materials Service Laboratory: Singapore, 2009. [Google Scholar]
- Yeh, C.C.; Li, Y.T.; Chiang, P.H.; Huang, C.H.; Wang, Y.; Chang, H.I. Characterizing Microporous PCL Matrices for Application of Tissue Engineering. J. Med. Biol. Eng. 2009, 29, 92–97. [Google Scholar]
- Gea, S.; Reynolds, C.T.; Roohpour, N.; Wirjosentono, B.; Soykeabkaew, N.; Bilotti, E.; Peijs, T. Investigation into the Structural, Morphological, Mechanical and Thermal Behaviour of Bacterial Cellulose after a Two-Step Purification Process. Bioresour. Technol. 2011, 102, 9105–9110. [Google Scholar] [CrossRef]
- Ezati, P.; Bang, Y.-J.; Rhim, J.-W. Preparation of a Shikonin-Based PH-Sensitive Color Indicator for Monitoring the Freshness of Fish and Pork. Food Chem. 2021, 337, 127995. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ren, Y.; Gong, J.; Fu, R.; Li, Z.; Yu, Z.; Lou, J.; Wang, F.; Zhang, J. Dyeing and Functional Properties of Polyester Fabric Dyed with Prodigiosins Nanomicelles Produced by Microbial Fermentation. J. Clean. Prod. 2017, 148, 375–385. [Google Scholar] [CrossRef]
- Hasanbeigi, A.; Price, L. A Technical Review of Emerging Technologies for Energy and Water Efficiency and Pollution Reduction in the Textile Industry. J. Clean. Prod. 2015, 95, 30–44. [Google Scholar] [CrossRef] [Green Version]
- Aruldass, C.A.; Aziz, A.; Venil, C.K.; Khasim, A.R.; Ahmad, W.A. Utilization of Agro-Industrial Waste for the Production of Yellowish-Orange Pigment from Chryseobacterium Artocarpi CECT 8497. Int. Biodeterior. Biodegrad. 2016, 113, 342–349. [Google Scholar] [CrossRef]
- Jiménez, M.E.P.; Pinilla, C.M.B.; Rodrigues, E.; Brandelli, A. Extraction and Partial Characterisation of Antioxidant Pigment Produced by Chryseobacterium Sp. Kr6. Nat. Prod. Res. 2018, 6419, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Lien, C.C.; Yeh, H.J.; Yu, C.M.; Hsu, S.H. Bacterial Cellulose and Bacterial Cellulose-Chitosan Membranes for Wound Dressing Applications. Carbohydr. Polym. 2013, 94, 603–611. [Google Scholar] [CrossRef]
- Leal, S.; Cristelo, C.; Silvestre, S.; Fortunato, E.; Sousa, A.; Alves, A.; Correia, D.M.; Lanceros-Mendez, S.; Gama, M. Hydrophobic Modification of Bacterial Cellulose Using Oxygen Plasma Treatment and Chemical Vapor Deposition. Cellulose 2020, 27, 10733–10746. [Google Scholar] [CrossRef]
- Sumathi, C.; MohanaPriya, D.; Swarnalatha, S.; Dinesh, M.G.; Sekaran, G. Production of Prodigiosin Using Tannery Fleshing and Evaluating Its Pharmacological Effects. Sci. World J. 2014, 2014, 290327. [Google Scholar] [CrossRef]
- Arivizhivendhan, K.V.; Boopathy, R.; Maharaja, P.; Regina Mary, R.; Sekaran, G. Bioactive Prodigiosin-Impregnated Cellulose Matrix for the Removal of Pathogenic Bacteria from Aqueous Solution. RSC Adv. 2015, 5, 68621–68631. [Google Scholar] [CrossRef]
- Gamboa-Suasnavart, R.A.; Valdez-Cruz, N.A.; Gaytan-Ortega, G.; Reynoso-Cereceda, G.I.; Cabrera-Santos, D.; López-Griego, L.; Klöckner, W.; Büchs, J.; Trujillo-Roldán, M.A. The Metabolic Switch Can Be Activated in a Recombinant Strain of Streptomyces Lividans by a Low Oxygen Transfer Rate in Shake Flasks. Microb. Cell Factories 2018, 17, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sathishkumar, P.; Malathi, M.; Jayakumar, R.; Yusoff, A.R.M.; Ahmad, W.A.; Venil, C.K.; Usha, R. Synthesis of Flexirubin-Mediated Silver Nanoparticles Using Chryseobacterium Artocarpi CECT 8497 and Investigation of Its Anticancer Activity. Mater. Sci. Eng. C 2015, 59, 228–234. [Google Scholar]
- Yuan, Y.; Lee, T.R. Contact Angle and Wetting Properties. In Springer Series in Surface Sciences; Bracco, G., Holst, B., Eds.; Springer Series in Surface Sciences; Springer: Berlin/Heidelberg, Germany, 2013; Volume 51, pp. 3–34. ISBN 978-3-64-234242-4. [Google Scholar]
- da Silva, R.; Sierakowski, M.R.; Bassani, H.P.; Zawadzki, S.F.; Pirich, C.L.; Ono, L.; de Freitas, R.A. Hydrophilicity Improvement of Mercerized Bacterial Cellulose Films by Polyethylene Glycol Graft. Int. J. Biol. Macromol. 2016, 86, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Karim, M.; Ahmadi, N. Nanostructures of Cellulose for Encapsulation of Food Ingredients. In Biopolymer Nanostructures for Food Encapsulation Purposes; Academic Press: Cambridge, MA, USA, 2019; pp. 493–519. ISBN 978-0-12-815663-6. [Google Scholar]
- Missoum, K.; Belgacem, M.N.; Bras, J. Nanofibrillated Cellulose Surface Modification: A Review. Materials 2013, 6, 1745–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.; Jia, S.; Jia, Y.; Yang, H. The Influence of Fermentation Conditions and Post-Treatment Methods on Porosity of Bacterial Cellulose Membrane. World J. Microbiol. Biotechnol. 2010, 26, 125–131. [Google Scholar] [CrossRef]
- Al-Shamary, E.; Al-Darwash, A. Influence of Fermentation Condition and Alkali Treatment on the Porosity and Thickness of Bacterial Cellulose Membranes. TOJSAT 2013, 3, 194–203. [Google Scholar]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial Cellulose Production, Properties and Applications with Different Culture Methods—A Review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Yin, N.; Du, R.; Zhao, F.; Han, Y.; Zhou, Z. Characterization of Antibacterial Bacterial Cellulose Composite Membranes Modified with Chitosan or Chitooligosaccharide. Carbohydr. Polym. 2020, 229, 115520. [Google Scholar] [CrossRef]
- Blanco, A.; Monte, M.C.; Campano, C.; Balea, A.; Merayo, N.; Negro, C. Nanocellulose for Industrial Use. In Handbook of Nanomaterials for Industrial Applications; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 74–126. ISBN 978-0-12-813351-4. [Google Scholar]
- Santmarti, A.; Zhang, H.; Lappalainen, T.; Lee, K.-Y. Cellulose Nanocomposites Reinforced with Bacterial Cellulose Sheets Prepared from Pristine and Disintegrated Pellicle. Compos. Part A Appl. Sci. Manuf. 2020, 130, 105766. [Google Scholar] [CrossRef]
- Kamiński, K.; Jarosz, M.; Grudzień, J.; Pawlik, J.; Zastawnik, F.; Pandyra, P.; Kołodziejczyk, A.M. Hydrogel Bacterial Cellulose: A Path to Improved Materials for New Eco-Friendly Textiles. Cellulose 2020, 27, 5353–5365. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Ji, H.; Yang, Y.; Guo, B.; Luo, L.; Meng, Z.; Fan, L.; Xu, J. TEMPO-Oxidized Bacterial Cellulose Nanofiber Membranes as High-Performance Separators for Lithium-Ion Batteries. Carbohydr. Polym. 2020, 230, 115570. [Google Scholar] [CrossRef]
- Morena, A.G.; Roncero, M.B.; Valenzuela, S.V.; Valls, C.; Vidal, T.; Pastor, F.I.J.; Diaz, P.; Martínez, J. Laccase/TEMPO-Mediated Bacterial Cellulose Functionalization: Production of Paper-Silver Nanoparticles Composite with Antimicrobial Activity. Cellulose 2019, 26, 8655–8668. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Yu, T.; Wang, N.; Wang, C.; Wang, H. Preparation of Polylactic Acid/TEMPO-Oxidized Bacterial Cellulose Nanocomposites for 3D Printing via Pickering Emulsion Approach. Compos. Commun. 2019, 16, 162–167. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, J.; Chen, J.; Li, B.; Li, Y.; Liu, S. Edible Foam Based on Pickering Effect of Bacterial Cellulose Nanofibrils and Soy Protein Isolates Featuring Interfacial Network Stabilization. Food Hydrocoll. 2020, 100, 105440. [Google Scholar] [CrossRef]
- Matos, M.; Sousa, A.F.; Silva, N.H.C.S.; Freire, C.S.R.; Andrade, M.; Mendes, A.; Silvestre, A.J.D. Furanoate-Based Nanocomposites: A Case Study Using Poly(Butylene 2,5-Furanoate) and Poly(Butylene 2,5-Furanoate)-Co-(Butylene Diglycolate) and Bacterial Cellulose. Polymers 2018, 10, 810. [Google Scholar] [CrossRef] [Green Version]
- Ávila Ramírez, J.A.; Cerrutti, P.; Bernal, C.; Errea, M.I.; Foresti, M.L. Nanocomposites Based on Poly(Lactic Acid) and Bacterial Cellulose Acetylated by an α-Hydroxyacid Catalyzed Route. J. Polym. Environ. 2019, 27, 510–520. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, M.N.; Sukirah, A.R.; Maizatulnisa, O.; Ayuni, J.; Khalisanni, K.; Rosmamuhamadani, R. Effect of Growth Times on the Physical and Mechanical Properties of Hydrophobic and Oleophilic Silylated Bacterial Cellulose Membranes. In Proceedings of the AIP Conference Proceedings, Rome, Italy, 26–29 September 2017; AIP Publishing LLC: College Park, MD, USA, 2017; Volume 1885, p. 020160. [Google Scholar]
- Ebrahimi, Y.; Peighambardoust, S.J.; Peighambardoust, S.H.; Karkaj, S.Z. Development of Antibacterial Carboxymethyl Cellulose-Based Nanobiocomposite Films Containing Various Metallic Nanoparticles for Food Packaging Applications. J. Food Sci. 2019, 84, 2537–2548. [Google Scholar] [CrossRef]
- Suryawanshi, R.K.; Patil, C.D.; Koli, S.H.; Hallsworth, J.E.; Patil, S.V. Antimicrobial Activity of Prodigiosin Is Attributable to Plasma-Membrane Damage. Nat. Prod. Res. 2017, 31, 572–577. [Google Scholar] [CrossRef]
- Yip, C.-H.; Yarkoni, O.; Ajioka, J.; Wan, K.-L.; Nathan, S. Recent Advancements in High-Level Synthesis of the Promising Clinical Drug, Prodigiosin. Appl. Microbiol. Biotechnol. 2019, 103, 1667–1680. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, S.; Röcker, B. Sustainable Antimicrobial Packaging Technologies. In Sustainable Food Packaging Technology; Wiley: Hoboken, NJ, USA, 2021; pp. 323–348. [Google Scholar]
- Rather, L.J.; Shahid-Ul-Islam; Azam, M.; Shabbir, M.; Bukhari, M.N.; Shahid, M.; Khan, M.A.; Rizwanul Haque, Q.M.; Mohammad, F. Antimicrobial and Fluorescence Finishing of Woolen Yarn with Terminalia Arjuna Natural Dye as an Ecofriendly Substitute to Synthetic Antibacterial Agents. RSC Adv. 2016, 6, 39080–39094. [Google Scholar] [CrossRef]
- Marcillo-Parra, V.; Anaguano, M.; Molina, M.; Tupuna-Yerovi, D.S.; Ruales, J. Characterization and Quantification of Bioactive Compounds and Antioxidant Activity in Three Different Varieties of Mango (Mangifera Indica L.) Peel from the Ecuadorian Region Using HPLC-UV/VIS and UPLC-PDA. NFS J. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Oudjedi, K.; Manso, S.; Nerin, C.; Hassissen, N.; Zaidi, F. New Active Antioxidant Multilayer Food Packaging Films Containing Algerian Sage and Bay Leaves Extracts and Their Application for Oxidative Stability of Fried Potatoes. Food Control 2019, 98, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Ivanović, M.; Makoter, K.; Islamčević Razboršek, M. Comparative Study of Chemical Composition and Antioxidant Activity of Essential Oils and Crude Extracts of Four Characteristic Zingiberaceae Herbs. Plants 2021, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Nistor, M.; Diaconeasa, Z.; Frond, A.D.; Stirbu, I.; Socaciu, C.; Pintea, A.; Rugina, D. Comparative Efficiency of Different Solvents for the Anthocyanins Extraction from Chokeberries and Black Carrots, to Preserve Their Antioxidant Activity. Chem. Pap. 2021, 75, 813–822. [Google Scholar] [CrossRef]
- Dasgupta, A.; Klein, K. Methods for Measuring Oxidative Stress in the Laboratory. In Antioxidants in Food, Vitamins and Supplements; Elsevier: Amsterdam, The Netherlands, 2014; pp. 19–40. [Google Scholar]
- Amarasinghe, H.; Weerakkody, N.S.; Waisundara, V.Y. Evaluation of Physicochemical Properties and Antioxidant Activities of Kombucha “Tea Fungus” during Extended Periods of Fermentation. Food Sci. Nutr. 2018, 6, 659–665. [Google Scholar] [CrossRef] [Green Version]
- Kusznierewicz, B.; Staroszczyk, H.; Malinowska-Pańczyk, E.; Parchem, K.; Bartoszek, A. Novel ABTS-Dot-Blot Method for the Assessment of Antioxidant Properties of Food Packaging. Food Packag. Shelf Life 2020, 24, 100478. [Google Scholar] [CrossRef]
- Arivizhivendhan, K.V.; Mahesh, M.; Boopathy, R.; Swarnalatha, S.; Regina Mary, R.; Sekaran, G. Antioxidant and Antimicrobial Activity of Bioactive Prodigiosin Produces from Serratia Marcescens Using Agricultural Waste as a Substrate. J. Food Sci. Technol. 2018, 55, 2661–2670. [Google Scholar] [CrossRef]
- Sajjad, W.; Ahmad, S.; Aziz, I.; Azam, S.S.; Hasan, F.; Shah, A.A. Antiproliferative, Antioxidant and Binding Mechanism Analysis of Prodigiosin from Newly Isolated Radio-Resistant Streptomyces Sp. Strain WMA-LM31. Mol. Biol. Rep. 2018, 45, 1787–1798. [Google Scholar] [CrossRef]
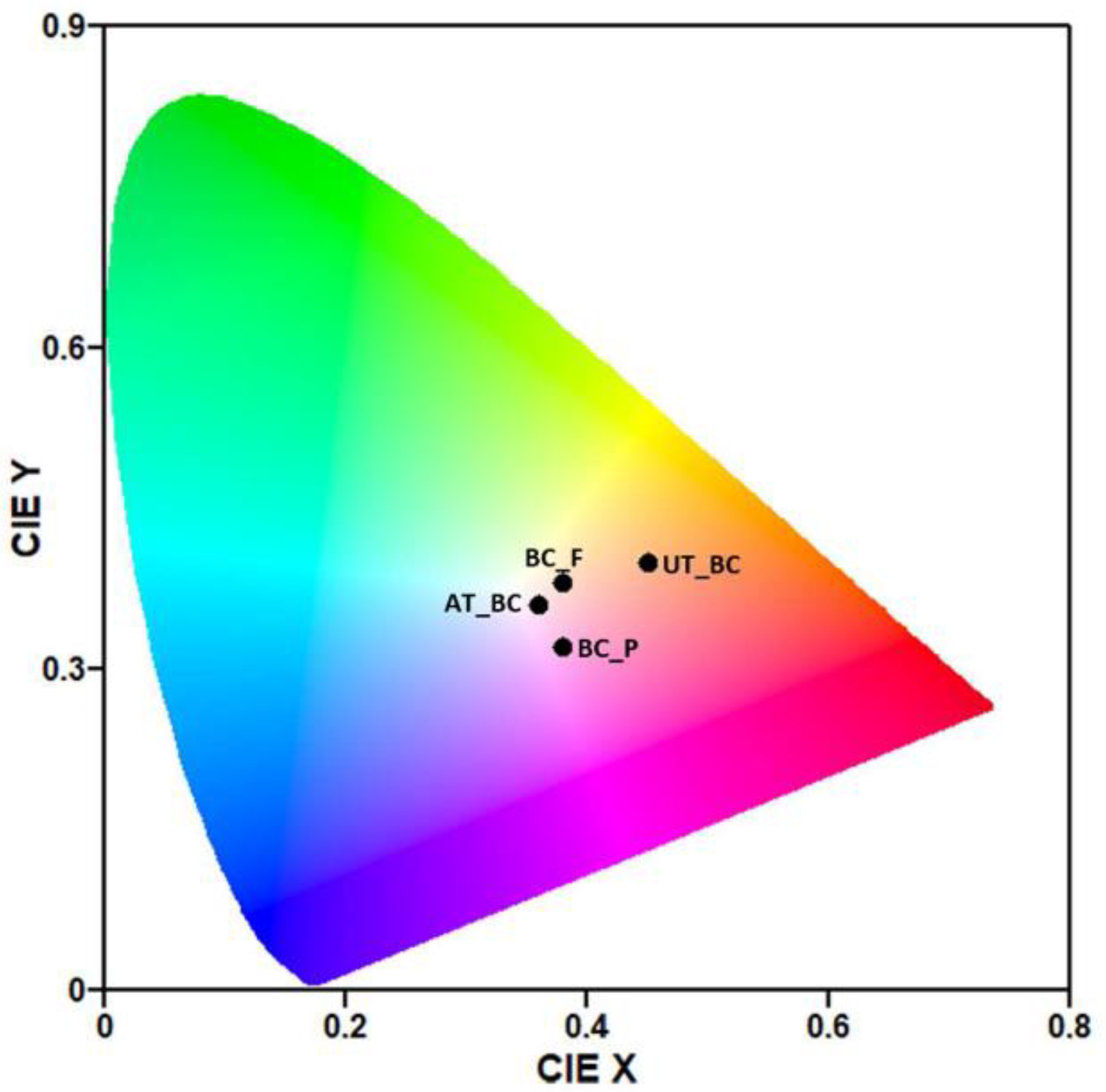
| UT_BC | AT_BC | BC_P | BC_F | ||
|---|---|---|---|---|---|
| Apparent color |  |  |  |  | |
| Reflectance (%R) | - | - | 26.8 ± 0.1 | 42.7 ± 1.0 | |
| Color strength (K/S) | - | - | 1.0 ± 0.0 | 0.4 ± 0.0 | |
| Whiteness Index (WI) | 40.3 ± 1.9 | 79.0 ± 1.2 | - | - | |
| CIELab and CIELch colorimetric parameters | L* | 51.5 ± 3.5 | 83.6 ± 1.0 | 71.1 ± 0.4 | 86.1 ± 0.4 |
| a* | 14.8 ± 0.6 | 1.5 ± 0.4 | 22.4 ± 0.1 | 3.1 ± 0.7 | |
| b* | 31.5 ± 2.1 | 13.1 ± 0.7 | 1.0 ± 0.3 | 24.1 ± 0.5 | |
| Chroma (C*) | 34.8 ± 1.7 | 13.2 ± 0.7 | 22.4 ± 0.1 | 24.3 ± 0.6 | |
| Hue angle (°) | 64.7 ± 2.4 | 83.4 ± 1.2 | 2.6 ± 0.7 | 82.6 ± 1.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amorim, L.F.A.; Fangueiro, R.; Gouveia, I.C. Characterization of Bioactive Colored Materials Produced from Bacterial Cellulose and Bacterial Pigments. Materials 2022, 15, 2069. https://doi.org/10.3390/ma15062069
Amorim LFA, Fangueiro R, Gouveia IC. Characterization of Bioactive Colored Materials Produced from Bacterial Cellulose and Bacterial Pigments. Materials. 2022; 15(6):2069. https://doi.org/10.3390/ma15062069
Chicago/Turabian StyleAmorim, Lúcia F. A., Raul Fangueiro, and Isabel C. Gouveia. 2022. "Characterization of Bioactive Colored Materials Produced from Bacterial Cellulose and Bacterial Pigments" Materials 15, no. 6: 2069. https://doi.org/10.3390/ma15062069
APA StyleAmorim, L. F. A., Fangueiro, R., & Gouveia, I. C. (2022). Characterization of Bioactive Colored Materials Produced from Bacterial Cellulose and Bacterial Pigments. Materials, 15(6), 2069. https://doi.org/10.3390/ma15062069








