3.1. Composition and Structure
After RF magnetron sputtering and low temperature ion sulfurizing, Mo/MoS
2-Pb-PbS multilayer thin film was exposed to the AO with different incident fluence, and the corresponding composition and structure changes were investigated by SEM and EDS.
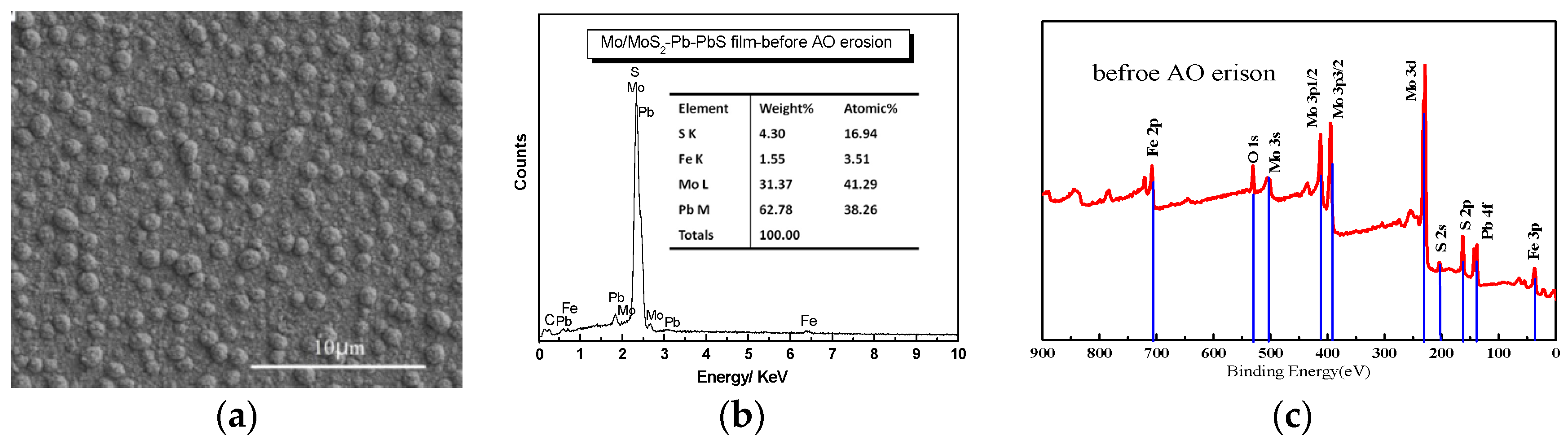
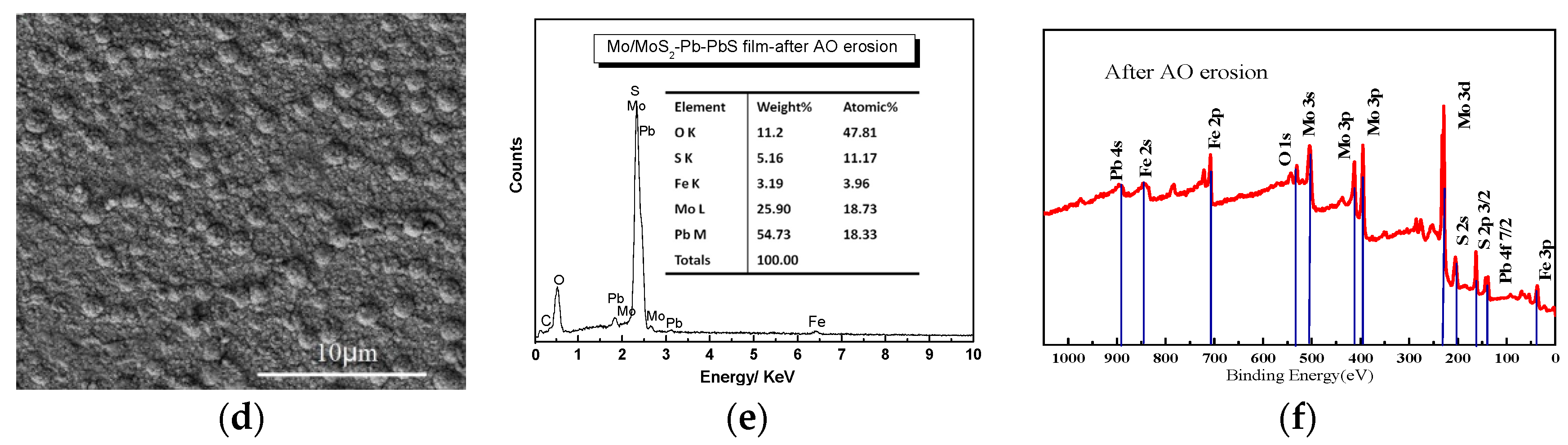
Figure 2 shows the surface morphology and composition of Mo/MoS
2-Pb-PbS film before and after AO exposure. The microstructure of the film without AO erosion was composed of nano-scale irregular particles with some pores between particles, as shown by
Figure 2a. After AO erosion for 15 h, about 1.35 × 10
20 atom/cm
2 incident fluence exposure, the surface morphology Mo/MoS
2-Pb-PbS film is shown by
Figure 2d. The composition Mo/MoS
2-Pb-PbS film without AO erosion is shown in
Figure 2b. The contents of Mo and Pb were 41.29% and 38.26%, respectively. After AO erosion, the number of cauliflower-like large particles on the surface of the film decreased because of flash etching of the AO beam. Therefore, the film surface became flat and full of pitting. In order to study the composition changes in Mo/MoS
2-Pb-PbS film further, XPS was used to RBS.
Figure 2c shows the XPS results of the valence of the Mo and Pb elements on the surface of Mo/MoS
2-Pb-PbS composite film before AO erosion. As shown in
Figure 2c, the main peaks of Mo element on the surface of the film are mostly located at 230 eV, corresponding to Mo, the molybdenum compound of MoS
2 and MoO
3, respectively. Due to the inevitable contact with air during the preparation of XPS samples, the Mo element on the surface of the original sample was also partially oxidized to MoO
3, but the high content of MoS
2 is still the main component. After AO erosion, the contents of Mo and Pb were decreased to 18.73% and 18.33%, respectively, while the content of O was increased to 47.81%, because of the oxidizing reaction shown by
Figure 2e. Combining with
Figure 2f, the content of MoS
2 decreased significantly because a large amount of Mo was oxidized to MoO
3 and metastable MoO
2 as shown in
Figure 2f. An obvious feature in the spectrum is the increase in the content of elemental Mo on the film surface caused by AO erosion because of the sputtering etching effect of high-energy AO removed the S-rich layer on the film surface. The Pb element on the original film surface mainly exists in the form of elemental Pb, PbS, and PbO, and the content of PbO was small. After AO erosion, the Pb element on the film surface was oxidized to Pb
3O
4 and minor PbSO
3, and the spectral peak of elemental Pb disappeared.
XPS results reveal clearly that mostly Mo and Pb elements in the film surface were both oxidized to MoO
3 and Pb
3O
4, respectively. There were rare amounts of the simple substance Pb, and the content of MoS
2 was decreased compared by Pb and Mo elements atomic percent form
Figure 2b,e. The Pb decreased to 18.33% from 38.26 without AO erosion, and Mo element decreased to 18.73% from 41.29 without AO erosion. In order to get more information about the structural evolution, Mo/MoS
2-Pb-PbS multilayer film before and after the AO exposure were investigated by TEM.
Figure 3a shows that there were long-range ordered structures and glassy substances with different orientations and plane spacings in the Mo/MoS
2-Pb-PbS film before AO erosion, and there were obvious interfaces between different structures. Glassy substances were one crystal structure of 2H, which is the stable state structure. Molybdenum disulfide crystal belonged to hexagonal system, with three crystal structures of 1T, 2H, and 3R. 1T-MoS
2 and 3R-MoS
2 crystal belonged to a metastable structure. After AO erosion, the original large-scale regular structure disappeared and the cluster size became smaller, while the glassy region became larger (the yellow circle region shown in
Figure 3), and a small-scale ordered structure region appeared (the green circle region shown in
Figure 3b), which is consistent with the test results of the decrease in metal (Mo, Pb) and metal sulfide (MoS
2, PbS) in
Figure 2, and the new generation of a large number of oxides (MoO
3, Pb
3O
4, etc.). Highly active atomic oxygen impacts caused great heat, whereby 1T-MoS
2 and 3R-MoS
2 of metastable states transformed into stable 2H-MoS
2. Hence, the glassy region became larger.
In summary, AO with high activity and high energy will cause serious oxidation on the surface of Mo/MoS2-Pb-PbS film, and the contents of lubricating components MoS2, PbS, and Pb were significantly reduced, and a large number of oxides was generated.
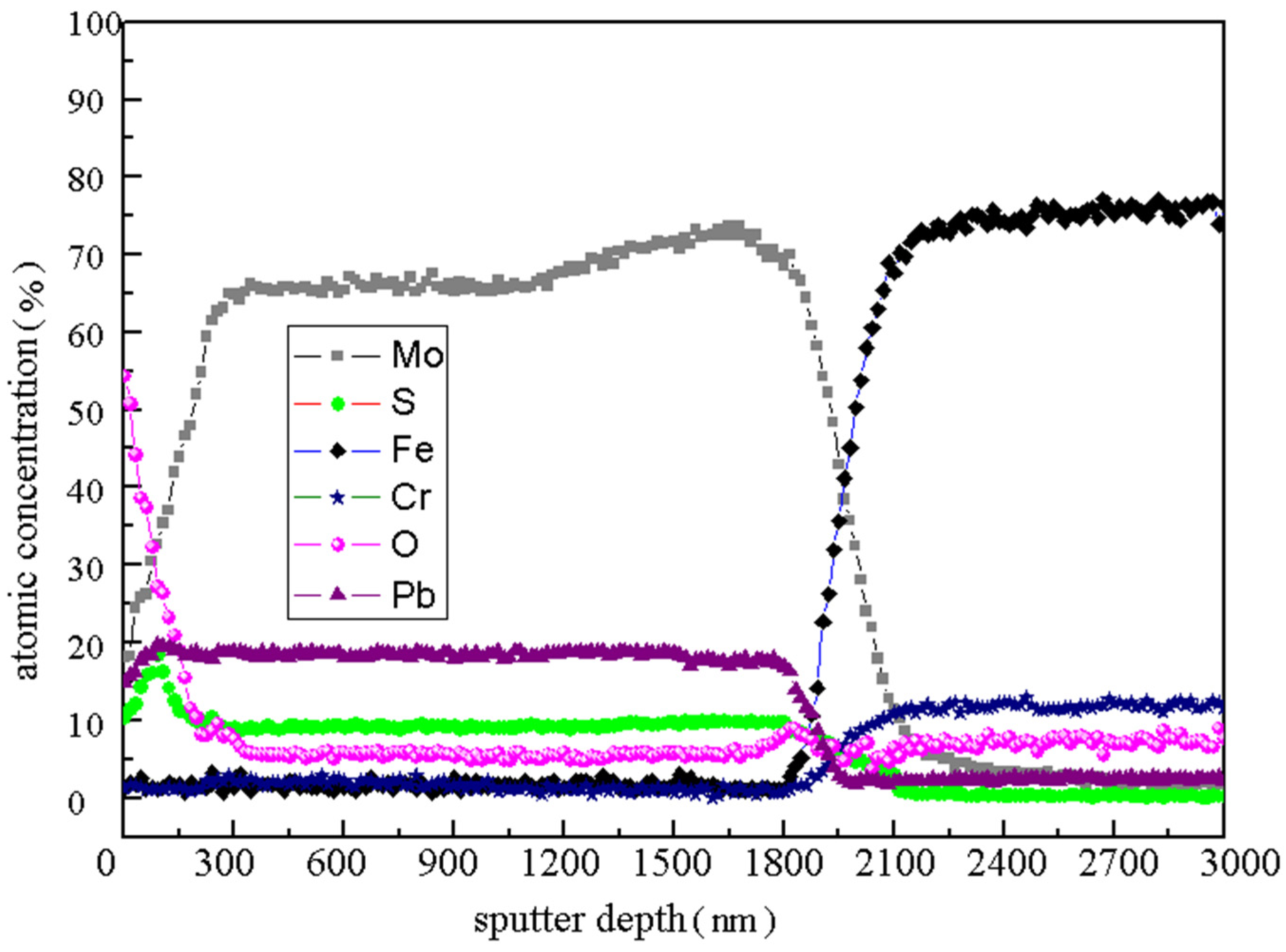
The element distribution along the depth direction of Mo/MoS
2-Pb-PbS composite film after 15 h AO erosion was shown in
Figure 4 by used AES analysis, when the Ar
+ sputtering rate is 30 nm/min. The contents of Mo, Pb, and S elements in the top layer of the film are 18.1, 14.8 and 10.3%, respectively, and the content of O element is as high as 54.4%. With the sputtering etching depth increasing, the content of the Mo, Pb, and S elements increased gradually. However, the content of the O element decreased sharply. When the etching time was 3 min (about 100 nm from the surface), the contents of the S and Pb elements reached their maximum values 18.52% and 19.72%, respectively. Subsequently, the content of the S element decreased slowly, and the content of the Pb element was stable at about 18.5%. After sputtering for 10 min (about 300 nm from the surface), the content of each element tended to be stable, and the content of the Mo, S, and O elements was stable at about 65, 9 and 5.5%, respectively. In the range of 60–70 min, the contents of the Mo, Pb, and S elements were mutated. In this region, the contents of Fe and Cr, which are the main elements constituting the 9Cr18 matrix, were rapidly increased to about 70% and 10%, respectively. There were 1800–2100 nm away from the surface of the sample, which was close to the Mo/MoS
2-Pb-PbS film thickness of 2000 nm. The position where the element mutation occurred was the film–substrate interface.
Previous research results showed that the S element content of ion-sulfurized film often had a decreased gradient in the depth direction, and the S element enrichment layer generally appeared near the surface in general [
23]. As shown in
Figure 4, the S element content of the film surface in the range of 300 nm was indeed relatively high, but the maximum S element content did not appear on the top surface. Because the standard formation free energy of oxides was low, it is very easy to form oxide. When the AO beam with high energy is on the surface of Mo/MoS
2-Pb-PbS film, AO will “grab” some metal ions from the metal sulfides. Then MoO
3 and Pb
3O
4 will be formed after AO enters the surface layer of the film. Therefore, it is obvious that the content of Mo, Pb, and S in the surface layer of the film will be low, and the content of O is high. However, with the increase in film depth, the oxidation decreased rapidly [
24,
25]
. Combined with the variation in the main element content, the Mo/MoS
2-Pb-PbS film has a certain self-protection ability in the AO environment. Oxides such as MoO
3 and Pb
3O
4 can hinder the deep oxidation of the lubricating material in the subsurface. The thickness of the oxidized area is clearly about 300 nm.
3.2. Tribological Properties
Because the remarkable tribological properties of Mo/MoS
2-Pb-PbS composite film in a vacuum, the effect of the space environment and especially AO erosion through the top surface to depth in determining the film tribological properties are also discussed. Tribological tests were performed on a ball-on-disk tribometer in the low-pressure vacuum of 5 × 10
−3 Pa, and the results are shown in
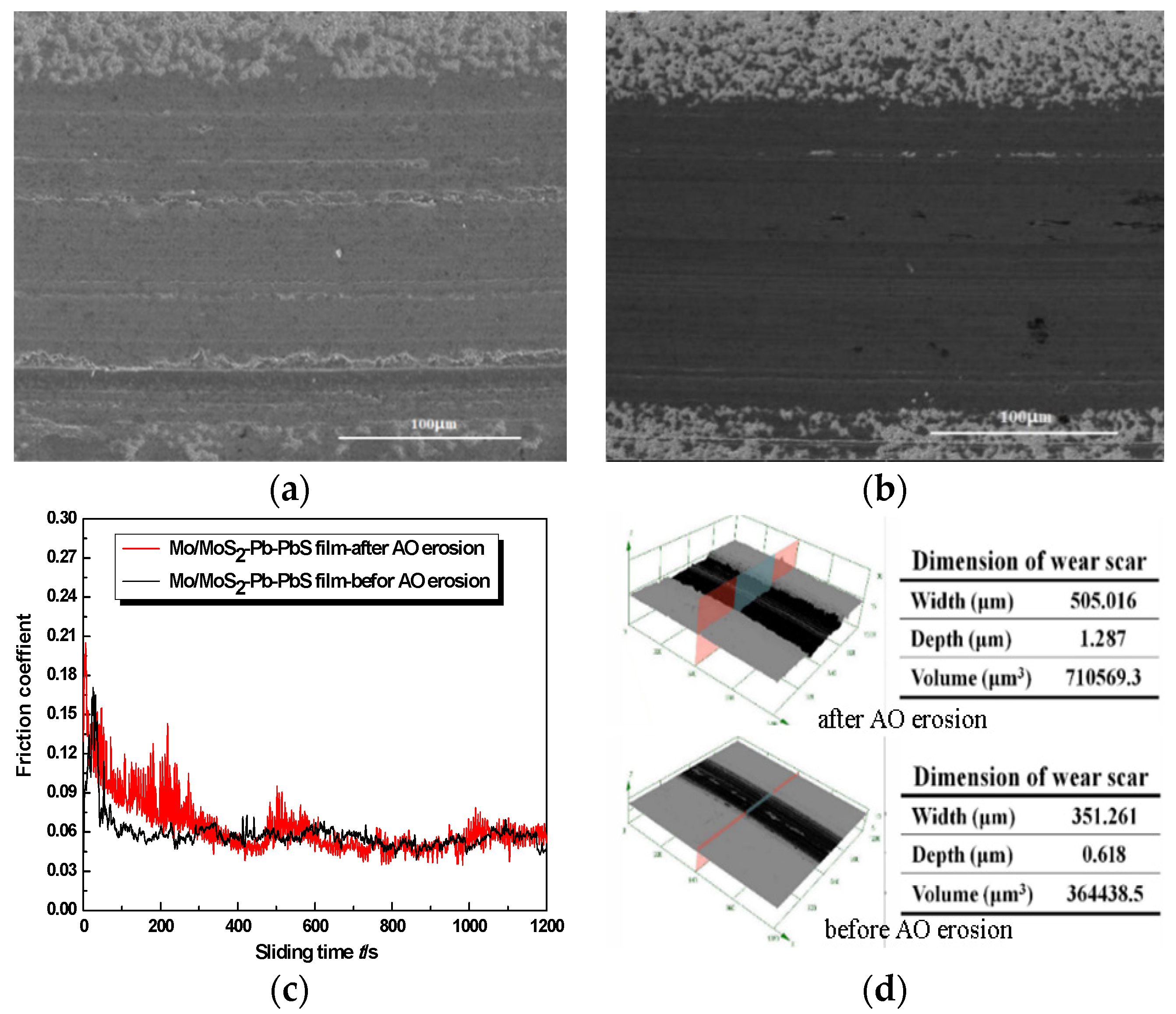
Figure 5. The variation in the friction coefficient of Mo/MoS
2-Pb-PbS film without AO erosion showed obvious three-stage characteristics of ‘starting-running-stability’. The starting friction coefficient was about 0.075, which rose rapidly to 0.175 when friction time was 30 s. Then, the friction coefficient decreased rapidly and stabilized to 0.05 when friction time was 80 s. After which, the fluctuation of the friction coefficient was very small, and the friction coefficient curve was very stable as shown in
Figure 5c. We found that the starting friction coefficient of Mo/MoS
2-Pb-PbS film after AO erosion increased to 0.2, and then the friction coefficient oscillated violently and decreased gradually. When the friction test time was about 330 s, the friction coefficient was stable at about 0.06. During the whole test process, the friction coefficient curve fluctuated slightly compared with the film before AO erosion.
Before AO erosion, obvious material transfer was observed on the worn surface of the film, which was seen as separate deep scars along the sliding direction. A large amount of material piled up outside the wear scar. In the vacuum environment, because the ambient medium was rarefied and there was no convection heat dissipation in air, the friction interface temperature was higher. The surface of the film was further softened during the friction process. The friction dual ball was embedded in the soft film surface under the positive pressure, and the soft film was pushed in the sliding to make plastic flow, and furrows were formed; meanwhile, a large amount of material accumulation was formed on both sides of the wear scar. Hence, the wear of Mo/MoS2-Pb-PbS film without AO erosion was dominated by plastic deformation and material transfer, and there were few lubricants separated from the friction orbit in the form of debris.
After AO erosion, the wear of the film became uneven, and the local material removal was serious. A plentiful supply of parallel grooves with different depths were distributed along the sliding direction. Irregular black particles could also be observed on the wear track. Compared with the three-dimensional morphology and profile data of the wear scars before and after AO erosion, the depth, width, and volume of the wear scars of the film after AO erosion were significantly increased, especially the depth of the wear scars, which increased from 0.618 to 1.287 µm.
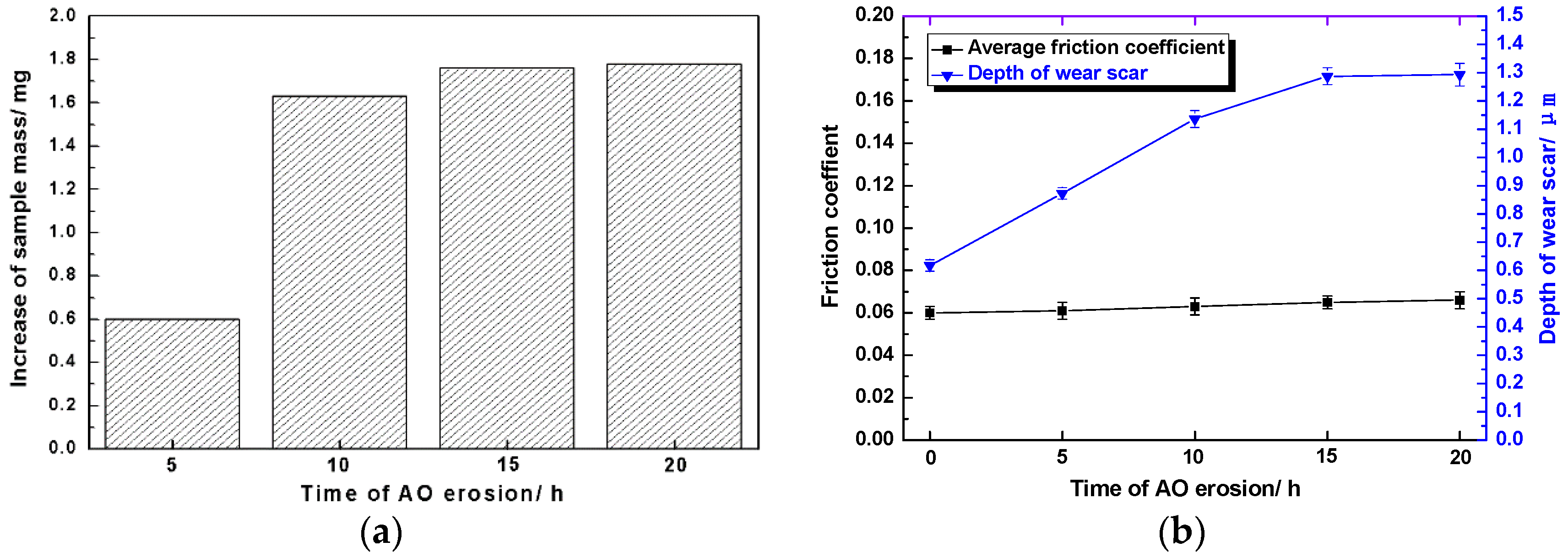
Sputtering and oxidation of spacecraft surface materials by AO with high speed and high activity often lead to significant changes in the quality of materials [
26,
27]. As shown in
Figure 6a, the quality of Mo/MoS
2-Pb-PbS thin film increased after AO erosion at different times, because the molecular weight of the newly formed oxides, namely, MoO
3 and Pb
3O
4, was larger than that Mo and Pb elements’ sulfides, and some elemental Mo and Pb were also oxidized. After AO erosion for 5 h, the sample mass increased by 0.6 mg. When the erosion time was extended to 10 h, the mass of the sample increased to 1.63 mg. When the erosion time continued to extend, the increase rate of sample mass decreased and stabilized at 1.7–1.8 mg.
As shown in
Figure 6b, after AO erosion for different time, the average friction coefficient of Mo/MoS
2-Pb-PbS film in the stable wear period remained around 0.06. When the erosion time was less than 15 h, the wear scar depth of the film increased approximately linearly with the extension of erosion time, but when the erosion time continued to increase (after 20 h), the wear scar depth no longer increased significantly.
3.3. Discussion
The microstructure of Mo/MoS2-Pb-PbS composite film changed significantly after AO erosion at different times, and the tribological properties were also degraded to varying degrees. In this section, we briefly analyze the mechanism of spatial AO on the structural change and tribological performance degradation of Mo/MoS2–Pb-PbS thin film.
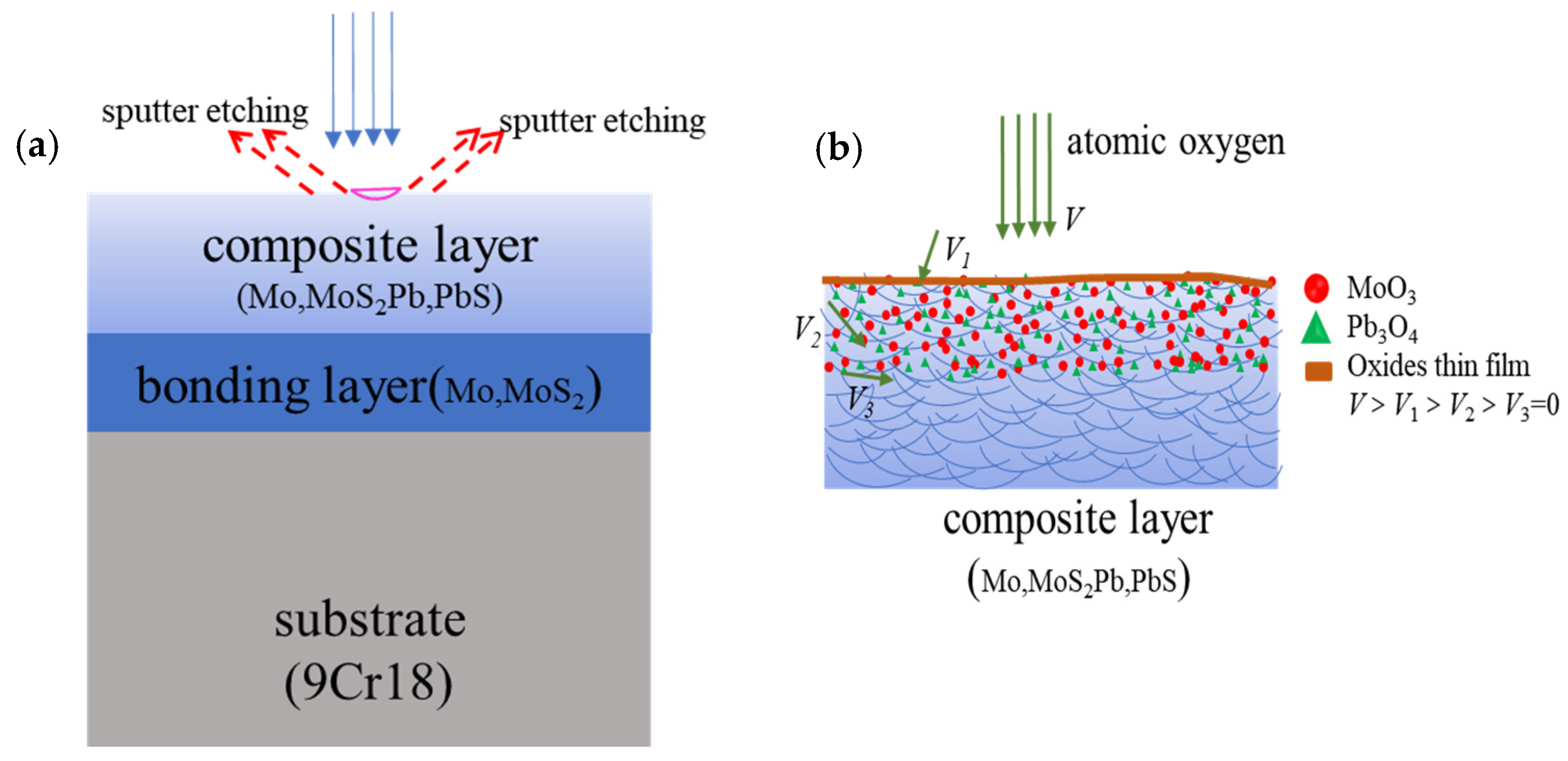
Firstly, the preparation process and composition compatibility of Mo/MoS2-Pb-PbS thin film determine that the surface roughness of the thin film is relatively large, and there is a certain gap between the structural unit particles. Mo/MoS2-Pb-PbS thin film were prepared by the two-step composite process of ‘RF magnetron sputtering and low temperature ion sulfurization’. The Mo-Pb thin film was formed of atomic groups of Mo and Pb elements, which were sputtered from the target, in the way of layer-by-layer stacking and mutual doping. After sulfurization treatment, Mo and Pb in the Mo-Pb thin film were partially sulfurized; thus, the special structure was formed. The two-phase of Mo and Pb mixed structure was the bulk of this special structure, and rich in MoS2 and PbS metal sulfide as lubricating phases. The microstructure of Mo/MoS2-Pb-PbS thin film was metal and metal compound particles with nano-sized, and their agglomerated micron-sized particles. However, the important difference from the sputtering deposition of pure MoS2 thin film was that the Mo/MoS2-Pb-PbS thin film lacked coarse columnar crystal growth, and that there were no structural defects, such as penetrating deep holes, on the surface of the thin film.
Secondly, AO with high energy and high activity will sputter and etch the film surface; meanwhile, AO will react with elements on the film surface when the film is irradiated by AO. The components in Mo/MoS
2-Pb-PbS thin film are metals or metal compounds, and each component is bound by strong chemical bonds. The atomic mass of Mo and Pb is large, and the kinetic energy is transferred to the surface of the thin film when the AO hits the surface of the film with high speed. These energies are not enough to destroy the chemical bonds inside the film, but the long-term physical sputtering will produce an etching effect on the surface of the film, making the film surface smoother and denser. After kinetic energy transfer, a lot of oxygen atoms are adsorbed on the surface of the film and the film surface is oxidized, as shown in
Figure 7.
When AO reached the film surface, it preferentially reacted with particles on the top of surface, so severe oxidation occurred at the top of the film. With the increased AO cumulative flux, some of the oxygen atoms will fill the gap of particles on the surface of the film, and even migrate to the film interior along the shallow defects of the film until the reaction with the film stops moving. The filling and migrating of AO also oxidizes the film interior. After AO reacts with Mo, Pb, MoS2, and PbS in the film, a thick and tightly bonded oxide layer is formed on surface of the film, and the quality of the film is significantly increased.
Thirdly, according to the molecular random dynamics model and chemical reaction dynamics model’ of the interaction between AO and the surface of the film, the oxidation degree of the film in the AO environment was closely related to the diffusion force of AO into the film and the activation energy of the oxidation reaction. The oxidation ability of AO in the thermal state was strong. With the increase in erosion, the continuous coverage of oxide thin layers such as MoO3 and Pb3O4 will be formed on the surface of the film. These formed dense oxide layers can prevent the diffusion of oxygen atoms into particles. There was also no penetrating deep hole in the film as a ‘channel’ for oxygen atoms to diffuse deeply. However, the Mo/MoS2-Pb-PbS thin film will undergo seriously oxidation under the action of AO, even if the erosion that increased the oxidation damage of the film was limited to hundreds of nanometers on the surface of the film.
After AO action, the starting friction coefficient of the film Mo/MoS2-Pb-PbS film increased due to the hard oxide shell formed on the surface of the film. As the sliding friction progresses, these oxide thin layers were broken and removed quickly. The hard oxide particles embedded in the surface of the soft lubrication film, which increases the fluctuation of the friction coefficient; however, the average friction coefficient will not change significantly in the stable period.