Functionally Graded Al2O3–CTZ Ceramics Fabricated by Spark Plasma Sintering
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of Raw Nanoparticles
2.2. Preparation and Fabrication of 4-Layered Al2O3–CTZ FGMs
2.3. Analysis Methods
3. Results and Discussion
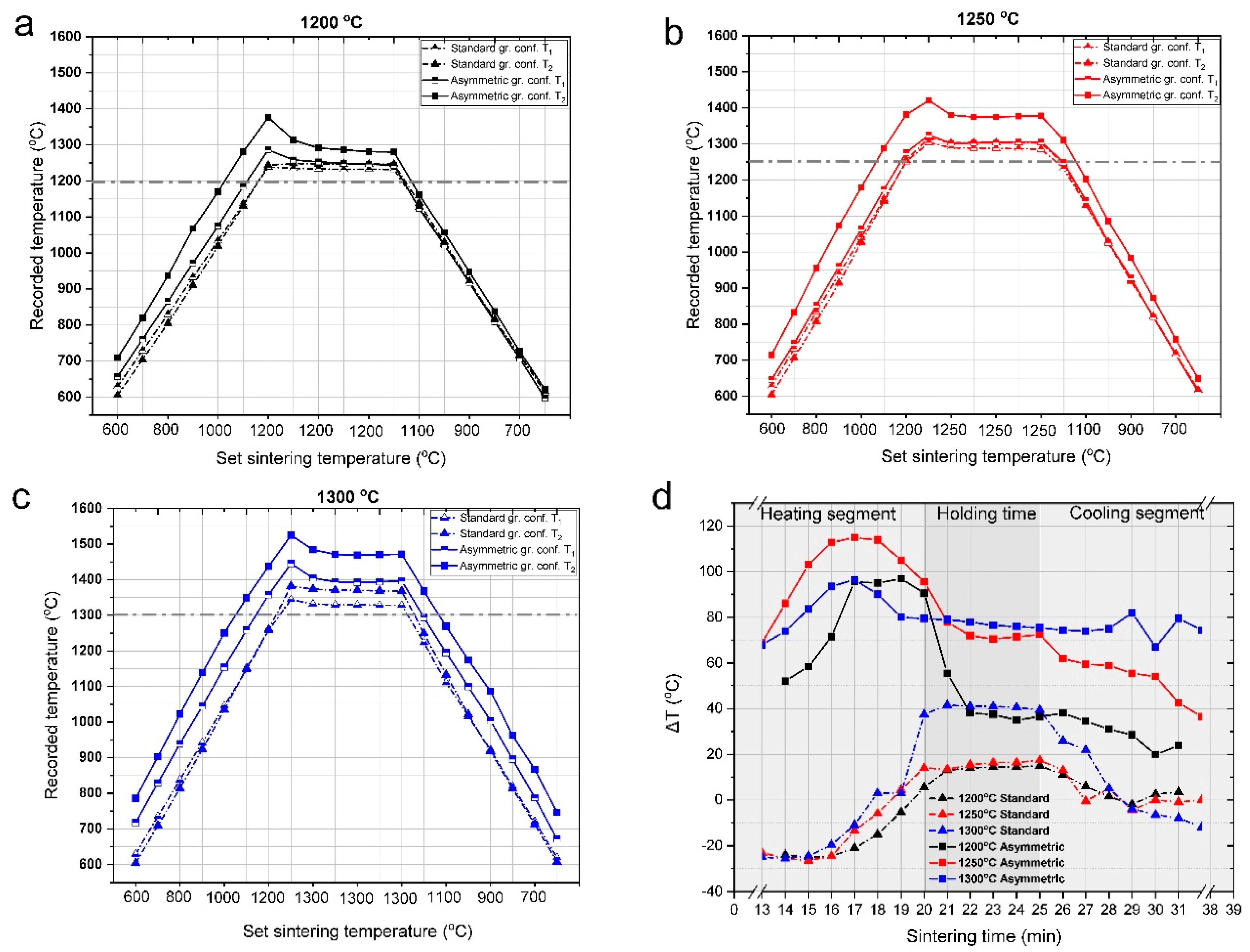
3.1. Temperature Gradient during the SPS Process
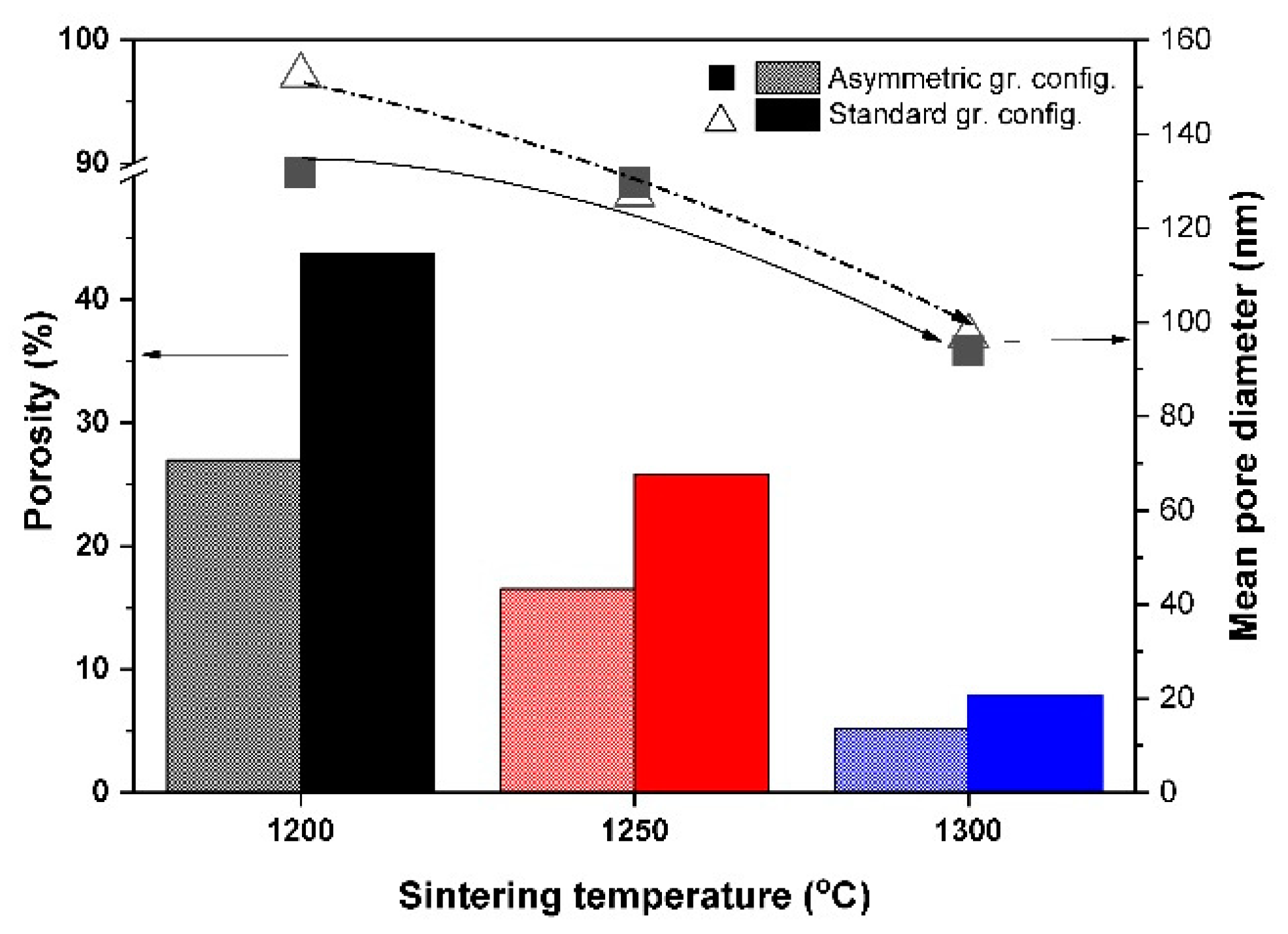
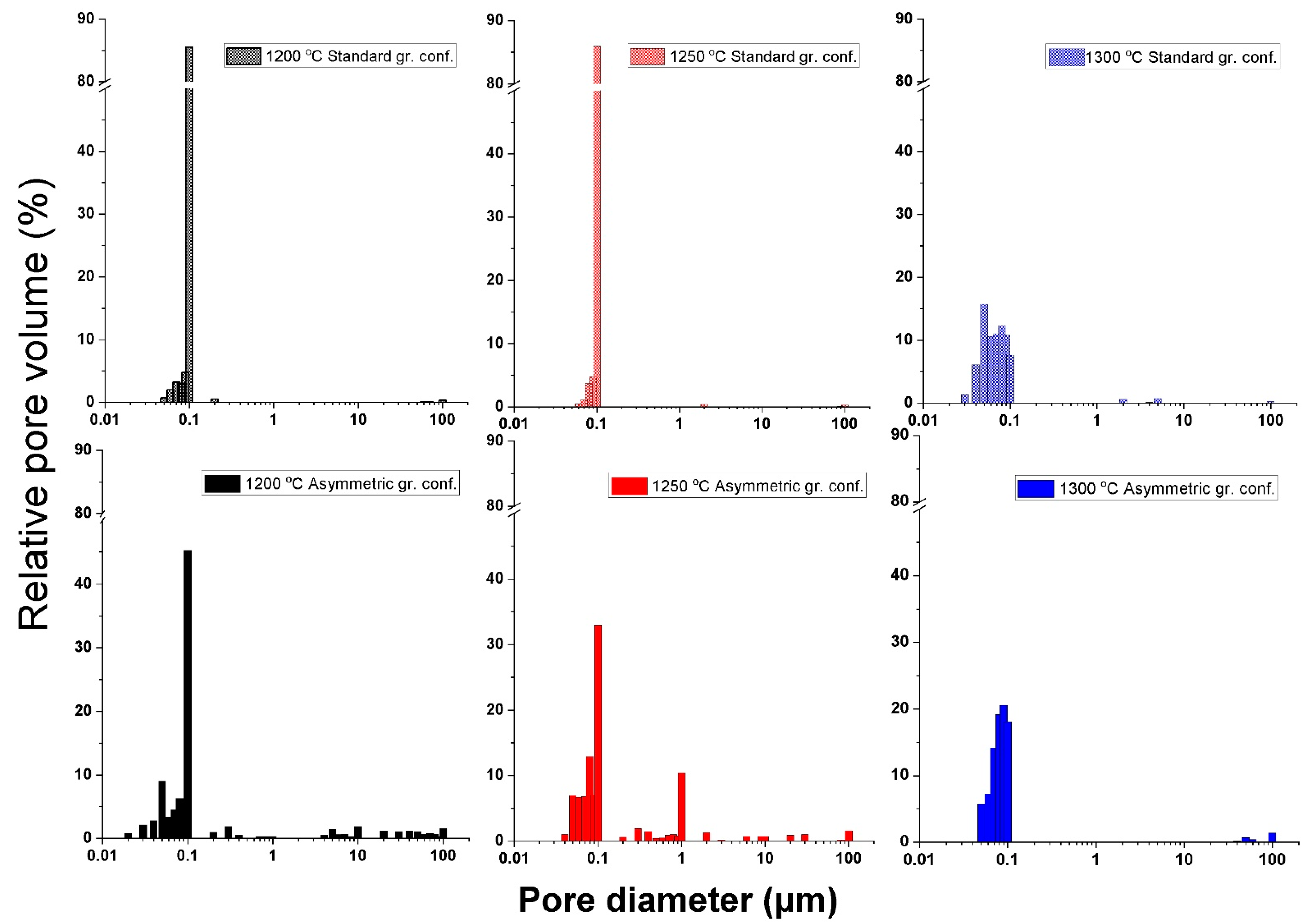
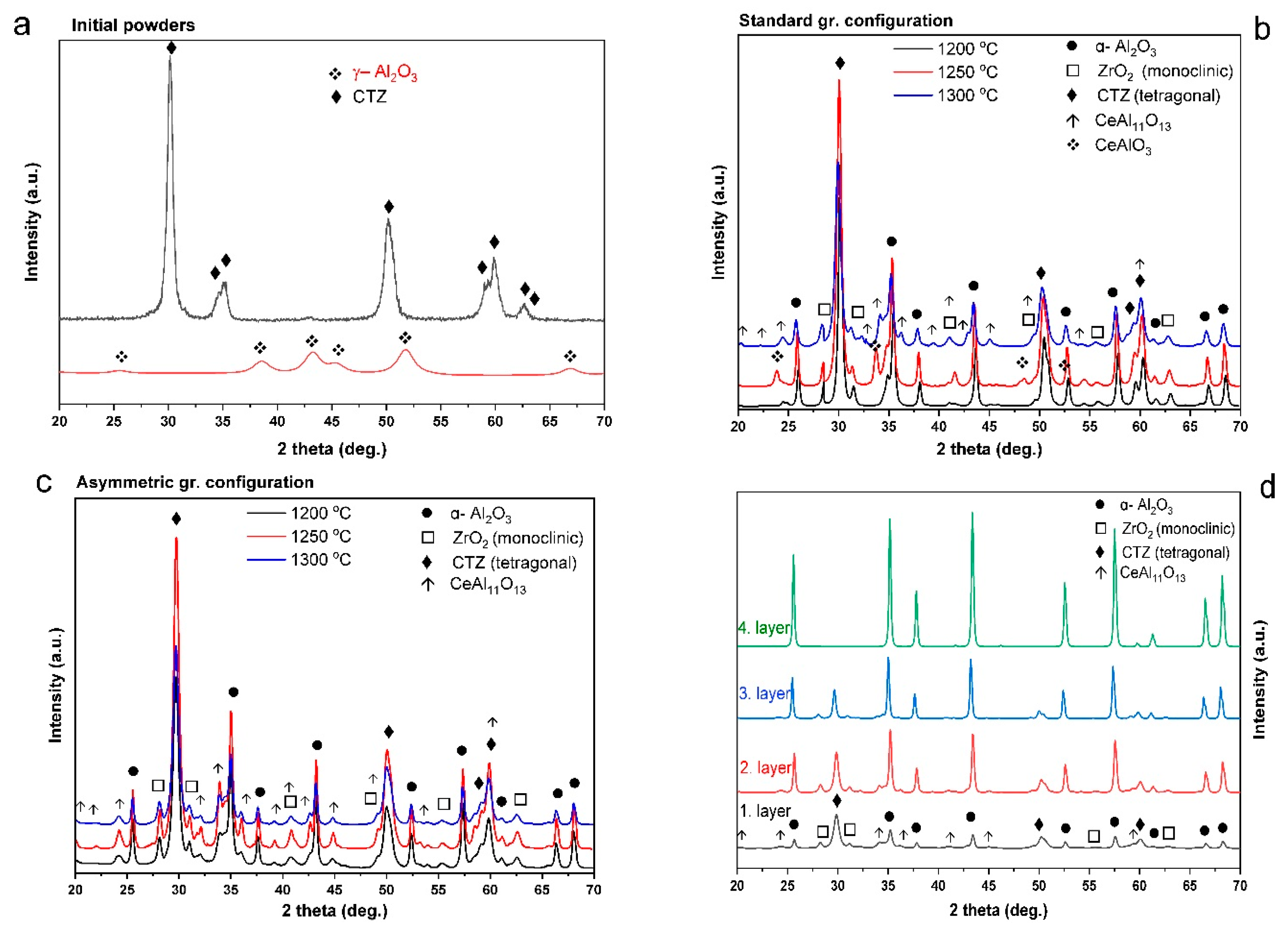
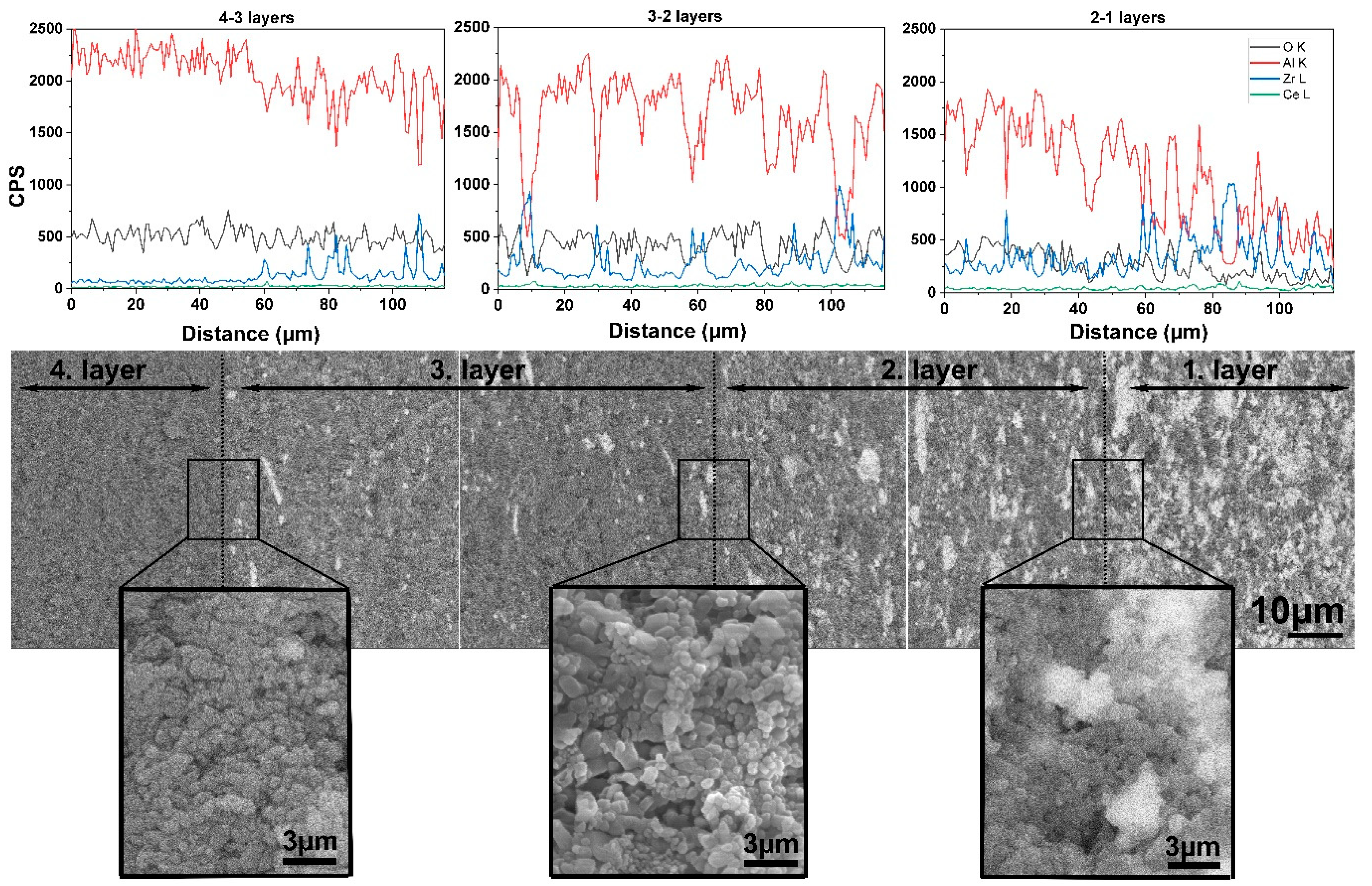
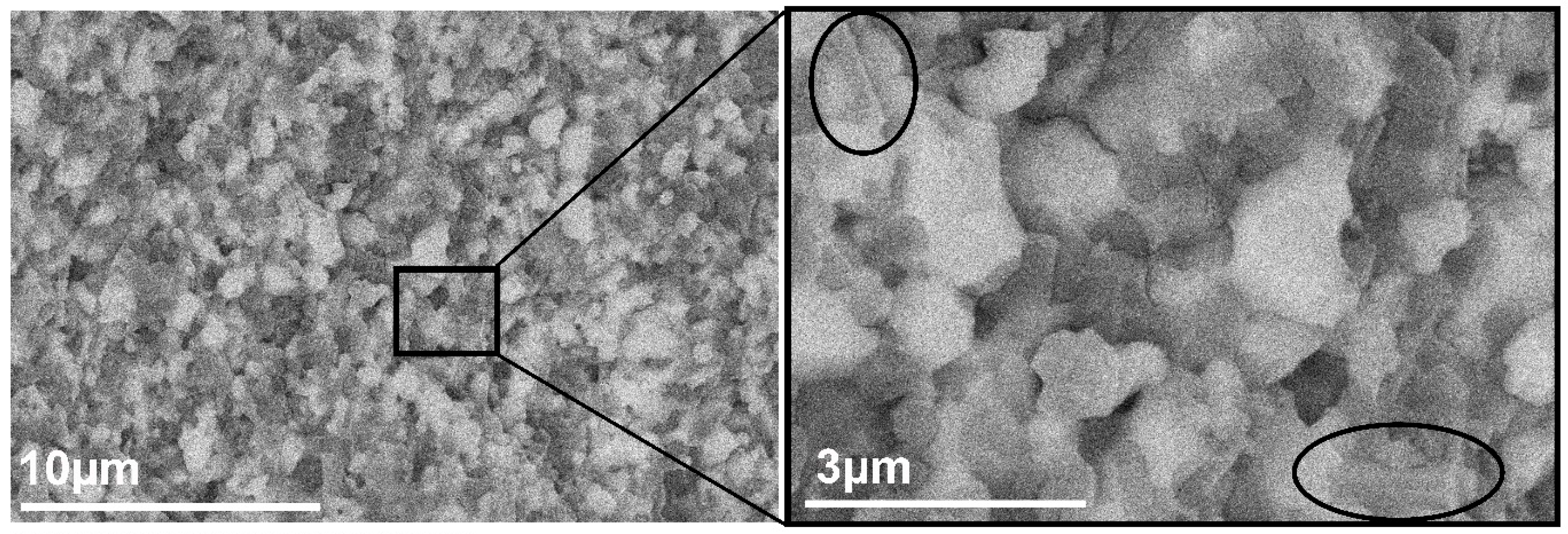
3.2. Microstructure Analysis of the Al2O3–CTZ FGMs
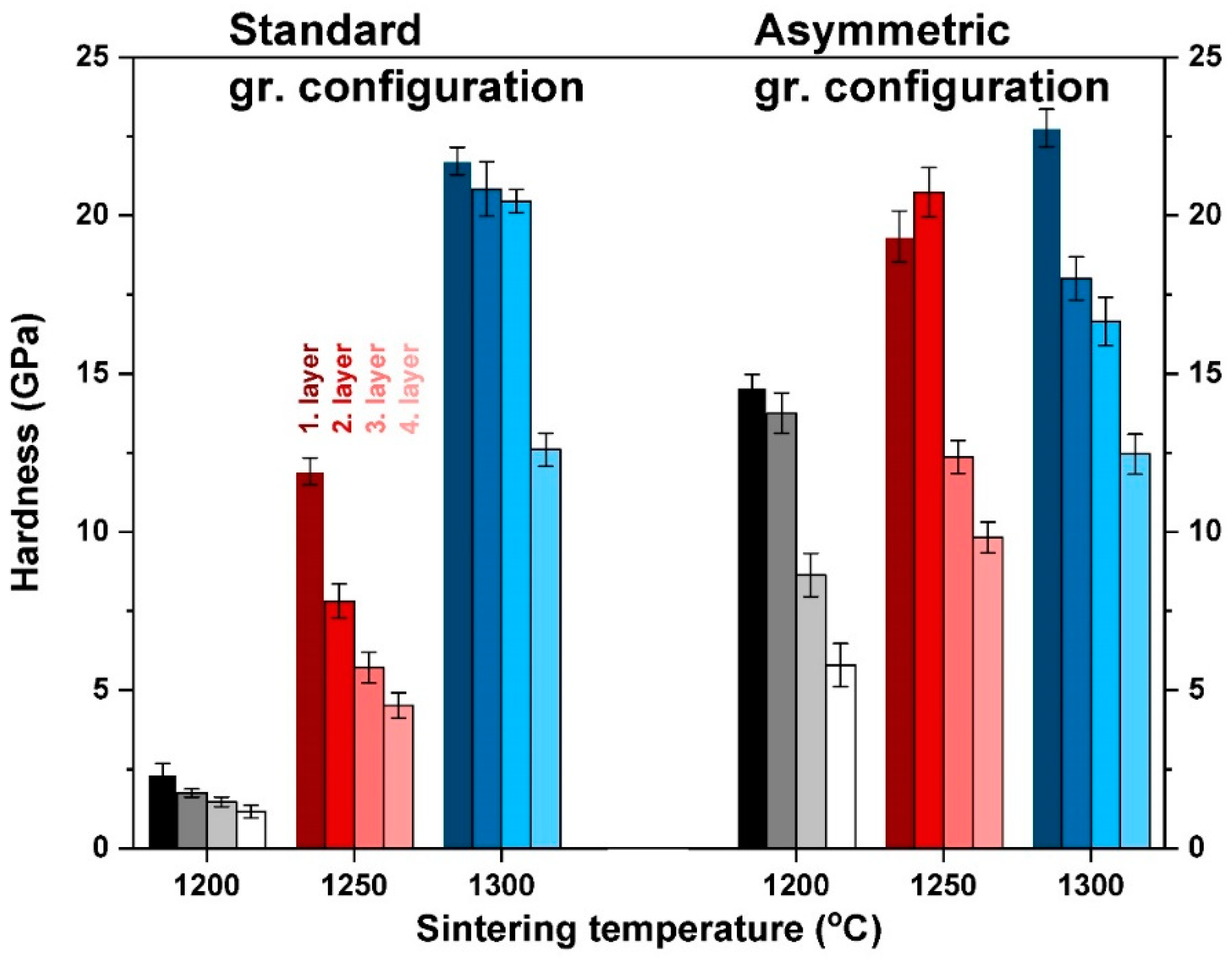
3.3. Mechanical Properties of Al2O3–CTZ FGMs
4. Conclusions
- In the asymmetric graphite configuration, there is a significant temperature difference between the top and bottom part of the samples. The difference increases with increasing sintering temperatures and reaches as much as 120 °C at a sintering temperature of 1300 °C. At the same time, the temperature at the top side was also invariably higher than the set temperature.
- Mercury porosimetry and SEM analysis showed the formation of a gradient pore structure for the samples heat treated in an asymmetric configuration. Total porosity decreased with higher sintering temperatures, regardless of the type of graphite configuration. However, it was still lower in the asymmetric graphite configuration. At the same time, the relative pore volume was distributed in a wide range, and pores of up to 100 µm were detected in a significant ratio, as well.
- SEM analysis also showed that porosity and pore size distribution is anisotropic—densification increased toward the bottom part of the samples compared with the reference material. Microstructural analysis at higher magnification confirmed a smooth transition of the material in terms of composition and porosity without any sign of delamination or cracks at the imaginary interfaces of the layers. XRD and SEM analysis revealed that the newly formed CeAl11O18 phase in structure, which has an elongated appearance, presumably affected the mechanical properties of the FGM.
- Microhardness and flexural test results also indicate the changes in the composition and porosity of the Al2O3–CTZ material. Hardness increased gradually along the axis, particularly for the asymmetric configuration samples. A difference of nearly 10 GP in hardness was achieved between the top and bottom part of the sample heat treated at 1300 °C. For these samples, hardness distribution was more homogenous within the sample, which prevents the formation of internal stresses inside the materials, therefore providing better load capacity.
- The flexural strength of the samples for both series shows an increasing tendency with higher sintering temperatures. However, the samples sintered in asymmetric configuration achieved significantly higher strength due to the lower total porosity of the sample on one hand, and the newly formed elongated CeAl11O18 particles on the other. The FGM materials produced possess better mechanical properties than natural bones.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahamood, R.M.; Akinlabi, E.T.; Shukla, M.; Pityana, S. Functionally graded material: An overview. Lect. Notes Eng. Comput. Sci. 2012, 3, 1593–1597. [Google Scholar]
- Bakar, W.Z.W.; Basri, S.; Jamaludin, S.N.S.; Sajjad, A. Functionally graded materials: An overview of dental applications. World J. Dent. 2018, 9, 137–144. [Google Scholar] [CrossRef]
- Van der Biest, O.; Neubrand, A. Functionally Graded Ceramics. Encycl. Mater. Tech. Ceram. Glas. 2021, 1, 374–398. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Kaysser, W.A.; Rabin, B.H.; Kawasaki, A.; Ford, R.G. Functionally Graded Materials: Design, Processing and Application; Kluwer Academic Publishers: Boston, MA, USA, 1999; ISBN 978-1-4200-9256-1. [Google Scholar]
- Madfa, A.A.; Yue, X.G. Dental prostheses mimic the natural enamel behavior under functional loading: A review article. Jpn. Dent. Sci. Rev. 2016, 52, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Dobrádi, A.; Enisz-Bódogh, M.; Kovács, K.; Balczár, I. Structure and properties of bio-glass–ceramics containing natural bones. Ceram. Int. 2015, 41, 4874–4881. [Google Scholar] [CrossRef]
- Magyari, K.; Pap, Z.; Tóth, Z.R.; Kása, Z.; Licarete, E.; Vodnar, D.C.; Hernadi, K.; Baia, L.; Magyari, K.; Baia, L. The impact of copper oxide nanoparticles on the structure and applicability of bioactive glasses. J. Sol-Gel Sci. Technol. 2019, 91, 634–643. [Google Scholar] [CrossRef]
- Sarkar, D.; Mandal, S.; Reddy, B.S.; Bhaskar, N.; Sundaresh, D.C.; Basu, B. ZrO2-toughened Al2O3-based near-net shaped femoral head: Unique fabrication approach, 3D microstructure, burst strength and muscle cell response. Mater. Sci. Eng. C 2017, 77, 1216–1227. [Google Scholar] [CrossRef]
- Shekhawat, D.; Singh, A.; Banerjee, M.K.; Singh, T.; Patnaik, A. Bioceramic composites for orthopaedic applications: A comprehensive review of mechanical, biological, and microstructural properties. Ceram. Int. 2021, 47, 3013–3030. [Google Scholar] [CrossRef]
- Bódis, E.; Molnár, K.; Mucsi, A.; Károly, Z.; Móczó, J.; Klébert, S.; Keszler, A.M.; Fazekas, P.; Szépvölgyi, J. Silicon nitride-based composites reinforced with zirconia nanofibres. Ceram. Int. 2017, 43, 16811–16818. [Google Scholar] [CrossRef]
- Chevalier, J.; Loh, J.; Gremillard, L.; Meille, S.; Adolfson, E. Low-temperature degradation in zirconia with a porous surface. Acta Biomater. 2011, 7, 2986–2993. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L.; Deville, S. Low-temperature degradation of zirconia and implications for biomedical implants. Annu. Rev. Mater. Res. 2007, 37, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, C.; Coutinho, I.F.; Amarante, J.E.V.; Alves, M.F.R.P.; Coutinho, M.M.; Moreira da Silva, C.R. Mechanical properties of ceramic composites based on ZrO2 co-stabilized by Y2O3–CeO2 reinforced with Al2O3 platelets for dental implants. J. Mech. Behav. Biomed. Mater. 2021, 116, 104372. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Tamura, J.; Kawanabe, K.; Nawa, M.; Oka, M.; Uchida, M.; Kokubo, T.; Nakamura, T. Ce-TZP/Al2O3 nanocomposite as a bearing material in total joint replacement. J. Biomed. Mater. Res. 2002, 63, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Meng, F.; Lin, D.T.; Yu, J.J.; Wang, H.J.; Xu, S.; Guo, W.M.; Cerecedo, C.; Valcárcel, V.; Lin, H.T. Influence of whisker-aspect-ratio on densification, microstructure and mechanical properties of Al2O3 whiskers-reinforced CeO2-stabilized ZrO2 composites. J. Eur. Ceram. Soc. 2018, 38, 1796–1801. [Google Scholar] [CrossRef]
- Palmero, P.; Fornabaio, M.; Montanaro, L.; Reveron, H.; Esnouf, C.; Chevalier, J. Towards long lasting zirconia-based composites for dental implants: Part I: Innovative synthesis, microstructural characterization and invitro stability. Biomaterials 2015, 50, 38–46. [Google Scholar] [CrossRef]
- Heness, G.; Ben-Nissan, B. (3) (PDF) Innovative Bioceramics. Mater. Forum 2007, 27, 104–114. [Google Scholar]
- Begand, S.; Oberbach, T.; Glien, W. Tribological Behaviour of an Alumina Toughened Zirconia Ceramic for an Application in Joint Prostheses. Key Eng. Mater. 2006, 309–311, 1261–1264. [Google Scholar] [CrossRef]
- Mott, M.; Evans, J.R.G. Zirconia/alumina functionally graded material made by ceramic ink jet printing. Mater. Sci. Eng. A 1999, 271, 344–352. [Google Scholar] [CrossRef]
- Salman, A.; Al-Ghaban, N.; Eesa, M.; Atiyah, A.; Farid, S. Osseointegration of Cylindrical Zirconia–Alumina Functionally Graded materials, Dental implant by Electrophoretic Deposition. Ziggurat J. Mater. Technol. 2020, 1, 2–12. [Google Scholar] [CrossRef]
- Salahi, E.; Esfahani, H.; Mobasherpour, I.; Bijarchi, M.A.; Taheri, M. Sintering behavior and mechanical properties of alumina/zirconia multilayers composite via nano-powder processing. Ceram. Int. 2014, 40, 2717–2722. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chen, Y.L. Design and impact resistant analysis of functionally graded Al2O3-ZrO2 ceramic composite. Mater. Des. 2016, 91, 294–305. [Google Scholar] [CrossRef]
- Cai, P.Z.; Green, D.J.; Messing, G.L. Constrained Densification of Alumina/Zirconia Hybrid Laminates, I: Experimental Observations of Processing Defects Various forms of damage were observed in pressure-less-sintered Al2O3/ZrO2 symmetric laminates and asym-metric laminates (bilayers) fab. J. Am. Ceram. Soc. 1997, 8, 1929–1939. [Google Scholar] [CrossRef]
- Sun, L.; Sneller, A.; Kwon, P. Fabrication of alumina/zirconia functionally graded material: From optimization of processing parameters to phenomenological constitutive models. Mater. Sci. Eng. A 2008, 488, 31–38. [Google Scholar] [CrossRef]
- Morales-Rodríguez, A.; Domínguez-Rodríguez, A.; De Portu, G.; Jiménez-Melendo, M. Creep mechanisms of laminated alumina/zirconia-toughened alumina composites. J. Eur. Ceram. Soc. 2009, 29, 1625–1630. [Google Scholar] [CrossRef]
- Marshall, D.B.; Ratto, J.J.; Lange, F.F. Enhanced Fracture Toughness in Layered Microcomposites of Ce-ZrO2 and Al2O3. J. Am. Ceram. Soc. 1991, 74, 2979–2987. [Google Scholar] [CrossRef]
- Anné, G.; Hecht-Mijic, S.; Richter, H.; Van der Biest, O.; Vleugels, J. Strength and residual stresses of functionally graded Al2O3/ZrO2 discs prepared by electrophoretic deposition. Scr. Mater. 2006, 54, 2053–2056. [Google Scholar] [CrossRef]
- Yu, C.; Zhuang, J.; Dong, L.; Cheng, K.; Weng, W. Effect of hierarchical pore structure on ALP expression of MC3T3-E1 cells on bioglass films. Colloids Surf. B Biointerfaces 2017, 156, 213–220. [Google Scholar] [CrossRef]
- Salimi, E. Functionally graded calcium phosphate bioceramics: An overview of preparation and properties. Ceram. Int. 2020, 46, 19664–19668. [Google Scholar] [CrossRef]
- Živcová-Vlčkova, Z.; Locs, J.; Keuper, M.; Sedlářová, I.; Chmelíčková, M. Microstructural comparison of porous oxide ceramics from the system Al2O3-ZrO2 prepared with starch as a pore-forming agent. J. Eur. Ceram. Soc. 2012, 32, 2163–2172. [Google Scholar] [CrossRef]
- Xu, C.; Liu, H.; Yang, H.; Yang, L. A Green Biocompatible Fabrication of Highly Porous Functional Ceramics with High Strength and Controllable Pore Structures. J. Mater. Sci. Technol. 2016, 32, 729–732. [Google Scholar] [CrossRef]
- Chen, R.; Jia, W.; Shan, Q.; Ling, Y.; Lao, D.; Wang, Y.; Li, S.; Hei, D. A novel design of Al2O3-ZrO2 reticulated porous ceramics with hierarchical pore structures and excellent properties. J. Eur. Ceram. Soc. 2019, 39, 1877–1886. [Google Scholar] [CrossRef]
- Kamyshnaya, K.S.; Khabas, T.A. Fabrication of Al2O3–ZrO2 ceramics with high porosity and strength. Ceram. Int. 2021, 47, 1666–1671. [Google Scholar] [CrossRef]
- Dudina, D.V.; Bokhonov, B.B.; Olevsky, E.A. Fabrication of porous materials by spark plasma sintering: A review. Materials 2019, 12, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anselmi-Tamburini, U.; Gennari, S.; Garay, J.E.; Munir, Z.A. Fundamental investigations on the spark plasma sintering/synthesis process: II. Modeling of current and temperature distributions. Mater. Sci. Eng. A 2005, 394, 139–148. [Google Scholar] [CrossRef]
- Vanmeensel, K.; Laptev, A.; Hennicke, J.; Vleugels, J.; Vanderbiest, O. Modelling of the temperature distribution during field assisted sintering. Acta Mater. 2005, 53, 4379–4388. [Google Scholar] [CrossRef]
- Morin, C.; Le Gallet, S.; Ariane, M.; Bernard, F. Spark Plasma Sintering tool design for preparing alumina-based Functionally Graded Materials. Ceram. Int. 2016, 42, 3056–3063. [Google Scholar] [CrossRef]
- Sweidan, F.B.; Ryu, H.J. One-step functionally graded materials fabrication using ultra-large temperature gradients obtained through finite element analysis of field-assisted sintering technique. Mater. Des. 2020, 192, 108714. [Google Scholar] [CrossRef]
- Belmonte, M.; Miranzo, P.; Osendi, I. Enhanced microstructural and mechanical gradients on silicon nitride ceramics. Ceram. Int. 2015, 41, 2594–2598. [Google Scholar] [CrossRef]
- Hulbert, D.M.; Jiang, D.; Dudina, D.V.; Mukherjee, A.K. The synthesis and consolidation of hard materials by spark plasma sintering. Int. J. Refract. Met. Hard Mater. 2009, 27, 367–375. [Google Scholar] [CrossRef]
- Suk, M.-J.; Choi, S.-I.; Kim, J.-S.; Do Kim, Y.; Kwon, Y.-S. Fabrication of a porous material with a porosity gradient by a pulsed electric current sintering process. Met. Mater. Int. 2003, 9, 599–603. [Google Scholar] [CrossRef]
- Bódis, E.; Károly, Z. Fabrication of graded alumina by spark plasma sintering. Int. J. Adv. Manuf. Technol. 2021, 117, 2835–2843. [Google Scholar] [CrossRef]
- Nono, M.D. Tetragonal-to-Monoclinic Transformation Influence on the Mechanical Properties of CeO2-ZrO2 Ceramics. Mater. Sci. Forum 2005, 498–499, 506–511. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.-M.; Zeng, L.-Y.; Wu, L.-X.; Wang, H.-J.; Sun, S.-K.; Lin, H.-T.; Wu, S.-H.; Wang, C.-Y. Effect of CeO2 and Al2O3 contents on Ce-ZrO2/Al2O3 composites. J. Am. Ceram. Soc. 2017, 101, 2066–2073. [Google Scholar] [CrossRef]
- Nawa, M.; Nakamoto, S.; Sekino, T.; Niihara, K. Tough and strong Ce-TZP/Alumina nanocomposites doped with titania. Ceram. Int. 1998, 24, 497–506. [Google Scholar] [CrossRef]
- Ding, H.; Zhao, Z.; Jin, J.; Deng, L.; Gong, P.; Wang, X. Densification mechanism of Zr-based bulk metallic glass prepared by two-step spark plasma sintering. J. Alloys Compd. 2021, 850, 156724. [Google Scholar] [CrossRef]
- Ding, H.; Bao, X.; Jamili-Shirvan, Z.; Jin, J.; Deng, L.; Yao, K.; Gong, P.; Wang, X. Enhancing strength-ductility synergy in an ex situ Zr-based metallic glass composite via nanocrystal formation within high-entropy alloy particles. Mater. Des. 2021, 210, 110108. [Google Scholar] [CrossRef]
- Munir, Z.A.; Quach, D.V.; Ohyanagi, M. Electric current activation of sintering: A review of the pulsed electric current sintering process. J. Am. Ceram. Soc. 2011, 94, 24–31. [Google Scholar] [CrossRef]
- Udenni Gunathilake, T.M.S.; Chee Ching, Y.; Yong Ching, K.; Hock Chuah, C.; Chuah Abdullah, L. L-polymers Biomedical and Microbiological Applications of Bio-Based Porous Materials: A Review. Polymers 2017, 9, 160. [Google Scholar] [CrossRef]
- Tampieri, A.; Celotti, G.; Sprio, S.; Delcogliano, A.; Franzese, S. Porosity-graded hydroxyapatite ceramics to replace natural bone. Biomaterials 2001, 22, 1365–1370. [Google Scholar] [CrossRef]
- Akin, I.; Yilmaz, E.; Sahin, F.; Yucel, O.; Goller, G. Effect of CeO2 addition on densification and microstructure of Al2O3–YSZ composites. Ceram. Int. 2011, 37, 3273–3280. [Google Scholar] [CrossRef]
- Yin, L.-J.; Chen, G.-Z.; Wang, C.; Xu, X.; Hao, L.-Y.; Hintzen, H.T.B. Tunable Luminescence of CeAl11O18 Based Phosphors by Replacement of (AlO) + by (SiN) + and Co-Doping with Eu. ECS J. Solid State Sci. Technol. 2014, 3, R131–R138. [Google Scholar] [CrossRef] [Green Version]
- Bódis, E.; Tapasztó, O.; Károly, Z.; Fazekas, P.; Klébert, S.; Keszler, A.M.; Balázsi, K.; Szépvölgyi, J. Spark plasma sintering of Si3N4/multilayer graphene composites. Open Chem. 2015, 13, 13. [Google Scholar] [CrossRef]
- Bódis, E.; Cora, I.; Németh, P.; Tapasztó, O.; Mohai, M.; Tóth, S.; Károly, Z.; Szépvölgyi, J. Toughening of silicon nitride ceramics by addition of multilayer graphene. Ceram. Int. 2019, 45, 4810–4816. [Google Scholar] [CrossRef]
- Mangalaraja, R.V.; Chandrasekhar, B.K.; Manohar, P. Effect of ceria on the physical, mechanical and thermal properties of yttria stabilized zirconia toughened alumina. Mater. Sci. Eng. A 2003, 343, 71–75. [Google Scholar] [CrossRef]
- Emence Petit, C.; Montanaro, L.; Palmero, P. Functionally graded ceramics for biomedical application: Concept, manufacturing, and properties. Int. J. Appl. Ceram. Technol. 2018, 15, 820–840. [Google Scholar] [CrossRef]
- Afzal, M.A.F.; Kesarwani, P.; Reddy, K.M.; Kalmodia, S.; Basu, B.; Balani, K. Functionally graded hydroxyapatite-alumina-zirconia biocomposite: Synergy of toughness and biocompatibility. Mater. Sci. Eng. C 2012, 32, 1164–1173. [Google Scholar] [CrossRef]
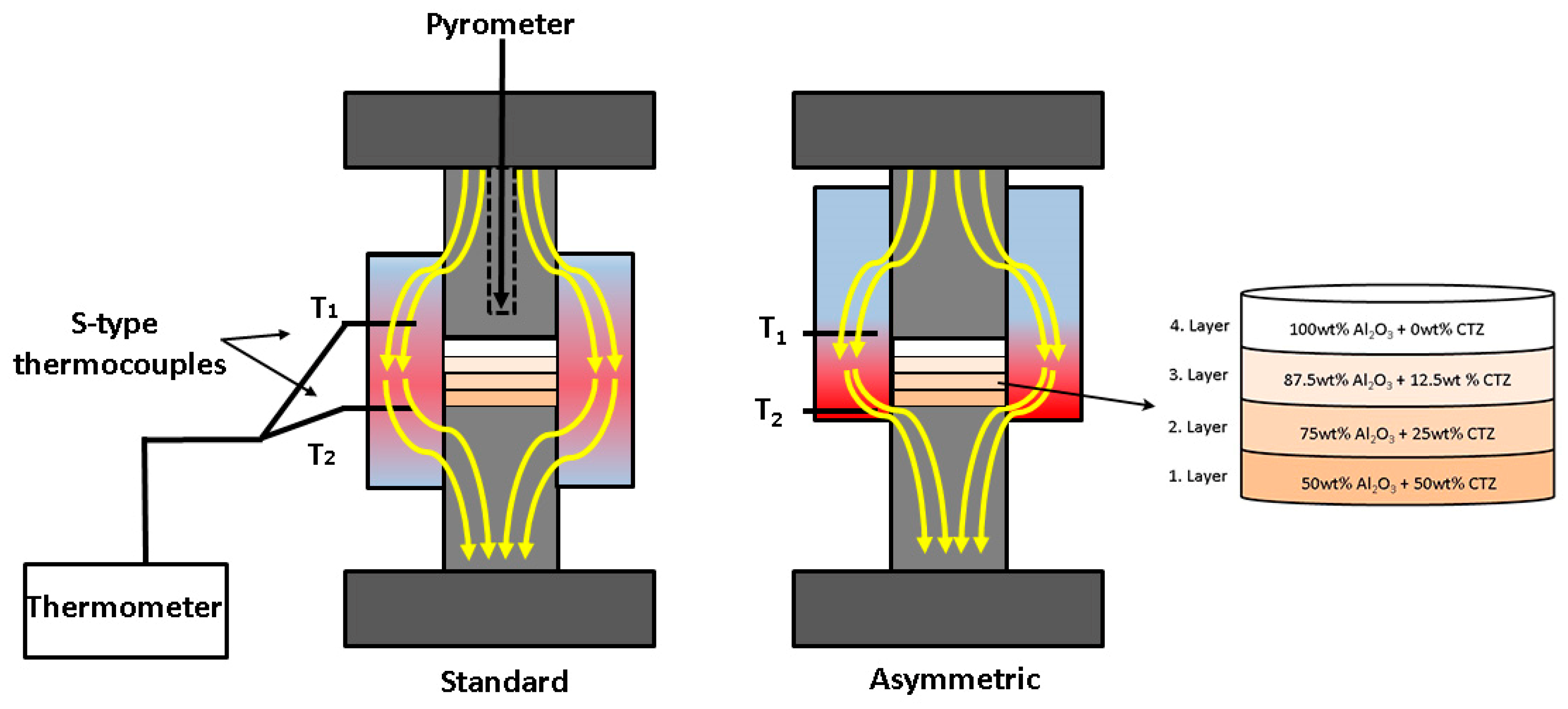



| Layer | Composition (wt%) | |
|---|---|---|
| Al2O3 | CTZ | |
| 4. | 100 | 0 |
| 3. | 87.5 | 12.5 |
| 2. | 75 | 25 |
| 1. | 50 | 50 |
| Sintering Conditions | |||||
|---|---|---|---|---|---|
| Temperature (°C) | Pressure (MPa) | Graphite Configurations | |||
| Standard (STD) | Sample name | Asymmetric (ASY) | Sample name | ||
| 1200 | 25 | STD1200 | ASY1200 | ||
| 1250 | STD1250 | ASY1250 | |||
| 1300 | STD1300 | ASY1300 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bódis, E.; Jakab, M.; Bán, K.; Károly, Z. Functionally Graded Al2O3–CTZ Ceramics Fabricated by Spark Plasma Sintering. Materials 2022, 15, 1860. https://doi.org/10.3390/ma15051860
Bódis E, Jakab M, Bán K, Károly Z. Functionally Graded Al2O3–CTZ Ceramics Fabricated by Spark Plasma Sintering. Materials. 2022; 15(5):1860. https://doi.org/10.3390/ma15051860
Chicago/Turabian StyleBódis, Eszter, Miklós Jakab, Krisztián Bán, and Zoltán Károly. 2022. "Functionally Graded Al2O3–CTZ Ceramics Fabricated by Spark Plasma Sintering" Materials 15, no. 5: 1860. https://doi.org/10.3390/ma15051860
APA StyleBódis, E., Jakab, M., Bán, K., & Károly, Z. (2022). Functionally Graded Al2O3–CTZ Ceramics Fabricated by Spark Plasma Sintering. Materials, 15(5), 1860. https://doi.org/10.3390/ma15051860






