Study of Nano h-BN Impact on Lubricating Properties of Selected Oil Mixtures
Abstract
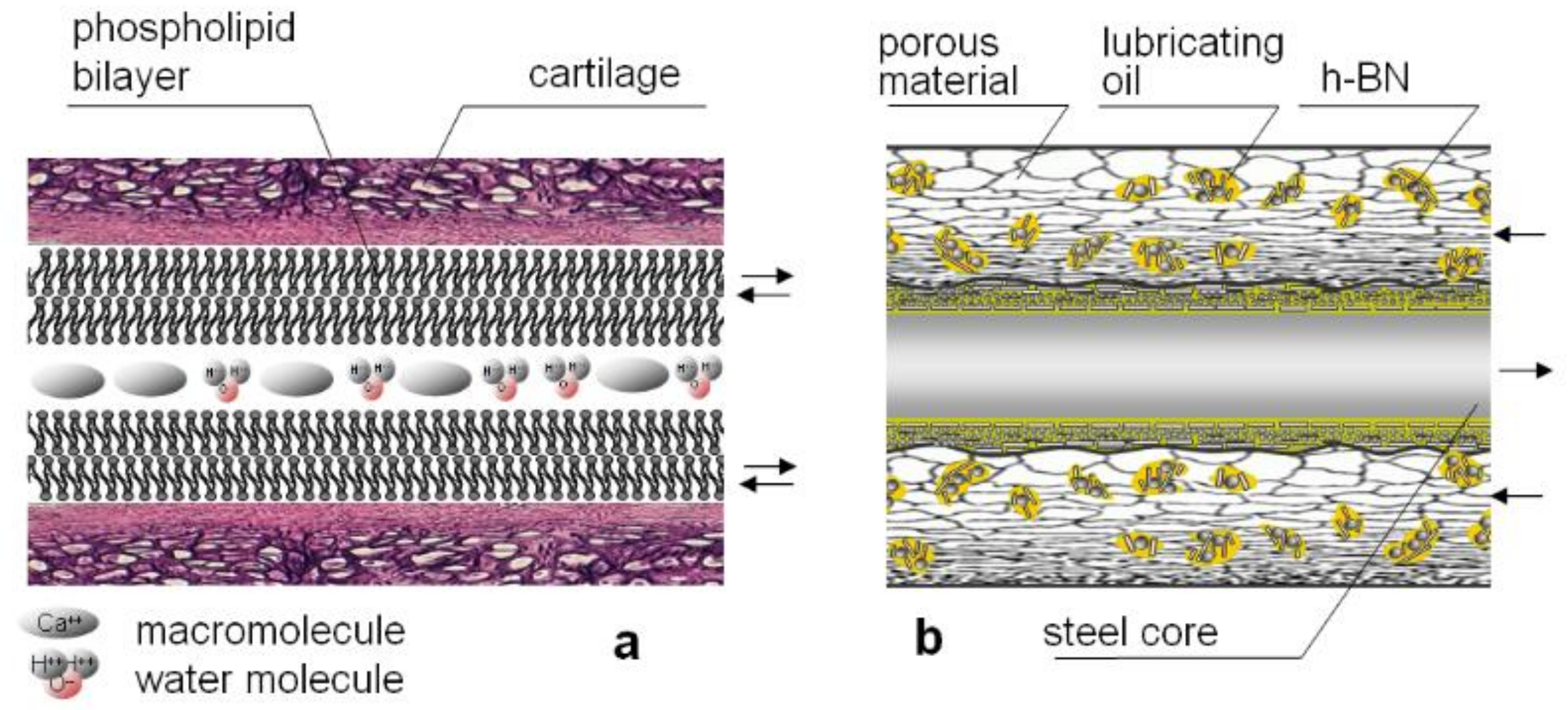
:1. Introduction
- ensure the lowest possible friction;
- fill the surface discontinuities that have arisen or appeared (smoothing out roughness);
- dissipate heat well from the friction node;
- be as least chemically aggressive as possible.
2. Materials and Methods
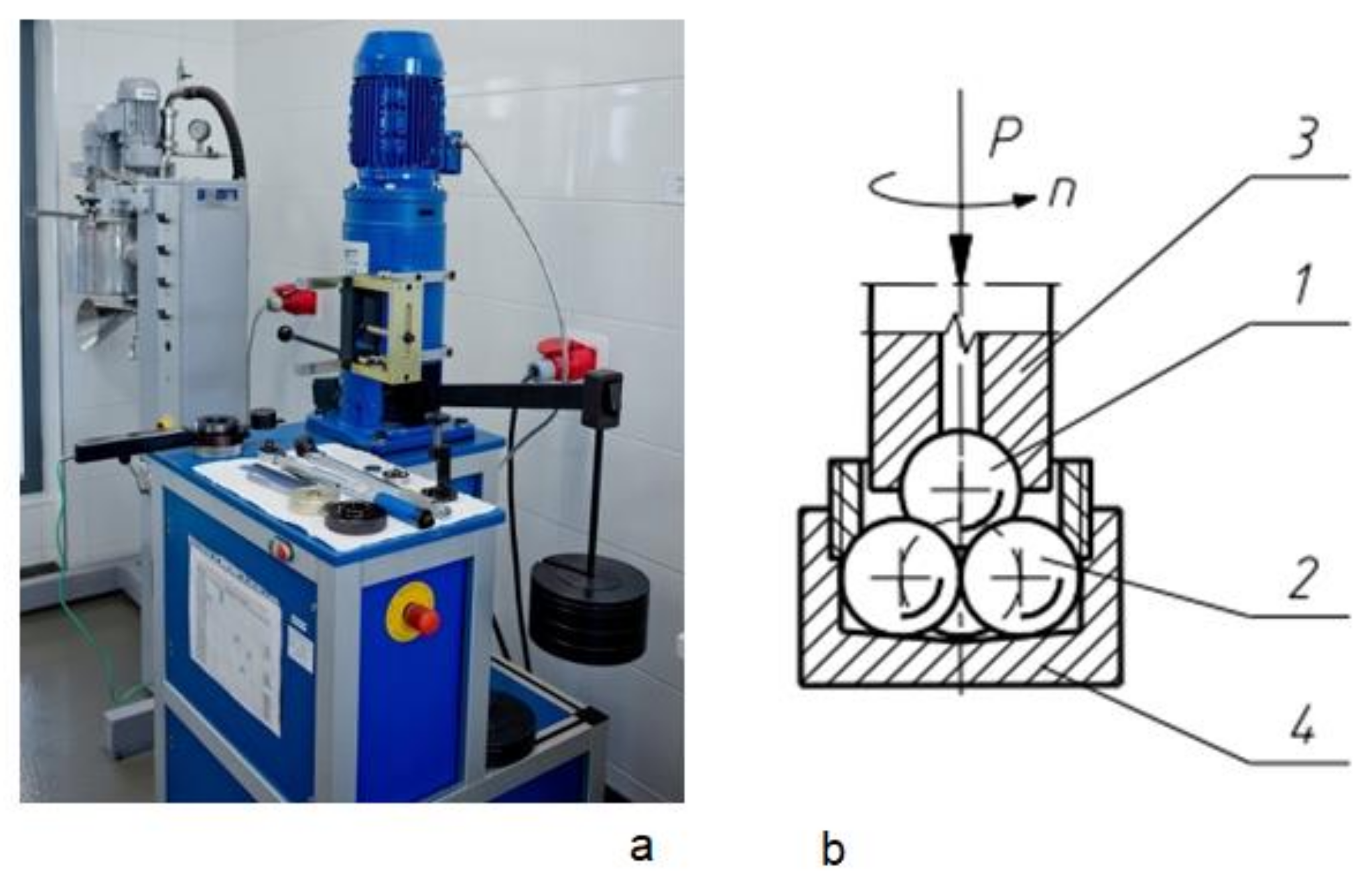
2.1. Test Equipment
- a WTW pH-meter (Mettler Toledo, Columbus, USA) with a SenTix®41 pH electrode (Mettler Toledo, Columbus, USA), which was used to determine the pH values for oils;
- an Abbe refractometer (KERN Optics, Balingen, Germany), which was used to determine the critical angle of refraction when light rays pass from the air to the test oil;
- a Nima tensiometer (Nima Technology Ltd., Coventry, England), which was employed to determine surface tension using the tensiometric method;
- an apparatus comprising a Höppler viscosimeter (RHEOTEST, Medingen, Germany) and a thermostat was used for viscosity testing, and the test itself was conducted for temperatures of 40 and 95 °C;
- pycnometers (Archem, Kielce, Poland) placed in a thermostat (Merazet, Poznań, Poland) were used for oil density testing, and the test was conducted at temperatures of 20 °C.
2.2. Test Materials
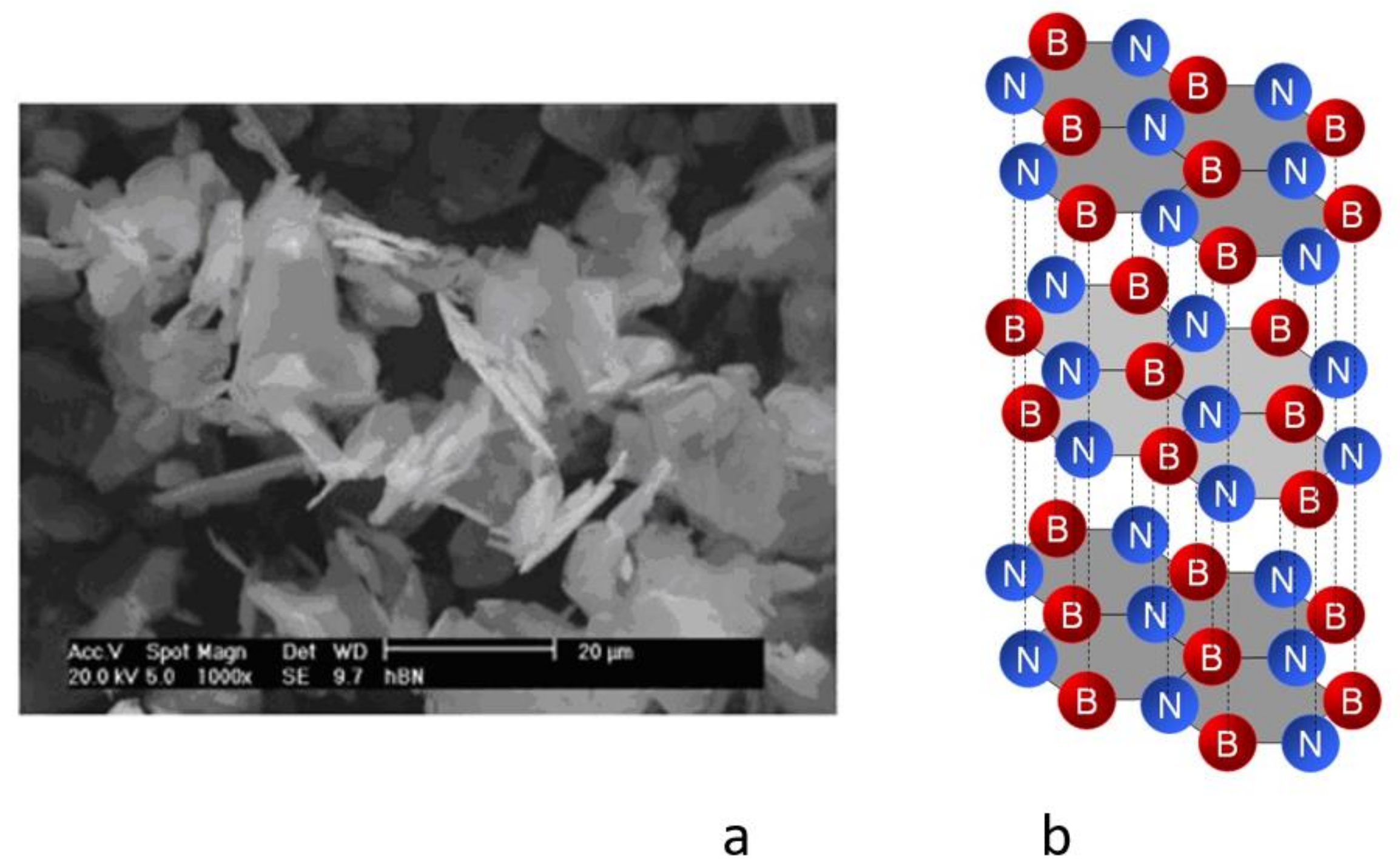
2.2.1. Hexagonal Boron Nitride
2.2.2. Oils Used to Prepare the Test Samples
2.2.3. Anti-Caking Agent
2.3. Sample Preparation for Testing
2.4. Measuring Procedure
3. Results and Discussion
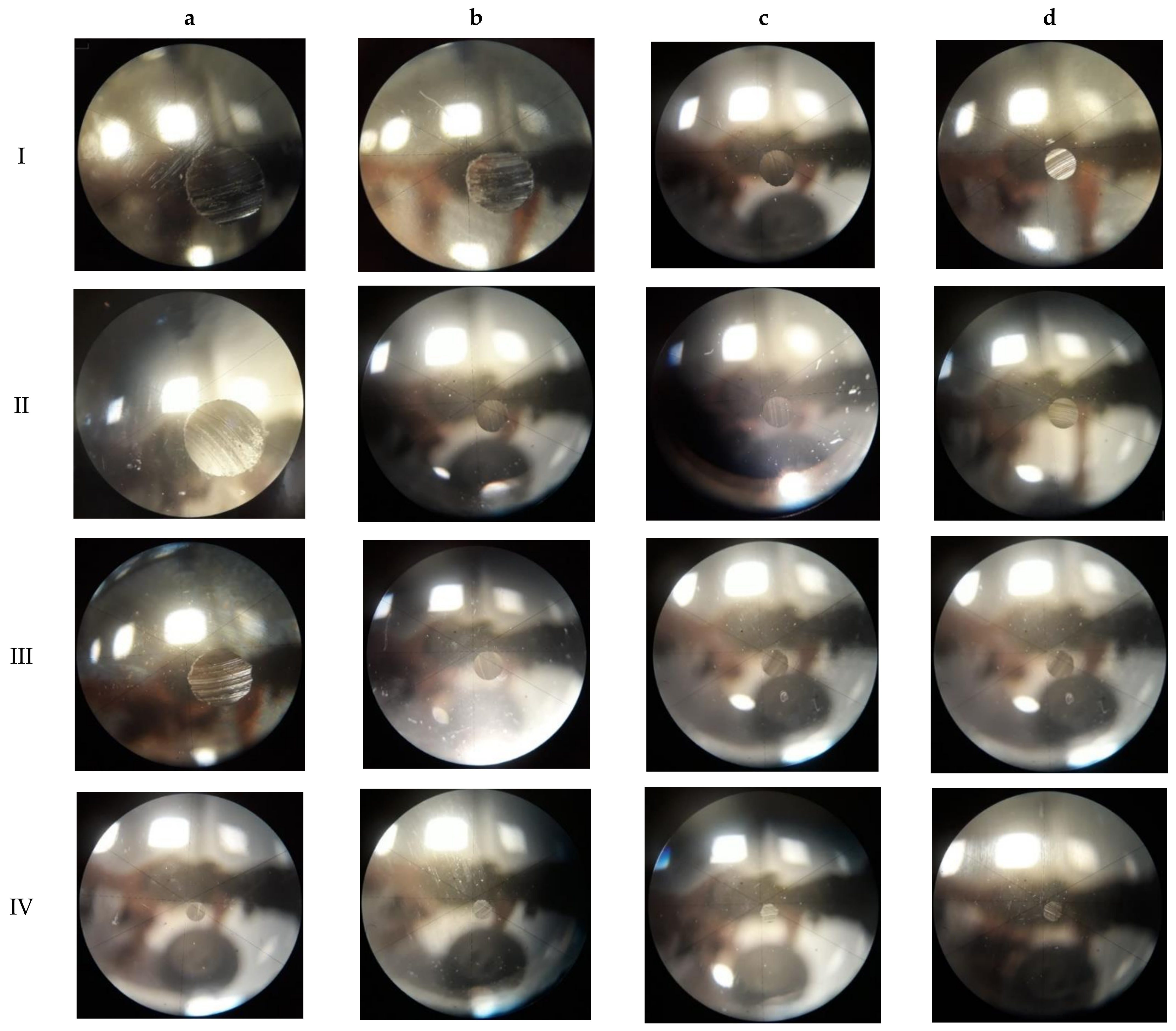
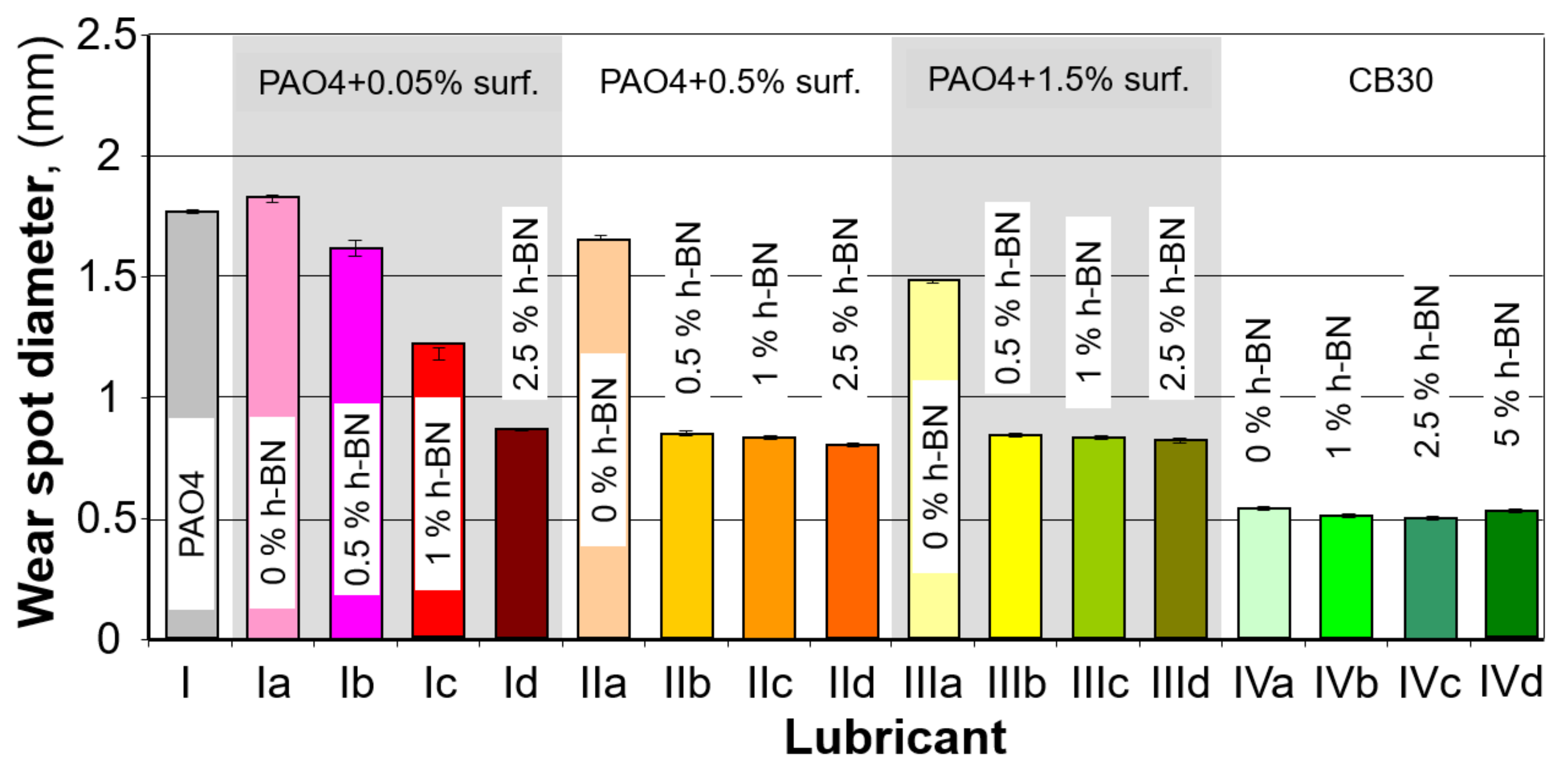
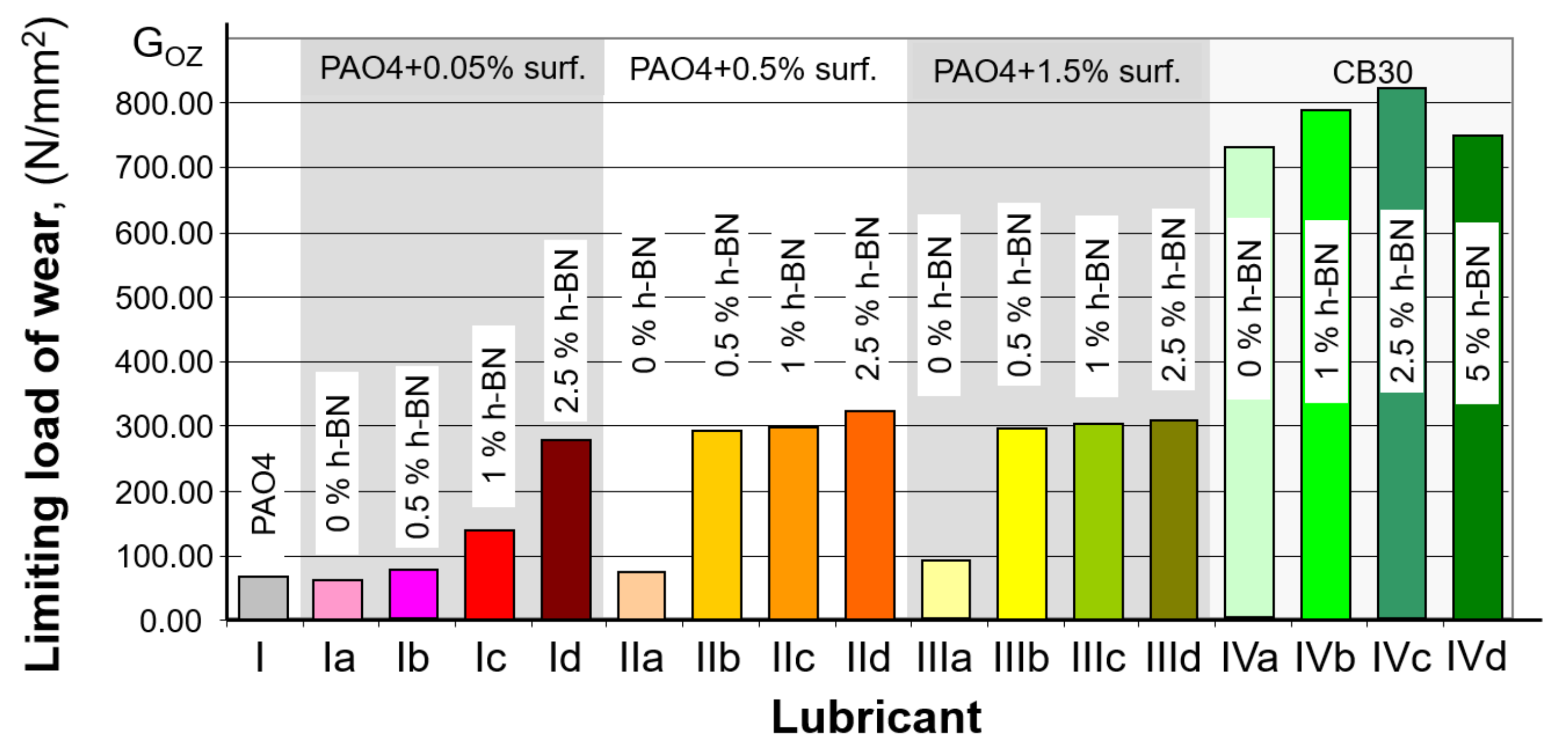
3.1. Wear Spot Measurement
- F is the friction node load [N], F = 392 N;
- D is the average wear spot diameter [mm];
- 0.52 is the factor accounting for the distribution of forces in the friction node.
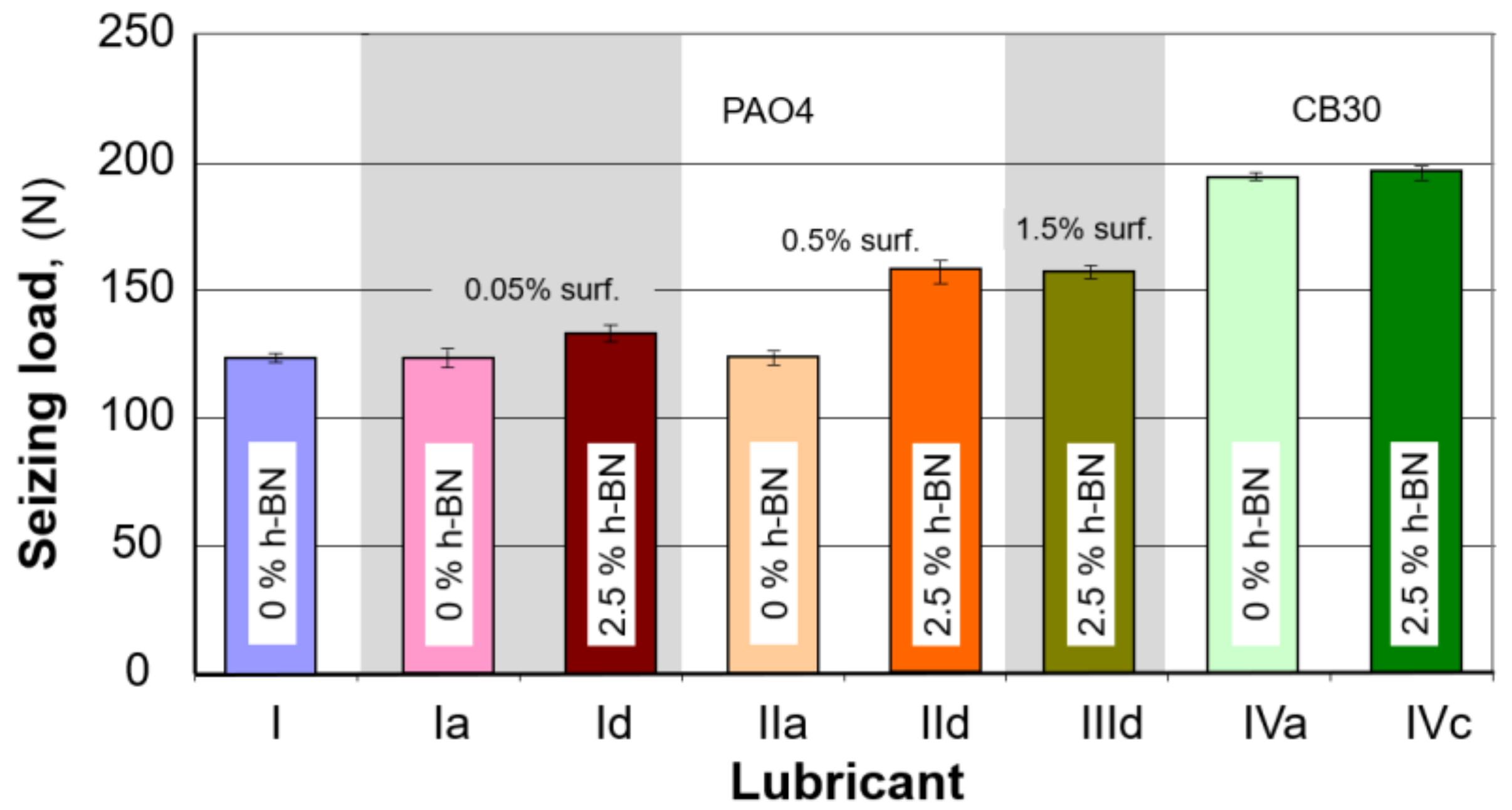
3.2. Seizure Load Determination
3.3. Physicochemical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Archard, J.E. Contact and rubbing of flat surfaces. J. Appl. Phys. 1953, 24, 981. [Google Scholar] [CrossRef]
- Bayer, R.G.; Clinton, W.C.; Nelson, C.W.; Schumacher, R.A. Engineering model for wear. Wear 1962, 5, 378–391. [Google Scholar] [CrossRef]
- Zum Gahr, K.H. (Ed.) Microstructure and Wear of Materials; Elsevier B.V.: Amsterdam, The Netherlands, 1987. [Google Scholar] [CrossRef]
- Kuczmaszewski, J. Fundamentals of Metal-Metal Adhesive Joint Design; Lublin University of Technology: Lublin, Poland, 2006. [Google Scholar]
- Kałdoński, T. Podstawowe Problemy Analizowania Procesów Tribologicznych (Basic Problems of Analyzing Tribological Processes); WAT: Warszawa, Poland, 2015. [Google Scholar]
- Pawlak, Z.; Urbaniak, W.; Kałdoński, T.; Oloyede, A. Importance of Bearing Porosity in Engineering and Natural Lubrication; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Urbaniak, W. Smarowanie Powierzchni Biologicznych i Inżynieryjnych (Lubrication of Biological and Engineering Surfaces); KWU: Bydgoszcz, Poland, 2015; ISBN 978-83-8018-023-9. [Google Scholar]
- Rabaso, P.; Dassenoy, F.; Ville, F.; Diaby, M.; Vacher, B.; Le Mogne, T.; Beli, M.; Cavoret, J. An investigation on the reduced ability of IF-MoS2 nanoparticles to reduce friction and wear in the presence of dispersants. Tribol. Lett. 2014, 55, 503–516. [Google Scholar] [CrossRef]
- Urbaniak, W.; Kałdoński, T.; Hagner-Derengowska, M.; Kałdoński, T.J.; Madhani, J.T.; Kruszewski, A.; Pawlak, Z. Impregnated porous bearings textured with a pocket on sliding surfaces: Comparison of h-BN with graphite and MoS2 up to 150 °C. Meccanica 2015, 50, 1343–1349. [Google Scholar] [CrossRef]
- Urbaniak, W.; Majewski, T.; Woźniak, R.; Sienkiewicz, J.; Kubik, J.; Petelska, A.D. Research on the influence of the manufacturing process conditions of iron sintered with the addition of layered lubricating materials on its selected properties. Materials 2020, 13, 4782. [Google Scholar] [CrossRef]
- Maharaj, D.; Bhusahan, B. Effect of MoS2 and WS2 Nanotubes on Nanofriction and Wear Reduction in Dry and Liquid Environments. Tribol. Lett. 2013, 49, 323–339. [Google Scholar] [CrossRef]
- Kimura, Y.; Wakabayashi, T.; Okada, K.; Wada, T.; Nishikawa, H. Boron nitride as a lubricant additive. Wear 1999, 232, 199–206. [Google Scholar] [CrossRef]
- Wan, Q.; Jin, Y.; Sun, P.; Ding, Y. Tribological Behaviour of a Lubricant Oil Containing Boron Nitride Nanoparticles. Procedia Eng. 2015, 102, 1038–1045. [Google Scholar] [CrossRef] [Green Version]
- Ramteke, S.; Chelladurai, H. Examining the role of hexagonal boron nitride nanoparticles as an additive in the lubricating oil and studying its application. Proc. Inst. Mech. Eng. Part N. J. Nanomater. Nanoeng. Nanosyst. 2020, 234, 19–36. [Google Scholar] [CrossRef]
- Lelonis, D.A.; Tereshko, J.W.; Andersen, C.M. Boron nitride powder-a high performance alternative for solid lubrication. GE Adv. Ceram. 2003, 4, 81506. [Google Scholar]
- Carrión, F.J.; Ginés, M.N.; Iglesias, P.; Sanes, J.; Bermúdez, M.D. Liquid crystals in tribology. Int. J. Mol. Sci. 2009, 10, 4102–4115. [Google Scholar] [CrossRef] [PubMed]
- Kałdoński, T.; Król, A.; Giemza, B.; Gocman, K.; Kałdońnski, T.J. Porowate łożyska ślizgowe spiekane z proszku żelaza z dodatkiem heksagonalnego azotku boru h-BN (Porous slide bearings sintered from iron powder with addition of hexagonal boron nitride h-BN). Patent P401050, 4 October 2012. [Google Scholar]
- Pawlak, Z.; Kałdoński, T.; Lisewski, M.; Urbaniak, W.; Oloyede, A. The effect of hexagonal boron nitride additive on the effectiveness of grease-based lubrication of a steel surface. Ind. Lubr. Tribol. 2012, 64, 84–89. [Google Scholar] [CrossRef]
- Groszek, A.J.; Whiteridge, R.E. Surface properties and lubricating action of graphite MoS2. Am. Soc. Lubr. Eng. Trans. 1971, 14, 254–266. [Google Scholar] [CrossRef]
- Urbaniak, W.; Kałdonski, T.; Kałdonski, T.J.; Pawlak, Z. Hexagonal boron nitride as a component of the iron porous bearing: Friction on the porous sinters up to 150 °C. Meccanica 2016, 51, 1157–1165. [Google Scholar] [CrossRef]
- Ay, N.; Ay, G.M.; Göncü, Y. Environmentally friendly material: Hexagonal boron nitride. J. Boron 2016, 1, 66–73. [Google Scholar]
- Wang, J.; Li, T.; Yan, T.; Zhang, L.; Zhang, K.; Qu, X. Role of Magnesium Perrhenate in an Oil/Solid Mixed System for Tribological Application at Various Temperatures. Materials 2018, 11, 1754. [Google Scholar] [CrossRef] [Green Version]
- Senyk, S.; Chodkiewicz, A.; Gocman, K.; Szczęśniak, B.; Kałdoński, T. Hexagonal Nano and Micro Boron Nitride: Properties and Lubrication Applications. Materials 2022, 15, 955. [Google Scholar] [CrossRef]
- Del Río, J.M.L.; López, E.R.; Fernández, J. Synergy between boron nitride or graphene nanoplatelets and tri(butyl)ethylphosphonium diethylphosphate ionic liquid as lubricant additives of triisotridecyltrimellitate oil. J. Mol. Liq. 2020, 301, 112442. [Google Scholar] [CrossRef]
- Kałdoński, T. Tribologiczne zastosowania azotku boru. In Tribological Applications of Boron Nitride, 2nd ed.; MUT: Warsaw, Poland, 2013. (In Polish) [Google Scholar]
- Senyk, S.; Perehubka, M.; Kałdoński, T. Badania smarności olejów zawierających heksagonalny azotek boru. Biul. Wojsk. Akad. Tech. 2019, 68, 131–151. (In Polish) [Google Scholar] [CrossRef]
- Reeves, C.J.; Menezes, P.L.; Lovell, M.R.; Jen, T.-C.H. The size effect of boron nitride particles on the tribological performance of biolubricants for energy conservation and sustainability. Tribol. Lett. 2013, 51, 437–452. [Google Scholar] [CrossRef]
- Gupta, M.K.; Bijwe, J.; Padhan, M. Role of size of hexagonal boron nitride particles on tribo-performance of nano and micro oils. Lubr. Sci. 2018, 30, 441–456. [Google Scholar] [CrossRef]
- Raina, A.; Haq, M.I.U.; Anand, A.; Mohan, S.; Kumar, R.; Jayalakshmi, S.; Singh., R.A. Nanodiamond particles as secondary additive for polyalphaolefin oil lubrication of steel–aluminium contact. Nanomaterials 2021, 11, 1438. [Google Scholar] [CrossRef] [PubMed]
- Michalczewski, R.; Szczerek, M.; Tuszyński, W.; Wulczyński, J. A four-ball machine for testing antiwear, extreme-pressure properties, and surface fatigue life with a possiblility to increase the lubricant. Tribologia 2009, 1, 113–127. [Google Scholar]
- Balmain, W.H. On ethogen and ethonides. Philos. Mag. J. Sci. 1843, 12, 466–470. [Google Scholar]
- Rudnick, R.L.; Shubkin, L.R. Synthetic Lubricants and High-Performance Functional Fluids; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Bizkjak, M.; Zalar, A.; Panjan, P.; Zorko, B.; Pracek, B. Characterization of iron oxide layer using auger electron spectroscopy. Appl. Surf. Sci. 2007, 253, 3977–3981. [Google Scholar] [CrossRef]
| Parameter | Character/Value |
|---|---|
| Movement Type | Sliding or rolling |
| Contact Geometry | Point |
| Friction Node | Four ½″ balls |
| Test Material | Lubricants and construction materials |
| Friction Node Temperature | Stabilized from ambient temperature to 75 (°C) ± 2 °C, possible temperature range up to 175 (°C) |
| Spindle Rotation Speed | Seamless adjustable from 300 to 1800 (RPM) |
| Contact Load | Adjustable from 0 to 7850 (N) using a lever with a weight |
| Measured Parameter | Friction node temperature, load, movement resistance, rotation speed, vibration amplitude, time |
| Power Consumption | ~2 (kW) |
| Power Supply | 230 [V] 50 (Hz) |
| Properties of Hexagonal Boron Nitride | |
|---|---|
| Molecular Weight (g/mol) | 24.82 |
| Metallic Properties | non-metal |
| Appearance | white |
| Mohs scale of Hardness | 1.5 ÷ 2 |
| Density, (g/cm3) | 1.7 ÷ 2.2 |
| Melting Point, (°C) | 1185 |
| Lubrication Temperature Range, (°C) | −40 ÷ 870 |
| Physicochemical Properties | PAO4 | CB30 |
|---|---|---|
| Flash Point, min (°C) | 204 | 210 |
| Pour Point, max (°C) | −57 | −24 |
| pH | 6.86 | 6.32 |
| Aqueous Reaction pH | 6.92 | 6.52 |
| Refractive Index | 1.4553 | 1.4765 |
| Acid Number, (mg KOH/g of oil) | 0.01 | 3.5 |
| Dynamic Viscosity at 40 °C (mPa·s) | 12.58 | 44.37 |
| Dynamic Viscosity at 95 °C (mPa·s) | 3.26 | 8.33 |
| Kinematic Viscosity at 40 °C, (mm2/s) | 16.39 | 51.48 |
| Kinematic Viscosity at 95 °C, (mm2/s) | 3.94 | 9.66 |
| Surface Tension (mN/m) | 45.20 | 44.70 |
| Viscosity Index | 105 | 95 |
| Density at 20 °C (g/cm3) | 0.8282 | 0.8619 |
| Physicochemical Properties | Succinic Acid Imide |
|---|---|
| Flashpoint, min (°C) | 190 |
| Acid Number, (mg KOH/g of oil) | 42 |
| Kinematic Viscosity at 100 °C, (mm2/s) | 440 |
| Kinematic Viscosity at 40 °C, (mm2/s) | 105 |
| Pour Point, max (°C) | −24 |
| Density (g/cm3) | 0.927 |
| Appearance | Dark brown, viscous liquid |
| Oil and % wt of Surfactant | h-BN Content (% wt) | |||
|---|---|---|---|---|
| 0.0 | 0.5 | 1.0 | 2.5 | |
| PA04, 0% | I | Not included | Not included | Not included |
| PA04, 0.05% | Ia | Ib | Ic | Id |
| PA04, 0.5% | IIa | IIb | IIc | IId |
| PA04, 1.5% | IIIa | IIIb | IIIc | IIId |
| Oil and % wt of surfactant | 0.0 | 1.0 | 2.5 | 5 |
| CB30, 0% | IVa | IVb | IVc | IVd |
| Sample No. | Composition | Average Wear Spot Diameter (mm) | Standard Deviation (Sx) | Limit Wear Load (N/mm2) | |||
|---|---|---|---|---|---|---|---|
| Measurement | Average | ||||||
| 1 | 2 | 3 | |||||
| I | PAO 4 | 1.76 | 1.76 | 1.77 | 1.76 | 0.01 | 65.58 |
| Ia | PAO 4 + 0.05% surf | 1.80 | 1.93 | 1.85 | 1.86 | 0.07 | 58.92 |
| Ib | PAO 4 + 0.05% surf + 0.5% h-BN | 1.60 | 1.70 | 1.60 | 1.63 | 0.05 | 76.41 |
| Ic | PAO 4 + 0.05% surf + 1.0% h-BN | 1.18 | 1.20 | 1.26 | 1.21 | 0.04 | 138.46 |
| Id | PAO 4 + 0.05% surf + 2.5% h-BN | 0.87 | 0.86 | 0.86 | 0.86 | 0.01 | 273.48 |
| IIa | PAO 4 + 0.5% surf | 1.64 | 1.74 | 1.66 | 1.68 | 0.05 | 72.22 |
| IIb | PAO 4 + 0.5% surf + 0.5% h-BN | 0.86 | 0.84 | 0.83 | 0.84 | 0.02 | 286.84 |
| IIc | PAO 4 + 0.5% surf + 1.0% h-BN | 0.84 | 0.84 | 0.82 | 0.83 | 0.01 | 293.53 |
| IId | PAO 4 + 0.5% surf + 2.5% h-BN | 0.80 | 0.79 | 0.81 | 0.80 | 0.01 | 318.50 |
| IIIa | PAO 4 + 1.5% surf | 1.50 | 1.55 | 1.51 | 1.52 | 0.03 | 88.23 |
| IIIb | PAO 4 + 1.5% surf + 0.5% h-BN | 0.84 | 0.84 | 0.83 | 0.84 | 0.01 | 291.20 |
| IIIc | PAO 4 + 1.5% surf + 1.0% h-BN | 0.82 | 0.84 | 0.82 | 0.83 | 0.01 | 298.28 |
| IIId | PAO 4 + 1.5% surf + 2.5% h-BN | 0.83 | 0.83 | 0.8 | 0.82 | 0.02 | 303.15 |
| IVa | CB 30 | 0.53 | 0.53 | 0.54 | 0.53 | 0.01 | 716.63 |
| IVb | CB 30 + 1.0% h-BN | 0.50 | 0.52 | 0.51 | 0.51 | 0.01 | 783.70 |
| IVc | CB 30 + 2.5% h-BN | 0.50 | 0.51 | 0.49 | 0.50 | 0.01 | 815.36 |
| IVd | CB 30 + 5.0% h-BN | 0.51 | 0.53 | 0.53 | 0.52 | 0.01 | 744.27 |
| Sample No. | Composition | Weld Point (daN) | Standard Deviation (Sx) | |||
|---|---|---|---|---|---|---|
| Measurement | ||||||
| 1 | 2 | 3 | Average | |||
| I | PAO4 | 12,3.43 | 12,3.65 | 12,3.60 | 12,3.56 | 0.12 |
| Ia | PAO 4 + 0.05% surf | 12,3.86 | 12,3.38 | 12,3.41 | 12,3.55 | 0.27 |
| Id | PAO 4 + 0.05% surf + 2.5% h-BN | 13,3.79 | 13,3.90 | 13,4.25 | 13,3.98 | 0.24 |
| IIa | PAO 4 + 0.5% surf | 12,3.56 | 12,3.37 | 12,3.75 | 12,3.56 | 0.19 |
| IId | PAO 4 + 0.5% surf + 2.5% h-BN | 15,8.39 | 15,8.40 | 15,7.84 | 15,8.21 | 0.32 |
| IIId | PAO 4 + 1.5% surf + 2.5% h-BN | 15,7.97 | 15,8.32 | 15,8.25 | 15,8.18 | 0.19 |
| IVa | CB 30 | 19,4.23 | 19,4.37 | 19,4.15 | 19,4.25 | 0.11 |
| IVc | CB 30 + 2.5% h-BN | 19,6.40 | 19,6.17 | 19,5.17 | 19,6.19 | 0.20 |
| Sample No. | Composition | Measured Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | Refractive Index | Surface Tension (mN/m) | Dynamic Viscosity (mPa·s) | Kinematic Viscosity (mm2/s) | Density at 20 °C (g/cm3) | ||||
| At 40 °C | At 95 °C | At 40 °C | At 95 °C | ||||||
| I | PAO4 | 6.86 | 1,4553 | 45.2 | 12.58 | 3.26 | 16.39 | 3.94 | 0,8282 |
| IIa | PAO 4 + 0.5% surf | 7.08 | 1,4550 | 43.6 | 13.08 | 3.35 | 17.22 | 4.69 | 0,8293 |
| IIb | PAO 4 + 0.5% surf + 0.5% h-BN | - | 1,4553 | 45.1 | 13.80 | 3.41 | 17.02 | 4.34 | 0,8299 |
| IIc | PAO 4 + 0.5% surf + 1.0% h-BN | - | 1,4556 | 43.2 | 14.45 | 3.49 | 16.67 | 4.01 | 0,8310 |
| IId | PAO 4 + 0.5% surf + 2.5% h-BN | - | 1,4556 | 40.0 | 15.50 | 3.90 | 17.07 | 5.23 | 0,8322 |
| IIIa | PAO 4 + 1.5% surf | 7.65 | 1,4555 | 41.2 | 14.03 | 3.72 | 16.23 | 4.89 | 0,8333 |
| IIIb | PAO 4 + 1.5% surf + 0.5% h-BN | - | 1,4560 | 50.7 | 14.66 | 3.78 | 16.22 | 4.88 | 0,8345 |
| IIIc | PAO 4 + 1.5% surf + 1.0% h-BN | - | 1,4562 | 53.1 | 14.79 | 3.81 | 15.81 | 4.68 | 0,8364 |
| IIId | PAO 4 + 1.5% surf + 2.5% h-BN | - | 1,4562 | 51.7 | 15.08 | 3.88 | 14.70 | 4.57 | 0,8376 |
| Iva | CB30 | 6.32 | 1,4765 | 44.7 | 44.37 | 8.33 | 51.48 | 9.66 | 0,8619 |
| IVb | CB 30 + 0.5% h-BN | - | 1,4774 | 48.5 | 47.40 | 10.26 | 42.38 | 9.18 | 0,8655 |
| IVc | CB 30 + 1.0% h-BN | - | 1,4774 | 44.0 | 51.67 | 11.80 | 46.37 | 9.55 | 0,8780 |
| IVd | CB 30 + 2.5% h-BN | - | 1,4777 | 42.8 | 52.99 | 12.05 | 52.61 | 10.65 | 0,8889 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbaniak, W.; Majewski, T.; Powązka, I.; Śmigielski, G.; Petelska, A.D. Study of Nano h-BN Impact on Lubricating Properties of Selected Oil Mixtures. Materials 2022, 15, 2052. https://doi.org/10.3390/ma15062052
Urbaniak W, Majewski T, Powązka I, Śmigielski G, Petelska AD. Study of Nano h-BN Impact on Lubricating Properties of Selected Oil Mixtures. Materials. 2022; 15(6):2052. https://doi.org/10.3390/ma15062052
Chicago/Turabian StyleUrbaniak, Wiesław, Tomasz Majewski, Iwona Powązka, Grzegorz Śmigielski, and Aneta D. Petelska. 2022. "Study of Nano h-BN Impact on Lubricating Properties of Selected Oil Mixtures" Materials 15, no. 6: 2052. https://doi.org/10.3390/ma15062052
APA StyleUrbaniak, W., Majewski, T., Powązka, I., Śmigielski, G., & Petelska, A. D. (2022). Study of Nano h-BN Impact on Lubricating Properties of Selected Oil Mixtures. Materials, 15(6), 2052. https://doi.org/10.3390/ma15062052






