Strontium Functionalization of Biomaterials for Bone Tissue Engineering Purposes: A Biological Point of View
Abstract
:1. Introduction
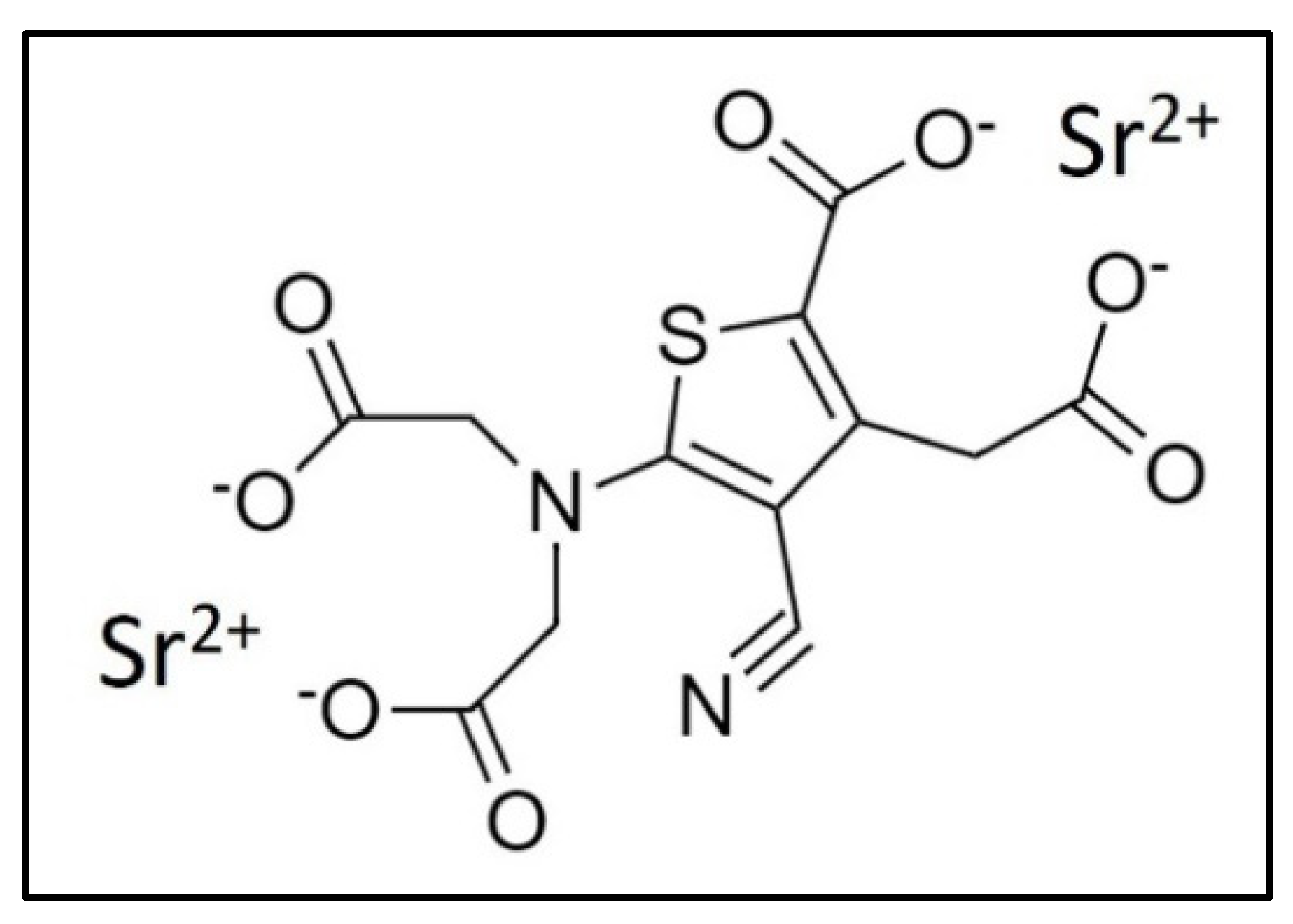
2. Strontium and Strontium Ranelate
3. Calcium-Sensing Receptors and Strontium Binding
4. Incorporation of Strontium in Bone Tissue and Factors Influencing the Process
5. Effect of Strontium on Mesenchymal Stem Cells
6. Effect of Strontium on Osteoblasts
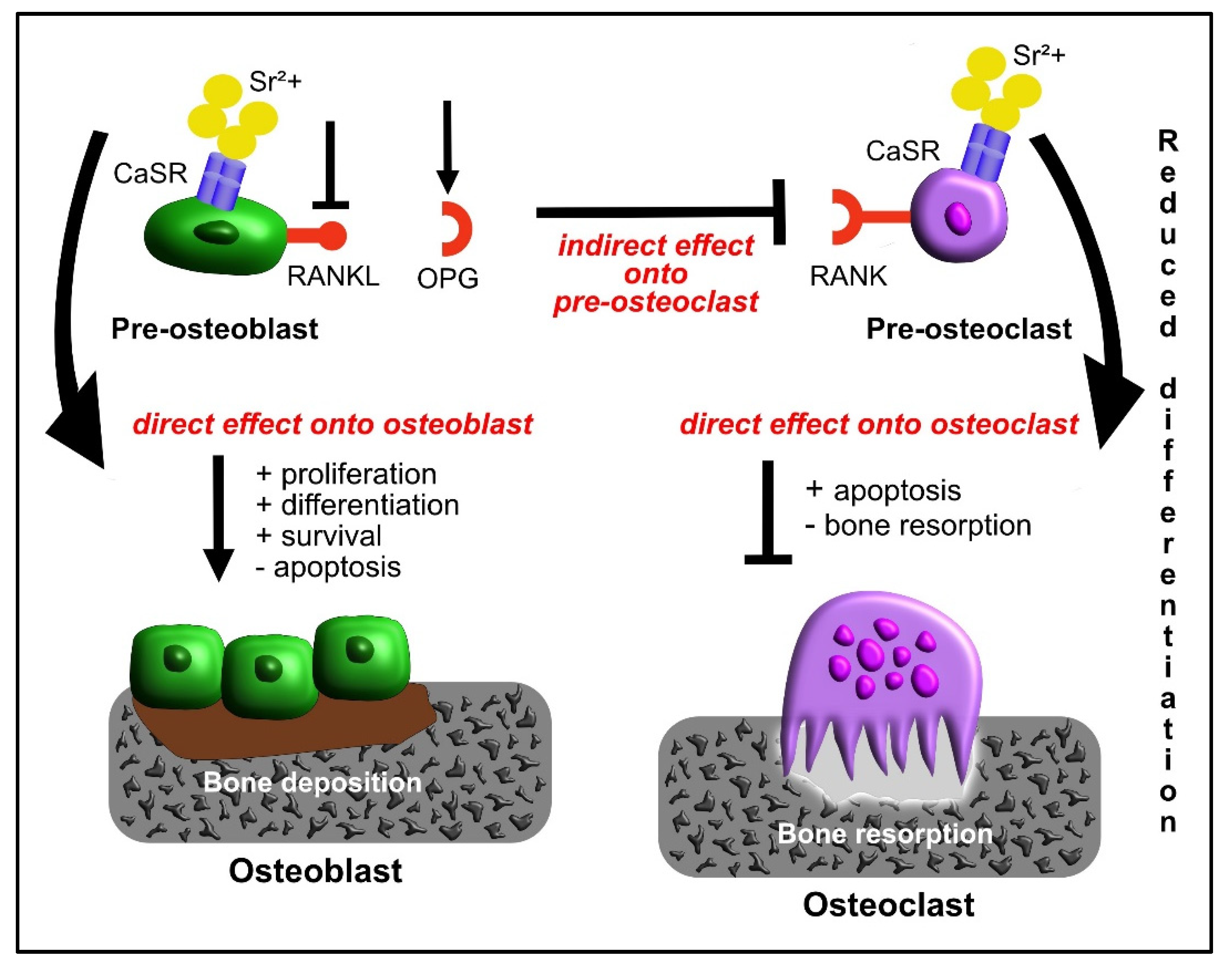
7. Effect of Strontium on Osteoclasts
8. Effect of Strontium on Osteoblast-Osteoclast Crosstalk
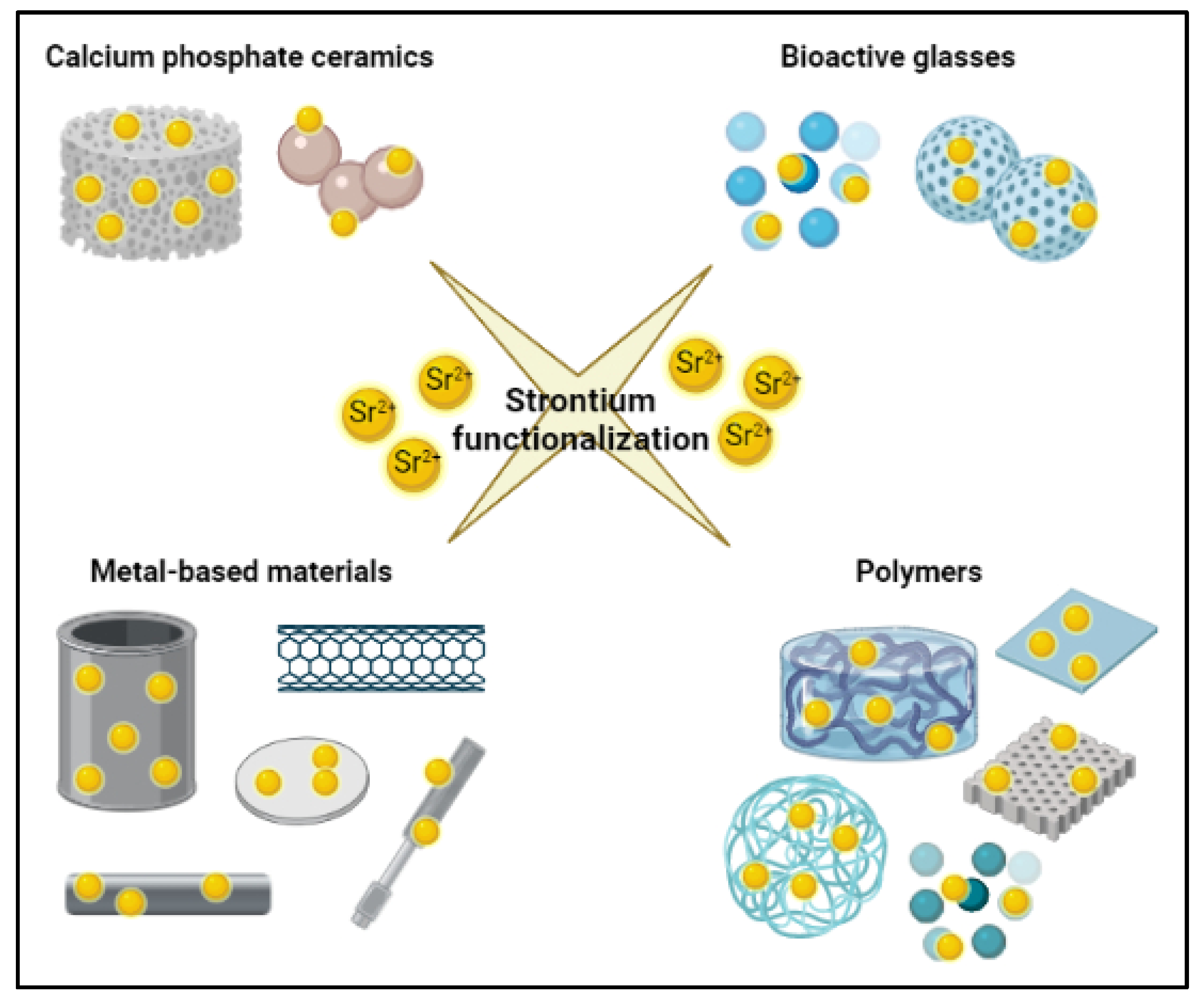
9. Biomaterials for Bone Tissue Engineering Approach and Their Functionalization with Strontium Ions
9.1. Calcium Phosphate Ceramics
| Material | In Vivo/In Vitro Evaluation | Results | Reference |
|---|---|---|---|
| HA-based cements containing Sr | In vivo → goat revision hip hemi-arthroplasty model (medullary cavity of proximal femur rasped and Sr-HA cement injected) | New bone bonded to the surface of Sr-HA cement and grew along its surface | [102] |
| Sr-containing HA (Sr-HA) cement | In vivo → hip replacement in 12–14-months-old and 4.5–5.5 kg weighed rabbit | After 6 months from implantation, good bioactivity, stability, and bone-bonding ability under weight-bearing conditions | [103] |
| Porous Sr-doped calcium polyphosphate scaffolds | In vivo → implantation in segmental defects of rabbit left foreleg radius (defect size: 15 mm) | Induction of an active bone formation and extensive osteoconductivity | [104] |
| Sr-modified calcium phosphate cement (SrCPC) | In vivo → critical-size metaphyseal defect in the femur of ovariectomized rats | Higher new bone formation both at the biomaterial-bone interface, with increased expression of ALP, OCN and COL10 | [105] |
| Sr-doped calcium polyphosphate (SCPP) | In vitro → endothelial cells (ECs) seeding | The surface of SCPP promotes the adhesion and spreading of ECs, improving the angiogenic behaviors | [106] |
| Synthesized HA coatings with different proportions of Sr substitution for Ca (0, 1, 3 or 7%) | In vitro → osteoblast-like MG-63 cells and human osteoclasts cultured on the materials | Enhanced MG-63 activity and differentiation alongside the inhibition of osteoclast differentiation | [107] |
| Sr-substituted HA-graft-poly(γ-benzyl-L-glutamate) hybrid nanocomposite | In vitro → cellular evaluation with rabbit adipose-derived stem cells (ADSCs) In vivo → bone repair potential in a critical bone defect in a rabbit model | In vitro → ADSCs adhesion, infiltration, proliferation, and promotion of osteogenic differentiation In vivo→ successful healing of critical bone defect in rabbits | [108] |
| Sr-doped HA microspheres shielded with Sr-incorporated RGD-alginate | In vivo → critical-sized metaphyseal bone defect in Wistar Han male rats | Higher new bone formation and higher cell invasion | [19] |
| Porous-core shell biphasic microspheres with 4 wt% Sr-substituted calcium silicate (CSi-Sr4) and beta-tricalcium phosphate (CaP) | In vivo → skull bone defect of rabbits | Bone regeneration | [109] |
| 3D-printed Sr-HA/PCL scaffold | In vitro → rat bone marrow-derived mesenchymal stem cells (BMSCs) In vivo → implantation in Sprague Dawley rat skull defect model | In vitro → enhanced cell proliferation and osteogenic differentiation In vivo → promotion of bone regeneration after 12 weeks | [110] |
| Porous Sr-doped calcium phosphate cement scaffolds | In vivo → trabecular bone defects in sheep | Enhanced bone formation | [111] |
| Porous Sr-doped calcium polyphosphate (SCPP) | In vivo → critical size defect in rabbit calvarial bone | Sr accelerated bone formation in a highly Ca-enriched microenvironment | [112] |
| Sr-β-tricalcium phosphate | In vivo → scaffold seeded with undifferentiated mesenchymal stem cells from bone marrow and implanted in spinal fusion bone defect model in rats | Significant spinal fusion | [113] |
| Sr-loaded deproteinized bovine bone with 5%, 25% and 50% Sr | In vivo → implantation in rat calvarial critical size defect (5 mm in diameter) | A minor inflammation and a higher amount of new bone formation in bone defect site at 60 days in comparison to Sr-free counterpart | [115] |
| Deproteinized bovine bone functionalized with strontium-doped HA | In vivo → implantation in a bone defect in rat femoral epiphysis (trabecular bone region) | Larger amount of bone, reduced expression of osteoclastic genes (CR and CatK), and osteoblast–osteoclast coupling gene (RANKL) in the SrHA-filled defect | [116] |
9.2. Bioactive Glasses
| Material | In Vivo/In Vitro Evaluation | Results | Reference |
|---|---|---|---|
| Sr-incorporated MBGs scaffold | In vitro → bone marrow-derived stromal cells In vivo → implantation of a scaffold in critical sized femur defects in ovariectomized rats | In vitro → stimulation of proliferation and expression of osteoblast commitment markers (ALP, COL1, RUNX2, and BGLAP) In vivo → significant stimulation of new bone formation | [125] |
| Sr-MBG microspheres and nanoparticles | In vitro → biocomaptibility with L929 cells, inflammatory response on J774a.1 cells, and pro-osteogenic effect on Saos-2 cells | Absence of cytotoxic effect on L929 cells, absence of inflammatory response on J774a.1 cells and pro-osteogenic effect on Soas-2 cells with the stimulation of the expression of COL1, SPARC, and OPG and the downregulation of RANKL | [104] |
| Sr-MBGs co-grafted with hydrolysable short chain silanes containing amino (aminopropylsilanetriol) and carboxylate (carboxyethylsilanetriol) moieties | In vitro → biocompatibility with MC3T3-E1 cells and evaluation of non-specific protein adsorption | Absence of cytotoxic effect on MC3T3-E1 cells and reduction of non-specific serum protein adsorption | [105] |
| Sr-MBGs bio-functionalized with ICOS-Fc | In vitro → biocomaptibility with MC3T3 cells, inhibitory effect of grafted ICOS-Fc on cell migratory activity of PC-3 and U2OS cells, inhibition of osteoclast differentiation and function on monocyte-derived osteoclasts (MDOCs) | Absence of cytotoxic effects on MC3T3 cells, inhibition of PC-3 and U2OS cell migration, decrease of TRAP+ cells, and decrease of DC-STAMP, OSCAR, and NFATc1 mRNA expression | [128] |
| 3D porous Sr-releasing, BG-based scaffold (pSrBG) | In vitro → ability of bone marrow-derived human mesenchymal stem cells to grow onto the scaffold In vivo → implantation of the scaffold in critical-sized femoral condyle defects in sheep (8 mm) | In vitro → cells attachment to scaffold inner and outer surfaces and good cell invasion and growth In vivo → promotion of the formation of mature-like well-organized lamellar neo-bone tissue | [129] |
| Porous nanocomposite PCL scaffolds coated with chitosan containing 15 wt% Sr-substituted BG nanoparticles (nanoparticles containing 7 wt% Sr) | In vitro → biocompatibility with MG-63 cell line | Absence of cytotoxic effects, enhanced ALP activity, and cell adhesion with healthy cell morphology | [130] |
| BG granules combining Sr and Mg | In vitro → biocompatibility with L929 fibroblasts and with 3D model of human BM-MSCs to predict the impact of the BG granules on bone tissue | Confirmation of material biocompatibility with L929 fibroblast cell line. Adhesion, proliferation, and osteo-lineage differentiation with 3D model of BM-MSCs | [16] |
| Temperature-sensitive p(N-isopropylacrylamide-co-butyl methylacrylate) nanogel with Sr containing MBGs | In vitro → preliminary evaluation with primary rat MSCs In vivo → implantation of the scaffold into femur defect in osteopenic rats | In vitro → enhanced cell proliferation and ALP activity In vivo → regeneration of critical-sized bone defects | [131] |
| 3D bioactive composite PCL scaffolds containing 45S5 Bioglass or Sr-substituted BGs | In vitro → biocompatibility test with MC3T3 cell line | Confirmed biocompatibility and positive influence of cell attachment and proliferation. No difference in ALP activity. | [132] |
| Sr-containing MBG scaffold | In vitro → evaluation of stimulation of osteogenic/cementogenic differentiation of periodontal ligament cells (PDLCs) | Stimulation of ALP activity and osteogenesis/cementogenesis-related gene expression of PDLCs | [133] |
| 3D Sr-containing MBG scaffold | In vitro → biological evaluation with MC3T3-E1 cell line | High ALP activity, enhanced expression of osteogenic markers RUNX2, OCN, BMP-2, COL1, BSP, and ECM mineralized nodules | [134] |
| PCL composite scaffold incorporating 10% (weight) of Sr-substituted BG particles by melt electrospinning | In vitro → biological evaluation with MC3T3-E1 cell line | Enhanced ALP activity, high expression of ALP and OCN gene, and high ECM formation | [135] |
| 3D printed bone constructs of silk-gelatin with Sr-BG | In vitro → biological evaluation with MSCs (TVA-MSC: a specialized, immortal BMSC cell line) | Induction of osteogenic differentiation that is the up-regulation of RUNX, ALP, OPN, ON, BSP and OCN expression | [136] |
| PCL-based composite scaffolds containing 50 wt% of 45S5 Bioglass (45S5) or Sr-BG particles, with calcium phosphate coating | In vitro → biological evaluation with sheep-derived BMSCs In vivo → implantation of the scaffolds subcutaneously into nude rats | In vitro → positive cell adhesion, growth and proliferation and up-regulation of osteogenic gene expression In vivo → host tissue well infiltrated into the scaffolds but no mature bone formation | [137] |
| Gelatin-Sr-BG scaffolds (Gel-BG/Sr) | In vitro → antibacterial evaluation with Escherichia coli and Staphylococcus aureus In vivo → implantation in a rabbit calvarial bone defect | In vitro → antibacterial properties on Escherichia coli and, compared to counterparts having no Sr, also on Staphylococcus aureus In vivo → enhanced deposition of newly formed bone tissue in comparison to Sr-free counterpart | [138] |
| Poly(methylmethacrylate) cements with Sr-containing borate BG | In vitro → biological evaluation with MC3T3-E1 cell line In vivo → implantation in a tibia defect in Sprague–Dawley rats | In vitro → promotion of cell adhesion, migration, proliferation, and collagen secretion In vivo → good osseointegration after 12 weeks | [140] |
| Composite bioactive PLLA membrane loaded with 10% (w/w) of Sr-borosilicate BG particles using electrospinning | In vitro → biological evaluation with bone marrow-derived mesenchymal stem cells | Promotion of osteogenic differentiation with increased ALP activity and up-regulated osteogenic gene expression (ALP, SP7, and BGLAP) in comparison to PLLA alone | [141] |
| Discs and microspheres made of Sr (0, 4, 8, 12 and 16 mol%)-substituted phosphate-based glass (PBGs) | In vitro → biological evaluation of discs with MG-63 cells and microspheres with a 3D culture of human MSCs | Cell attachment and spreading confirmed for MG-63 cells with ALP activity. HMSCs attachment and colonization of the microsphere surfaces | [145] |
9.3. Metal-Based Materials
| Material | In Vivo/In Vitro Evaluation | Results | Reference |
|---|---|---|---|
| Bioactive SrTiO3 nanotube array on Ti implant | In vitro → biological evaluation with bone cells | Confirmed biocompatibility and promotion of bone cell attachment and growth | [148] |
| Coatings containing TiO2 nanotubes with Sr on titanium surfaces through hydrothermal treatment | In vitro → biological evaluation with mouse BMMCs and RAW264.7 cells In vivo → implantation in tibia defect in ovariectomized Sprague Dawley rats | In vitro → osteoclast differentiation inhibition In vivo → prevention of bone loss | [149] |
| Laser sintered porous cylindrical Ti6Al4V implants with 700 μm and 1500 μm pore sizes, electrochemically coated with HA, silicon-substituted HA, and Sr-substituted HA | In vivo → implantation in ovine femoral condylar defects | Coated implants significantly promoted bone attachment to the implant surface and improved osseointegration | [150] |
| Surface-treated Ti disks with Sr (Sr-Ti) | In vitro → biological evaluation with MC3T3-E1 cell line In vivo → implantation in tibia defect in a rabbit model | In vitro → enhanced proliferation and osteogenic differentiation with the expression of integrin β1, β-catenin, and cyclin D1, and osteogenic gene, ALP activity, extracellular mineralization In vivo → major biomechanical strength and bone-implant contact for Sr-Ti in comparison to Sr-free counterpart | [151] |
| Commercially pure Ti disks with surface functionalized with Sr ions | In vitro → biological evaluation with mouse J774.A1 macrophages | Induction of regenerative M2 macrophage phenotype of J774.A1 cells in nanostructured Ti surfaces | [152] |
| Commercially pure Ti disks with a wet-abraded smooth or grit-blasted micro rough surface functionalized with Sr ions | In vitro → biological evaluation with mesenchymal stem cells (MSCs)—primary murine BMSCs and human ASCs— | Cell spreading, focal adhesion development, ALP activity, and gene expression of integrins enhanced in mBMSCs grown on the nano Sr surface; enhanced osteogenic differentiation of hASCs in the presence of Sr | [153] |
| Microporous titania coatings containing Sr ions deposited onto Ti implants | In vitro → biological evaluation with bone marrow MSCs from New Zeland rabbits In vivo → implantation in femoral shafts of New Zealand male rabbits | In vitro → Sr enhanced MSCs proliferation and osteogenic differentiation In vivo → Sr enhanced implant osseointegration and new bone formation | [154] |
| Sr-functionalized Ti implants | In vivo → implantation in femoral condyle defect of male New Zealand White rabbits | Acceleration of bone apposition | [155] |
| Alkali-heat treated Ti coated with SrTiO3 nanolayer with different Sr content: AH-Ti/Sr30, AH-Ti/Sr90, AH-Ti/Sr150 | In vitro → biological evaluation with MC3T3-E1 cell line In vivo → implantation in pile road of the femur of normal and osteoporotic female adult Sprague Dawley rats | In vitro → cytocompatibility, stimulation of osteogenic differentiation while hindering osteoclastogenesis In vivo → promotion of osseointegration both in normal and osteoporotic rat models | [156] |
| SrRan loaded mesoporous titania thin coatings deposited on mini-screws made of cp Ti grade IV | In vivo → implantation in bone tibia defect of Sprague Dawley female rats | Woven bone formation around the surface of all implants already after 2 weeks | [157] |
| Porous scaffold made of Ti with Sr and Ag ions (AH-Sr-AgNPs) | In vitro → biological evaluation with Raw 264.7 cells and MC3T3 cells and antibacterial property with Escherichia coli and Staphylococcus aureus In vivo → implantation on infected New Zealand rabbit femoral metaphysis defect | In vitro → M2 polarization of macrophages using Raw 264.7 cells and promotion of pre-osteoblast differentiation of MC3T3 cells with higher expression of ALP, RUNX2, and COL1. Promotion of an adverse microenvironment for bacterial survival In vivo → complete bone coverage and penetration into the pores of AH-Sr-AgNPs | [158] |
| Topologically ordered porous implant by additive manufacturing made from Ti-6Al-4V functionalized with Sr ions | In vitro → biological evaluation with MC3T3-E1 cells Ex vivo → antibacterial evaluation with highly virulent and multidrug-resistant Staphylococcus aureus by intraosseous infection model consisting of murine femora | In vitro → higher levels of ALP activity in MC3T3-E1 cells Ex vivo → Bactericidal effects with total eradication of both planktonic and adherent bacteria | [159] |
| Sr and Ag loaded nanotubular structures with controlled and prolonged release | In vitro → biological evaluation with MC3T3-E1 cells and antibacterial evaluation with methicillin-resistant Staphylococcus aureus, methicillin-sensitive Staphylococcus aureus, and Escherichia coli In vivo → implantation in bone defect below the epiphyseal plate of both normal and osteoporotic Sprague Dawley rats | In vitro → enhanced cell adhesion, migration, and proliferation of MC3T3-E1 cells with the up-regulated expression of osteogenic genes and induced mineralization. Antibacterial activity in vitro due to the release of Ag In vivo → accelerated formation of new bone in both osteoporotic and bone defect models | [160] |
9.4. Polymers
| Material | In Vivo/In Vitro Evaluation | Results | Reference |
|---|---|---|---|
| Collagen scaffold reinforced with Sr−graphene oxide | In vitro → biological evaluation with human adipose-derived stem cells and human umbilical vein endothelial cell In vivo → implantation in a critical-size bone defect in rat | In vitro → cell adhesion and spreading, marked mineralization and enhanced ALP activity, with enhanced expression of VEGF and BMP-2, tube formation and angiogenesis In vivo → enhancement of bone regeneration after 12 weeks of implantation | [166] |
| Collagen-based material with Sr-doped MBGs | In vitro → biological evaluation with MG-63 cells | High biocompatibility | [167] |
| Collagen-based material with Sr-doped MBGs | In vitro → biological evaluation with MG-63 and Saos-2 cells | High biocompatibility | [168] |
| Collagen-based material with Sr-doped MBGs | In vitro → biological evaluation with an indirect co-culture of human osteoblasts and osteoclast precursors | High biocompatibility and ability to support viability and proliferation of human bone-derived cells | [169] |
| Chondroitin sulfate/silk fibroin blended membrane with microporous structure loaded with different concentrations of Sr | In vitro → biological evaluation with RAW 264.7 cells and human osteoblasts | Downregulation of pro-inflammatory cytokines in RAW 264.7 cells and upregulation of osteogenic factors in human osteoblasts | [170] |
| Sr-loaded silk fibroin nanofibrous membrane (Sr-SFM) (1%, 5%, and 10% Sr) | In vitro → biological evaluation with rat bone marrow stromal cells In vivo → implantation in rat calvarial defect model | In vitro → enhancement in cell numbers, cell adhesion and ALP activity in Sr-SFM in comparison to Sr-free counterpart In vivo → pronounced bone formation after 6 weeks (especially in 10% Sr-SFM group) | [171] |
| Porous calcined porcine bone scaffold coated with SrCl2 and PCL | In vitro → biological evaluation with human fetal mesenchymal stem cells (MSCs) In vivo → implantation in a bone defect in the tibia of male SD rats | In vitro → osteogenic differentiation of MSCs In vivo → a better new bone formation in the presence of Sr | [173] |
| Blend of PCL and poly(diisopropyl fumarate) enriched with 1% or 5% Sr | In vitro → biological evaluation with bone marrow stromal cells from young male WKAH/Hok Wistar rats In vivo → implantation in a circular bone defect in parietal bones of WKAH/Hok Wistar rats | In vitro → better proliferation and COL1 and ALP expression for blend + 1% Sr in comparison to Blend + 5% Sr In vivo → increased bone tissue regeneration and improved fibrous bridging for blend + 1% Sr | [174] |
| PCL–laponite–SrRan composite scaffold | In vitro → biological evaluation with human telomerase immortalized bone marrow derived skeletal stem cell line (hMSC-TERT) In vivo → implantation of hMSC-seeded PLS3 subcutaneously in SCID mice | In vitro → cell growth and osteogenic differentiation In vivo → vascularized ectopic bone formation | [175] |
| Microparticles composed of PLLA and PEG copolymer containing vancomycin and strontium-doped apatite | In vitro → biological evaluation with bone marrow mesenchymal stromal cells (BMSCs) derived from Sprague-Dawley rat In vivo → subcutaneous implantation in pockets in rabbit backs (ectopic site); implantation in a cylindrical infected bone defect in rabbit’s lateral femoral condyle | In vitro → antibacterial effect against Staphylococcus aureus and excellent biocompatibility with BMSCs In vivo → induction of neovascularization and ectopic osteogenesis; significant antibacterial activity and efficient new bone deposition | [177] |
| Membrane scaffold composed of a matrix of ionically cross-linked chitosan and microparticles of PCL containing 5 wt% Sr salts | In vitro → biological evaluation with MG-63 cells and hBMSCs In vivo → implantation in a subcutaneous model in rats | In vitro → absence of cytotoxicity, better adhesion and spreading, and higher ALP activity with MG-63 cells; good adhesion and proliferation together with higher ALP level for hBMSCs In vivo → biocompatible behaviour especially for Sr-containing membrane: less development of fibrosis with a thinner fibrous tissue | [178] |
| Black phosphorus (BPs) and SrCl2 with PLGA microspheres (BP-SrCl2/PLGA microspheres) as a near-infrared light-triggered drug delivery system | In vitro → biological evaluation with hMSCs In vivo → implantation in femoral defects of Wistar rats | In vitro → excellent cell viability, osteoblastic differentiation, and biodegradability In vivo → good bone regeneration capability | [179] |
| Sr encapsulated in PLA microcapsules | In vitro → biological evaluation with MG-63 cells | Absence of cytotoxic effect of microcapsule extracts | [180] |
10. Discussion
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Murray, T. Elementary Scots the Discovery of Strontium. Scott. Med. J. 1993, 38, 188–189. [Google Scholar] [CrossRef]
- Watts, P.; Howe, P. Strontium and Strontium Compounds; World Health Organization: Geneva, Switzerland, 2010; ISBN 978-92-4-153077-4. [Google Scholar]
- Nielsen, S.P. The biological role of strontium. Bone 2004, 35, 583–588. [Google Scholar] [CrossRef]
- Andersen, O.Z.; Offermanns, V.; Sillassen, M.; Almtoft, K.P.; Andersen, I.H.; Sørensen, S.; Jeppesen, C.S.; Kraft, D.C.; Bøttiger, J.; Rasse, M.; et al. Accelerated bone ingrowth by local delivery of strontium from surface functionalized titanium implants. Biomaterials 2013, 34, 5883–5890. [Google Scholar] [CrossRef]
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Trace element doping in calcium phosphate ceramics to Understand osteogenesis and angiogenesis. Trends Biotechnol. 2013, 31, 594–605. [Google Scholar] [CrossRef] [Green Version]
- Reginster, J.-Y.; Lecart, M.-P.; Deroisy, R.; Lousberg, C. Strontium ranelate: A new paradigm in the treatment of osteoporosis. Expert Opin. Investig. Drugs 2004, 13, 857–864. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Seeman, E.; De Vernejoul, M.C.; Adami, S.; Compston, J.; Phenekos, C.; Devogelaer, J.P.; Curiel, M.D.; Sawicki, A.; Goemaere, S.; et al. Strontium Ranelate Reduces the Risk of Nonvertebral Fractures in Postmenopausal Women with Osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) Study. J. Clin. Endocrinol. Metab. 2005, 90, 2816–2822. [Google Scholar] [CrossRef] [Green Version]
- Marie, P.J. Strontium as therapy for osteoporosis. Curr. Opin. Pharmacol. 2005, 5, 633–636. [Google Scholar] [CrossRef]
- Marie, P.J.; Hott, M.; Modrowski, D.; De Pollak, C.; Guillemain, J.; Deloffre, P.; Tsouderos, Y. An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen-deficient rats. J. Bone Miner. Res. 1993, 8, 607–615. [Google Scholar] [CrossRef]
- Meunier, P.J.; Roux, C.; Seeman, E.; Ortolani, S.; Badurski, J.E.; Spector, T.D.; Cannata-Andía, J.B.; Balogh, A.; Lemmel, E.-M.; Pors-Nielsen, S.; et al. The Effects of Strontium Ranelate on the Risk of Vertebral Fracture in Women with Postmenopausal Osteoporosis. N. Engl. J. Med. 2004, 350, 459–468. [Google Scholar] [CrossRef] [Green Version]
- Shorr, E.; Carter, A.C. The usefulness of strontium as an adjuvant to calcium in the remineralization of the skeleton in man. Bull. Hosp. Jt. Dis. 1952, 13, 59–66. [Google Scholar]
- Morohashi, T.; Izumisawa, T.; Matsumoto, A.; Yamada, S. The effects of stable strontium on calcium metabolism: I. Kinetic analysis of calcium metabolism in strontium-fed rats. J. Bone Miner. Metab. 1993, 11, 31–38. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Deroisy, R.; Dougados, M.; Jupsin, I.; Colette, J.; Roux, C. Prevention of Early Postmenopausal Bone Loss by Strontium Ranelate: The Randomized, Two-Year, Double-Masked, Dose-Ranging, Placebo-Controlled PREVOS Trial. Osteoporos. Int. 2002, 13, 925–931. [Google Scholar] [CrossRef]
- Pilmane, M.; Salma-Ancane, K.; Loca, D.; Locs, J.; Berzina-Cimdina, L. Strontium and strontium ranelate: Historical review of some of their functions. Mater. Sci. Eng. C 2017, 78, 1222–1230. [Google Scholar] [CrossRef]
- Kołodziejska, B.; Stępień, N.; Kolmas, J. The Influence of Strontium on Bone Tissue Metabolism and Its Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2021, 22, 6564. [Google Scholar] [CrossRef]
- Bellucci, D.; Veronesi, E.; Strusi, V.; Petrachi, T.; Murgia, A.; Mastrolia, I.; Dominici, M.; Cannillo, V. Human Mesenchymal Stem Cell Combined with a New Strontium-Enriched Bioactive Glass: An ex-vivo Model for Bone Regeneration. Materials 2019, 12, 3633. [Google Scholar] [CrossRef] [Green Version]
- Kruppke, B.; Ray, S.; Alt, V.; Rohnke, M.; Kern, C.; Kampschulte, M.; Heinemann, C.; Budak, M.; Adam, J.; Döhner, N.; et al. Gelatin-Modified Calcium/Strontium Hydrogen Phosphates Stimulate Bone Regeneration in Osteoblast/Osteoclast Co-Culture and in Osteoporotic Rat Femur Defects—In Vitro to In Vivo Translation. Molecules 2020, 25, 5103. [Google Scholar] [CrossRef]
- Xue, W.; Moore, J.L.; Hosick, H.L.; Bose, S.; Bandyopadhyay, A.; Lu, W.W.; Cheung, K.M.C.; Luk, K.D.K. Osteoprecursor cell response to strontium-containing hydroxyapatite ceramics. J. Biomed. Mater. Res. Part A 2006, 79A, 804–814. [Google Scholar] [CrossRef]
- Lourenço, A.H.; Neves, N.; Machado, C.; Sousa, S.R.; Lamghari, M.; Barrias, C.; Cabral, A.T.; Barbosa, M.A.; Ribeiro, C.C. Injectable hybrid system for strontium local delivery promotes bone regeneration in a rat critical-sized defect model. Sci. Rep. 2017, 7, 5098. [Google Scholar] [CrossRef] [Green Version]
- Marx, D.; Yazdi, A.R.; Papini, M.; Towler, M. A review of the latest insights into the mechanism of action of strontium in bone. Bone Rep. 2020, 12, 100273. [Google Scholar] [CrossRef]
- Blake, G.M.; Fogelman, I. Strontium ranelate: A novel treatment for postmenopausal osteoporosis: A review of safety and efficacy. Clin. Interv. Aging 2006, 1, 367–375. [Google Scholar] [CrossRef]
- Stepan, J.J. Strontium ranelate: In search for the mechanism of action. J. Bone Miner. Metab. 2013, 31, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Blake, G.M.; Compston, J.E.; Fogelman, I. Could strontium ranelate have a synergistic role in the treatment of osteoporosis? J. Bone Miner. Res. 2009, 24, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Donneau, A.-F.; Reginster, J.-Y. Cardiovascular safety of strontium ranelate: Real-life assessment in clinical practice. Osteoporos. Int. 2013, 25, 397–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Medicines Agency Protelos/Osseor to Remain Available but with Further Restrictions. 2014. Available online: https://www.ema.europa.eu/en/news/recommendation-restrict-use-protelos-osseor-strontium-ranelate (accessed on 28 December 2021).
- European Medicines Agency (2014) Strontium Ranelate. Summary of Product Characteristics. 2014. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/protelos (accessed on 28 December 2021).
- Compston, J. Strontium ranelate lives to fight another day. Maturitas 2014, 78, 75–76. [Google Scholar] [CrossRef]
- Audran, M.; Jakob, F.J.; Palacios, S.; Brandi, M.-L.; Bröll, H.; Hamdy, N.A.T.; Mccloskey, E.V. A large prospective European cohort study of patients treated with strontium ranelate and followed up over 3 years. Rheumatol. Int. 2013, 33, 2231–2239. [Google Scholar] [CrossRef] [Green Version]
- Reginster, J.-Y.; Brandi, M.-L.; Cannata-Andía, J.B.; Cooper, C.; Cortet, B.; Feron, J.-M.; Genant, H.; Palacios, S.; Ringe, J.D.; Rizzoli, R. The position of strontium ranelate in today’s management of osteoporosis. Osteoporos. Int. 2015, 26, 1667–1671. [Google Scholar] [CrossRef]
- Pin, J.-P.; Galvez, T.; Prézeau, L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol. Ther. 2003, 98, 325–354. [Google Scholar] [CrossRef]
- Brown, E.M.; MacLeod, R.J. Extracellular Calcium Sensing and Extracellular Calcium Signaling. Physiol. Rev. 2001, 81, 239–297. [Google Scholar] [CrossRef]
- Hannan, F.M.; Kallay, E.; Chang, W.; Brandi, M.L.; Thakker, R.V. The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases. Nat. Rev. Endocrinol. 2018, 15, 33–51. [Google Scholar] [CrossRef]
- Geng, Y.; Mosyak, L.; Kurinov, I.; Zuo, H.; Sturchler, E.; Cheng, T.C.; Subramanyam, P.; Brown, A.P.; Brennan, S.C.; Mun, H.-C.; et al. Author response: Structural mechanism of ligand activation in human calcium-sensing receptor. Elife 2016, 5, 1–25. [Google Scholar] [CrossRef]
- Chattopadhyay, N.; Quinn, S.J.; Kifor, O.; Ye, C.; Brown, E.M. The calcium-sensing receptor (CaR) is involved in strontium ranelate-induced osteoblast proliferation. Biochem. Pharmacol. 2007, 74, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.C.; Thiem, U.; Roth, S.; Aggarwal, A.; Fetahu, I.S.; Tennakoon, S.; Gomes, A.R.; Brandi, M.L.; Bruggeman, F.; Mentaverri, R.; et al. Calcium sensing receptor signalling in physiology and cancer. Biochim. Biophys. Acta—Mol. Cell Res. 2013, 1833, 1732–1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goltzman, D.; Hendy, G.N. The calcium-sensing receptor in bone—Mechanistic and therapeutic insights. Nat. Rev. Endocrinol. 2015, 11, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M. Is the calcium receptor a molecular target for the actions of strontium on bone? Osteoporos. Int. 2003, 14, 25–34. [Google Scholar] [CrossRef]
- Fromigué, O.; Haã¿, E.; Barbara, A.; Petrel, C.; Traiffort, E.; Ruat, M.; Marie, P.J. Calcium sensing receptor-dependent and receptor-independent activation of osteoblast replication and survival by strontium ranelate. J. Cell. Mol. Med. 2009, 13, 2189–2199. [Google Scholar] [CrossRef]
- Pi, M.; Quarles, L.D. A Novel Cation-Sensing Mechanism in Osteoblasts Is a Molecular Target for Strontium. J. Bone Miner. Res. 2004, 19, 862–869. [Google Scholar] [CrossRef]
- Marie, P.J.; Ammann, P.; Boivin, G.; Rey, C. Mechanisms of Action and Therapeutic Potential of Strontium in Bone. Calcif. Tissue Res. 2001, 69, 121–129. [Google Scholar] [CrossRef]
- Dahl, S.G.; Allain, P.; Marie, P.; Mauras, Y.; Boivin, G.; Ammann, P.; Tsouderos, Y.; Delmas, P.; Christiansen, C. Incorporation and distribution of strontium in bone. Bone 2001, 28, 446–453. [Google Scholar] [CrossRef]
- O’Flaherty, E.J. Modeling bone mineral metabolism, with special reference to calcium and lead. Neurotoxicology 1992, 13, 789–797. [Google Scholar]
- Johnson, A.R.; Armstrong, W.D.; Singer, L. The incorporation and removal of large amounts of strontium by physiologic mechanisms in mineralized tissues of the rat. Calcif. Tissue Res. 1968, 2, 242–252. [Google Scholar] [CrossRef]
- Boivin, G.; Deloffre, P.; Perrat, B.; Panczer, G.; Boudeulle, M.; Mauras, Y.; Allain, P.; Tsouderos, Y.; Meunier, P.J. Strontium distribution and interactions with bone mineral in monkey iliac bone after strontium salt (S 12911) administration. J. Bone Miner. Res. 2009, 11, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Doublier, A.; Farlay, D.; Khebbab, M.T.; Jaurand, X.; Meunier, P.J.; Boivin, G. Distribution of strontium and mineralization in iliac bone biopsies from osteoporotic women treated long-term with strontium ranelate. Eur. J. Endocrinol. 2011, 165, 469–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, D.G.A.; Featherstone, J.D.B.; Duncan, J.F.; Cutress, T.W. Paracrystalline Disorder of Biological and Synthetic Carbonate-substituted Apatites. J. Dent. Res. 1982, 61, 1274–1281. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Chemical and Crystallographic Events in the Caries Process. J. Dent. Res. 1990, 69, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Berebichez-Fridman, R.; Gómez-García, R.; Granados-Montiel, J.; Berebichez-Fastlicht, E.; Olivos-Meza, A.; Granados, J.; Velasquillo, C.; Ibarra, C. The Holy Grail of Orthopedic Surgery: Mesenchymal Stem Cells—Their Current Uses and Potential Applications. Stem Cells Int. 2017, 2017, 2638305. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Kamali, A.; Moshiri, A.; Eslaminejad, M.B. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? Cells Tissues Organs 2017, 204, 59–83. [Google Scholar] [CrossRef]
- Yang, F.; Yang, D.; Tu, J.; Zheng, Q.; Cai, L.; Wang, L. Strontium Enhances Osteogenic Differentiation of Mesenchymal Stem Cells and In Vivo Bone Formation by Activating Wnt/Catenin Signaling. Stem Cells 2011, 29, 981–991. [Google Scholar] [CrossRef]
- Su, T.-R.; Huang, T.-H.; Kao, C.-T.; Ng, H.Y.; Chiu, Y.-C.; Hsu, T.-T. The Calcium Channel Affect Osteogenic Differentiation of Mesenchymal Stem Cells on Strontium-Substituted Calcium Silicate/Poly-ε-Caprolactone Scaffold. Processes 2020, 8, 198. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, J.; Zhu, S.; Luo, E.; Feng, G.; Chen, Q.; Hu, J. Effects of strontium on proliferation and differentiation of rat bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2012, 418, 725–730. [Google Scholar] [CrossRef]
- Sila-Asna, M.; Bunyaratvej, A.; Maeda, S.; Kitaguchi, H.; Bunyaratavej, N. Osteoblast differentiation and bone formation gene expression in strontium-inducing bone marrow mesenchymal stem cell. Kobe J. Med. Sci. 2007, 53, 25–35. [Google Scholar] [PubMed]
- Canalis, E. The divalent strontium salt S12911 enhances bone cell replication and bone formation in vitro. Bone 1996, 18, 517–523. [Google Scholar] [CrossRef]
- Peng, S.; Liu, X.S.; Huang, S.; Li, Z.; Pan, H.; Zhen, W.; Luk, K.D.K.; Guo, X.E.; Lu, W.W. The cross-talk between osteoclasts and osteoblasts in response to strontium treatment: Involvement of osteoprotegerin. Bone 2011, 49, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-L.; Zaidi, S.; Peng, Y.; Zhou, H.; Moonga, B.S.; Blesius, A.; Dupin-Roger, I.; Zaidi, M.; Sun, L. Induction of a program gene expression during osteoblast differentiation with strontium ranelate. Biochem. Biophys. Res. Commun. 2007, 355, 307–311. [Google Scholar] [CrossRef]
- Choudhary, S.; Halbout, P.; Alander, C.; Raisz, L.; Pilbeam, C. Strontium Ranelate Promotes Osteoblastic Differentiation and Mineralization of Murine Bone Marrow Stromal Cells: Involvement of Prostaglandins. J. Bone Miner. Res. 2007, 22, 1002–1010. [Google Scholar] [CrossRef]
- Brennan, T.C.; Rybchyn, M.S.; Green, W.; Atwa, S.; Conigrave, A.D.; Mason, R.S. Osteoblasts play key roles in the mechanisms of action of strontium ranelate. J. Cereb. Blood Flow Metab. 2009, 157, 1291–1300. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, S.; Wadhwa, S.; Raisz, L.G.; Alander, C.; Pilbeam, C.C. Extracellular Calcium Is a Potent Inducer of Cyclo-oxygenase-2 in Murine Osteoblasts Through an ERK Signaling Pathway. J. Bone Miner. Res. 2003, 18, 1813–1824. [Google Scholar] [CrossRef]
- Barbara, A.; Delannoy, P.; Denis, B.G.; Marie, P.J. Normal matrix mineralization induced by strontium ranelate in MC3T3-E1 osteogenic cells. Metabolism 2004, 53, 532–537. [Google Scholar] [CrossRef]
- Fonseca, J.E. Rebalancing bone turnover in favour of formation with strontium ranelate: Implications for bone strength. Rheumatology 2008, 47, iv17–iv19. [Google Scholar] [CrossRef] [Green Version]
- Bonnelye, E.; Chabadel, A.; Saltel, F.; Jurdic, P. Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 2008, 42, 129–138. [Google Scholar] [CrossRef]
- Wornham, D.P.; Hajjawi, M.O.; Orriss, I.R.; Arnett, T.R. Strontium potently inhibits mineralisation in bone-forming primary rat osteoblast cultures and reduces numbers of osteoclasts in mouse marrow cultures. Osteoporos. Int. 2014, 25, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Verberckmoes, S.C.; De Broe, M.E.; D’Haese, P.C. Dose-dependent effects of strontium on osteoblast function and mineralization. Kidney Int. 2003, 64, 534–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybchyn, M.S.; Slater, M.; Conigrave, A.D.; Mason, R.S. An Akt-dependent Increase in Canonical Wnt Signaling and a Decrease in Sclerostin Protein Levels Are Involved in Strontium Ranelate-induced Osteogenic Effects in Human Osteoblasts. J. Biol. Chem. 2011, 286, 23771–23779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saidak, Z.; Marie, P.J. Strontium signaling: Molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol. Ther. 2012, 136, 216–226. [Google Scholar] [CrossRef]
- Marie, P.J.; Miraoui, H.; Sévère, N. FGF/FGFR signaling in bone formation: Progress and perspectives. Growth Factors 2012, 30, 117–123. [Google Scholar] [CrossRef]
- Caverzasio, J. Strontium ranelate promotes osteoblastic cell replication through at least two different mechanisms. Bone 2008, 42, 1131–1136. [Google Scholar] [CrossRef]
- Caverzasio, J.; Thouverey, C. Activation of FGF Receptors is a new Mechanism by which Strontium Ranelate Induces Osteoblastic Cell Growth. Cell. Physiol. Biochem. 2011, 27, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Pi, M.; Faber, P.; Ekema, G.; Jackson, P.D.; Ting, A.; Wang, N.; Fontilla-Poole, M.; Mays, R.W.; Brunden, K.R.; Harrington, J.J.; et al. Identification of a Novel Extracellular Cation-sensing G-protein-coupled Receptor. J. Biol. Chem. 2005, 280, 40201–40209. [Google Scholar] [CrossRef] [Green Version]
- Pi, M.; Chen, L.; Huang, M.-Z.; Zhu, W.; Ringhofer, B.; Luo, J.; Christenson, L.; Li, B.; Zhang, J.; Jackson, P.D.; et al. GPRC6A Null Mice Exhibit Osteopenia, Feminization and Metabolic Syndrome. PLoS ONE 2008, 3, e3858. [Google Scholar] [CrossRef]
- Pi, M.; Zhang, L.; Lei, S.-F.; Huang, M.-Z.; Zhu, W.; Zhang, J.; Shen, H.; Deng, H.-W.; Quarles, L.D. Impaired Osteoblast Function inGPRC6ANull Mice. J. Bone Miner. Res. 2009, 25, 1092–1137. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Zhang, B.; Zhu, X.; Ao, P.; Guo, H.; Yi, W.; Zhou, G.-Q. Deregulation of Bone Forming Cells in Bone Diseases and Anabolic Effects of Strontium-Containing Agents and Biomaterials. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Baron, R.; Tsouderos, Y. In vitro effects of S12911-2 on osteoclast function and bone marrow macrophage differentiation. Eur. J. Pharmacol. 2002, 450, 11–17. [Google Scholar] [CrossRef]
- Descartes, M.; Sillence, D.O. Disorders of Bone Density, Volume, and Mineralization. In Emery and Rimoin’s Principles and Practice of Medical Genetics; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–20. ISBN 9780123838346. [Google Scholar]
- Billecocq, A.; Emanuel, J.R.; Levenson, R.; Baron, R. 1 alpha,25-dihydroxyvitamin D3 regulates the expression of carbonic anhydrase II in nonerythroid avian bone marrow cells. Proc. Natl. Acad. Sci. USA 1990, 87, 6470–6474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lomri, A.; Baron, R. 1 alpha,25-dihydroxyvitamin D3 regulates the transcription of carbonic anhydrase II mRNA in avian myelomonocytes. Proc. Natl. Acad. Sci. USA 1992, 89, 4688–4692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakkakorpi, P.T.; Väänänen, H.K. Cytoskeletal changes in osteoclasts during the resorption cycle. Microsc. Res. Tech. 1996, 33, 171–181. [Google Scholar] [CrossRef]
- Schumacher, M.; Wagner, A.S.; Kokesch-Himmelreich, J.; Bernhardt, A.; Rohnke, M.; Wenisch, S.; Gelinsky, M. Strontium substitution in apatitic CaP cements effectively attenuates osteoclastic resorption but does not inhibit osteoclastogenesis. Acta Biomater. 2016, 37, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Sasaki, T.; Tsouderos, Y.; Suda, T. S 12911-2 Inhibits Osteoclastic Bone Resorption In Vitro. J. Bone Miner. Res. 2003, 18, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Caudrillier, A.; Hurtel-Lemaire, A.-S.; Wattel, A.; Cournarie, F.; Godin, C.; Petit, L.; Petit, J.-P.; Terwilliger, E.; Kamel, S.; Brown, E.M.; et al. Strontium Ranelate Decreases Receptor Activator of Nuclear Factor-κB Ligand-Induced Osteoclastic Differentiation In Vitro: Involvement of the Calcium-Sensing Receptor. Mol. Pharmacol. 2010, 78, 569–576. [Google Scholar] [CrossRef]
- Coulombe, J.; Faure, H.; Robin, B.; Ruat, M. In vitro effects of strontium ranelate on the extracellular calcium-sensing receptor. Biochem. Biophys. Res. Commun. 2004, 323, 1184–1190. [Google Scholar] [CrossRef]
- Hurtel-Lemaire, A.S.; Mentaverri, R.; Caudrillier, A.; Cournarie, F.; Wattel, A.; Kamel, S.; Terwilliger, E.F.; Brown, E.M.; Brazier, M. The Calcium-sensing Receptor Is Involved in Strontium Ranelate-induced Osteoclast Apoptosis. J. Biol. Chem. 2009, 284, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Boyce, B.F.; Xing, L. The RANKL/RANK/OPG pathway. Curr. Osteoporos. Rep. 2007, 5, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Atkins, G.J.; Welldon, K.J.; Halbout, P.; Findlay, D.M. Strontium ranelate treatment of human primary osteoblasts promotes an osteocyte-like phenotype while eliciting an osteoprotegerin response. Osteoporos. Int. 2008, 20, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shen, Y.; Pan, H.; Lin, K.; Liu, X.; Darvell, B.W.; Lu, W.W.; Chang, J.; Deng, L.; Wang, D.; et al. Effects of strontium in modified biomaterials. Acta Biomater. 2011, 7, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Forsgren, J.; Engqvist, H. A novel method for local administration of strontium from implant surfaces. J. Mater. Sci. Mater. Med. 2010, 21, 1605–1609. [Google Scholar] [CrossRef]
- Pina, S.; Torres, P.M.C.; Goetz-Neunhoeffer, F.; Neubauer, J.; Ferreira, J.M.F. Newly developed Sr-substituted α-TCP bone cements. Acta Biomater. 2010, 6, 928–935. [Google Scholar] [CrossRef]
- Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R.; et al. Applications of Metals for Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef] [Green Version]
- Jiwoon, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Genetos, D.C.; Shao, Y.; Geist, D.J.; Li, J.; Ke, H.Z.; Turner, C.H.; Duncan, R.L. Activation of extracellular-signal regulated kinase (ERK1/2) by fluid shear is Ca2+- and ATP-dependent in MC3T3-E1 osteoblasts. Bone 2008, 42, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Danciu, T.E.; Adam, R.M.; Naruse, K.; Freeman, M.R.; Hauschka, P.V. Calcium regulates the PI3K-Akt pathway in stretched osteoblasts. FEBS Lett. 2003, 536, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, Y.; Hisatsune, C.; Nakamura, T.; Matsuo, K.; Mikoshiba, K. Osteoblasts induce Ca2+ oscillation-independent NFATc1 activation during osteoclastogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 8643–8648. [Google Scholar] [CrossRef] [Green Version]
- Julien, M.; Khoshniat, S.; Lacreusette, A.; Gatius, M.; Bozec, A.; Wagner, E.F.; Wittrant, Y.; Masson, M.; Weiss, P.; Beck, L.; et al. Phosphate-Dependent Regulation of MGP in Osteoblasts: Role of ERK1/2 and Fra-1. J. Bone Miner. Res. 2009, 24, 1856–1868. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Nemoto, E.; Foster, B.L.; Somerman, M.J.; Shimauchi, H. Phosphate increases bone morphogenetic protein-2 expression through cAMP-dependent protein kinase and ERK1/2 pathways in human dental pulp cells. Bone 2011, 48, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Mozar, A.; Haren, N.; Chasseraud, M.; Louvet, L.; Mazière, C.; Wattel, A.; Mentaverri, R.; Morlière, P.; Kamel, S.; Brazier, M.; et al. High extracellular inorganic phosphate concentration inhibits RANK-RANKL signaling in osteoclast-like cells. J. Cell. Physiol. 2007, 215, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lu, Y.; Ye, L.; Yuan, B.; Yu, S.; Qin, C.; Xie, Y.; Gao, T.; Drezner, M.K.; Bonewald, L.F.; et al. Unique roles of phosphorus in endochondral bone formation and osteocyte maturation. J. Bone Miner. Res. 2010, 26, 1047–1056. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.J.; Kasper, F.K.; Mikos, A.G. Biomaterials for Tissue Engineering. Ann. Biomed. Eng. 2014, 42, 323–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laskus, A.; Kolmas, J. Ionic Substitutions in Non-Apatitic Calcium Phosphates. Int. J. Mol. Sci. 2017, 18, 2542. [Google Scholar] [CrossRef] [Green Version]
- Chandran, S.; John, A. Osseointegration of osteoporotic bone implants: Role of stem cells, Silica and Strontium—A concise review. J. Clin. Orthop. Trauma 2018, 10, S32–S36. [Google Scholar] [CrossRef]
- Ni, G.X.; Chiu, K.-Y.; Lu, W.W.; Wang, Y.; Zhang, Y.G.; Hao, L.B.; Li, Z.Y.; Lam, W.M.; Lu, S.B.; Luk, K.D.K. Strontium-containing hydroxyapatite bioactive bone cement in revision hip arthroplasty. Biomaterials 2006, 27, 4348–4355. [Google Scholar] [CrossRef]
- Ni, G.X.; Lu, W.W.; Chiu, K.Y.; Li, Z.Y.; Fong, D.Y.T.; Luk, K.D.K. Strontium-containing hydroxyapatite (Sr-HA) bioactive cement for primary hip replacement: An in vivo study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 77B, 409–415. [Google Scholar] [CrossRef]
- Tian, M.; Chen, F.; Song, W.; Song, Y.; Chen, Y.; Wan, C.; Yu, X.; Zhang, X. In vivo study of porous strontium-doped calcium polyphosphate scaffolds for bone substitute applications. J. Mater. Sci. Mater. Med. 2009, 20, 1505–1512. [Google Scholar] [CrossRef]
- Thormann, U.; Ray, S.; Sommer, U.; El Khassawna, T.; Rehling, T.; Hundgeburth, M.; Henß, A.; Rohnke, M.; Janek, J.; Lips, K.S.; et al. Bone formation induced by strontium modified calcium phosphate cement in critical-size metaphyseal fracture defects in ovariectomized rats. Biomaterials 2013, 34, 8589–8598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.W.; Feng, T.; Shi, G.Q.; Ding, Y.L.; Yu, X.X.; Zhang, X.H.; Zhang, Z.B.; Wan, C.X. Interaction of endothelial cells with biodegradable strontium-doped calcium polyphosphate for bone tissue engineering. Appl. Surf. Sci. 2008, 255, 331–335. [Google Scholar] [CrossRef]
- Capuccini, C.; Torricelli, P.; Sima, F.; Boanini, E.; Ristoscu, C.; Bracci, B.; Socol, G.; Fini, M.; Mihailescu, I.N.; Bigi, A. Strontium-substituted hydroxyapatite coatings synthesized by pulsed-laser deposition: In vitro osteoblast and osteoclast response. Acta Biomater. 2008, 4, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Xia, P.; Xu, S.; Zhang, K.; Li, G.; Cui, L.; Yin, J. Nanocomposite Porous Microcarriers Based on Strontium-Substituted HA-g-Poly(γ-benzyl-l-glutamate) for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2018, 10, 16270–16281. [Google Scholar] [CrossRef]
- Fu, J.; Zhuang, C.; Qiu, J.; Ke, X.; Yang, X.; Jin, Z.; Zhang, L.; Yang, G.; Xie, L.; Xu, S.; et al. Core–Shell Biphasic Microspheres with Tunable Density of Shell Micropores Providing Tailorable Bone Regeneration. Tissue Eng. Part A 2019, 25, 588–602. [Google Scholar] [CrossRef]
- Liu, D.; Nie, W.; Li, D.; Wang, W.; Zheng, L.; Zhang, J.; Zhang, J.; Peng, C.; Mo, X.; He, C. 3D printed PCL/SrHA scaffold for enhanced bone regeneration. Chem. Eng. J. 2019, 362, 269–279. [Google Scholar] [CrossRef]
- Reitmaier, S.; Kovtun, A.; Schuelke, J.; Kanter, B.; Lemm, M.; Hoess, A.; Heinemann, S.; Nies, B.; Ignatius, A. Strontium(II) and mechanical loading additively augment bone formation in calcium phosphate scaffolds. J. Orthop. Res. 2018, 36, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Gu, Z.; He, Y.; Xu, J.; Xu, C.; Li, L.; Ye, Q. Microenvironment construction of strontium–calcium-based biomaterials for bone tissue regeneration: The equilibrium effect of calcium to strontium. J. Mater. Chem. B 2018, 6, 2332–2339. [Google Scholar] [CrossRef]
- Salamanna, F.; Giavaresi, G.; Contartese, D.; Bigi, A.; Boanini, E.; Parrilli, A.; Lolli, R.; Gasbarrini, A.; Brodano, G.B.; Fini, M. Effect of strontium substituted ß-TCP associated to mesenchymal stem cells from bone marrow and adipose tissue on spinal fusion in healthy and ovariectomized rat. J. Cell. Physiol. 2019, 234, 20046–20056. [Google Scholar] [CrossRef]
- Chakar, C.; Naaman, N.; Soffer, E.; Cohen, N.; El Osta, N.; Petite, H.; Anagnostou, F. Bone Formation with Deproteinized Bovine Bone Mineral or Biphasic Calcium Phosphate in the Presence of Autologous Platelet Lysate: Comparative Investigation in Rabbit. Int. J. Biomater. 2014, 2014, 367265. [Google Scholar] [CrossRef] [Green Version]
- Aroni, M.A.T.; De Oliveira, G.J.P.L.; Spolidorio, L.C.; Andersen, O.Z.; Foss, M.; Marcantonio, R.A.C.; Stavropoulos, A. Loading deproteinized bovine bone with strontium enhances bone regeneration in rat calvarial critical size defects. Clin. Oral Investig. 2018, 23, 1605–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elgali, I.; Turri, A.; Xia, W.; Norlindh, B.; Johansson, A.; Dahlin, C.; Thomsen, P.; Omar, O. Guided bone regeneration using resorbable membrane and different bone substitutes: Early histological and molecular events. Acta Biomater. 2016, 29, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Pickrell, G.; Sriranganathan, N.; Kumar, V.; Homa, D. Review and the state of the art: Sol-gel and melt quenched bioactive glasses for tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 104, 1248–1275. [Google Scholar] [CrossRef] [PubMed]
- Arcos, D.; Vallet-Regí, M. Sol–gel silica-based biomaterials and bone tissue regeneration. Acta Biomater. 2010, 6, 2874–2888. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Salinas, A. Mesoporous bioactive glasses for regenerative medicine. Mater. Today Bio 2021, 11, 100121. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, V.; Baino, F.; Mauro, J.C.; Pickrell, G.; Evans, I.; Bretcanu, O. Mechanical properties of bioactive glasses, ceramics, glass-ceramics and composites: State-of-the-art review and future challenges. Mater. Sci. Eng. C 2019, 104, 109895. [Google Scholar] [CrossRef]
- Bari, A.; Molino, G.; Fiorilli, S.; Vitale-Brovarone, C. Novel multifunctional strontium-copper co-substituted mesoporous bioactive particles. Mater. Lett. 2018, 223, 37–40. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Niu, W.; Lei, B.; Boccaccini, A.R. Immunomodulatory bioactive glasses for tissue regeneration. Acta Biomater. 2021, 133, 168–186. [Google Scholar] [CrossRef]
- O’Donnell, M.D.; Hill, R.G. Influence of strontium and the importance of glass chemistry and structure when designing bioactive glasses for bone regeneration. Acta Biomater. 2010, 6, 2382–2385. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, L.; Chang, J.; Miron, R.J.; Shi, B.; Yi, S.; Wu, C. Strontium-incorporated mesoporous bioactive glass scaffolds stimulating in vitro proliferation and differentiation of bone marrow stromal cells and in vivo regeneration of osteoporotic bone defects. J. Mater. Chem. B 2013, 1, 5711–5722. [Google Scholar] [CrossRef] [PubMed]
- Fiorilli, S.; Molino, G.; Pontremoli, C.; Iviglia, G.; Torre, E.; Cassinelli, C.; Morra, M.; Vitale-Brovarone, C.; Fiorilli, S.; Molino, G.; et al. The Incorporation of Strontium to Improve Bone-Regeneration Ability of Mesoporous Bioactive Glasses. Materials 2018, 11, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pontremoli, C.; Izquierdo-Barba, I.; Montalbano, G.; Vallet-Regí, M.; Vitale-Brovarone, C.; Fiorilli, S. Strontium-releasing mesoporous bioactive glasses with anti-adhesive zwitterionic surface as advanced biomaterials for bone tissue regeneration. J. Colloid Interface Sci. 2020, 563, 92–103. [Google Scholar] [CrossRef]
- Fiorilli, S.; Pagani, M.; Boggio, E.; Gigliotti, C.L.; Dianzani, C.; Gauthier, R.; Pontremoli, C.; Montalbano, G.; Dianzani, U.; Vitale-Brovarone, C. Sr-Containing Mesoporous Bioactive Glasses Bio-Functionalized with Recombinant ICOS-Fc: An In Vitro Study. Nanomaterials 2021, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Autefage, H.; Allen, F.; Tang, H.M.; Kallepitis, C.; Gentleman, E.; Reznikov, N.; Nitiputri, K.; Nommeots-Nomm, A.; O’Donnell, M.D.; Lange, C.; et al. Multiscale analyses reveal native-like lamellar bone repair and near perfect bone-contact with porous strontium-loaded bioactive glass. Biomaterials 2019, 209, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Shaltooki, M.; Dini, G.; Mehdikhani, M. Fabrication of chitosan-coated porous polycaprolactone/strontium-substituted bioactive glass nanocomposite scaffold for bone tissue engineering. Mater. Sci. Eng. C 2019, 105, 110138. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Geng, S.; Wei, L.; Miron, R.J.; Zhao, Y.; Zhang, Y. Nanogel-based scaffolds fabricated for bone regeneration with mesoporous bioactive glass and strontium: In vitro and in vivo characterization. J. Biomed. Mater. Res. Part A 2017, 105, 1175–1183. [Google Scholar] [CrossRef]
- Poh, P.S.P.; Hutmacher, D.W.; Stevens, M.M.; Woodruff, M.A. Fabrication and in vitro characterization of bioactive glass composite scaffolds for bone regeneration. Biofabrication 2013, 5, 045005. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Zhou, Y.; Lin, C.; Chang, J.; Xiao, Y. Strontium-containing mesoporous bioactive glass scaffolds with improved osteogenic/cementogenic differentiation of periodontal ligament cells for periodontal tissue engineering. Acta Biomater. 2012, 8, 3805–3815. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, S.; Zhu, Y.; Huang, Y.; Zhu, M.; Tao, C.; Zhang, C. Three-dimensional printing of strontium-containing mesoporous bioactive glass scaffolds for bone regeneration. Acta Biomater. 2014, 10, 2269–2281. [Google Scholar] [CrossRef]
- Ren, J.; Blackwood, K.A.; Doustgani, A.; Poh, P.P.; Steck, R.; Stevens, M.M.; Woodruff, M.A. Melt-electrospun polycaprolactone strontium-substituted bioactive glass scaffolds for bone regeneration. J. Biomed. Mater. Res. Part A 2013, 102, 3140–3153. [Google Scholar] [CrossRef] [PubMed]
- Midha, S.; Kumar, S.; Sharma, A.; Kaur, K.; Shi, X.; Naruphontjirakul, P.; Jones, J.R.; Ghosh, S. Silk fibroin-bioactive glass based advanced biomaterials: Towards patient-specific bone grafts. Biomed. Mater. 2018, 13, 055012. [Google Scholar] [CrossRef] [PubMed]
- Poh, P.S.P.; Hutmacher, D.W.; Holzapfel, B.M.; Solanki, A.K.; Stevens, M.M.; Woodruff, M.A. In vitro and in vivo bone formation potential of surface calcium phosphate-coated polycaprolactone and polycaprolactone/bioactive glass composite scaffolds. Acta Biomater. 2016, 30, 319–333. [Google Scholar] [CrossRef]
- Baheiraei, N.; Eyni, H.; Bakhshi, B.; Najafloo, R.; Rabiee, N. Effects of strontium ions with potential antibacterial activity on in vivo bone regeneration. Sci. Rep. 2021, 11, 8745. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Montazerian, M.; Fiume, E.; Baino, F. Multiple and Promising Applications of Strontium (Sr)-Containing Bioactive Glasses in Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, X.; Huang, C.; Zhang, M.; Ruan, C.; Peng, S.; Li, L.; Liu, W.; Wang, T.; Li, B.; Huang, W.; et al. Enhanced osteointegration of poly(methylmethacrylate) bone cements by incorporating strontium-containing borate bioactive glass. J. R. Soc. Interface 2017, 14, 20161057. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.S.; Gentile, P.; Martins, M.; Neves, N.M.; Miller, C.; Crawford, A.; Pires, R.A.; Hatton, P.; Reis, R.L. Reinforcement of poly-l-lactic acid electrospun membranes with strontium borosilicate bioactive glasses for bone tissue engineering. Acta Biomater. 2016, 44, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, J.S.; Gentile, P.; Crawford, A.; Pires, R.A.; Hatton, P.V.; Reis, R.L. Substituted Borosilicate Glasses with Improved Osteogenic Capacity for Bone Tissue Engineering. Tissue Eng. Part A 2017, 23, 1331–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brow, R.K. Review: The structure of simple phosphate glasses. J. Non-Cryst. Solids 2000, 263–264, 1–28. [Google Scholar] [CrossRef]
- Lakhkar, N.J.; Lee, I.-H.; Kim, H.-W.; Salih, V.; Wall, I.B.; Knowles, J.C. Bone formation controlled by biologically relevant inorganic ions: Role and controlled delivery from phosphate-based glasses. Adv. Drug Deliv. Rev. 2013, 65, 405–420. [Google Scholar] [CrossRef]
- Patel, U.; Macri-Pellizzeri, L.; Hossain, K.M.Z.; Scammell, B.E.; Grant, D.M.; Scotchford, C.A.; Hannon, A.C.; Kennedy, A.R.; Barney, E.R.; Ahmed, I.; et al. In vitro cellular testing of strontium/calcium substituted phosphate glass discs and microspheres shows potential for bone regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 396–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanawa, T. Titanium–Tissue Interface Reaction and Its Control with Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, M.; Lagneau, C.; Moreno, J.; Lissac, M.; Dalard, F.; Grosgogeat, B. Corrosion resistance and biocompatibility of a new porous surface for titanium implants. Eur. J. Oral Sci. 2005, 113, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Jiang, J.; Huo, K.; Hu, T.; Chu, P.K. Bioactive SrTiO3 Nanotube Arrays: Strontium Delivery Platform on Ti-Based Osteoporotic Bone Implants. ACS Nano 2009, 3, 3228–3234. [Google Scholar] [CrossRef] [PubMed]
- Mi, B.; Xiong, W.; Xu, N.; Guan, H.; Fang, Z.; Liao, H.; Zhang, Y.; Gao, B.; Xiao, X.; Fu, J.; et al. Strontium-loaded titania nanotube arrays repress osteoclast differentiation through multiple signalling pathways: In vitro and in vivo studies. Sci. Rep. 2017, 7, 2328. [Google Scholar] [CrossRef] [PubMed]
- Mumith, A.; Cheong, V.S.; Fromme, P.; Coathup, M.J.; Blunn, G.W. The effect of strontium and silicon substituted hydroxyapatite electrochemical coatings on bone ingrowth and osseointegration of selective laser sintered porous metal implants. PLoS ONE 2020, 15, e0227232. [Google Scholar] [CrossRef] [PubMed]
- Okuzu, Y.; Fujibayashi, S.; Yamaguchi, S.; Yamamoto, K.; Shimizu, T.; Sono, T.; Goto, K.; Otsuki, B.; Matsushita, T.; Kokubo, T.; et al. Strontium and magnesium ions released from bioactive titanium metal promote early bone bonding in a rabbit implant model. Acta Biomater. 2017, 63, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-H.; Kim, Y.-J.; Jang, J.-H.; Park, J.-W. Modulating macrophage polarization with divalent cations in nanostructured titanium implant surfaces. Nanotechnology 2016, 27, 085101. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, Y.-J.; Jang, J.-H.; Park, J.-W. Surface Engineering of Nanostructured Titanium Implants with Bioactive Ions. J. Dent. Res. 2016, 95, 558–565. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.; Zhao, L. Antibacterial, angiogenic, and osteogenic activities of Ca, P, Co, F, and Sr compound doped titania coatings with different Sr content. Sci. Rep. 2019, 9, 14203. [Google Scholar] [CrossRef]
- Offermanns, V.; Andersen, O.Z.; Riede, G.; Sillassen, M.; Jeppesen, C.S.; Almtoft, K.P.; Talasz, H.; Öhman-Mägi, C.; Lethaus, B.; Tolba, R.; et al. Effect of strontium surface-functionalized implants on early and late osseointegration: A histological, spectrometric and tomographic evaluation. Acta Biomater. 2018, 69, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Q.; Hu, H.; Shi, C.; Lin, Z.; Jiang, H.; Dong, H.; Guo, J. The Fabrication and Function of Strontium-modified Hierarchical Micro/Nano Titanium Implant. Int. J. Nanomed. 2020, 15, 8983–8998. [Google Scholar] [CrossRef]
- Alenezi, A.; Galli, S.; Atefyekta, S.; Andersson, M.; Wennerberg, A. Osseointegration effects of local release of strontium ranelate from implant surfaces in rats. J. Mater. Sci. Mater. Med. 2019, 30, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Li, Y.; Shrestha, A.; Wang, S.; Wu, Q.; Li, L.; Guan, C.; Wang, C.; Fu, T.; Liu, W.; et al. Effects of Programmed Local Delivery from a Micro/Nano-Hierarchical Surface on Titanium Implant on Infection Clearance and Osteogenic Induction in an Infected Bone Defect. Adv. Healthc. Mater. 2019, 8, e1900002. [Google Scholar] [CrossRef] [PubMed]
- van Hengel, I.A.J.; Gelderman, F.S.A.; Athanasiadis, S.; Minneboo, M.; Weinans, H.; Fluit, A.C.; van der Eerden, B.C.J.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Functionality-packed additively manufactured porous titanium implants. Mater. Today Bio 2020, 7, 100060. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xiong, W.; Fang, Z.; Guan, H.; Wu, W.; Li, Y.; Zhang, Y.; Alvarez, M.M.; Gao, B.; Huo, K.; et al. Strontium (Sr) and silver (Ag) loaded nanotubular structures with combined osteoinductive and antimicrobial activities. Acta Biomater. 2016, 31, 388–400. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef]
- Bonani, W.; Singhatanadgige, W.; Pornanong, A.; Motta, A. Natural Origin Materials for Osteochondral Tissue Engineering. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1058, pp. 3–30. ISBN 9783319767116. [Google Scholar]
- Rao, S.H.; Harini, B.; Shadamarshan, R.P.K.; Balagangadharan, K.; Selvamurugan, N. Natural and synthetic polymers/bioceramics/bioactive compounds-mediated cell signalling in bone tissue engineering. Int. J. Biol. Macromol. 2018, 110, 88–96. [Google Scholar] [CrossRef]
- Lee, S.-H.; Shin, H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 339–359. [Google Scholar] [CrossRef]
- Lei, B.; Guo, B.; Rambhia, K.J.; Ma, P.X. Hybrid polymer biomaterials for bone tissue regeneration. Front. Med. 2018, 13, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zheng, Z.; Zhou, R.; Zhang, H.; Chen, C.; Xiong, Z.; Liu, K.; Wang, X. Developing a Strontium-Releasing Graphene Oxide-/Collagen-Based Organic–Inorganic Nanobiocomposite for Large Bone Defect Regeneration via MAPK Signaling Pathway. ACS Appl. Mater. Interfaces 2019, 11, 15986–15997. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, G.; Borciani, G.; Pontremoli, C.; Ciapetti, G.; Mattioli-Belmonte, M.; Fiorilli, S.; Vitale-Brovarone, C. Development and Biocompatibility of Collagen-Based Composites Enriched with Nanoparticles of Strontium Containing Mesoporous Glass. Materials 2019, 12, 3719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montalbano, G.; Borciani, G.; Cerqueni, G.; Licini, C.; Banche-Niclot, F.; Janner, D.; Sola, S.; Fiorilli, S.; Mattioli-Belmonte, M.; Ciapetti, G.; et al. Collagen Hybrid Formulations for the 3D Printing of Nanostructured Bone Scaffolds: An Optimized Genipin-Crosslinking Strategy. Nanomaterials 2020, 10, 1681. [Google Scholar] [CrossRef]
- Borciani, G.; Montalbano, G.; Melo, P.; Baldini, N.; Ciapetti, G.; Vitale-Brovarone, C. Assessment of Collagen-Based Nanostructured Biomimetic Systems with a Co-Culture of Human Bone-Derived Cells. Cells 2021, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Fenbo, M.; Xingyu, X.; Bin, T. Strontium chondroitin sulfate/silk fibroin blend membrane containing microporous structure modulates macrophage responses for guided bone regeneration. Carbohydr. Polym. 2019, 213, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, P.; Zhang, F.; Zhou, X.; Zuo, B.; You, X.; Gao, Y.; Liu, H.; Tang, H. A novel silk fibroin nanofibrous membrane for guided bone regeneration: A study in rat calvarial defects. Am. J. Transl. Res. 2015, 7, 2244–2253. [Google Scholar] [PubMed]
- Luz, E.P.C.G.; Borges, M.D.F.; Andrade, F.K.; Rosa, M.D.F.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Vieira, R.S. Strontium delivery systems based on bacterial cellulose and hydroxyapatite for guided bone regeneration. Cellulose 2018, 25, 6661–6679. [Google Scholar] [CrossRef] [Green Version]
- Cheng, D.; Liang, Q.; Li, Y.; Fan, J.; Wang, G.; Pan, H.; Ruan, C. Strontium incorporation improves the bone-forming ability of scaffolds derived from porcine bone. Colloids Surfaces B Biointerfaces 2018, 162, 279–287. [Google Scholar] [CrossRef]
- Lino, A.B.; McCarthy, A.D.; Fernández, J.M. Evaluation of Strontium-Containing PCL-PDIPF Scaffolds for Bone Tissue Engineering: In Vitro and In Vivo Studies. Ann. Biomed. Eng. 2019, 47, 902–912. [Google Scholar] [CrossRef]
- Prabha, R.D.; Nair, B.P.; Ditzel, N.; Kjems, J.; Nair, P.D.; Kassem, M. Strontium functionalized scaffold for bone tissue engineering. Mater. Sci. Eng. C 2019, 94, 509–515. [Google Scholar] [CrossRef]
- Jiménez, M.; Abradelo, C.; Román, J.S.; Rojo, L. Bibliographic review on the state of the art of strontium and zinc based regenerative therapies. Recent developments and clinical applications. J. Mater. Chem. B 2019, 7, 1974–1985. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Jing, W.; Yuan, Z.; Huang, Y.; Guan, B.; Zhang, W.; Zhang, X.; Mao, J.; Cai, Q.; Chen, D.; et al. Vancomycin- and Strontium-Loaded Microspheres with Multifunctional Activities against Bacteria, in Angiogenesis, and in Osteogenesis for Enhancing Infected Bone Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 30596–30609. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Méndez, I.; Fernández-Gutiérrez, M.; Rodríguez-Navarrete, A.; Rosales-Ibañez, R.; Benito-Garzón, L.; Vázquez-Lasa, B.; Román, J.S. Bioactive Sr(II)/Chitosan/Poly(ε-caprolactone) Scaffolds for Craniofacial Tissue Regeneration. In Vitro and In Vivo Behavior. Polymers 2018, 10, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Shao, J.; El Raouf, M.A.; Xie, H.; Huang, H.; Wang, H.; Chu, P.; Yu, X.-F.; Yang, Y.; AbdEl-Aal, A.M.; et al. Near-infrared light-triggered drug delivery system based on black phosphorus for in vivo bone regeneration. Biomaterials 2018, 179, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Loca, D.; Smirnova, A.; Locs, J.; Dubnika, A.; Vecstaudza, J.; Stipniece, L.; Makarova, E.; Dambrova, M. Development of local strontium ranelate delivery systems and long term in vitro drug release studies in osteogenic medium. Sci. Rep. 2018, 8, 16754. [Google Scholar] [CrossRef]
- O’Neill, E.; Awale, G.; Daneshmandi, L.; Umerah, O.; Lo, K.W.-H. The roles of ions on bone regeneration. Drug Discov. Today 2018, 23, 879–890. [Google Scholar] [CrossRef]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast Differentiation and Bone Matrix Formation In Vivo and In Vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef] [Green Version]
- Girón, J.; Kerstner, E.; Medeiros, T.; Oliveira, L.; Machado, G.M.; Malfatti, C.F.; Pranke, P. Biomaterials for bone regeneration: An orthopedic and dentistry overview. Braz. J. Med. Biol. Res. 2021, 54, e11055. [Google Scholar] [CrossRef]
- Naruphontjirakul, P.; Tsigkou, O.; Li, S.; Porter, A.E.; Jones, J.R. Human mesenchymal stem cells differentiate into an osteogenic lineage in presence of strontium containing bioactive glass nanoparticles. Acta Biomater. 2019, 90, 373–392. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borciani, G.; Ciapetti, G.; Vitale-Brovarone, C.; Baldini, N. Strontium Functionalization of Biomaterials for Bone Tissue Engineering Purposes: A Biological Point of View. Materials 2022, 15, 1724. https://doi.org/10.3390/ma15051724
Borciani G, Ciapetti G, Vitale-Brovarone C, Baldini N. Strontium Functionalization of Biomaterials for Bone Tissue Engineering Purposes: A Biological Point of View. Materials. 2022; 15(5):1724. https://doi.org/10.3390/ma15051724
Chicago/Turabian StyleBorciani, Giorgia, Gabriela Ciapetti, Chiara Vitale-Brovarone, and Nicola Baldini. 2022. "Strontium Functionalization of Biomaterials for Bone Tissue Engineering Purposes: A Biological Point of View" Materials 15, no. 5: 1724. https://doi.org/10.3390/ma15051724
APA StyleBorciani, G., Ciapetti, G., Vitale-Brovarone, C., & Baldini, N. (2022). Strontium Functionalization of Biomaterials for Bone Tissue Engineering Purposes: A Biological Point of View. Materials, 15(5), 1724. https://doi.org/10.3390/ma15051724










