Incorporation of Optical Density into the Blending Design for a Biocement Solution
Abstract
:1. Introduction
2. Materials and Methods
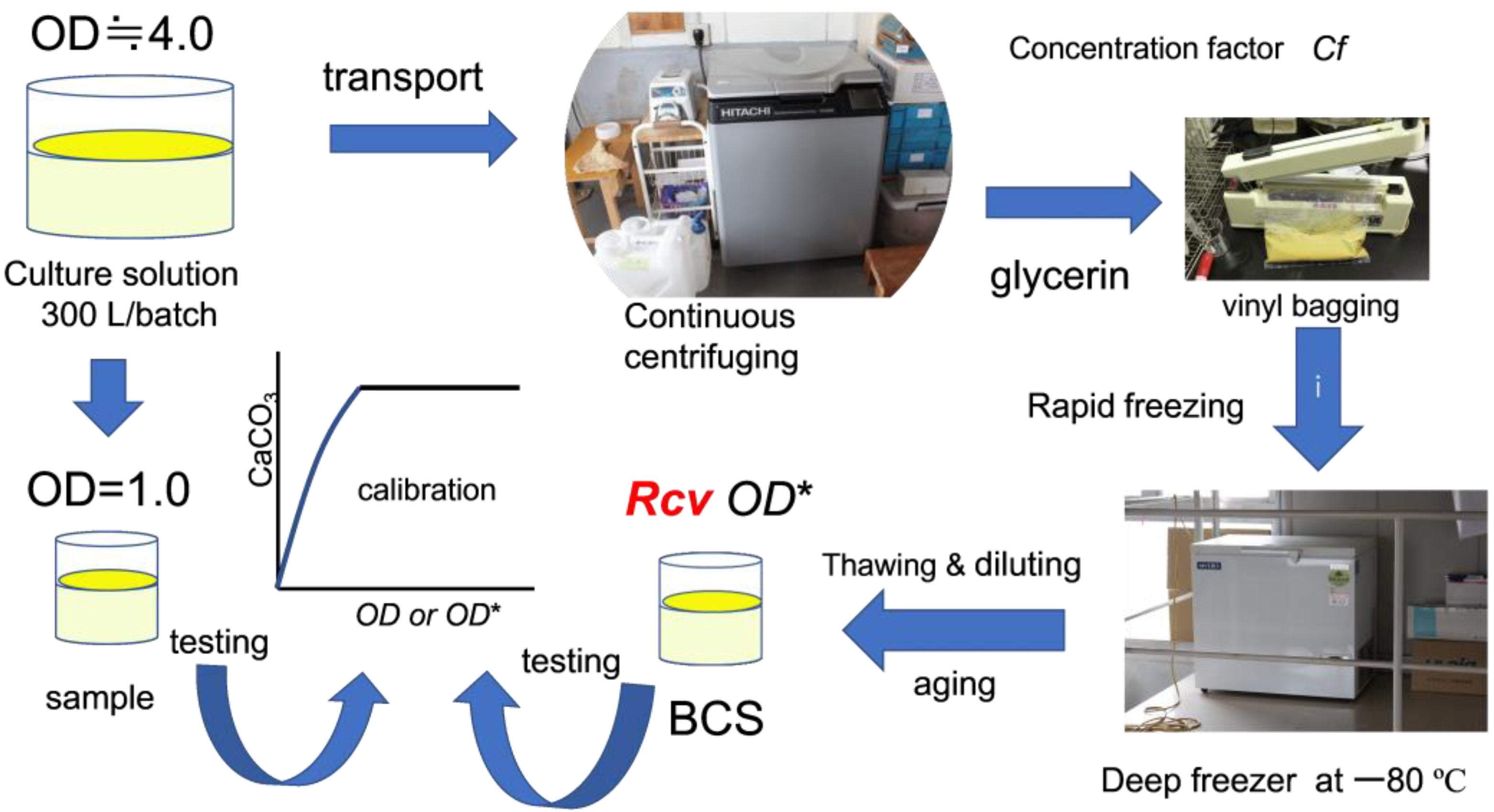
2.1. Microbial Preparation
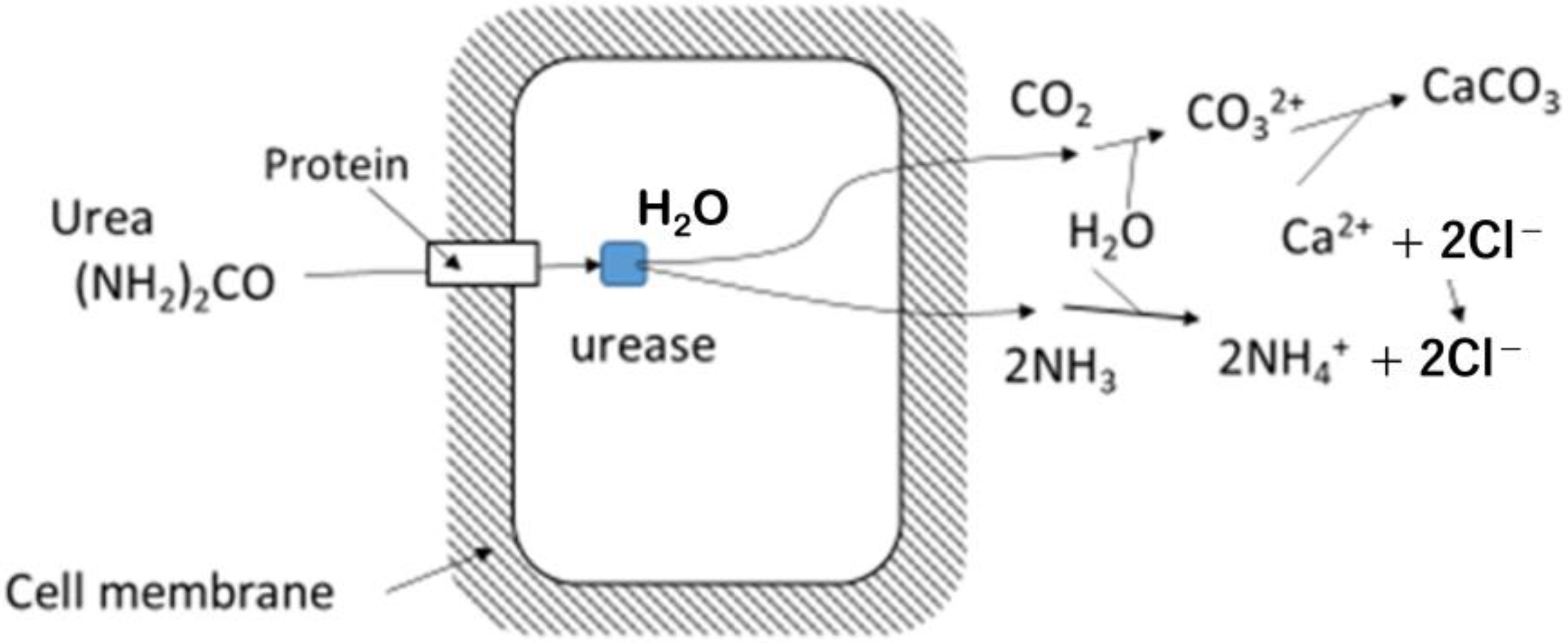
2.2. Limitation of Urease Activity for Carbonate Precipitation
2.3. Preparation of Sample for Obtaining Standard Precipitation Rate
- (1)
- Prescribed quantities of reagents, i.e., urea, calcium chloride, etc., were prepared;
- (2)
- Ten milliliter reactive solutions were added to each test tube containing the centrifuged microbes. Note that the volume of microbes is negligible, as the centrifugal concentration factor by volume is approximately 250–300;
- (3)
- The test tubes were left for 24 h, and the precipitated CaCO3 was measured by dividing into suspended particles and adsorbed carbonates.
2.4. Calibration Curves
2.5. Materials
2.6. Methods
3. Results and Discussion
3.1. CPR Controlled by Ca2+
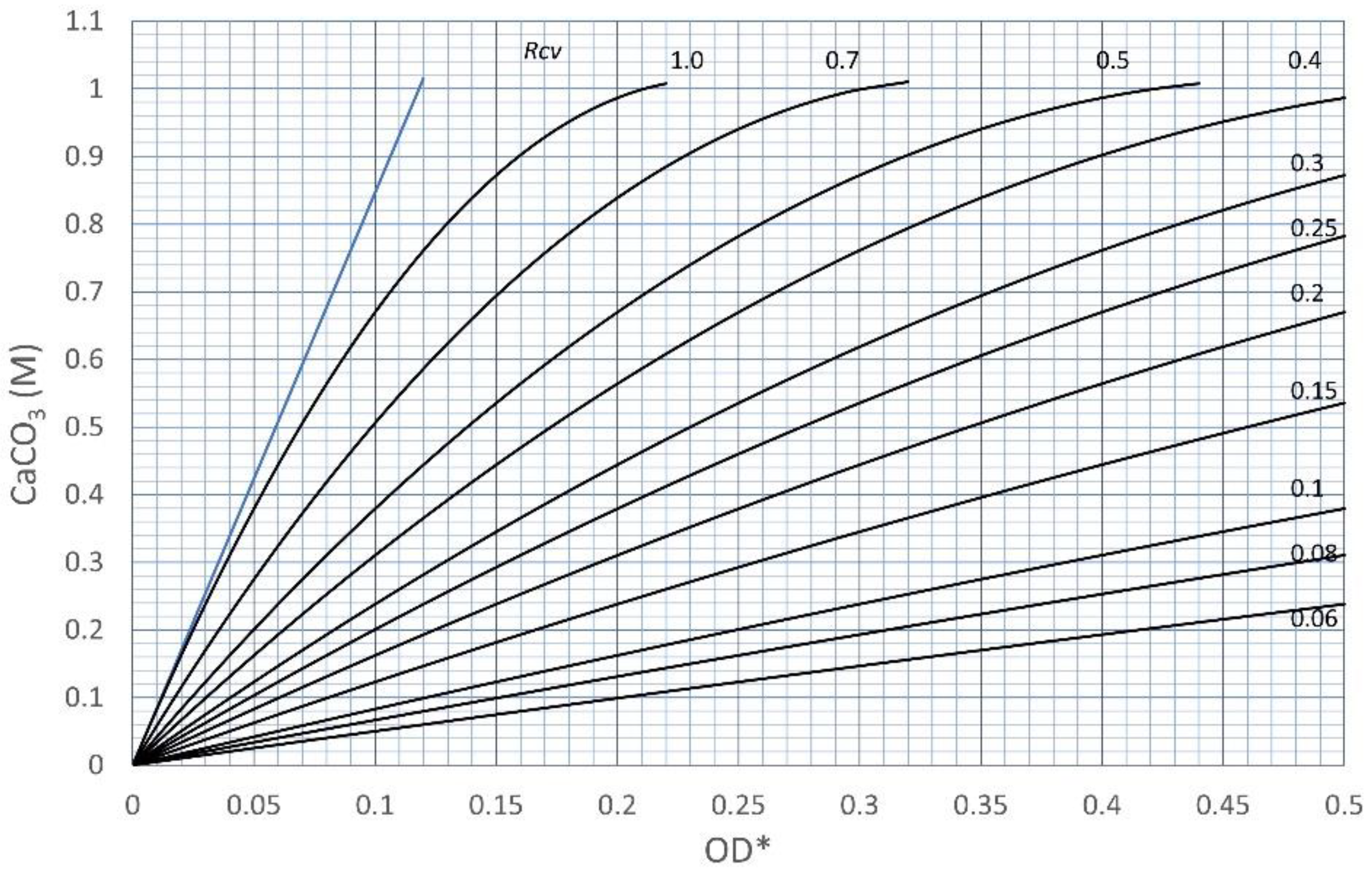
3.2. Effects of Increasing Ca2+/OD*
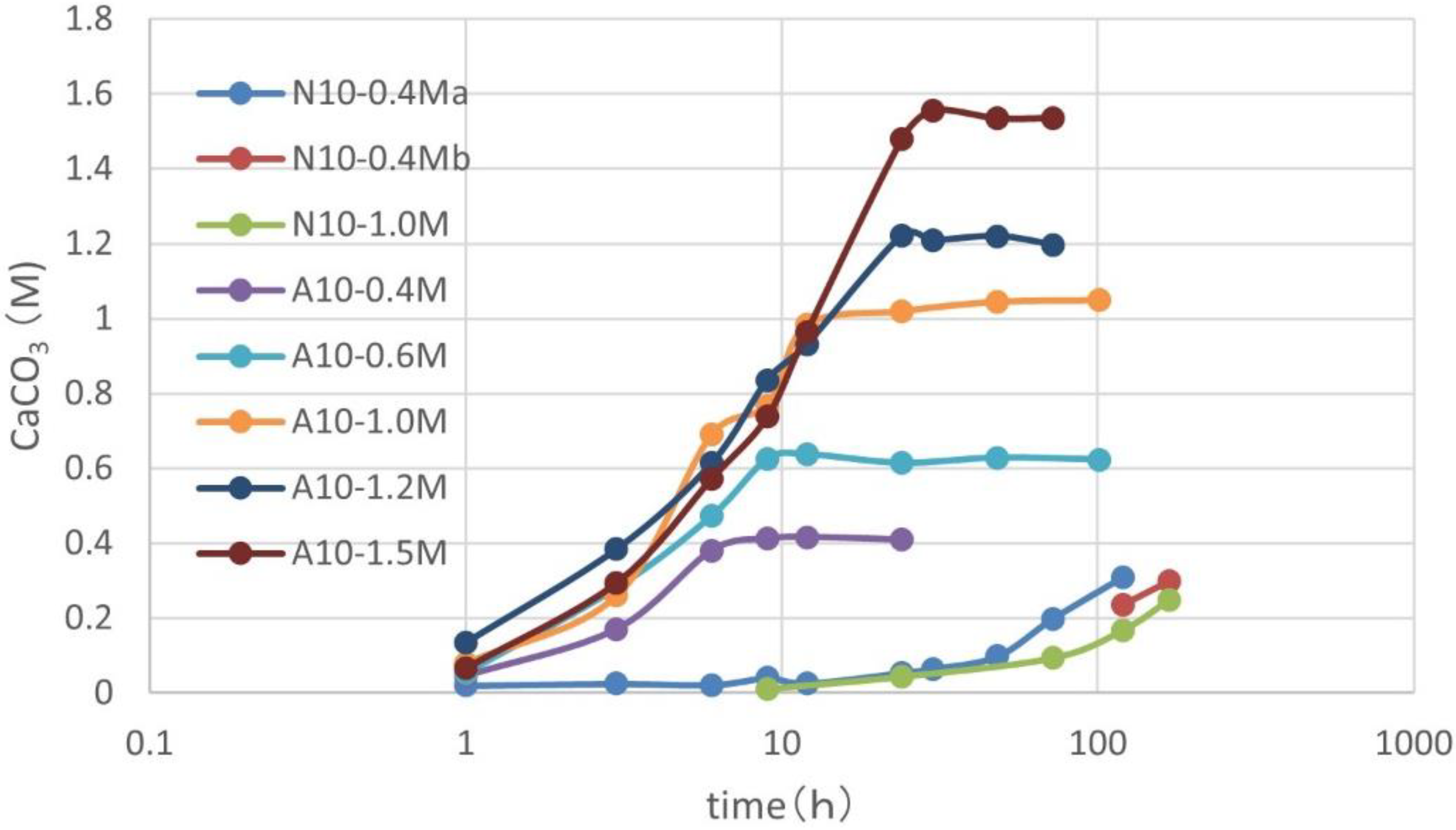
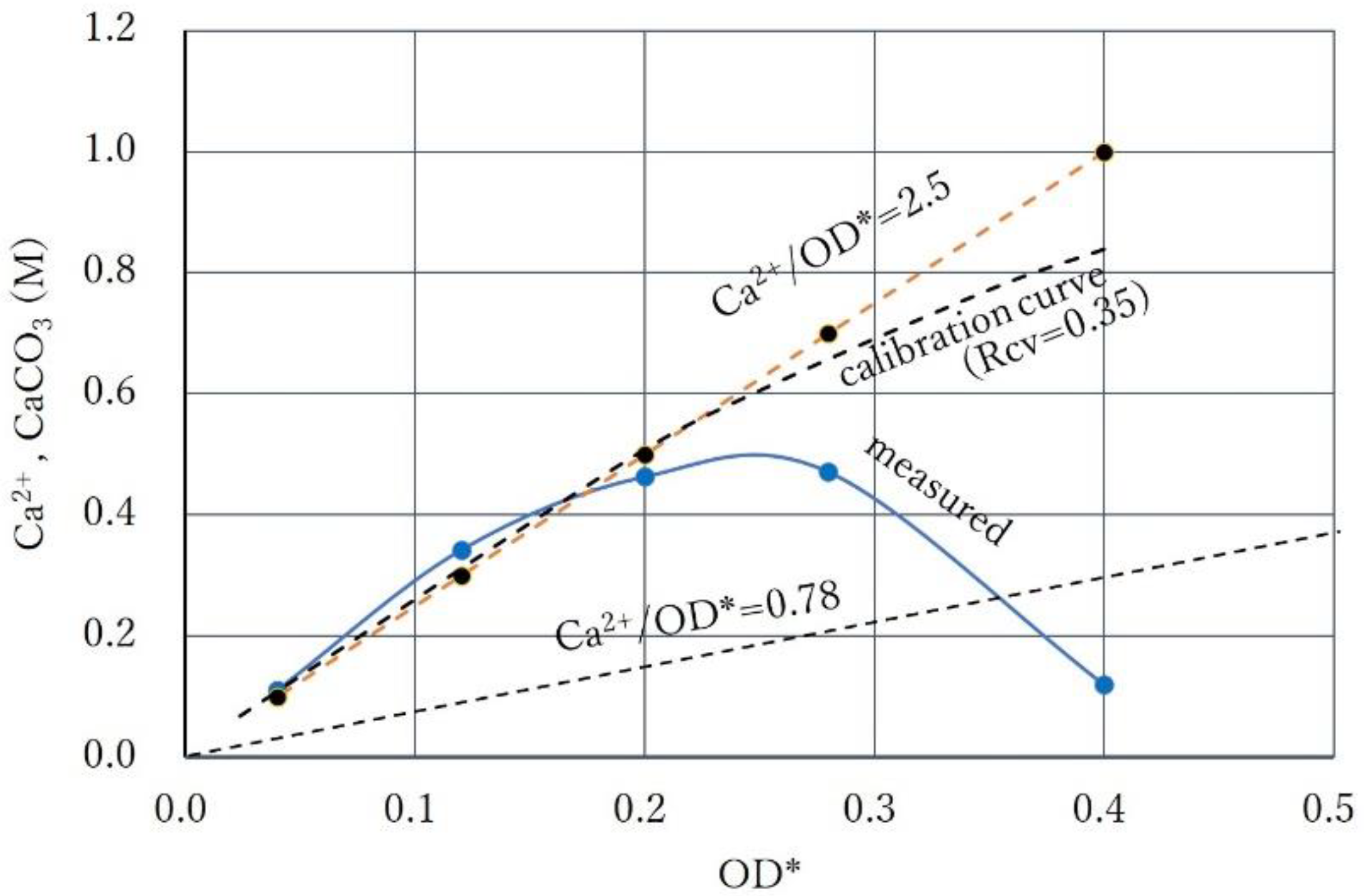
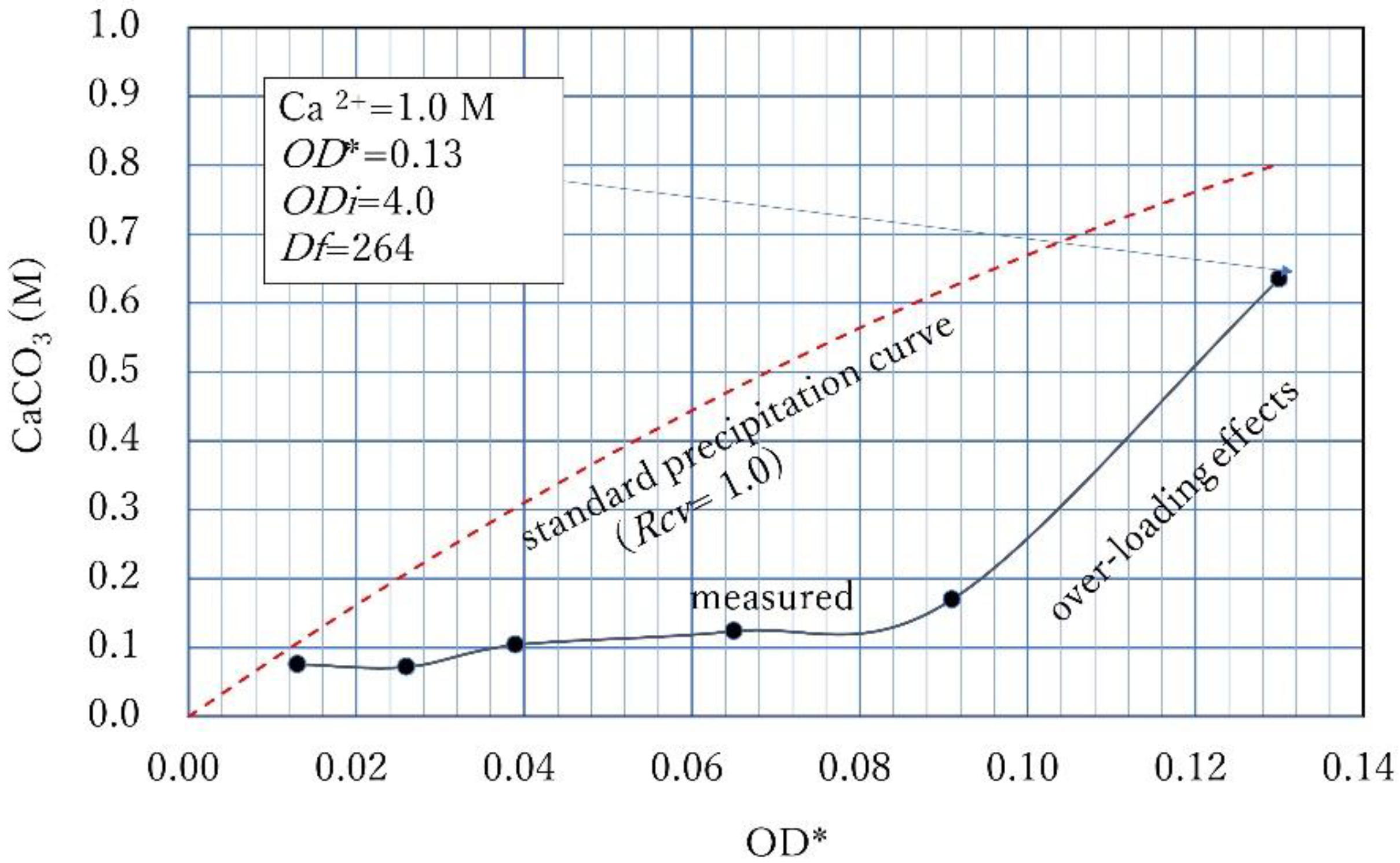
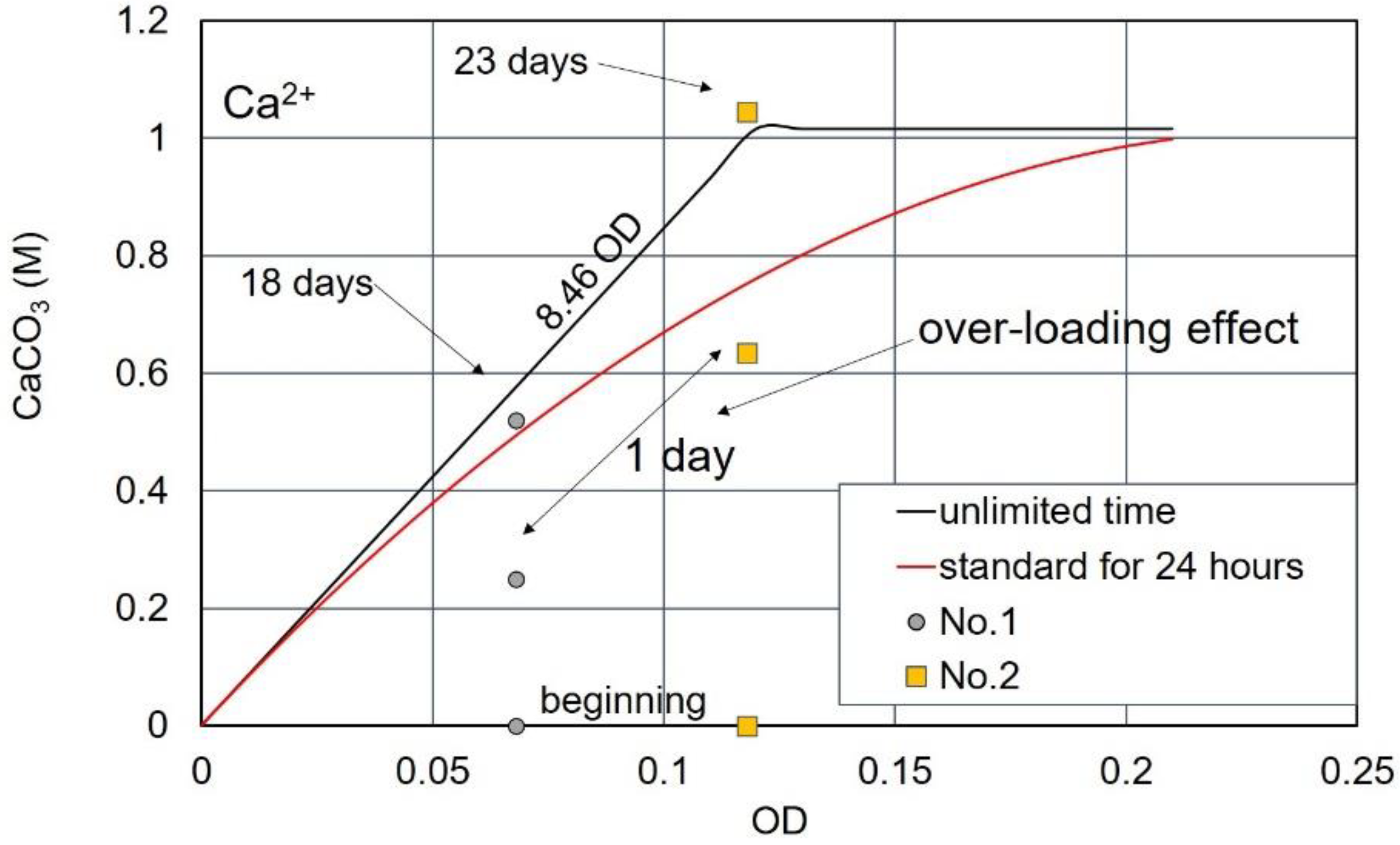
3.3. Over-Loading Effect
3.4. Over-loading Effects for Low OD Values
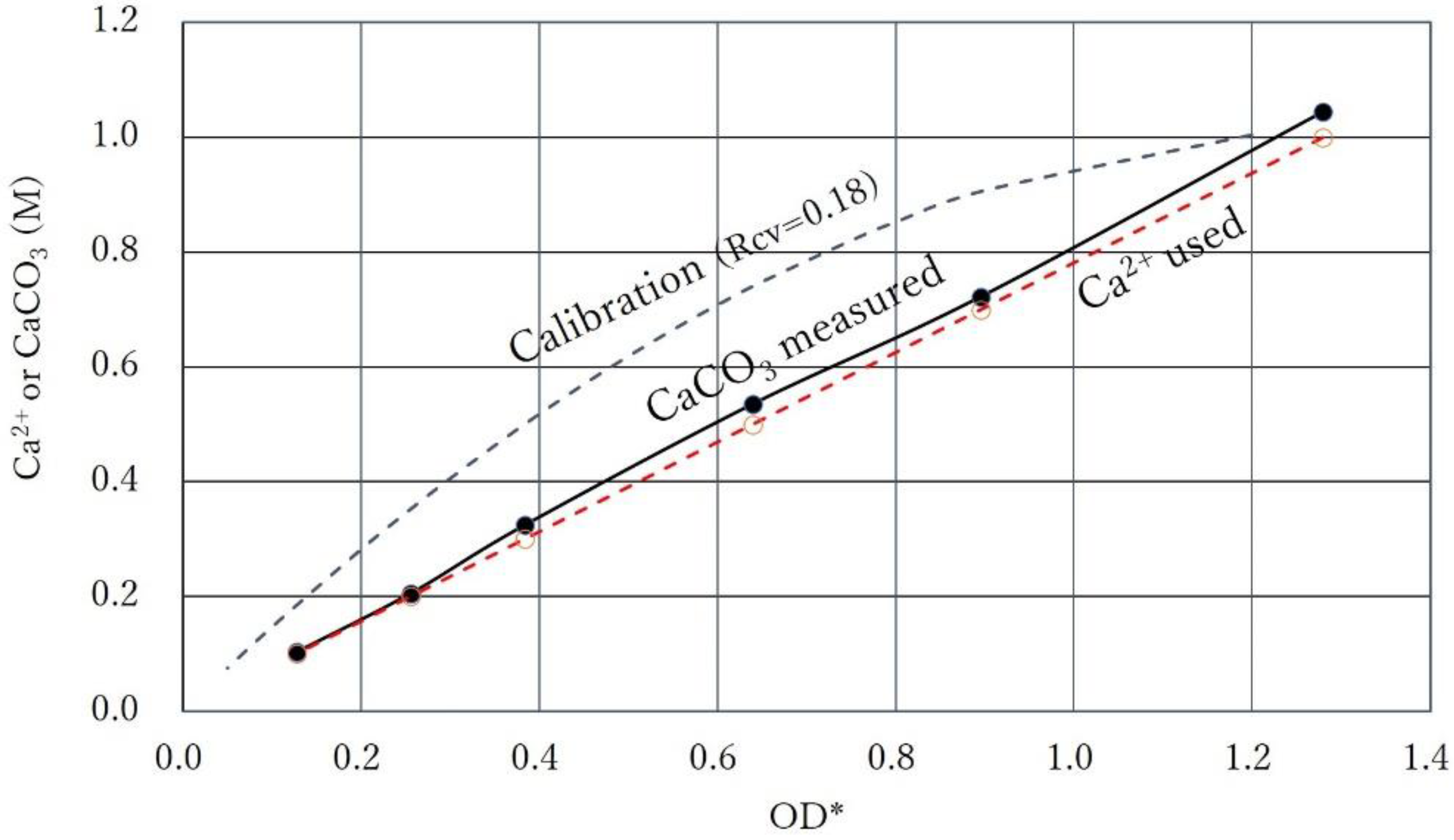
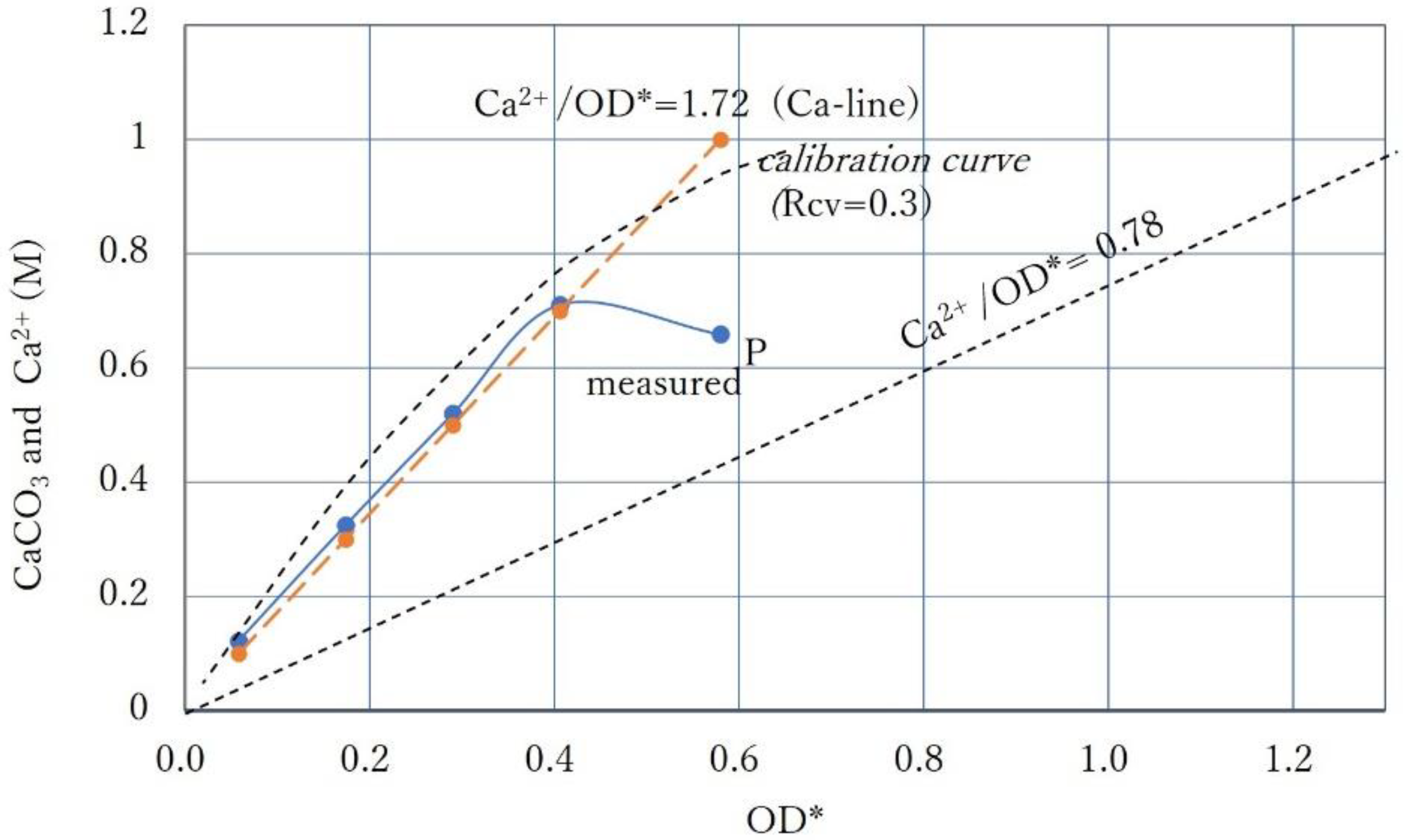
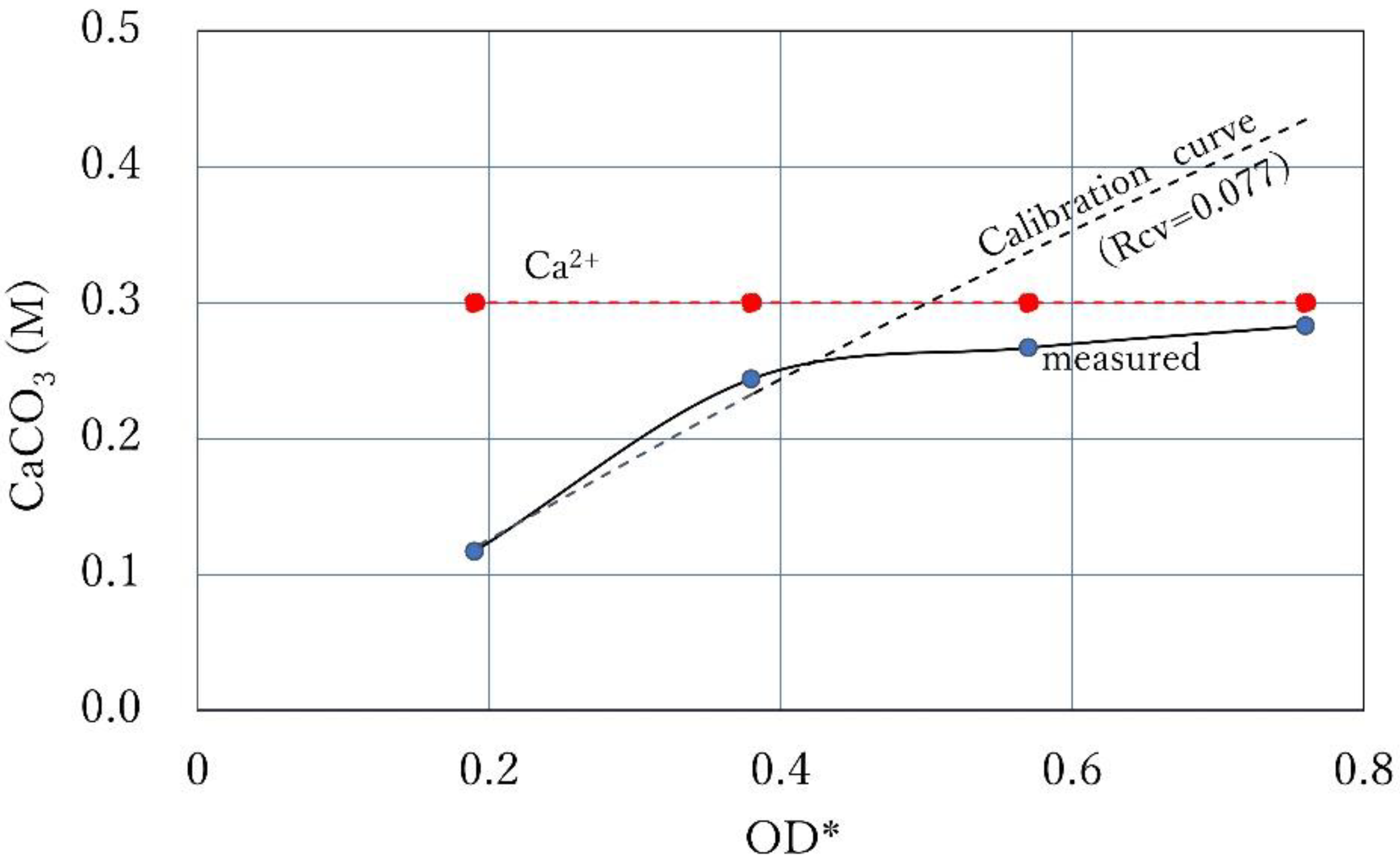
3.5. Intersection of Constant Ca2+ Line and Calibration Curve
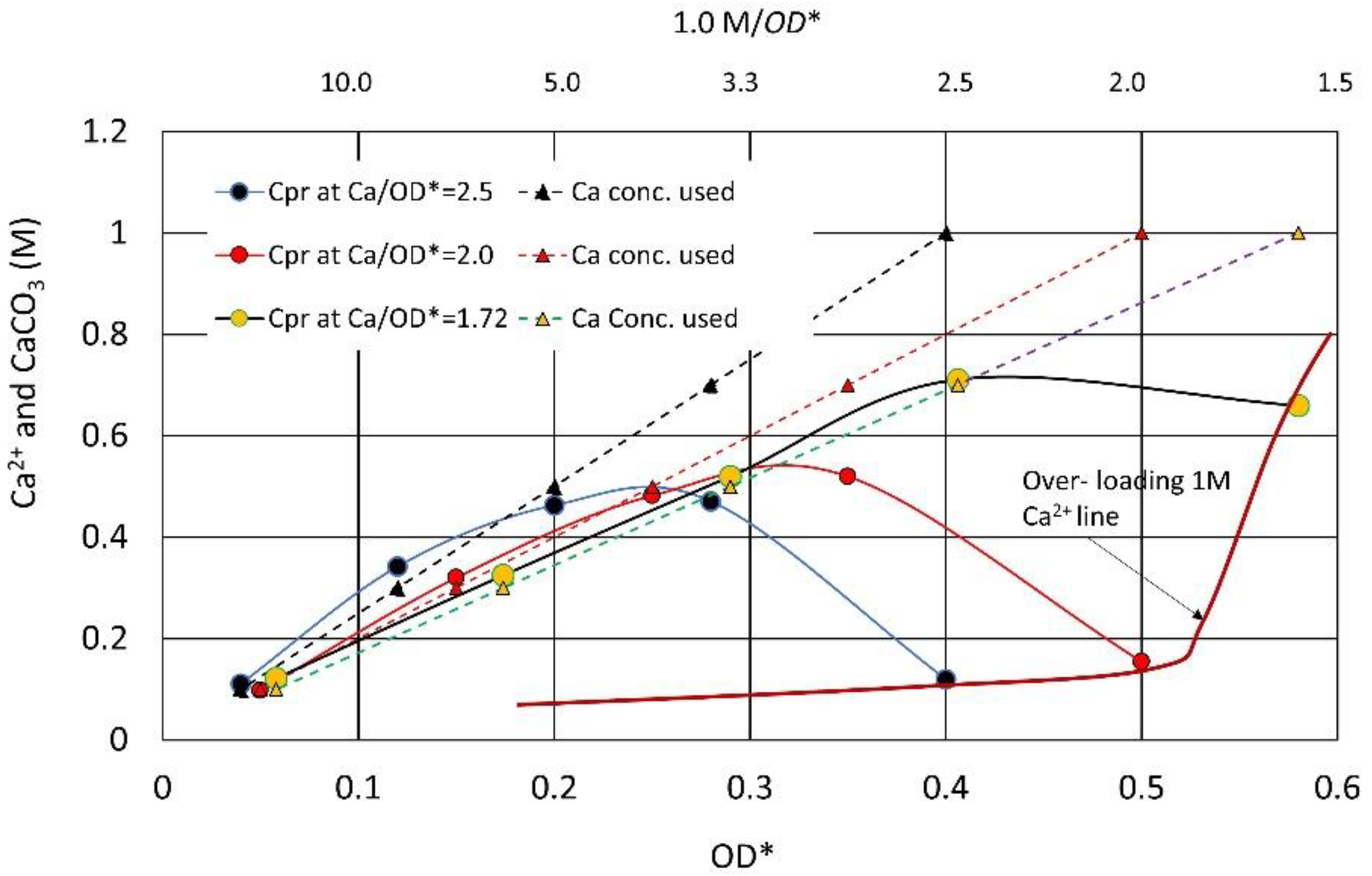
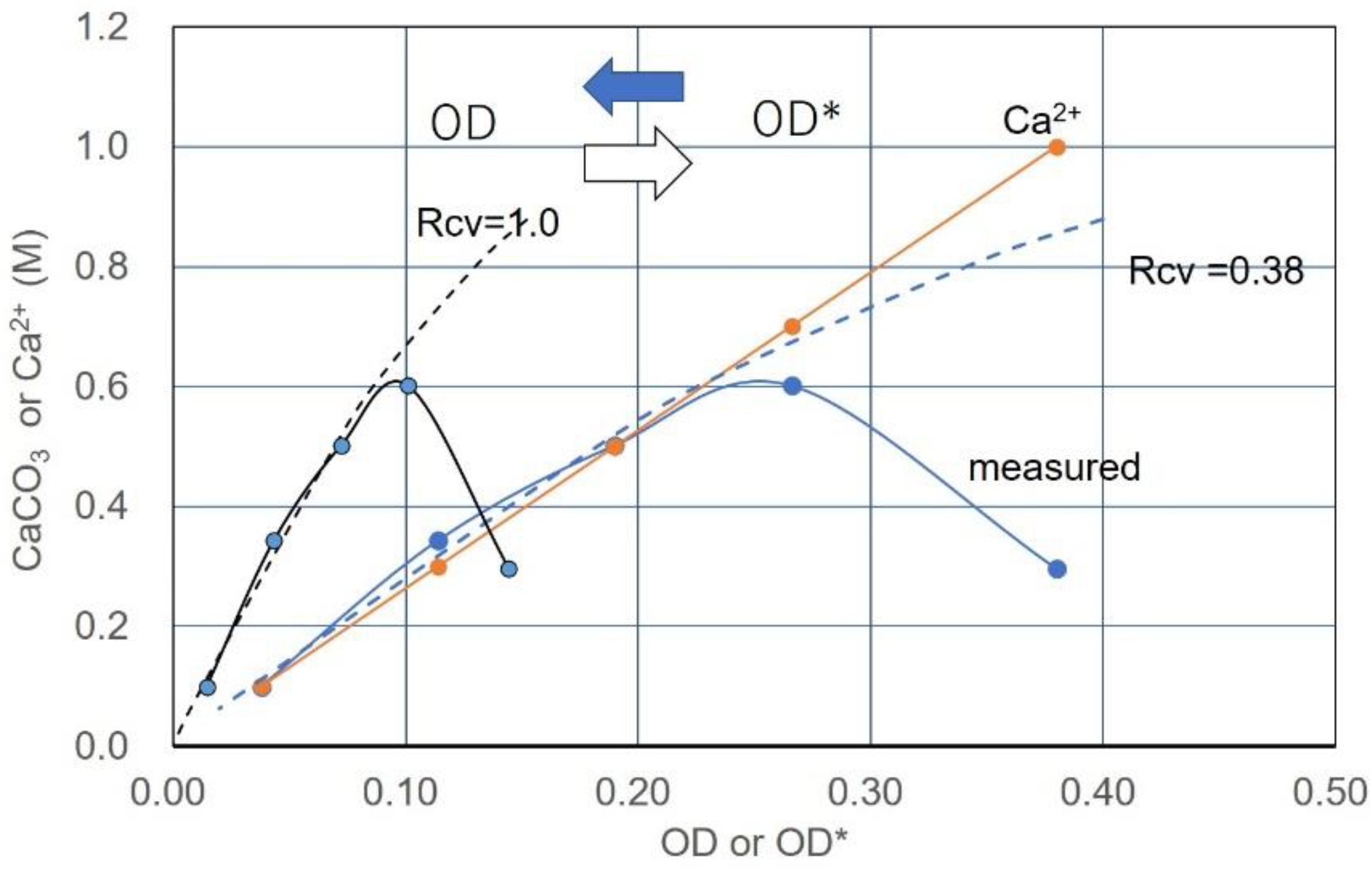
3.6. Example of Translation from CPR-OD* to CPR-OD Relations
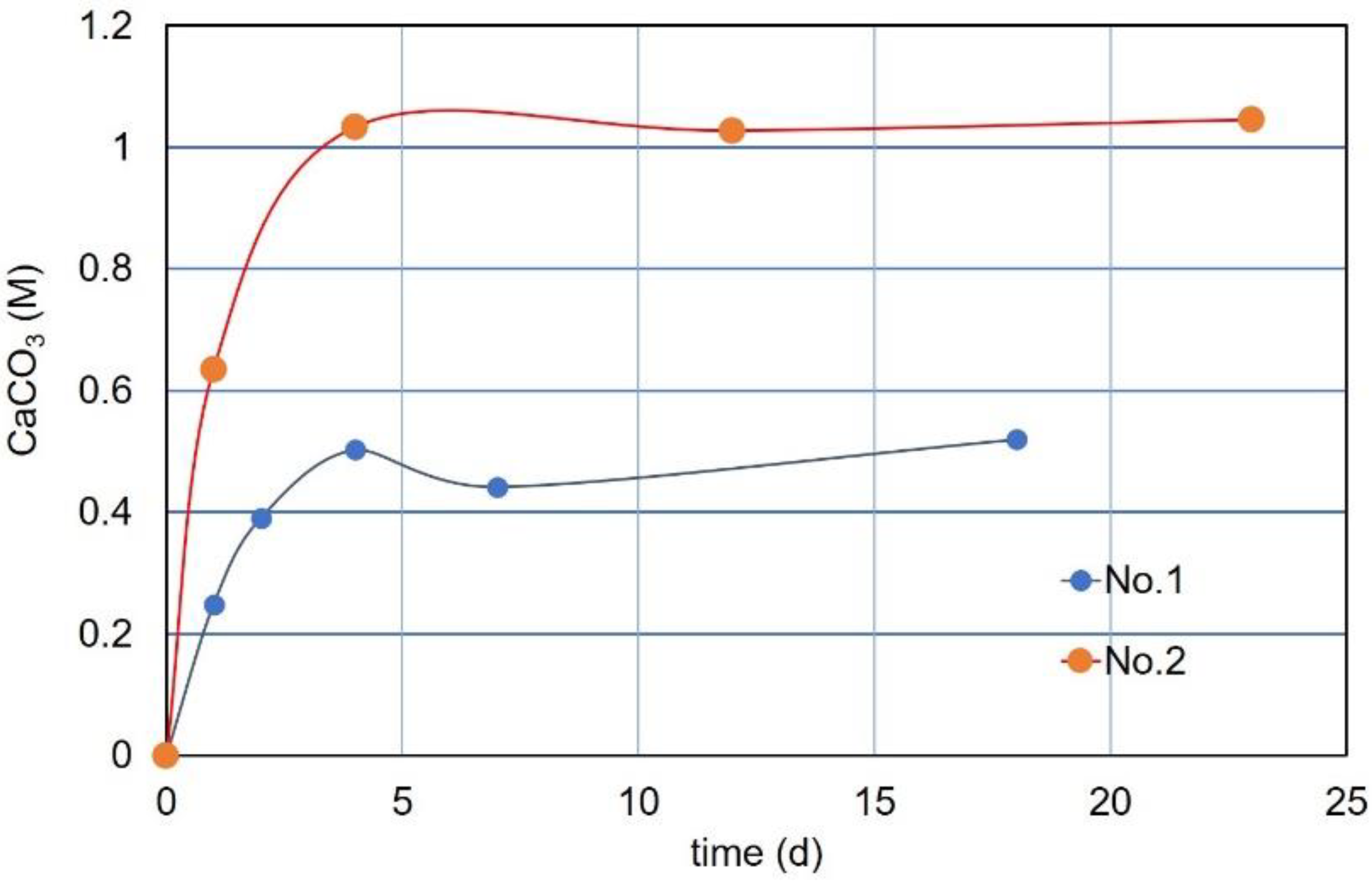
3.7. CPR after Over-Loading Effects
4. Conclusions
- (1)
- The microbial strain (NO-A10) is mainly examined in this study. Note that the quantitative results presented are dependent on microbes and strains;
- (2)
- The CPR desired can be blended in terms of OD and Ca2+;
- (3)
- CPR is governed by OD or Ca2+. If the OD is dominant, the CPR (24 h) is expressed as CPR = 8460 OD − 17.633 OD2;
- (4)
- If Ca2+ is dominant, CPR= a CaCO3 (M/24h) for the case of NO-A10 strains and a M Ca2+;
- (5)
- If Ca2+ is high and OD is relatively low, the over-loading effect, which causes exceptionally low CPR, occurs. However, the carbonate precipitation increases according to the following extension of the concept with time;
- (6)
- Extending the concept of the unique standard precipitation rate regarding time, the CPR-OD relationship was simply expressed by CPR = l OD, where l was 8.46 M for NO-A10 strains. Note that the CPR does not necessarily mean CaCO3 precipitated for 24 h.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedman, G.M. Rapidity of marine carbonate cementation-implications for carbonate diagenesis and sequence stratigraphy: Perspective. Sed. Geol. 1998, 119, 1–4. [Google Scholar] [CrossRef]
- Fukue, M.; Nakamura, T.; Kato, Y.; Naoe, K. Correlation among carbonate content, accumulation rate and topography of seabed. Soils Found. 1996, 36, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Fukue, M.; Nakamura, T.; Kato, Y. Cementation of soils due to calcium carbonate. Soils Found. 1999, 39, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Moore, C.H. Developments in Sedimentology. In Carbonate Reservoirs, Porosity Evolution and Diagenesis in a Sequence Stratigraphic Framework; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Castanier, S.; Métayer-Levrel, G.L.; Perthuisot, J.-P. Ca-carbonates precipitation and limestone genesis-the microbiogeologist point of view. Sed. Geol. 1999, 126, 9–23. [Google Scholar] [CrossRef]
- Neumeier, U. Experimental modelling of beachrock cementation under microbial influence. Sed. Geol. 1999, 126, 35–46. [Google Scholar] [CrossRef]
- Mobley, H.L.T.; Hausinger, R.P. Microbial urease: Significance, regulation, and molecular characterization. Microbiol. Rev. 1989, 53, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Fenn, L.B.; Miyamoto, S. Ammonia loss and associated reactions of urea in calcareous soils. Soil. Sci. Soc. Am. J. 1981, 45, 537–540. [Google Scholar] [CrossRef]
- Akiyama, M.; Kawasaki, S. Biogeochemical simulation of microbially induced calcite precipitation with Pararhodobacter sp. strain SO1. Acta Geotechnol. 2019, 14, 685–696. [Google Scholar] [CrossRef]
- Azadi, M.; Ghayoomi, M.; Sgamskia, N.; Kalantari, H. Physical and mechanical properties of reconstructed bio-cemented sand. Soils Found. 2017, 57, 698–706. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.; Chu, J. Soil bio-cementation using a new one-phase low-pH injection method. Acta Geotechnol. 2019, 14, 615–626. [Google Scholar] [CrossRef] [Green Version]
- Jiang, N.-J.; Soga, K. Erosional behavior of gravel-sand mixtures stabilized by microbially induced calcite precipitation (MICP). Soils Found. 2019, 59, 699–709. [Google Scholar] [CrossRef]
- Kahani, M.; Kalantary, F.; Soudi, M.R.; Pakdel, L.; Aghaalizadeh, S. Optimization of cost-effective culture medium for Sporosarcina pasteurii as biocementing agent using response surface methodology, Up cycling dairy waste and seawater. J. Clean. Prod. 2020, 253. [Google Scholar] [CrossRef]
- Lin, H.; Suleiman, M.; Jabbour, H.M.; Brown, D. Bio-grouting to enhance axial pull-out response of pervious concrete ground improvement piles. Can. Geotechnol. J. 2018, 55, 119–130. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Tong, T.; Wong, C. Study of the effect of temperature on microbially induced carbonate precipitation. Acta Geotechnol. 2019, 14, 627–638. [Google Scholar] [CrossRef]
- Kim, D.; Park, K.; Kim, D. Effects of Ground Conditions on Microbial Cementation in Soils. Materials 2014, 7, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gowthaman, S.; Nakashima, K.; Komatsu, S.; Kawasaki, S. Experimental Study on Sand Stabilization Using Bio-Cementation with Wastepaper Fiber Integration. Materials 2021, 14, 5164. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, C.; Biscontin, G.; Logothetis, F. Tensile Strength of Artificially Cemented Sandstone Generated via Microbially Induced Carbonate Precipitation. Materials 2021, 14, 4735. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Shahin, M.A.; Mujah, D. Influence of key environmental conditions on microbially induced cementation for soil stabilization. J. Geotechnol. Geoenviron. Eng. 2016. [Google Scholar] [CrossRef] [Green Version]
- Van Paassen, L.A. Biogrout, Ground Improvement by Microbial Induced Carbonate Precipitation. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2009. [Google Scholar]
- DeJong, J.T.; Fritzges, M.B.; Nüsslein, K. Microbially induced cementation to control sand response to undrained shear. J. Geotechnol. Geoenviron. Eng. 2006, 132, 1381–1392. [Google Scholar] [CrossRef]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-mediated soil improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Fukue, M.; Lechowicz, Z. Strength of biocemented sandy soils using simple devices. In Proceedings of the International Congress Natural Sciences and Engineering, Kuala Lumpur, Malaysia, 6–8 July 2018; pp. 17–28, ISBN 978-986-87417-8-2. [Google Scholar]
- Choi, S.-G.; Chang, I.; Lee, M.; Lee, J.-H.; Han, J.-T.; Kwon, T.-H. Review on geotechnical engineering properties of sands treated by microbially induced calcium carbonate precipitation (MICP) and biopolymers. Constr. Build. Mater. 2020, 246, 118415. [Google Scholar] [CrossRef]
- Benini, S.; Gessa, C.; Ciurli, S. Bacillus Pasteurii urease: A Heteropolymeric enzyme with a binuclear nickel active site. Soil Biol. Biochem. 1996, 28, 819–821. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Khoshdelnezamiha, G.; Senian, N.; Ong, D.E.L.; Nissom, P.M. Experimental optimization of various cultural conditions on urease activity for isolated Sporosarcina pasteurii strains and evaluation of their biocement potentials. Ecol. Eng. 2017, 109, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Omoregie, A.I.; Ginjom, R.H.; Nissom, P.M. Microbially induced carbonate precipitation via ureolysis process: A Mini- Review. Trans. Sci. Technol. 2018, 5, 245–256. [Google Scholar]
- Fukue, M.; Ono, S.; Sato, Y. Cementation of sands due to Microbiologically induced Carbonate. Soils Found. 2011, 51, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.; Farnum, J.L.; Oke, M.A. Protective action of Glycerol in the freezing of Shigella sonnel. Nature 1962, 4926, 405. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Sand, K.K.; Benning, L.G. ACC and vaterite as intermediates in the solution-based crystallization of CaCO3. In New Perspectives on Mineral Nucleation and Growth: Chapter 5; Van Driessche, A., Kellermeier, M., Benning, L., Gebauer, D., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 93–111. [Google Scholar]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Okwadha, G.D.O.; Li, J. Technical Note: Optimum conditions for microbial carbonate precipitation. Chemosphere 2010, 81, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Francois, K.; Devlieghere, F.; Standaert, A.R.; Geeraerd, A.H.; Cools, I.; Van Impe, J.F.; Debevere, J. Environmental factors influencing the relationship between optical density and cell count for Listeria monocytogenes. J. Appl. Microbiol. 2005, 99, 1503–1515. [Google Scholar] [CrossRef] [PubMed]
- Beal, J.; Farny, N.G.; Haddock-Angelli, T.; Selvarajah, V.; Baldwin, G.S.; Buckley-Taylor, R.; Gershater, M.; Kiga, D.; Marken, J.; Sanchania, V.; et al. the iGEM Interlab Study Contributors, Robust estimation of bacterial cell count from optical density. bioRxiv 2019, preprint. [Google Scholar] [CrossRef] [Green Version]
- Garcia, S.; Limón, J.C.; Heredia, N.L. Cross protection by heat and cold shock to lethal temperatures in clostridium perfringens. Brazil. J. Microbiol. 2001, 32, 110–112. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Navarro, C.; Jimenez-Lopez, C.; Rodriguez-Navarro, A.; Gonzalez-Muñoz, M.T.; Rodriguez-Gallego, M. Bacterially mediated mineralization of vaterite. Geochim. Cosmochim. Acta 2007, 71, 1197–1213. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, Q.; Zhoui, G.; Fu, S. Transformation of amorphous calcium carbonate into monohydrocalcite in aqueous solution: A biomimetic mineralization study. Eur. J. Mineral 2015, 27, 717–729. [Google Scholar] [CrossRef]
- Wen, K.; Yang, L.; Farshad, A.; Lin, L. Impact of bacteria and urease concentration on precipitation kinetics and crystal morphology of calcium carbonate. Acta Geotechnol. 2020, 15, 17–27. [Google Scholar] [CrossRef]
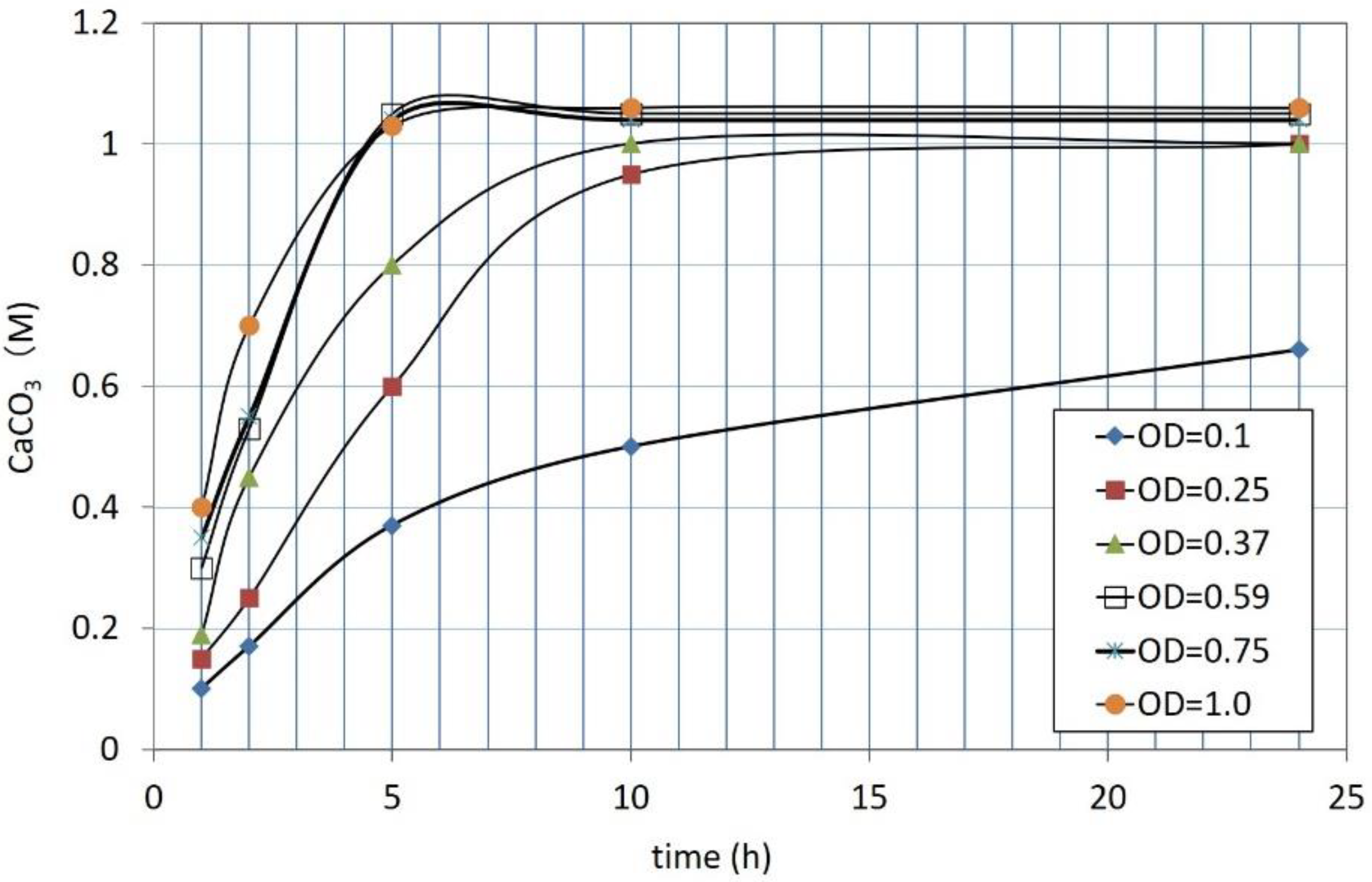
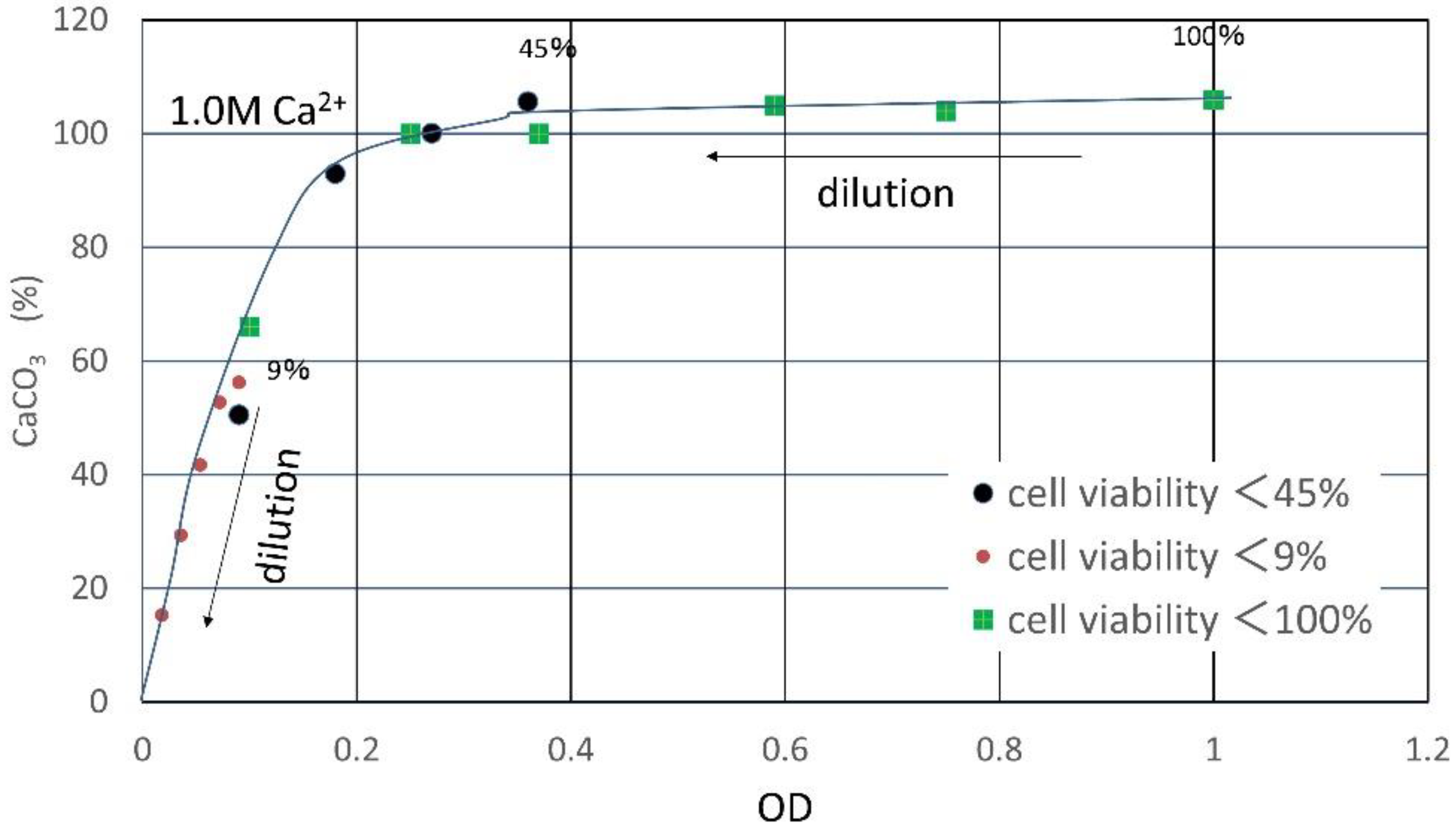
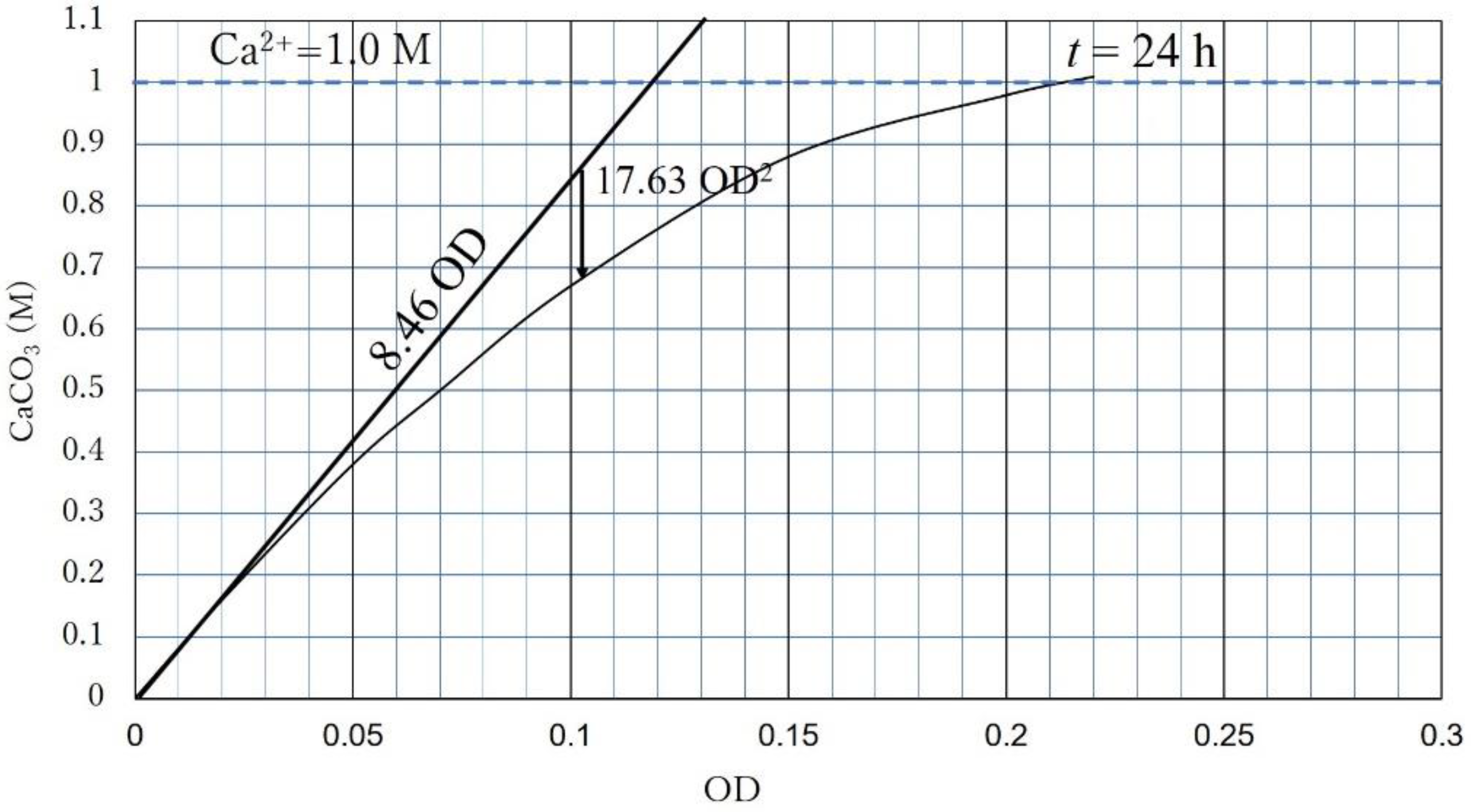
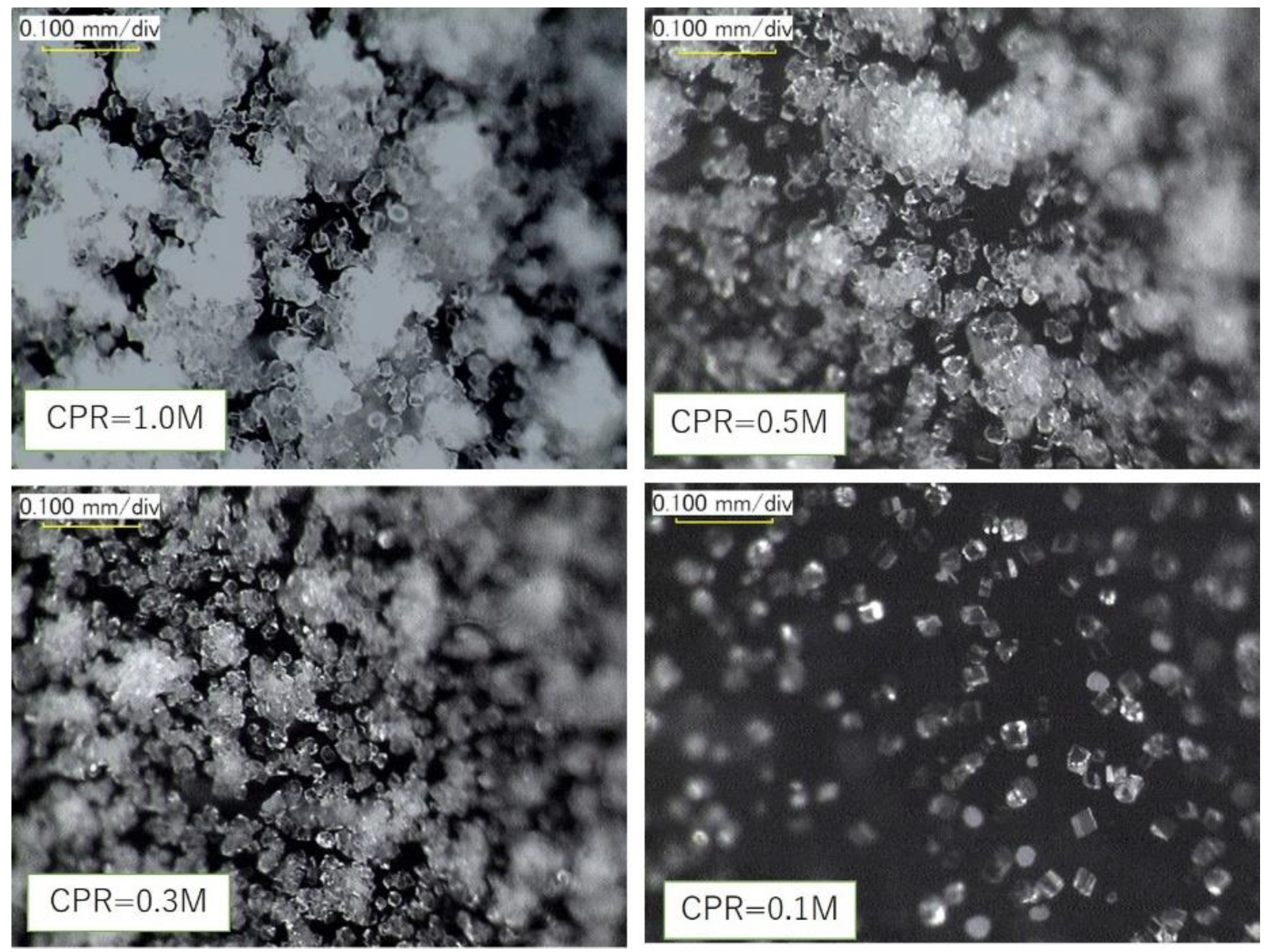
| Flask No. | Temp. (°C) | Age | Spec. No. | Cell Viability (%) | OD | CPR (M) |
|---|---|---|---|---|---|---|
| 1 | 25 | 1 day | 1 | 100 | 1.0 | 1.06 |
| 2 | 0.75 | 1.04 | ||||
| 3 | 0.59 | 1.05 | ||||
| 4 | 0.37 | 1.00 | ||||
| 5 | 0.25 | 1.00 | ||||
| 6 | 0.10 | 0.66 | ||||
| 2 | 4 | 1 month | 7 | 45 | 0.45 | — |
| 8 | 0.36 | 1.06 | ||||
| 9 | 0.27 | 1.00 | ||||
| 10 | 0.18 | 0.93 | ||||
| 11 | 0.09 | 0.51 | ||||
| 3 | 25 | 1 month | 12 | 9 | 0.09 | 0.56 |
| 13 | 0.072 | 0.53 | ||||
| 14 | 0.054 | 0.42 | ||||
| 15 | 0.036 | 0.29 | ||||
| 16 | 0.018 | 0.15 |
| No. | OD* | Rcv | OD | Ca2+ | Ca2+/OD* | Ca2+/OD |
|---|---|---|---|---|---|---|
| 1 | 0.180 | 0.38 | 0.068 | 1.0 | 5.56 | 14.62 |
| 2 | 0.310 | 0.38 | 0.118 | 1.0 | 3.23 | 8.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukue, M.; Lechowicz, Z.; Fujimori, Y.; Emori, K.; Mulligan, C.N. Incorporation of Optical Density into the Blending Design for a Biocement Solution. Materials 2022, 15, 1951. https://doi.org/10.3390/ma15051951
Fukue M, Lechowicz Z, Fujimori Y, Emori K, Mulligan CN. Incorporation of Optical Density into the Blending Design for a Biocement Solution. Materials. 2022; 15(5):1951. https://doi.org/10.3390/ma15051951
Chicago/Turabian StyleFukue, Masaharu, Zbigniew Lechowicz, Yuichi Fujimori, Kentaro Emori, and Catherine N. Mulligan. 2022. "Incorporation of Optical Density into the Blending Design for a Biocement Solution" Materials 15, no. 5: 1951. https://doi.org/10.3390/ma15051951
APA StyleFukue, M., Lechowicz, Z., Fujimori, Y., Emori, K., & Mulligan, C. N. (2022). Incorporation of Optical Density into the Blending Design for a Biocement Solution. Materials, 15(5), 1951. https://doi.org/10.3390/ma15051951







