Self-Powered Electrical Impulse Chemotherapy for Oral Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. TENG Fabrication
2.2. 2D Stimulation Device Preparation
2.3. Cell Culture
2.4. 2D Cell Toxicity
2.5. Microneedle Electrode Preparation
2.6. Apoptosis Assay of the MCTS
2.7. Growth Inhibition of the MCTS
3. Results and Discussion
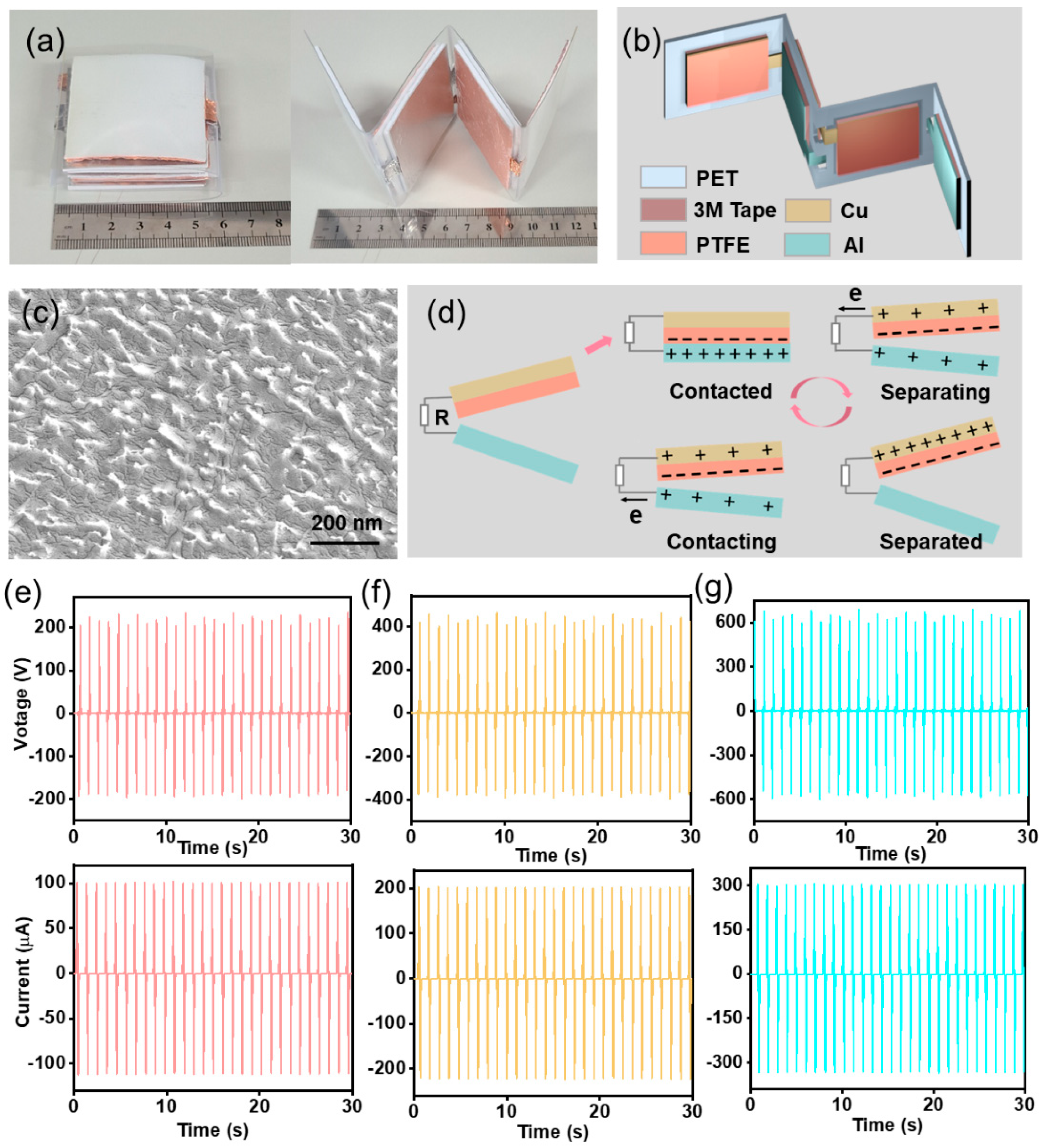
3.1. Fabrication and Characterization of the TENG
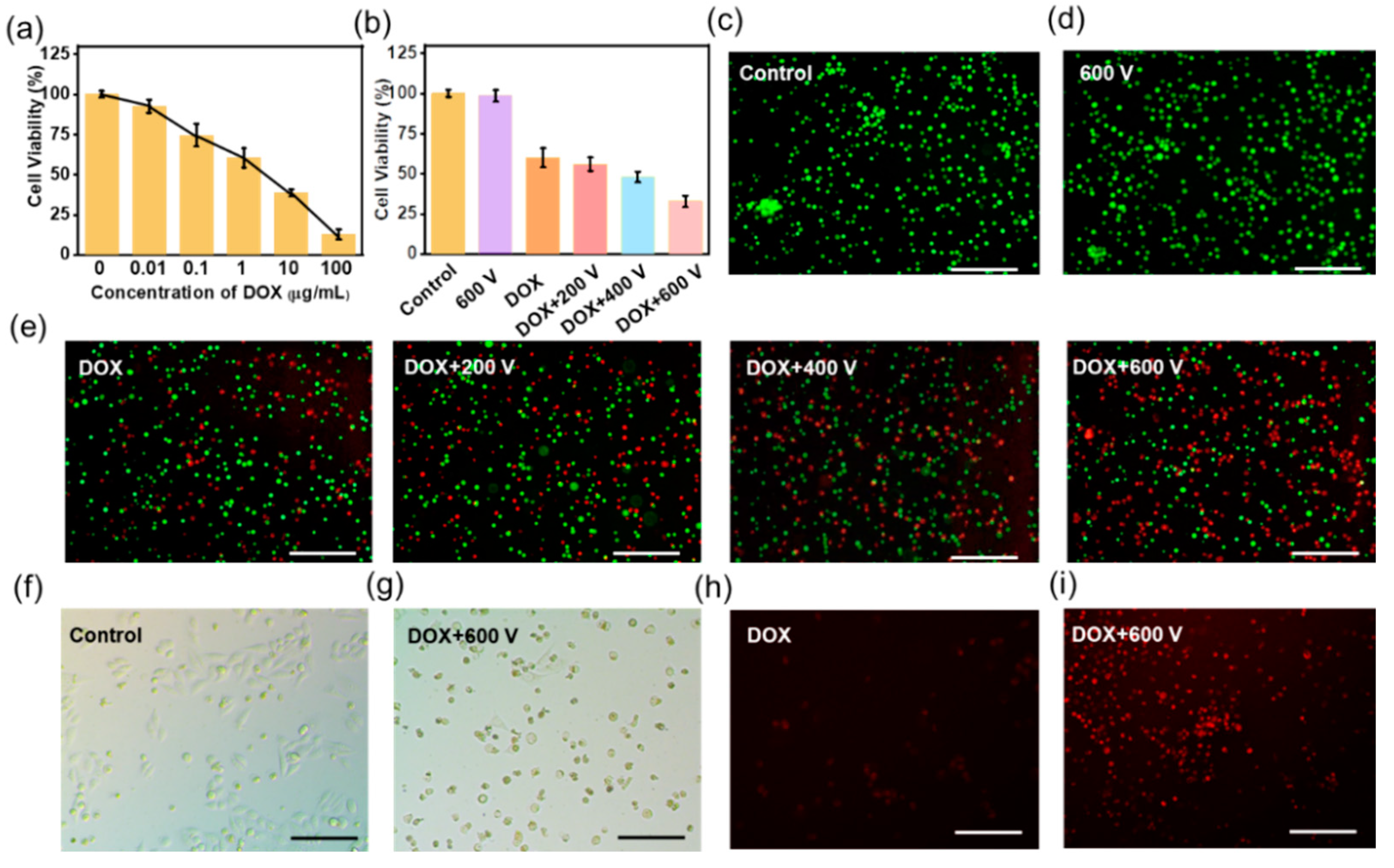
3.2. TENG Controlled EIC to Treat 2D Tca-8113 Cells
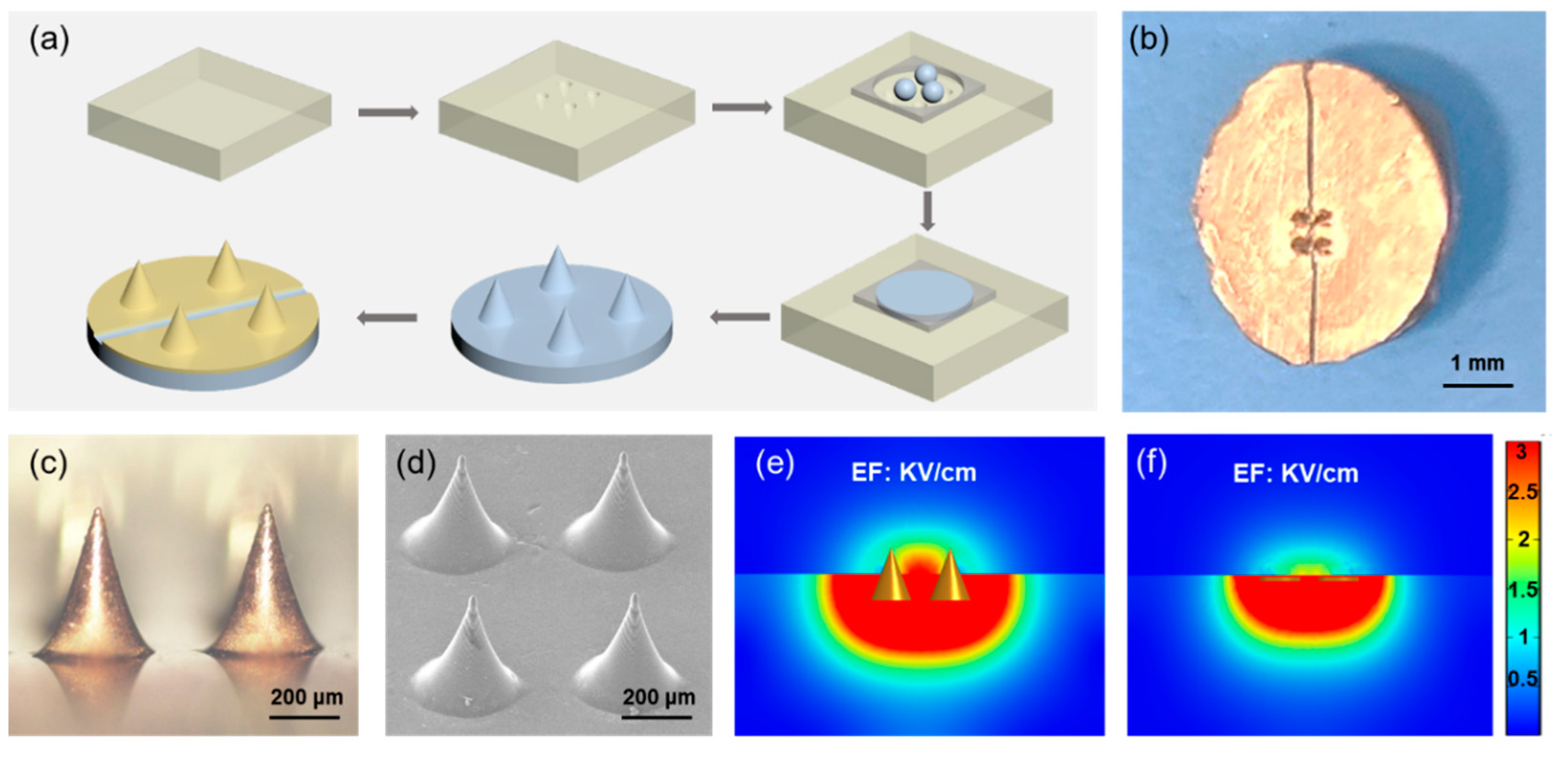
3.3. Microneedle Electrode Fabrication
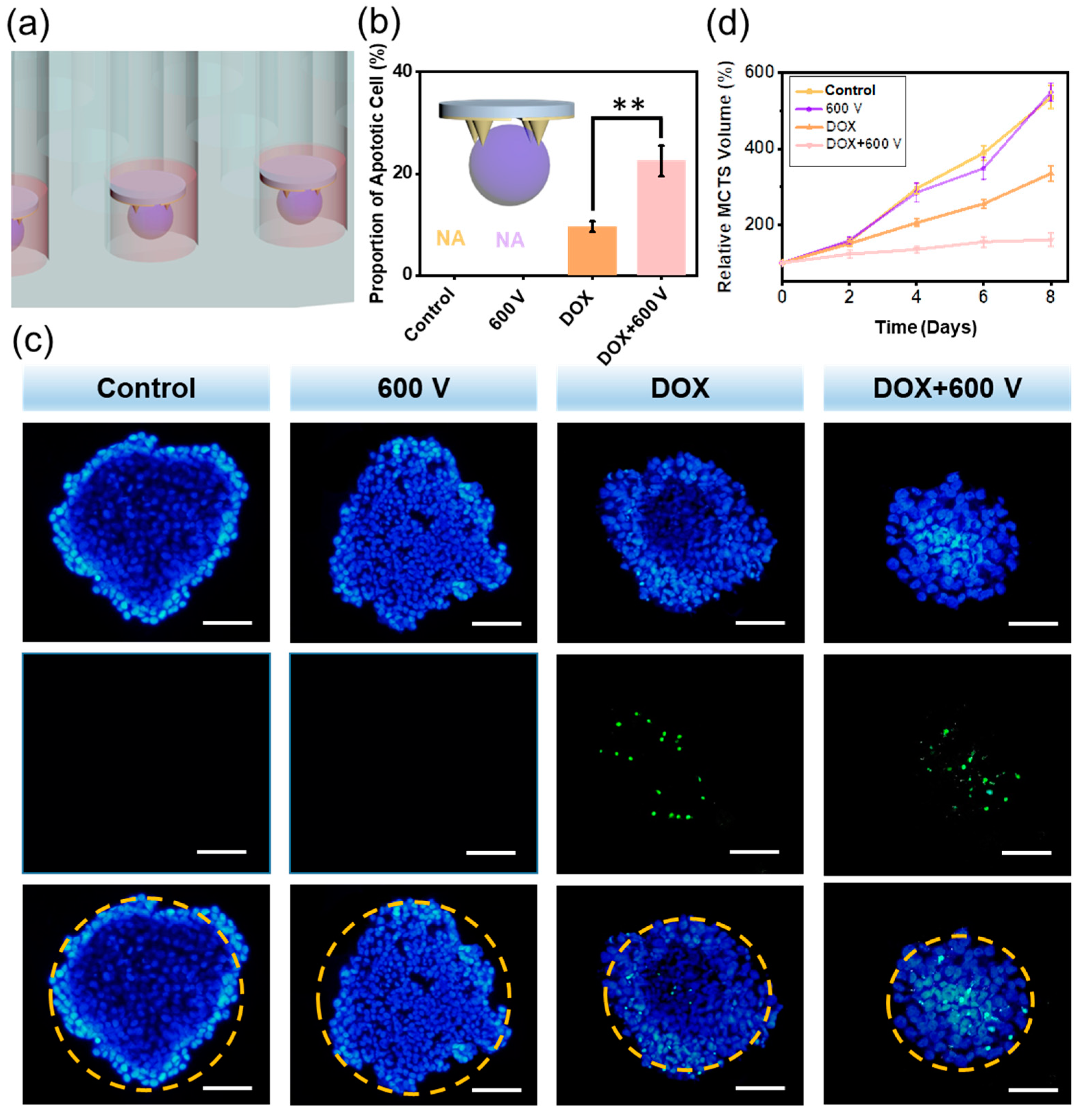
3.4. The Self-Powered EIC to Treat 3D MCTS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panta, P.; Dhopathi, S.R.; Gilligan, G.; Seshadri, M. Invasive oral squamous cell carcinoma induced by concurrent smokeless tobacco and creamy snuff use: A case report. Oral Oncol. 2021, 118, 105354. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Ebadi, M.; Vo, K.; Novak, J.; Govindarajan, A.; Amini, A. An Updated Review on Head and Neck Cancer Treatment with Radiation Therapy. Cancers 2021, 13, 4912. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma—An update. CA Cancer J. Clin. 2015, 65, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Auguste, A.; Deloumeaux, J.; Joachim, C.; Gaete, S.; Michineau, L.; Herrmann-Storck, C.; Duflo, S.; Luce, D. Joint effect of tobacco, alcohol, and oral HPV infection on head and neck cancer risk in the French West Indies. Cancer Med. 2020, 9, 6854–6863. [Google Scholar] [CrossRef] [PubMed]
- Thomson, P.J. Perspectives on oral squamous cell carcinoma prevention-proliferation, position, progression and prediction. J. Oral Pathol. Med. 2018, 47, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zeng, L.; Wang, S.; Wang, R.; Yang, R.; Jin, Z.; Tao, H. LncRNA FER1L4 Promotes Oral Squamous Cell Carcinoma Progression via Targeting miR-133a-5p/Prx1 Axis. Oncol. Targets Ther. 2021, 14, 795–806. [Google Scholar] [CrossRef]
- Cheraghlou, S.; Schettino, A.; Zogg, C.K.; Judson, B.L. Changing prognosis of oral cancer: An analysis of survival and treatment between 1973 and 2014. Laryngoscope 2018, 128, 2762–2769. [Google Scholar] [CrossRef]
- Bonifacio, B.V.; Silva, P.B.; Ramos, M.A.; Negri, K.M.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar]
- Okino, M.; Tomie, H.; Kanesada, H.; Marumoto, M.; Esato, K.; Suzuki, H. Optimal electric conditions in electrical impulse chemotherapy. Jpn. J. Cancer Res. 1992, 83, 1095–1101. [Google Scholar] [CrossRef]
- Mir, L.M.; Belehradek, M.; Domenge, C.; Orlowski, S.; Poddevin, B.; Belehradek, J., Jr.; Schwaab, G.; Luboinski, B.; Paoletti, C. Electrochemotherapy, a new antitumor treatment: First clinical trial. Comptes Rendus Acad. Sci. III 1991, 313, 613–618. [Google Scholar]
- Belehradek, M.; Domenge, C.; Luboinski, B.; Orlowski, S.; Belehradek, J., Jr.; Mir, L.M. Electrochemotherapy, a new antitumor treatment. First clinical phase I-II trial. Cancer 1993, 72, 3694–3700. [Google Scholar] [CrossRef]
- Sersa, G.; Miklavcic, D.; Cemazar, M.; Rudolf, Z.; Pucihar, G.; Snoj, M. Electrochemotherapy in treatment of tumours. Eur. J. Surg. Oncol. 2008, 34, 232–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campana, L.G.; Clover, A.J.; Valpione, S.; Quaglino, P.; Gehl, J.; Kunte, C.; Snoj, M.; Cemazar, M.; Rossi, C.R.; Miklavcic, D.; et al. Recommendations for improving the quality of reporting clinical electrochemotherapy studies based on qualitative systematic review. Radiol. Oncol. 2016, 50, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Coletti, L.; Battaglia, V.; De Simone, P.; Turturici, L.; Bartolozzi, C.; Filipponi, F. Safety and feasibility of electrochemotherapy in patients with unresectable colorectal liver metastases: A pilot study. Int. J. Surg. 2017, 44, 26–32. [Google Scholar] [CrossRef]
- Glass, L.F.; Fenske, N.A.; Jaroszeski, M.; Perrott, R.; Harvey, D.T.; Reintgen, D.S.; Heller, R. Bleomycin-Mediated electrochemotherapy of basal cell carcinoma. J. Am. Acad. Dermatol. 1996, 34, 82–86. [Google Scholar] [CrossRef]
- Bazzolo, B.; Mittal, L.; Sieni, E.; Piovan, A.; Filippini, R.; Conconi, M.T.; Camarillo, I.G.; Sundararajan, R. The electrical pulse application enhances intra-cellular localization and potentiates cytotoxicity of curcumin in breast cancer cells. Bioelectrochemistry 2021, 140, 107817. [Google Scholar] [CrossRef]
- Landstrom, F.; Kristiansson, S.; Reizenstein, J. The Role of Electrochemotherapy in Curative Treatment of Head and Neck Cancer and Advanced Skin Cancer: A Need for New Treatment Protocols? Anticancer Res. 2021, 41, 3977–3982. [Google Scholar] [CrossRef]
- Stefano, M.; Prosperi, E.; Fugazzola, P.; Benini, B.; Bisulli, M.; Coccolini, F.; Mastronardi, C.; Palladino, A.; Tomasoni, M.; Agnoletti, V.; et al. Case Report: Cytoreductive Surgery and HIPEC Associated with Liver Electrochemotherapy in a Cholangiocarcinoma Patient with Peritoneal Carcinomatosis and Liver Metastasis Case Report. Front. Surg. 2021, 8, 624817. [Google Scholar] [CrossRef]
- Feng, H.Q.; Zhao, C.C.; Tan, P.C.; Liu, R.P.; Chen, X.; Li, Z. Nanogenerator for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, e1701298. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, J.; Wang, Z.Z.; Ji, L.H.; Wang, Z.L. Triboelectric nanogenerators for human-health care. Sci. Bull. 2021, 66, 490–511. [Google Scholar] [CrossRef]
- Shao, J.J.; Willatzen, M.; Wang, Z.L. Theoretical modeling of triboelectric nanogenerators (TENGs). J. Appl. Phys. 2020, 128, 111101. [Google Scholar] [CrossRef]
- Zhao, C.C.; Cui, X.; Wu, Y.X.; Li, Z. Recent Progress of Nanogenerators Acting as Self-Powered Drug Delivery Devices. Adv. Sustain. Syst. 2021, 5, 2000268. [Google Scholar] [CrossRef]
- Sun, J.Y.; Yang, A.P.; Zhao, C.C.; Liu, F.; Li, Z. Recent progress of nanogenerators acting as biomedical sensors in vivo. Sci. Bull. 2019, 64, 1336–1347. [Google Scholar] [CrossRef] [Green Version]
- Han, K.; Luo, J.J.; Feng, Y.W.; Xu, L.; Tang, W.; Wang, Z.L. Self-powered electrocatalytic ammonia synthesis directly from air as driven by dual triboelectric nanogenerators. Energ. Environ. Sci. 2020, 13, 2450–2458. [Google Scholar] [CrossRef]
- Fan, F.R.; Tian, Z.Q.; Wang, Z.L. Flexible triboelectric generator! Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Zhao, L.M.; Zheng, Q.; Ouyang, H.; Li, H.; Yan, L.; Shi, B.J.; Li, Z. A size-unlimited surface microstructure modification method for achieving high performance triboelectric nanogenerator. Nano Energy 2016, 28, 172–178. [Google Scholar] [CrossRef]
- Wang, X.D.; Song, J.H.; Liu, J.; Wang, Z.L. Direct-current nanogenerator driven by ultrasonic waves. Science 2007, 316, 102–105. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.S.; Jeong, C.K.; Seo, M.H.; Joe, D.J.; Han, J.H.; Yoon, J.B.; Lee, K.J. Performance-enhanced triboelectric nanogenerator enabled by wafer-scale nanogrates of multistep pattern downscaling. Nano Energy 2017, 35, 415–423. [Google Scholar] [CrossRef]
- Guo, H.Y.; Chen, J.; Wang, L.F.; Wang, A.C.; Li, Y.F.; An, C.H.; He, J.H.; Hu, C.G.; Hsiao, V.K.S.; Wang, Z.L. A highly efficient triboelectric negative air ion generator. Nat. Sustain. 2021, 4, 147–153. [Google Scholar] [CrossRef]
- Wang, H.M.; Li, D.; Zhong, W.; Xu, L.; Jiang, T.; Wang, Z.L. Self-Powered Inhomogeneous Strain Sensor Enabled Joint Motion and Three-Dimensional Muscle Sensing. ACS Appl. Mater. Inter. 2019, 11, 34251–34257. [Google Scholar] [CrossRef]
- Lim, G.H.; Kwak, S.S.; Kwon, N.; Kim, T.; Kim, H.; Kim, S.M.; Kim, S.W.; Lim, B. Fully stretchable and highly durable triboelectric nanogenerators based on gold-nanosheet electrodes for self-powered human-motion detection. Nano Energy 2017, 42, 300–306. [Google Scholar] [CrossRef]
- Dai, J.Y.; Li, L.L.; Shi, B.J.; Li, Z. Recent progress of self-powered respiration monitoring systems. Biosens. Bioelectron. 2021, 194, 113609. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qu, X.C.; Zheng, Q.; Liu, Y.; Tan, P.C.A.; Shi, B.J.; Ouyang, H.; Chao, S.Y.; Zou, Y.; Zhao, C.C.; et al. Stretchable, Self-Healing, and Skin-Mounted Active Sensor for Multipoint Muscle Function Assessment. ACS Nano 2021, 15, 10130–10140. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Jiang, D.J.; Qu, X.C.; Bai, Y.; Cao, Y.; Luo, R.Z.; Li, Z. A Stretchable, Self-Healable Triboelectric Nanogenerator as Electronic Skin for Energy Harvesting and Tactile Sensing. Materials 2021, 14, 1689. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhang, H.; Shi, B.J.; Xue, X.; Liu, Z.; Jin, Y.M.; Ma, Y.; Zou, Y.; Wang, X.X.; An, Z.; et al. In Vivo Self-Powered Wireless Cardiac Monitoring via Implantable Triboelectric Nanogenerator. ACS Nano 2016, 10, 6510–6518. [Google Scholar] [CrossRef]
- Zhao, C.C.; Feng, H.Q.; Zhang, L.J.; Li, Z.; Zou, Y.; Tan, P.C.; Ouyang, H.; Jiang, D.J.; Yu, M.; Wang, C.; et al. Highly Efficient In Vivo Cancer Therapy by an Implantable Magnet Triboelectric Nanogenerator. Adv. Funct. Mater. 2019, 29, 1808640. [Google Scholar] [CrossRef]
- Tian, J.J.; Shi, R.; Liu, Z.; Ouyang, H.; Yu, M.; Zhao, C.C.; Zou, Y.; Jiang, D.J.; Zhang, J.S.; Li, Z. Self-powered implantable electrical stimulator for osteoblasts’ proliferation and differentiation. Nano Energy 2019, 59, 705–714. [Google Scholar] [CrossRef]
- Jiang, W.; Li, H.; Liu, Z.; Li, Z.; Tian, J.J.; Shi, B.J.; Zou, Y.; Ouyang, H.; Zhao, C.C.; Zhao, L.M.; et al. Fully Bioabsorbable Natural-Materials-Based Triboelectric Nanogenerators. Adv. Mater. 2018, 30, 1801895. [Google Scholar] [CrossRef]
- Zheng, Q.; Zou, Y.; Zhang, Y.L.; Liu, Z.; Shi, B.J.; Wang, X.X.; Jin, Y.M.; Ouyang, H.; Li, Z.; Wang, Z.L. Biodegradable triboelectric nanogenerator as a life-time designed implantable power source. Sci. Adv. 2016, 2, 6510–6518. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Xu, L.L.; Jiang, D.J.; Chen, B.Z.; Luo, R.Z.; Liu, Z.; Qu, X.C.; Wang, C.; Shan, Y.Z.; Cui, Y.; et al. Self-Powered Controllable Transdermal Drug Delivery System. Adv. Funct. Mater. 2021, 31, 2104092. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Yang, Y.; Cui, X.; Shan, Y.; Xue, J.; Jiang, D.; Sun, J.; Li, N.; Li, Z.; Yang, A. Self-Powered Electrical Impulse Chemotherapy for Oral Squamous Cell Carcinoma. Materials 2022, 15, 2060. https://doi.org/10.3390/ma15062060
Zhao C, Yang Y, Cui X, Shan Y, Xue J, Jiang D, Sun J, Li N, Li Z, Yang A. Self-Powered Electrical Impulse Chemotherapy for Oral Squamous Cell Carcinoma. Materials. 2022; 15(6):2060. https://doi.org/10.3390/ma15062060
Chicago/Turabian StyleZhao, Chaochao, Yuan Yang, Xi Cui, Yizhu Shan, Jiangtao Xue, Dongjie Jiang, Jinyan Sun, Na Li, Zhou Li, and Anping Yang. 2022. "Self-Powered Electrical Impulse Chemotherapy for Oral Squamous Cell Carcinoma" Materials 15, no. 6: 2060. https://doi.org/10.3390/ma15062060
APA StyleZhao, C., Yang, Y., Cui, X., Shan, Y., Xue, J., Jiang, D., Sun, J., Li, N., Li, Z., & Yang, A. (2022). Self-Powered Electrical Impulse Chemotherapy for Oral Squamous Cell Carcinoma. Materials, 15(6), 2060. https://doi.org/10.3390/ma15062060






