Surface Functionalisation of Dental Implants with a Composite Coating of Alendronate and Hydrolysed Collagen: DFT and EIS Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Solutions, and Materials
2.2. Functionalisation of the Implant Surfaces
2.3. Characterisation of the Implants
2.4. Quantum Chemical Calculations
3. Results
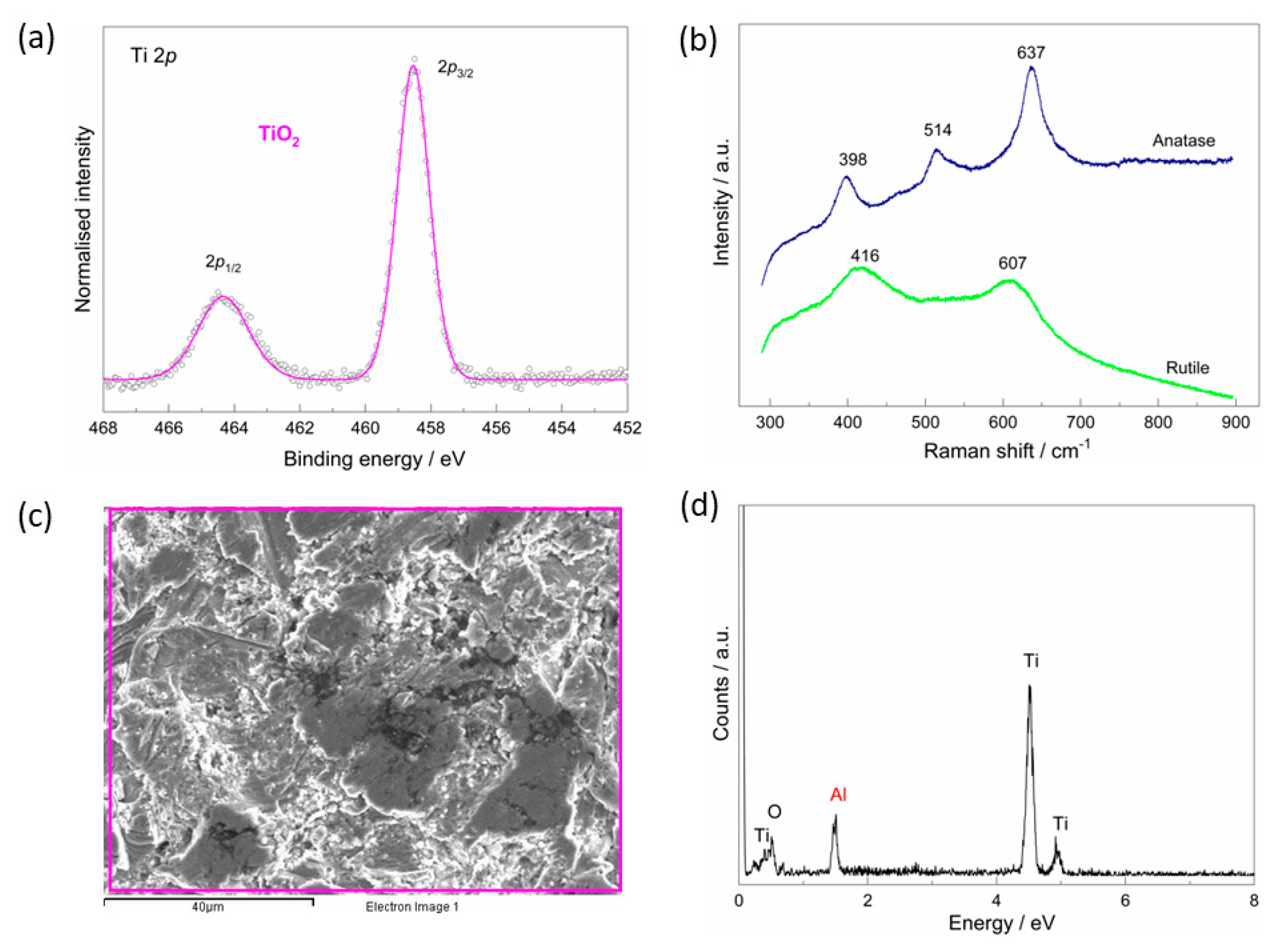
3.1. The As-Received Implant—Morphological, Chemical, and Phase Analysis
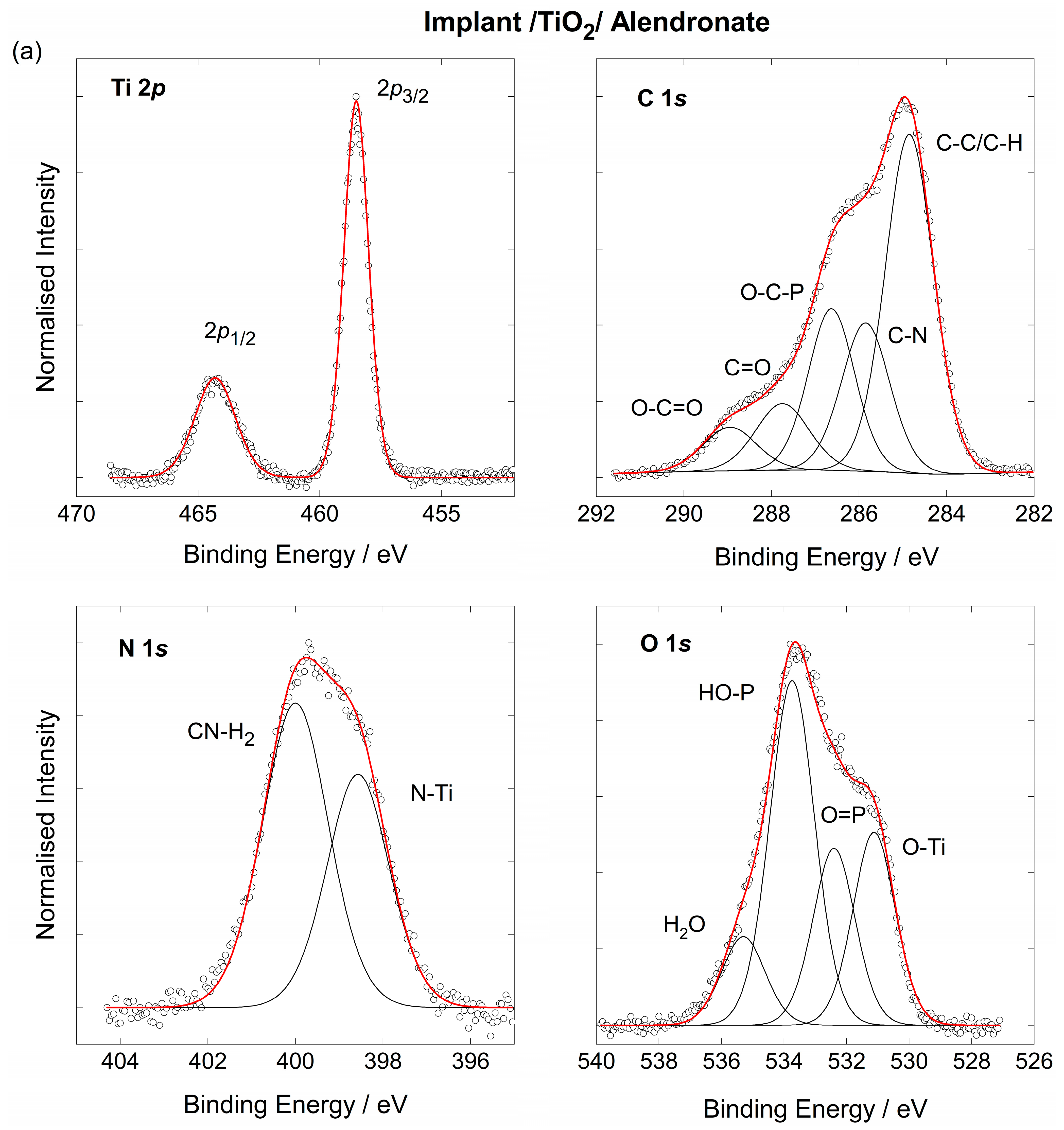
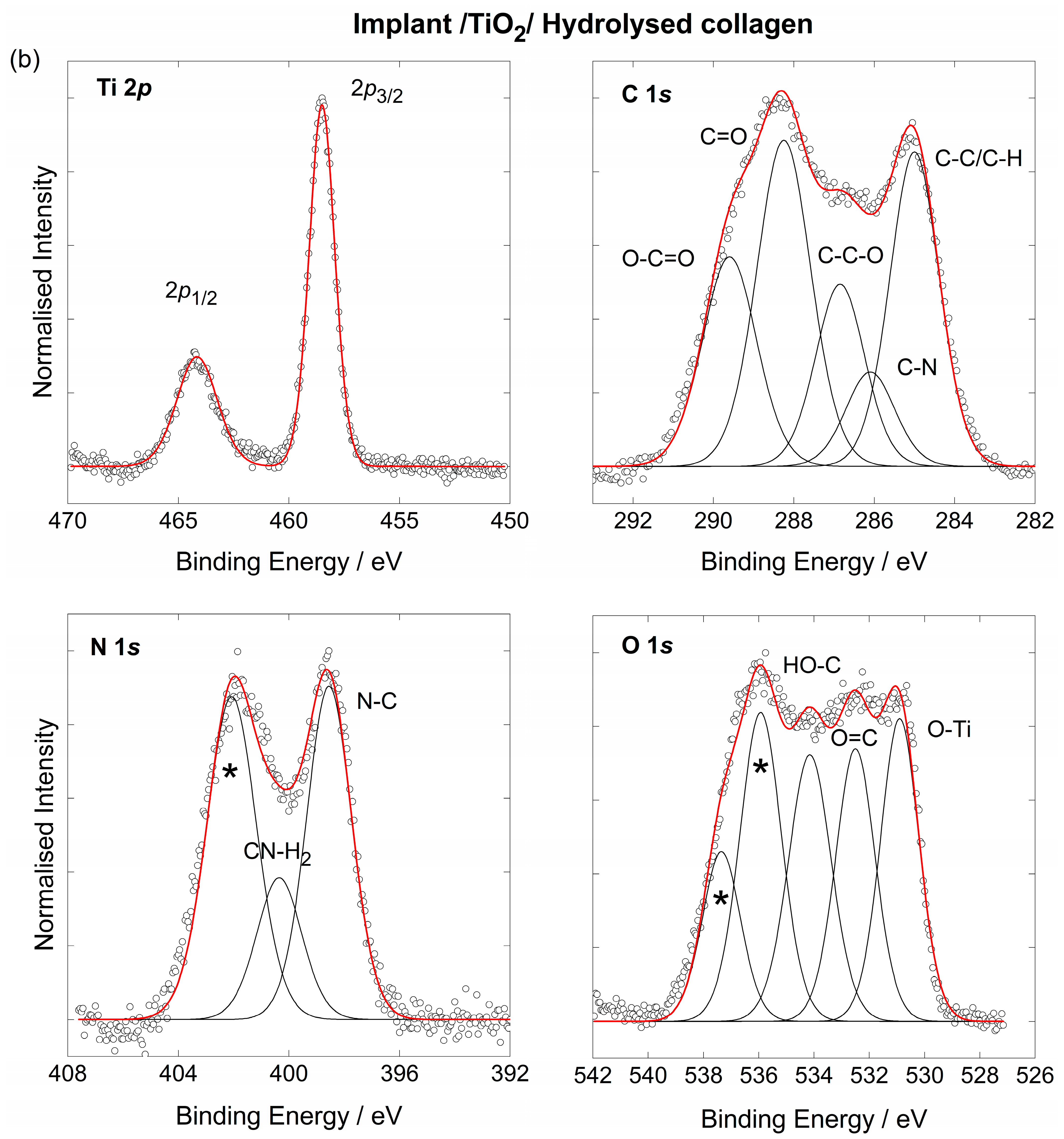
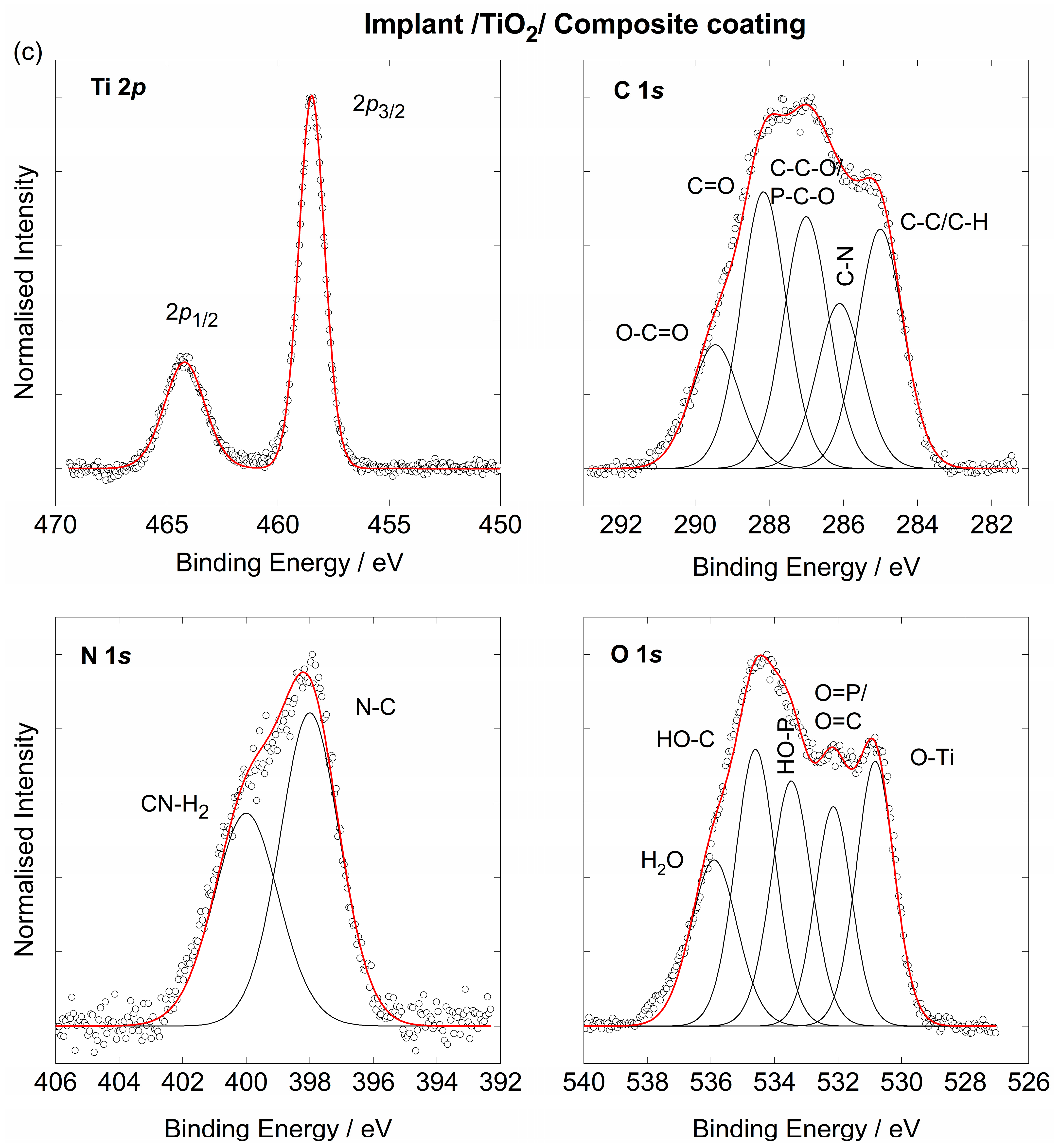
3.2. The Chemical Characterisation of the Implant
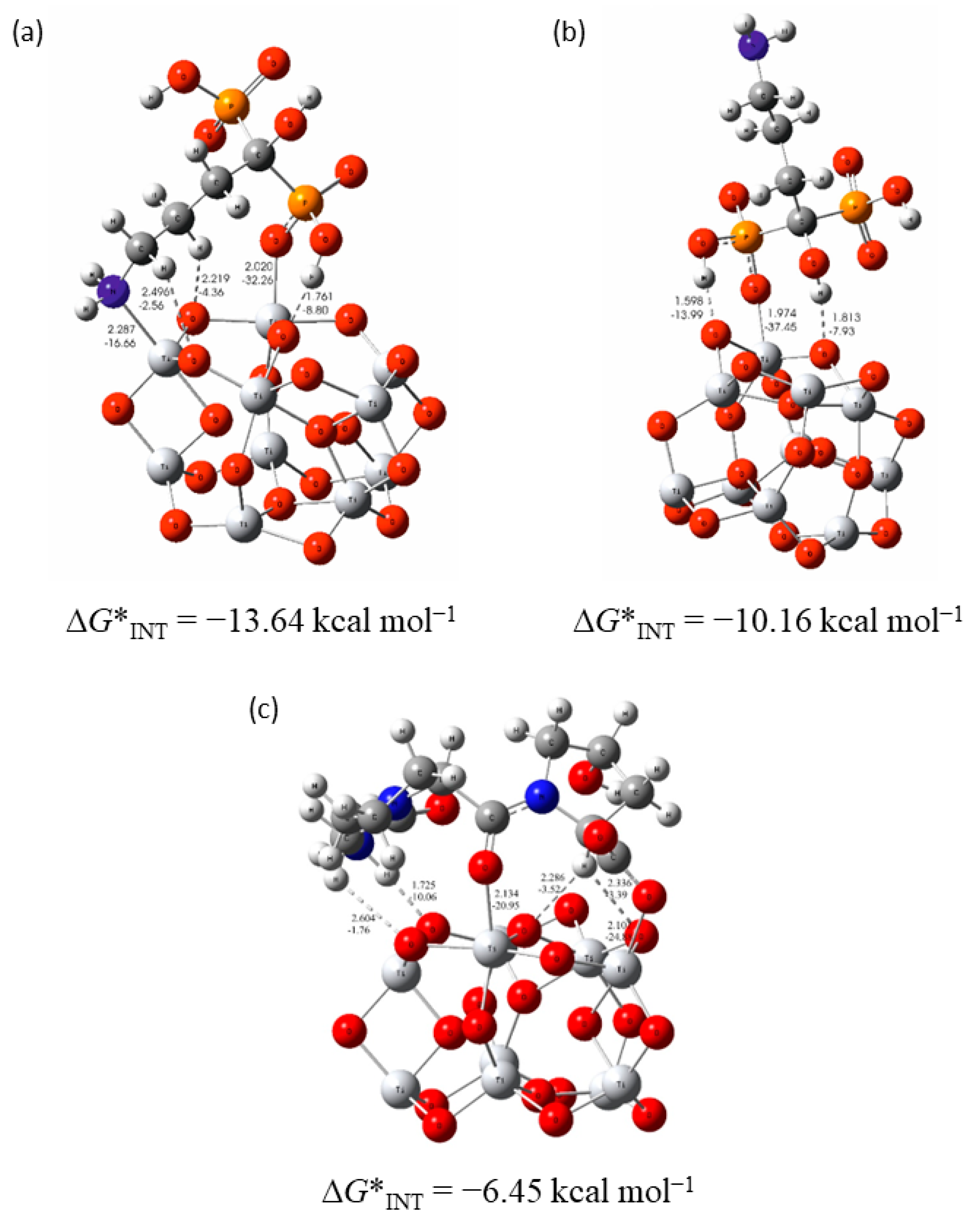
3.3. The Coating’s Formation Mechanism on the Implant
3.4. The Electrochemical Behaviour of Implants in Artificial Saliva Solution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duddeck, D.U.; Albrektsson, T.; Wennerberg, A.; Larsson, C.; Mouhyi, J.; Beuer, F. Quality Assessment of Five Randomly Chosen Ceramic Oral Implant Systems: Cleanliness, Surface Topography, and Clinical Documentation. Int. J. Oral Maxillofac. Implants 2021, 36, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.J.; Fuentes, R.; Navarro, P.; Weber, B.; Borie, E. Assessment of the Chemical Composition in Different Dental Implant Types: An Analysis through EDX System. Coatings 2020, 10, 882. [Google Scholar] [CrossRef]
- Semez, G.; Todea, C.; Mocuta, D.; Tuta, I.S.; Luca, R. Chemical and Morphologic Analysis of Titanium Dental Implants: X-ray Photoemission Techniques (XPS) and Scanning Electron Microscopy (SEM) with EDX Analysis. Rev. Chim. 2018, 69, 474–477. [Google Scholar] [CrossRef]
- Gil, J.; Pérez, R.; Herrero-Climent, M.; Rizo-Gorrita, M.; Torres-Lagares, D.; Gutierrez, J.L. Benefits of Residual Aluminum Oxide for Sand Blasting Titanium Dental Implants: Osseointegration and Bactericidal Effects. Materials 2022, 15, 178. [Google Scholar] [CrossRef]
- Dhaliwal, J.S.; David, S.R.N.; Zulhilmi, N.R.; Dhaliwal, J.S.; Knights, J.; de Albuquerque, R.F., Jr. Contamination of titanium dental implants: A narrative review. SN Appl. Sci. 2020, 2, 1011. [Google Scholar] [CrossRef]
- Schupbach, P.; Glauser, R.; Bauer, S. Al2O3 Particles on Titanium Dental Implant Systems Following Sandblasting and Acid-Etching Process. Int. J. Biomater. 2019, 2019, 6318429. [Google Scholar] [CrossRef] [Green Version]
- De Santis, D.; Guerriero, C.; Nocini, P.F.; Ungersbock, A.; Richards, G.; Gotte, P.; Armato, U. Adult Human Bone Cells from Jaw Bones Cultured on Plasma-Sprayed or Polished Surfaces of Titanium or Hydroxylapatite Discs. J. Mater. Sci. Mater. Med. 1996, 7, 21–28. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Alfonso-Rodríguez, C.; Monsalve-Guil, L.; España-López, A.; Jiménez-Guerra, A.; Garzón, I.; Alaminos, M.; Gil, F. Relevant aspects in the surface properties in titanium dental implants for the cellular viability. Mater. Sci. Eng. C 2016, 64, 1–10. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Dahlin, C. The Impact of Early Saliva Interaction on Dental Implants and Biomaterials for Oral Regeneration: An Overview. Int. J. Mol. Sci. 2022, 23, 2024. [Google Scholar] [CrossRef]
- Hu, C.; Ashok, D.; Nisbet, D.R.; Gautam, V. Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials 2019, 219, 119366. [Google Scholar] [CrossRef] [PubMed]
- Rojo, L.; Gharibi, B.; McLister, R.; Meenan, B.J.; Deb, S. Self-assembled monolayers of alendronate on Ti6Al4V alloy surfaces enhance osteogenesis in mesenchymal stem cells. Sci. Rep. 2016, 6, 30548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrović, Ž.; Šarić, A.; Despotović, I.; Katić, J.; Peter, R.; Petravić, M.; Petković, M. A new insight into alendronate coating’s formation mechanism on titanium dental implant. Materials 2020, 13, 3220. [Google Scholar] [CrossRef] [PubMed]
- León-López, A.; Morales-Peňaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen-Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dentsply Sirona. Croatia, N.D. Available online: https://www.dentsplysirona.com/content/dentsply-sirona/hr-hr.html (accessed on 9 May 2020).
- Quiñones, R.; Gawalt, E.S. Study of the Formation of Self-Assembled Monolayers on Nitinol. Langmuir 2007, 23, 10123–10130. [Google Scholar] [CrossRef] [PubMed]
- Hesse, R.; Chassé, T.; Szargan, R. Peak shape analysis of core level photoelectron spectra using UNIFIT for WINDOWS. Fresenius J. Anal. Chem. 1999, 365, 48–54. [Google Scholar] [CrossRef]
- Mellado-Valero, A.; Muñoz, A.I.; Pina, V.G.; Sola-Ruiz, M.F. Electrochemical Behaviour and Galvanic Effects of Titanium Implants Coupled to Metallic Suprastructures in Artificial Saliva. Materials 2018, 11, 171. [Google Scholar] [CrossRef] [Green Version]
- Boukamp, B.A. A Nonlinear Least Squares Fit procedure for analysis of immittance data of electrochemical systems. Solid State Ion. 1986, 20, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Account. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density Functional Theory for Reaction Energies: Test of Meta and Hybrid Meta Functionals, Range-Separated Functionals, and Other High-Performance Functionals. J. Chem. Theory Comput. 2011, 7, 669–676. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.R.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Keith, T.A. AIMAll (Version 17.01.25); TK Gristmill Software: Overland Park, KS, USA, 2017. [Google Scholar]
- Allard, M.M.; Merlos, S.N.; Springer, B.N.; Cooper, J.; Zhang, G.; Boskovic, D.S.; Kwon, S.R.; Nick, K.E.; Perry, C.C. Role of TiO2 Anatase Surface Morphology on Organophosphorus Interfacial Chemistry. J. Phys. Chem. C 2018, 122, 29237–29248. [Google Scholar] [CrossRef]
- Qu, Z.; Kroes, G.-J. Theoretical Study of Stable, Defect-Free (TiO2)n Nanoparticles with n = 10−16. J. Phys. Chem. C 2007, 111, 16808–16817. [Google Scholar] [CrossRef]
- Ramshaw, J.A.M.; Shah, N.K.; Brodsky, B. Gly-X-Y Tripeptide Frequencies in Collagen: A Context for Host–Guest Triple-Helical Peptides. J. Struct. Biol. 1998, 122, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Petravić, M.; Varašanec, M.; Peter, R.; Kavre, I.; Metikoš-Huković, M.; Yang, Y.-W. Electronic structure of nitinol surfaces oxidized by low-energy ion bombardment. J. Appl. Phys. 2014, 115, 243703. [Google Scholar] [CrossRef]
- Viornery, C.; Chevolot, Y.; Léonard, D.; Aronsson, B.-O.; Péchy, P.; Mathieu, H.-J.; Descouts, P.; Grätzel, M. Surface Modification of Titanium with Phosphonic Acid To Improve Bone Bonding: Characterization by XPS and ToF-SIMS. Langmuir 2002, 18, 2582–2589. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Local structure modification and phase transformation of TiO2 nanoparticles initiated by oxygen defects, grain size, and annealing temperature. Int. Nano Lett. 2013, 3, 55. [Google Scholar] [CrossRef]
- Ma, H.L.; Yang, J.Y.; Dai, Y.; Zhang, Y.B.; Lu, B.; Ma, G.H. Raman study of phase transformation of TiO2 rutile single crystal irradiated by infrared femtosecond laser. Appl. Surf. Sci. 2007, 253, 7497–7500. [Google Scholar] [CrossRef]
- Georgescu, D.; Baia, L.; Ersen, O.; Baia, M.; Simon, S. Experimental assessment of the phonon confinement in TiO2 anatase nanocrystallites by Raman spectroscopy. J. Raman Spect. 2012, 43, 876–883. [Google Scholar] [CrossRef]
- Sahoo, S.; Arora, A.K.; Sridharan, V. Raman line shapes of optical phonons of different symmetries in anatase TiO2 nanocrystals. J. Phys. Chem. C 2009, 113, 16927–16933. [Google Scholar] [CrossRef]
- Albano, C.S.; Gomes, A.M.; da Silva Feltran, G.; da Costa Fernandes, C., Jr.; Trino, L.D.; Zambuzzi, W.F.; Lisboa-Filho, P.N. Biofunctionalization of titanium surfaces with alendronate and albumin modulates osteoblast performance. Heliyon 2020, 6, e04455. [Google Scholar] [CrossRef] [PubMed]
- Ochiuz, L.; Grigoras, C.; Popa, M.; Stoleriu, I.; Munteanu, C.; Timofte, D.; Profire, L.; Grigoras, A.G. Alendronate-Loaded Modified Drug Delivery Lipid Particles Intended for Improved Oral and Topical Administration. Molecules 2016, 21, 858. [Google Scholar] [CrossRef] [Green Version]
- Lima, C.G.A.; de Oliveira, R.S.; Figueiró, S.D.; Wehmann, C.F.; Goes, J.C.; Sombra, A.S.B. DC conductivity and dielectric permittivity of collagen–chitosan films. Mater. Chem. Phys. 2006, 99, 284–288. [Google Scholar] [CrossRef]
- de Campos Vidal, B.; Mello, M.L.S. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef]
- Günzler, H.; Gremlich, H.-U. IR-Spektroskopie, Eine Einführung; Wiley-VCH Verlag GmbH& Co. KgaA: Weinheim, Germany, 2003. [Google Scholar]
- Spori, D.M.; Venkataraman, N.V.; Tosatti, S.G.P.; Durmaz, F.; Spencer, N.D.; Zurcher, S. Influence of Alkyl Chain Length on Phosphate Self-Assembled Monolayers. Langmuir 2007, 23, 8053–8060. [Google Scholar] [CrossRef]
- Mao, Y.; Zhao, C.; Ge, S.; Luo, T.; Chen, J.; Liu, J.; Xi, F.; Liu, J. Gram-scale synthesis of nitrogen doped graphene quantum dots for sensitive detection of mercury ions and L-cysteine. RSC Adv. 2019, 9, 32977–32983. [Google Scholar] [CrossRef] [Green Version]
- Ospina-Orejarena, A.; Vera-Graziano, R.; Castillo-Ortega, M.M.; Hinestroza, J.P.; Rodriguez-Gonzalez, M.; Palomares-Aguilera, L.; Morales-Moctezuma, M.; Maciel-Cerda, A. Grafting Collagen on Poly (Lactic Acid) by a Simple Route to Produce Electrospun Scaffolds, and Their Cell Adhesion Evaluation. Tissue Eng. Regen. Med. 2016, 13, 375–387. [Google Scholar] [CrossRef]
- Lee, M.-S.; Park, M.; Kim, H.Y.; Park, S.-J. Effects of Microporosity and Surface Chemistry on Separation Performances of N-Containing Pitch-Based Activated Carbons for CO2/N2 Binary Mixture. Sci. Rep. 2016, 6, 23224. [Google Scholar] [CrossRef]
- Mani, G.; Johnson, D.M.; Marton, D.; Dougherty, V.L.; Feldman, M.D.; Patel, D.; Ayon, D.D.; Agrawal, C.M. Stability of Self-Assembled Monolayers on Titanium and Gold. Langmuir 2008, 24, 6774–6784. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz-Gromiuk, J.; Rusinek, I.; Kurach, L.; Rzaczynska, Z. Thermal and spectroscopic (IR, XPS) properties of lanthanide(III) benzene-1,3,5-triacetate complexes. J. Therm. Anal. Calorim. 2016, 126, 327–342. [Google Scholar] [CrossRef] [Green Version]
- Katić, J.; Šarić, A.; Despotović, I.; Matijaković, N.; Petković, M.; Petrović, Ž. Bioactive Coating on Titanium Dental Implants for Improved Anticorrosion Protection: A Combined Experimental and Theoretical Study. Coatings 2019, 9, 612. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Thierry, D.; Leygraf, C. Electrochemical impedance spectroscopy study of the passive oxide film on titanium for implant application. Electrochim. Acta 1996, 41, 1143–1153. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy, 2nd ed.; Wiley: New York, NY, USA, 2020; Available online: https://www.wiley.com/en-us/Electrochemical+Impedance+Spectroscopy%2C+2ndEdition-p-9781118527399 (accessed on 7 May 2020).
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interf. Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Boubour, E.; Lennox, R.B. Insulating Properties of Self-Assembled Monolayers Monitored by Impedance Spectroscopy. Langmuir 2000, 16, 4222–4228. [Google Scholar] [CrossRef]
- Scully, J.R. Polarization Resistance Method for Determination of Instantaneous Corrosion Rates. Corrosion 2000, 56, 199–218. [Google Scholar] [CrossRef]
- Boyatzis, S.C.; Velivasaki, G.; Malea, E. A study of the deterioration of aged parchment marked with laboratory iron gall inks using FTIR-ATR spectroscopy and micro hot table. Herit. Sci. 2016, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Alendronic-acid (accessed on 15 February 2020).
- Bader, R.F.W. A Bond Path: A Universal Indicator of Bonded Interactions. J. Phys. Chem. A 1998, 102, 7314–7323. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Essén, H. The characterization of atomic interactions. J. Chem. Phys. 1984, 80, 1943–1960. [Google Scholar] [CrossRef]
- Cremer, D.; Kraka, E. A Description of the Chemical Bond in Terms of Local Properties of Electron Density and Energy. Croat. Chem. Acta 1984, 57, 1259–1281. [Google Scholar]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Rozas, I.; Elguero, J.; Molins, E. About the evaluation of the local kinetic, potential and total energy densities in closed-shell interactions. Chem. Phys. Lett. 2001, 336, 457–461. [Google Scholar] [CrossRef]
- Borissova, A.O.; Antipin, M.Y.; Karapetyan, H.A.; Petrosyan, A.M.; Lyssenko, K.A. Cooperativity effects of H-bonding and charge transfer in an L-nitroarginine crystal with Z’ > 1. Mendeleev Commun. 2010, 20, 260–262. [Google Scholar] [CrossRef]
- Baryshnikov, G.V.; Minaev, B.F.; Minaeva, V.A.; Nenajdenko, V.G. Single crystal architecture and absorption spectra of octathio[8]circulene and sym-tetraselenatetrathio[8]circulene: QTAIM and TD-DFT approach. J. Mol. Model. 2013, 19, 4511–4519. [Google Scholar] [CrossRef]
- Baryshnikov, G.V.; Minaev, B.F.; Korop, A.A.; Minaeva, V.A.; Gusev, A.N. Structure of zinc complexes with 3-(pyridin-2-yl)-5-(arylideneiminophenyl)-1HH-1,2,4-triazoles in different tautomeric forms: DFT and QTAIM study. Russ. J. Inorg. Chem. 2013, 58, 928–934. [Google Scholar] [CrossRef]
- Shahangi, F.; Chermahini, A.N.; Farrokhpour, H.; Teimouri, A. Selective complexation of alkaline earth metal ions with nanotubular cyclopeptides: DFT theoretical study. RSC Adv. 2014, 5, 2305–2317. [Google Scholar] [CrossRef]
- Puntus, L.N.; Lyssenko, K.A.; Antipin, M.Y.; Bünzli, J.C.G. Role of Inner- and Outer-Sphere Bonding in the Sensitization of EuIII-Luminescence Deciphered by Combined Analysis of Experimental Electron Density Distribution Function and Photophysical Data. Inorg. Chem. 2008, 47, 11095–11107. [Google Scholar] [CrossRef]




| Samples | Rs/Ω cm2 | CPE1∙106/ Ω−1 cm−2 sn1 | n1 | C1/µF cm−2 | R1/Ω cm2 | CPE2∙106/ Ω−1 cm−2 sn1 | n2 | C2/µF cm−2 | R2/MΩ cm2 | η/% |
|---|---|---|---|---|---|---|---|---|---|---|
| AS-RECEIVED IMPLANT | ||||||||||
| 1 h | 111 | 9.98 | 0.853 | 3.02 | 760 | 5.16 | 0.850 | 1.38 | 9.90 | |
| 1 day | 123 | 2.88 | 0.997 | 2.88 | 174 | 12.1 | 0.788 | 2.10 | 0.79 | |
| 7 days | 123 | 3.26 | 0.978 | 2.71 | 307 | 9.21 | 0.810 | 1.88 | 0.44 | |
| IMPLANT/TiO2/ALENDRONATE | ||||||||||
| 1 h | 109 | 2.00 | 1 | 2.00 | 307 | 9.21 | 0.820 | 2.03 | 39.0. | 74.6 |
| 1 day | 119 | 1.95 | 1 | 1.95 | 332 | 8.05 | 0.806 | 1.48 | 16.5 | 95.2 |
| 7 days | 109 | 1.98 | 1 | 1.98 | 302 | 7.23 | 0.795 | 1.15 | 5.88 | 92.5 |
| IMPLANT/TiO2/HYDROLYSED COLLAGEN | ||||||||||
| 1 h | 149 | 2.03 | 1 | 2.03 | 851 | 5.46 | 0.815 | 1.07 | 54.5 | 81.8 |
| 1 day | 147 | 2.09 | 1 | 2.09 | 641 | 6.45 | 0.809 | 0.91 | 24.0 | 96.7 |
| 7 days | 160 | 1.95 | 1 | 1.95 | 501 | 5.40 | 0.782 | 0.45 | 60.1 | 99.3 |
| IMPLANT/TiO2/COMPOSITE COATING | ||||||||||
| 1 h | 169 | 3.22 | 1 | 3.22 | 182 | 9.01 | 0.804 | 1.14 | 29.4 | 66.3 |
| 1 day | 137 | 3.30 | 1 | 3.30 | 176 | 9.25 | 0.801 | 0.99 | 23.7 | 96.0 |
| 7 days | 130 | 3.85 | 1 | 3.85 | 205 | 8.33 | 0.826 | 1.26 | 24.0 | 98.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrović, Ž.; Šarić, A.; Despotović, I.; Katić, J.; Peter, R.; Petravić, M.; Ivanda, M.; Petković, M. Surface Functionalisation of Dental Implants with a Composite Coating of Alendronate and Hydrolysed Collagen: DFT and EIS Studies. Materials 2022, 15, 5127. https://doi.org/10.3390/ma15155127
Petrović Ž, Šarić A, Despotović I, Katić J, Peter R, Petravić M, Ivanda M, Petković M. Surface Functionalisation of Dental Implants with a Composite Coating of Alendronate and Hydrolysed Collagen: DFT and EIS Studies. Materials. 2022; 15(15):5127. https://doi.org/10.3390/ma15155127
Chicago/Turabian StylePetrović, Željka, Ankica Šarić, Ines Despotović, Jozefina Katić, Robert Peter, Mladen Petravić, Mile Ivanda, and Marin Petković. 2022. "Surface Functionalisation of Dental Implants with a Composite Coating of Alendronate and Hydrolysed Collagen: DFT and EIS Studies" Materials 15, no. 15: 5127. https://doi.org/10.3390/ma15155127
APA StylePetrović, Ž., Šarić, A., Despotović, I., Katić, J., Peter, R., Petravić, M., Ivanda, M., & Petković, M. (2022). Surface Functionalisation of Dental Implants with a Composite Coating of Alendronate and Hydrolysed Collagen: DFT and EIS Studies. Materials, 15(15), 5127. https://doi.org/10.3390/ma15155127









