Treatment of Infection-Related Non-Unions with Bioactive Glass—A Promising Approach or Just Another Method of Dead Space Management?
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Infecting Agent
2.2. Bioactive Glass (S53P4)
2.3. Implants
2.4. Groups
2.5. Animals, Operative Procedure and Osteotomy Model
2.6. Follow-Up
2.7. μCT Scan Evaluation
2.8. Microbiological Evaluation
2.9. Sacrifice
2.10. Mechanical Testing
2.11. Histology
2.12. Statistical Analysis
3. Results
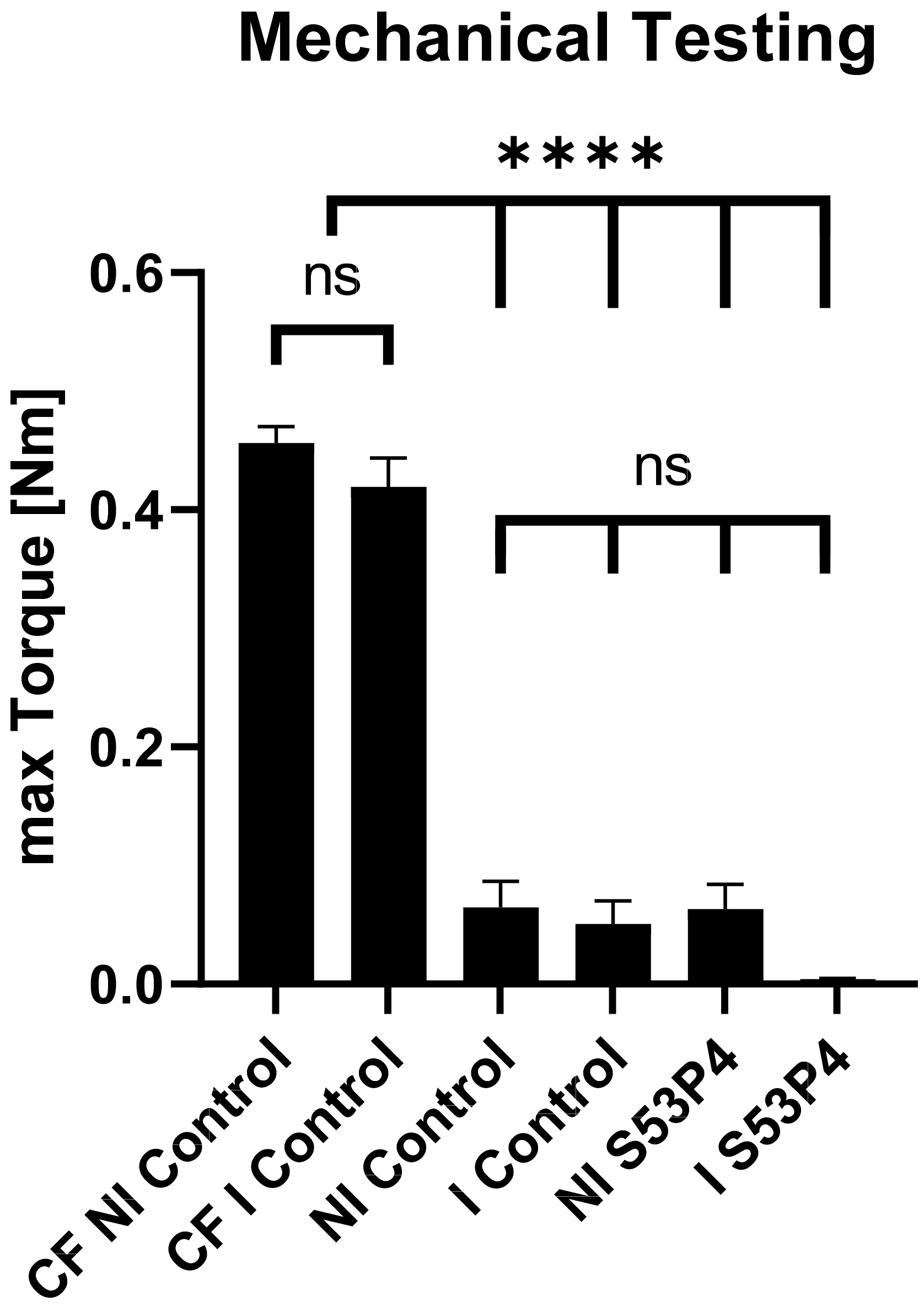
3.1. Failure Parameters
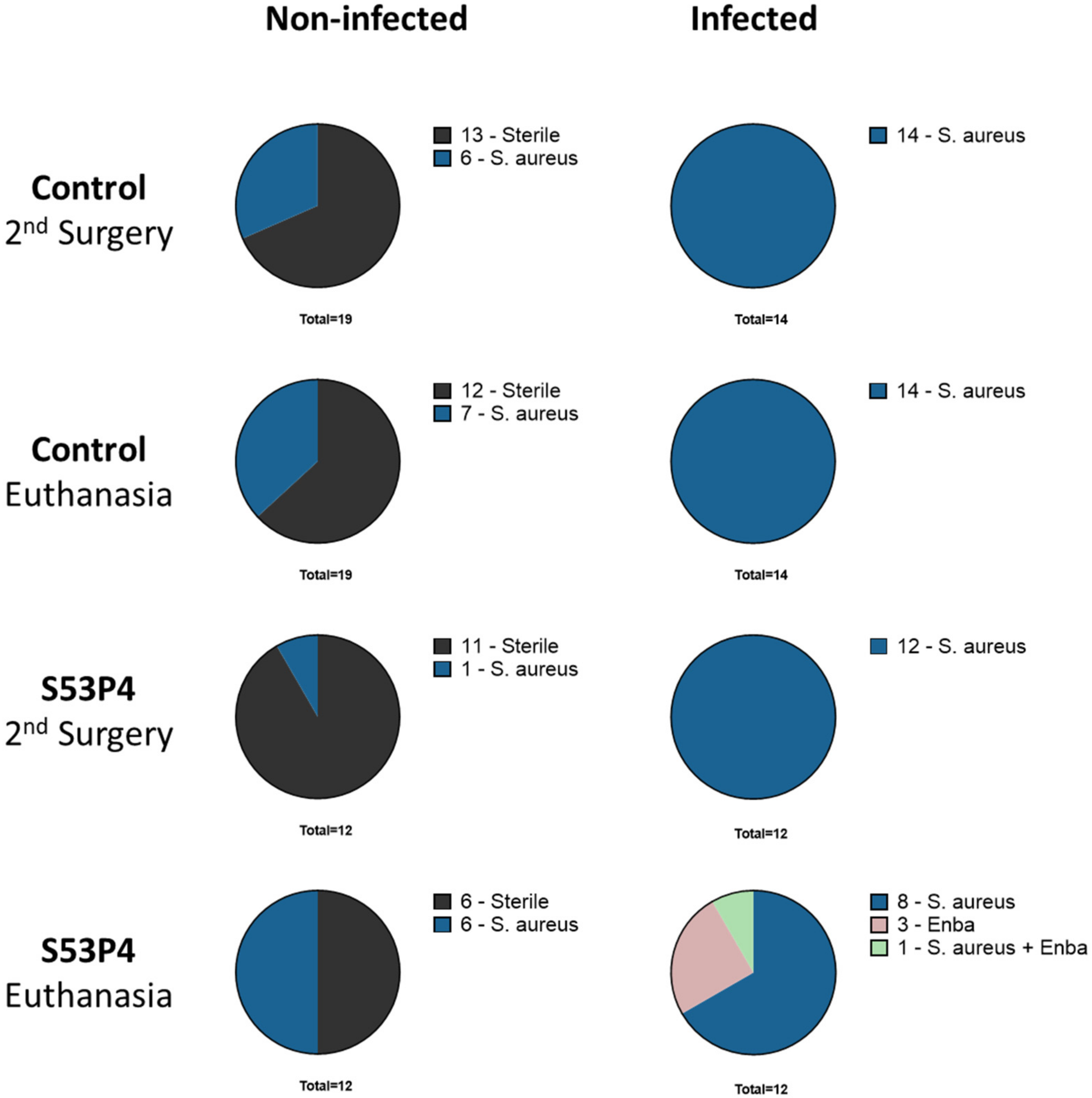
3.2. Microbiologic Results Showed No Anti-Infective Potential in S53P4
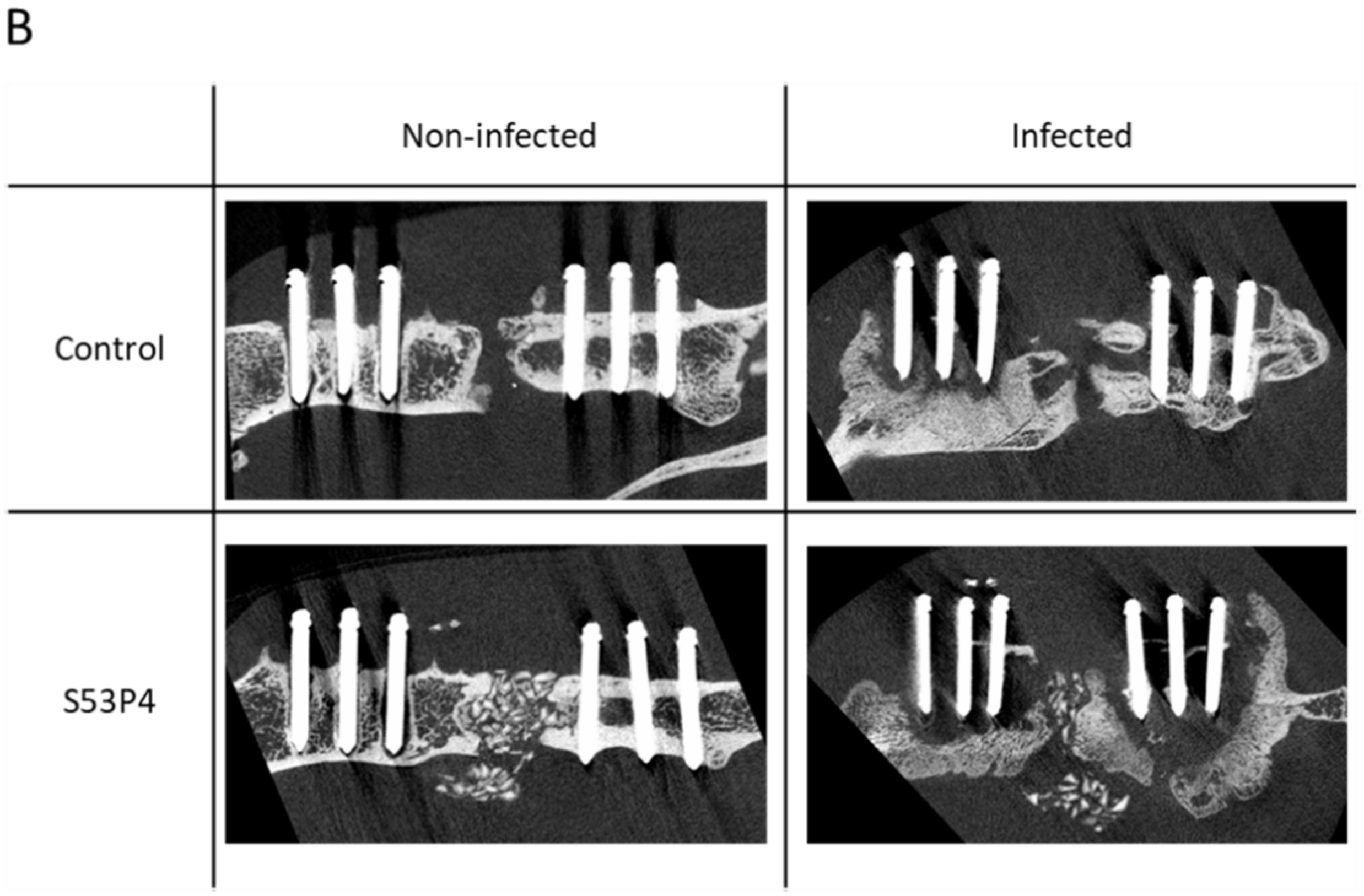
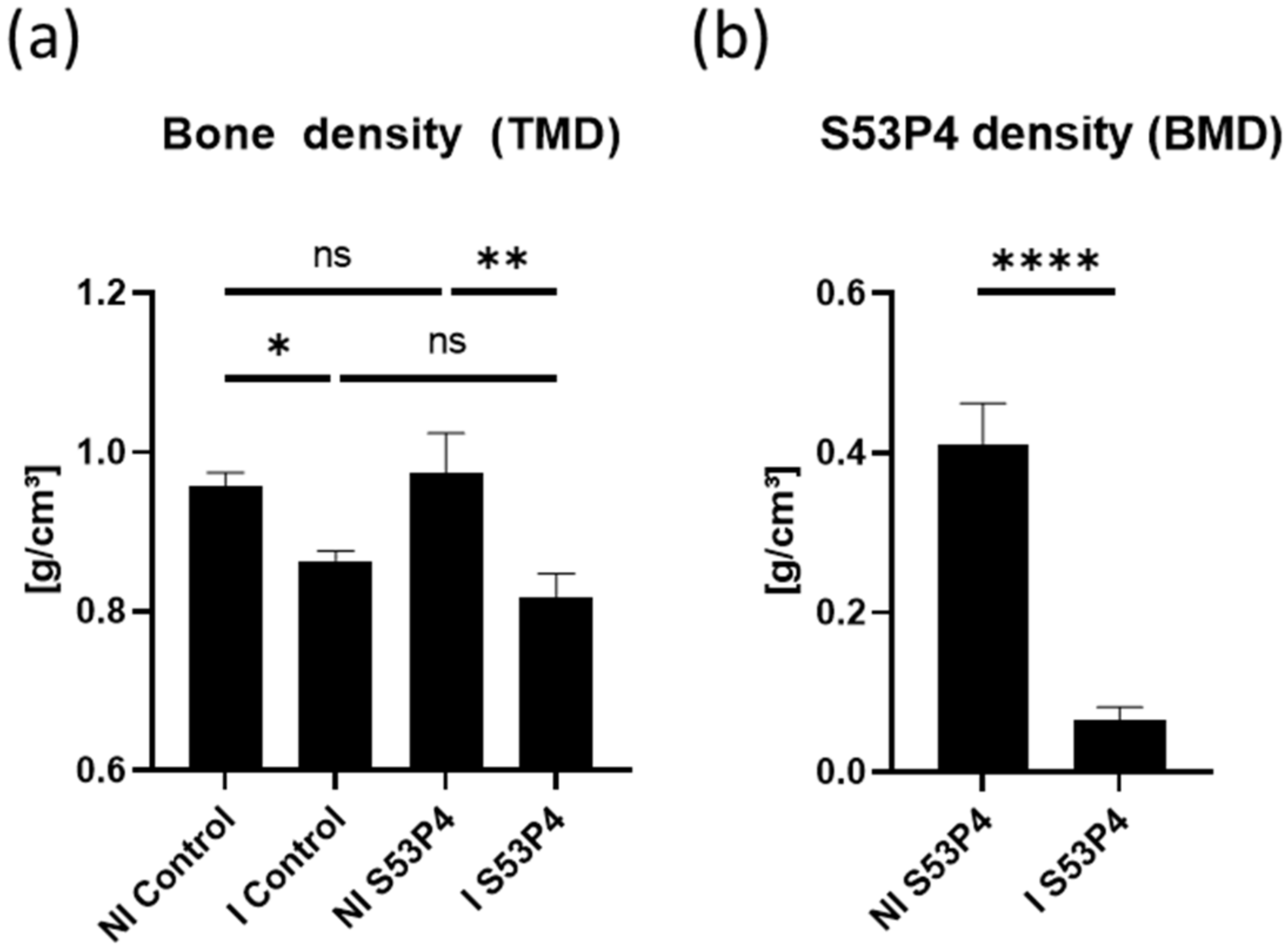
3.3. S53P4 Did Not Lead to a Stable Union in Non-Infected or Infected Conditions
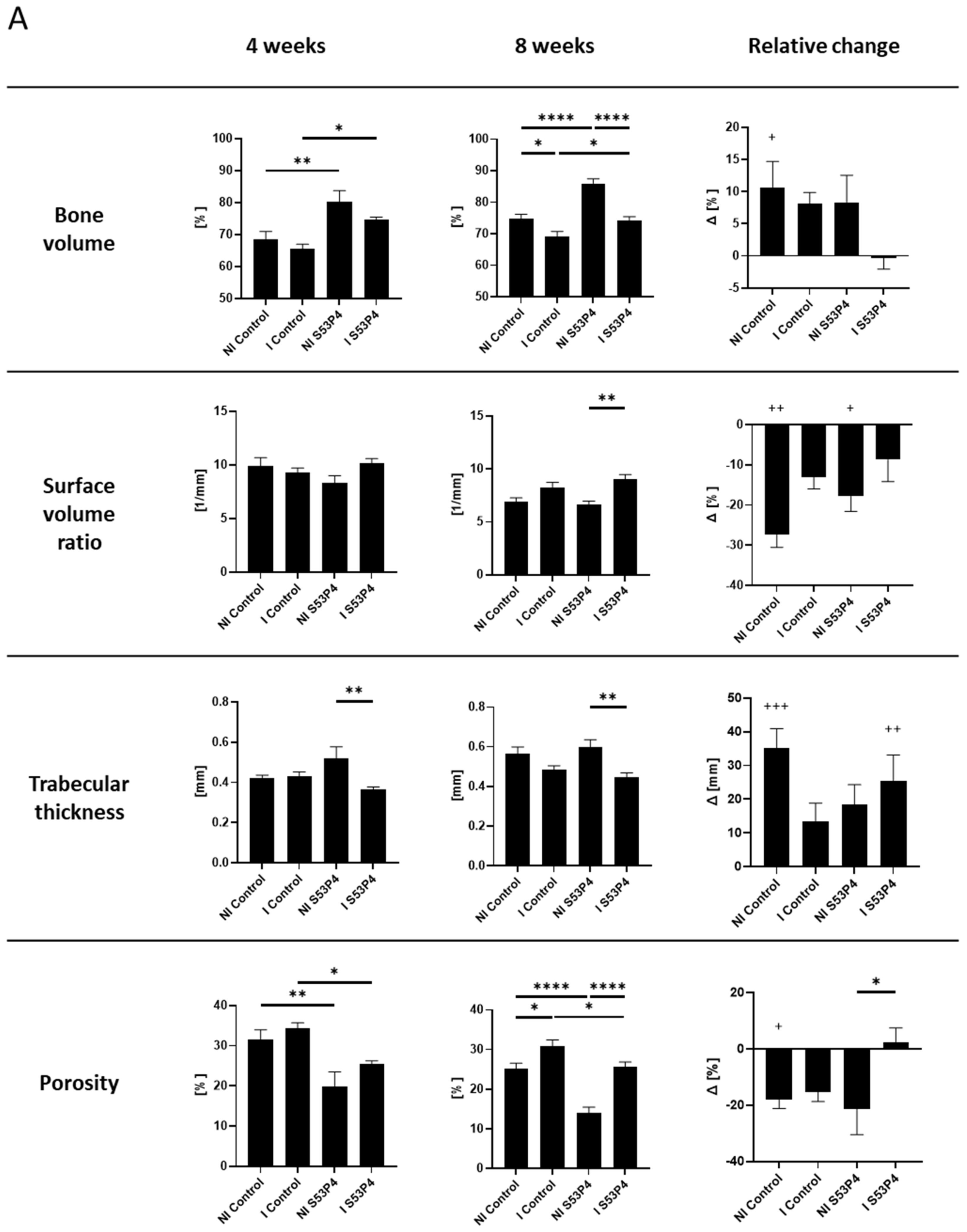
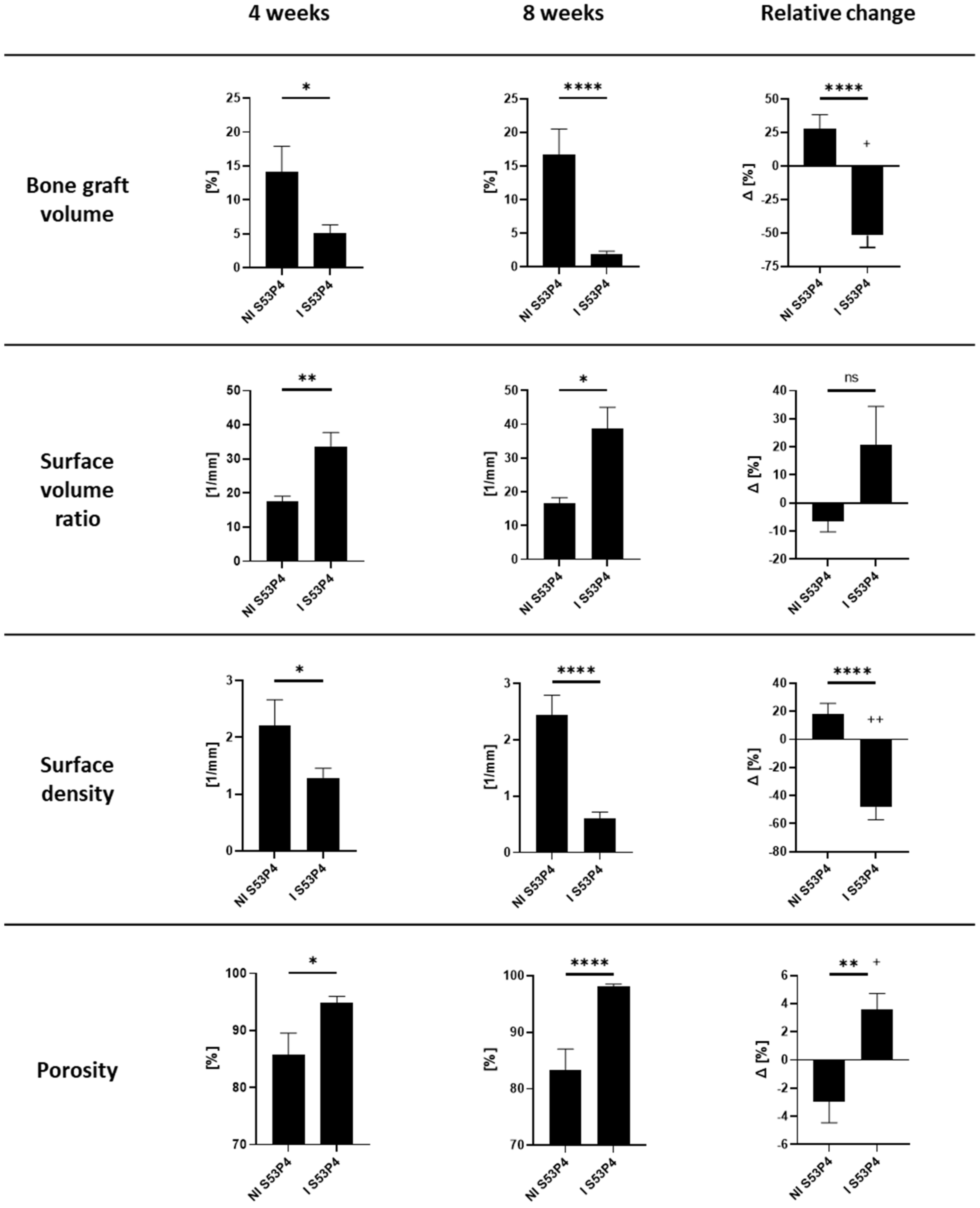
3.4. µCT Analysis of the Bone Showed an Osteoinductive Effect of S53P4 in Non-Infected Groups, Which Was Diminished in Infected Groups
3.5. µCT Analysis of S53P4 Showed the Detrimental Effects of Infection
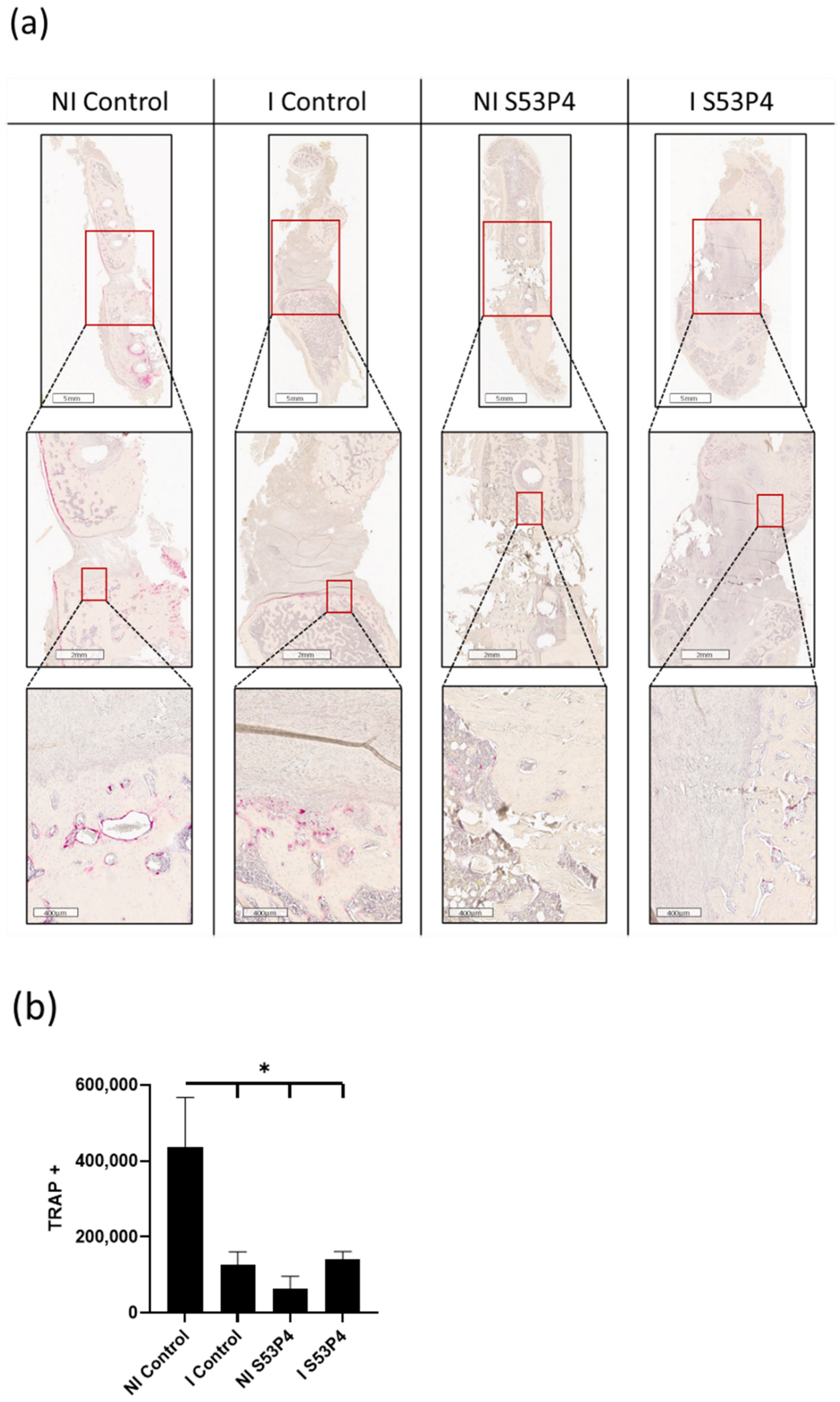
3.6. There Was a Lower Invasion of Osteoclasts into the Bone Defect in Infected Groups but Also in Non-Infected S53P4
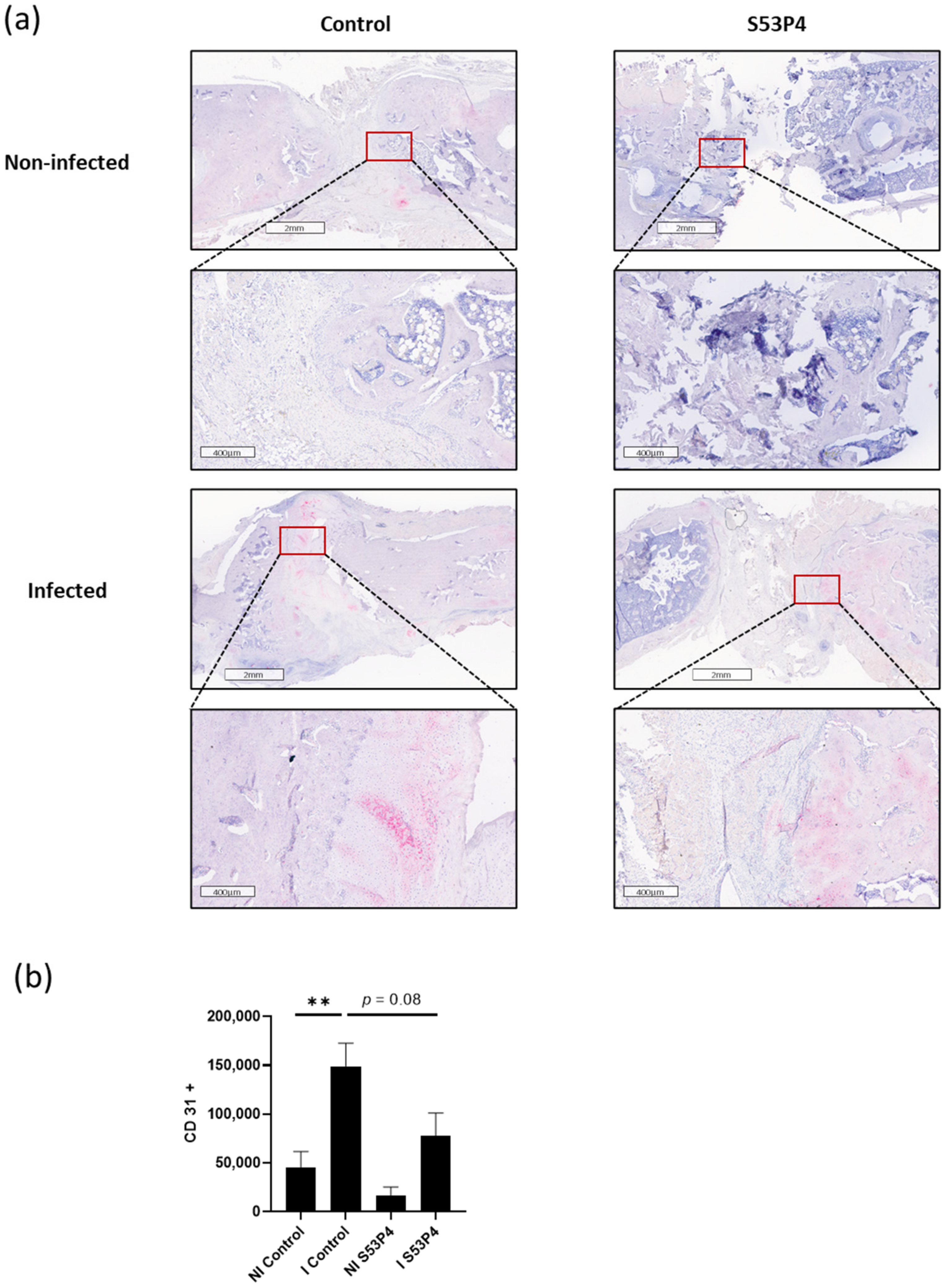
3.7. Infection-Triggered Hypervascularization Was Diminished in I S53P4
3.8. No Reduction in the Inflammatory Response in S53P4 in CD14 and CD68 Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moghaddam, A.; Zietzschmann, S.; Bruckner, T.; Schmidmaier, G. Treatment of atrophic tibia non-unions according to ‘diamond concept’: Results of one- and two-step treatment. Injury 2015, 46, 39–50. [Google Scholar] [CrossRef]
- Lerner, R.K.; Esterhai, J.L., Jr.; Polomano, R.C.; Cheatle, M.D.; Heppenstall, R.B. Quality of life assessment of patients with posttraumatic fracture nonunion, chronic refractory osteomyelitis, and lower-extremity amputation. Clin. Orthop. Relat. Res. 1993, 295, 28–36. [Google Scholar] [CrossRef]
- Westgeest, J.; Weber, D.; Dulai, S.K.; Bergman, J.W.; Buckley, R.; Beaupre, L.A. Factors Associated with Development of Nonunion or Delayed Healing after an Open Long Bone Fracture—A Prospective Cohort Study of 736 Subjects. J. Orthop. Trauma 2016, 30, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Lerner, R.K.; Esterhai, J.L., Jr.; Polomono, R.C.; Cheatle, M.C.; Heppenstall, R.B.; Brighton, C.T. Psychosocial, functional, and quality of life assessment of patients with posttraumatic fracture nonunion, chronic refractory osteomyelitis, and lower extremity amputation. Arch. Phys. Med. Rehabil. 1991, 72, 122–126. [Google Scholar]
- Hak, D.J.; Fitzpatrick, D.; Bishop, J.A.; Marsh, J.L.; Tilp, S.; Schnettler, R.; Simpson, H.; Alt, V. Delayed union and nonunions: Epidemiology, clinical issues, and financial aspects. Injury 2014, 45, S3–S7. [Google Scholar] [CrossRef]
- Zura, R.; Mehta, S.; Della Rocca, G.J.; Steen, R.G. Biological Risk Factors for Nonunion of Bone Fracture. JBJS Rev. 2016, 4, S1–S12. [Google Scholar] [CrossRef]
- Rupp, M.; Biehl, C.; Budak, M.; Thormann, U.; Heiss, C.; Alt, V. Diaphyseal long bone nonunions—Types, aetiology, economics, and treatment recommendations. Int. Orthop. 2017, 42, 247–258. [Google Scholar] [CrossRef]
- Society, C.O.T. Nonunion Following Intramedullary Nailing of the Femur with and without Reaming. J. Bone Jt. Surg. Am. 2003, 85, 2093–2096. [Google Scholar]
- Helland, P.; Bøe, A.; Mølster, A.O.; Solheim, E.; Hordvik, M. Open tibial fractures treated with the Ex-fi-re external fixation system. Clin. Orthop. Relat. Res. 1996, 326, 209–220. [Google Scholar] [CrossRef]
- Papakostidis, C.; Kanakaris, N.K.; Pretel, J.; Faour, O.; Morell, D.J.; Giannoudis, P.V. Prevalence of complications of open tibial shaft fractures stratified as per the Gustilo-Anderson classification. Injury 2011, 42, 1408–1415. [Google Scholar] [CrossRef]
- Teixeira, L.E.M.; Pádua, B.J.; Castilho, A.M.; Araújo, I.D.D.; Andrade, M.A.P.D.; Cardoso, V.N.; Diniz, S.O.; Leal, J.S.; Takenaka, I.K. Influence of biomaterials on scintigraphic diagnosis of periprosthetic infections. Ceftizoxime-99m technetium model. Acta Cir. Bras. 2018, 33, 14–21. [Google Scholar] [CrossRef]
- Gustilo, R.B.; Gruninger, R.P.; Davis, T. Classification of type III (severe) open fractures relative to treatment and results. Orthopedics 1987, 10, 1781–1788. [Google Scholar]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38, S3–S6. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Angoules, A.G.; Kanakaris, N.K.; Giannoudis, P.V. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: A systematic review. Injury 2011, 42, S3–S15. [Google Scholar] [CrossRef]
- Janicki, P.; Schmidmaier, G. What should be the characteristics of the ideal bone graft substitute? Combining scaffolds with growth factors and/or stem cells. Injury 2011, 42, S77–S81. [Google Scholar] [CrossRef]
- Gómez-Barrena, E.; Rosset, P.; Lozano, D.; Stanovici, J.; Ermthaller, C.; Gerbhard, F. Bone fracture healing: Cell therapy in delayed unions and nonunions. Bone 2015, 70, 93–101. [Google Scholar] [CrossRef]
- Kurien, T.; Pearson, R.G.; Scammell, B.E. Bone graft substitutes currently available in orthopaedic practice. Bone Jt. J. 2013, 95-B, 583–597. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Hupa, L. Melt-derived bioactive glasses. In Bioactive Glasses; Woodhead Publishing: Cambridge, UK, 2011; pp. 3–28. [Google Scholar] [CrossRef]
- Salinas, A.J.; Vallet-Regi, M.; Heikkilä, J. Use of bioactive glasses as bone substitutes in orthopedics and traumatology. In Bioactive Glasses; Woodhead Publishing: Cambridge, UK, 2018; pp. 337–364. [Google Scholar] [CrossRef]
- Aurégan, J.C.; Begue, T. Bioactive glass for long bone infection—A systematic review. Injury 2015, 46, 3–7. [Google Scholar] [CrossRef]
- Detsch, R.; Stoor, P.; Grünewald, A.; Roether, J.A.; Lindfors, N.C.; Boccaccini, A.R. Increase in VEGF secretion from human fibroblast cells by bioactive glass S53P4 to stimulate angiogenesis in bone. J. Biomed. Mater. Res. Part A 2014, 102, 4055–4061. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Sonny Bal, B.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Lindfors, N.C.; Heikkilä, J.T.; Koski, I.; Mattila, K.; Aho, A.J. Bioactive glass and autogenous bone as bone graft substitutes in benign bone tumors. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 90B, 131–136. [Google Scholar] [CrossRef]
- van Gestel, N.A.P.; Geurts, J.; Hulsen, D.J.W.; van Rietbergen, B.; Hofmann, S.; Arts, J.J. Clinical Applications of S53P4 Bioactive Glass in Bone Healing and Osteomyelitic Treatment: A Literature Review. BioMed Res. Int. 2015, 2015, 684826. [Google Scholar] [CrossRef]
- Westhauser, F.; Weis, C.; Prokscha, M.; Bittrich, L.A.; Li, W.; Xiao, K.; Kneser, U.; Kauczor, H.-U.; Schmidmaier, G.; Boccaccini, A.R.; et al. Three-dimensional polymer coated 45S5-type bioactive glass scaffolds seeded with human mesenchymal stem cells show bone formation in vivo. J. Mater. Sci. Mater. Med. 2016, 27, 119. [Google Scholar] [CrossRef]
- Morgenstern, M.; Vallejo, A.; McNally, M.A.; Moriarty, T.F.; Ferguson, J.Y.; Nijs, S.; Metsemakers, W.J. The effect of local antibiotic prophylaxis when treating open limb fractures: A systematic review and meta-analysis. Bone Jt. Res. 2018, 7, 447–456. [Google Scholar] [CrossRef]
- Solberg, B.D.; Gutow, A.; Baumgaertner, M.R. Efficacy of Gentamycin-Impregnated Resorbable Hydroxyapatite Cement in Treating Osteomyelitis in a Rat Model. J. Orthop. Trauma 1999, 13, 102–106. [Google Scholar] [CrossRef]
- Stravinskas, M.; Nilsson, M.; Horstmann, P.; Petersen, M.M.; Tarasevicius, S.; Lidgren, L. Antibiotic Containing Bone Substitute in Major Hip Surgery: A Long Term Gentamicin Elution Study. J. Bone Jt. Infect. 2018, 3, 68–72. [Google Scholar] [CrossRef][Green Version]
- Stoor, P.; Söderling, E.; Salonen, J.I. Antibacterial effects of a bioactive glass paste on oral microorganisms. Acta Odontol. Scand. 1998, 56, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Leppäranta, O.; Munukka, E.; Ylänen, H.; Viljanen, M.K.; Eerola, E.; Hupa, M.; Hupa, L. Antibacterial effects and dissolution behavior of six bioactive glasses. J. Biomed. Mater. Res. Part A 2010, 93A, 475–483. [Google Scholar] [CrossRef]
- Zhang, D.; Munukka, E.; Leppäranta, O.; Hupa, L.; Ylänen, H.O.; Salonen, J.I.; Eerola, E.; Viljanen, M.K.; Hupa, M. Comparison of Antibacterial Effect of Three Bioactive Glasses. Key Eng. Mater. 2006, 309–311, 345–348. [Google Scholar] [CrossRef]
- Bortolin, M.; De Vecchi, E.; Romanò, C.L.; Toscano, M.; Mattina, R.; Drago, L. Antibiofilm agents against MDR bacterial strains: Is bioactive glass BAG-S53P4 also effective? J. Antimicrob. Chemother. 2016, 71, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.C.; Heller, R.; Westhauser, F.; Miska, M.; Ferbert, T.; Fischer, C.; Gantz, S.; Schmidmaier, G.; Haubruck, P. Evaluation of the clinical effectiveness of bioactive glass (S53P4) in the treatment of non-unions of the tibia and femur- study protocol of a randomized controlled non-inferiority trial. Trials 2018, 19, 299. [Google Scholar] [CrossRef] [PubMed]
- Helbig, L.; Guehring, T.; Titze, N.; Nurjadi, D.; Sonntag, R.; Armbruster, J.; Wildemann, B.; Schmidmaier, G.; Gruetzner, A.P.; Freischmidt, H. A new sequential animal model for infection-related non-unions with segmental bone defect. BMC Musculoskelet. Disord. 2020, 21, 329. [Google Scholar] [CrossRef]
- Lucke, M.; Wildemann, B.; Sadoni, S.; Surke, C.; Schiller, R.; Stemberger, A.; Raschke, M.; Haas, N.P.; Schmidmaier, G. Systemic versus local application of gentamicin in prophylaxis of implant-related osteomyelitis in a rat model. Bone 2005, 36, 770–778. [Google Scholar] [CrossRef]
- Helbig, L.; Guehring, T.; Rosenberger, S.; Ivanova, A.; Kaeppler, K.; Fischer, C.A.; Moghaddam, A.; Schmidmaier, G. A new animal model for delayed osseous union secondary to osteitis. BMC Musculoskelet. Disord. 2015, 16, 362. [Google Scholar] [CrossRef]
- Lane, J.M.; Sandhu, H.S. Current approaches to experimental bone grafting. Orthop. Clin. N. Am. 1987, 18, 213–225. [Google Scholar] [CrossRef]
- An, Y.H.; Friedman, R.J. Animal models of orthopedic implant infection. J. Invest. Surg. 1998, 11, 139–146. [Google Scholar] [CrossRef]
- Nurjadi, D.; Friedrich-Janicke, B.; Schafer, J.; Van Genderen, P.J.; Goorhuis, A.; Perignon, A.; Neumayr, A.; Mueller, A.; Kantele, A.; Schunk, M.; et al. Skin and soft tissue infections in intercontinental travellers and the import of multi-resistant Staphylococcus aureus to Europe. Clin. Microbiol. Infect. 2015, 21, 567.e1–567.e10. [Google Scholar] [CrossRef]
- Gronseth, T.; Vestby, L.K.; Nesse, L.L.; von Unge, M.; Silvola, J.T. Bioactive glass S53P4 eradicates Staphylococcus aureus in biofilm/planktonic states in vitro. Ups. J. Med. Sci. 2020, 125, 217–225. [Google Scholar] [CrossRef]
- Van Vugt, T.A.G.; Heidotting, J.; Arts, J.J.; Ploegmakers, J.J.W.; Jutte, P.C.; Geurts, J.A.P. Mid-term clinical results of chronic cavitary long bone osteomyelitis treatment using S53P4 bioactive glass: A multi-center study. J. Bone Jt. Infect. 2021, 6, 413–421. [Google Scholar] [CrossRef]
- Thijssen, E.G.J.; van Gestel, N.A.P.; Bevers, R.; Hofmann, S.; Geurts, J.; van Loo, I.H.M.; Arts, J.J. Assessment of Growth Reduction of Five Clinical Pathogens by Injectable S53P4 Bioactive Glass Material Formulations. Front. Bioeng. Biotechnol. 2020, 8, 634. [Google Scholar] [CrossRef]
- Gaiarsa, G.P.; Dos Reis, P.R.; Kojima, K.E.; Silva, J.S.; Lima, A. A Retrospective Case-Series on the Use of S53p4 Bioactive Glass for the Adjunctive Treatment of Septic Diaphyseal Non-Union. Acta Ortop. Bras. 2019, 27, 273–275. [Google Scholar] [CrossRef]
- Brunello, G.; Elsayed, H.; Biasetto, L. Bioactive Glass and Silicate-Based Ceramic Coatings on Metallic Implants: Open Challenge or Outdated Topic? Materials 2019, 12, 2929. [Google Scholar] [CrossRef]
- Bjorkenheim, R.; Jamsen, E.; Eriksson, E.; Uppstu, P.; Aalto-Setala, L.; Hupa, L.; Eklund, K.K.; Ainola, M.; Lindfors, N.C.; Pajarinen, J. Sintered S53P4 bioactive glass scaffolds have anti-inflammatory properties and stimulate osteogenesis in vitro. Eur. Cells Mater. 2021, 41, 15–30. [Google Scholar] [CrossRef]
- Eriksson, E.; Bjorkenheim, R.; Stromberg, G.; Ainola, M.; Uppstu, P.; Aalto-Setala, L.; Leino, V.M.; Hupa, L.; Pajarinen, J.; Lindfors, N.C. S53P4 bioactive glass scaffolds induce BMP expression and integrative bone formation in a critical-sized diaphysis defect treated with a single-staged induced membrane technique. Acta Biomater. 2021, 126, 463–476. [Google Scholar] [CrossRef]
- van Gestel, N.A.P.; Schuiringa, G.H.; Hennissen, J.; Delsing, A.C.A.; Ito, K.; van Rietbergen, B.; Arts, J.J.; Hofmann, S. Resorption of the calcium phosphate layer on S53P4 bioactive glass by osteoclasts. J. Mater. Sci. Mater. Med. 2019, 30, 94. [Google Scholar] [CrossRef]
- Beamer, B.; Hettrich, C.; Lane, J. Vascular endothelial growth factor: An essential component of angiogenesis and fracture healing. HSS J. 2010, 6, 85–94. [Google Scholar] [CrossRef]
- Hausman, M.R.; Schaffler, M.B.; Majeska, R.J. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone 2001, 29, 560–564. [Google Scholar] [CrossRef]
- Song, E.; Mao, T.; Dong, H.; Boisserand, L.S.B.; Antila, S.; Bosenberg, M.; Alitalo, K.; Thomas, J.L.; Iwasaki, A. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 2020, 577, 689–694. [Google Scholar] [CrossRef]
- Freischmidt, H.; Armbruster, J.; Bonner, E.; Guehring, T.; Nurjadi, D.; Bechberger, M.; Sonntag, R.; Schmidmaier, G.; Grutzner, P.A.; Helbig, L. Systemic Administration of PTH Supports Vascularization in Segmental Bone Defects Filled with Ceramic-Based Bone Graft Substitute. Cells 2021, 10, 2058. [Google Scholar] [CrossRef] [PubMed]
- Boora, M.; Malik, S.; Kumar, V.; Bala, M.; Arora, S.; Rohilla, S.; Kumar, A.; Dalal, J. Investigation of structural and impedance spectroscopic properties of borate glasses with high Li+ concentration. Solid State Ion. 2021, 368, 115704. [Google Scholar] [CrossRef]
- Westhauser, F.; Decker, S.; Nawaz, Q.; Rehder, F.; Wilkesmann, S.; Moghaddam, A.; Kunisch, E.; Boccaccini, A.R. Impact of Zinc- or Copper-Doped Mesoporous Bioactive Glass Nanoparticles on the Osteogenic Differentiation and Matrix Formation of Mesenchymal Stromal Cells. Materials 2021, 14, 1864. [Google Scholar] [CrossRef] [PubMed]


| Groups | Procedure |
|---|---|
| 1: NI control group (non-infected group, n = 21) | K-wire osteosynthesis with intramedullary application of 10 µL PBS (non-infected)/103 KBE S. aureus in 10 µL PBS (infected). Debridement and re-osteosynthesis after 5 weeks with an angle-stable plate. |
| 2: I control group (infected control group, n = 14) | |
| 3: NI S53P4 (intervention group, non infected, n = 16) | K-wire osteosynthesis with intramedullary application of 10 µL PBS (non-infected)/103 KBE S. aureus in 10 µL PBS (infected). Debridement and Re-osteosynthesis after 5 weeks with an angle-stable plate and application of S53P4 to the bone defect. |
| 4: I S53P4 (intervention group, infected, n = 14) |
| Hue | Saturation | Brightness | |
|---|---|---|---|
| CD31 | 200/255 | 40/255 | 0/255 |
| CD14 | 200/255 | 60/255 | 0/255 |
| CD68 | 200/255 | 100/255 | 0/255 |
| TRAP | 200/255 | 90/255 | 0/255 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freischmidt, H.; Armbruster, J.; Rothhaas, C.; Titze, N.; Guehring, T.; Nurjadi, D.; Sonntag, R.; Schmidmaier, G.; Grützner, P.A.; Helbig, L. Treatment of Infection-Related Non-Unions with Bioactive Glass—A Promising Approach or Just Another Method of Dead Space Management? Materials 2022, 15, 1697. https://doi.org/10.3390/ma15051697
Freischmidt H, Armbruster J, Rothhaas C, Titze N, Guehring T, Nurjadi D, Sonntag R, Schmidmaier G, Grützner PA, Helbig L. Treatment of Infection-Related Non-Unions with Bioactive Glass—A Promising Approach or Just Another Method of Dead Space Management? Materials. 2022; 15(5):1697. https://doi.org/10.3390/ma15051697
Chicago/Turabian StyleFreischmidt, Holger, Jonas Armbruster, Catharina Rothhaas, Nadine Titze, Thorsten Guehring, Dennis Nurjadi, Robert Sonntag, Gerhard Schmidmaier, Paul Alfred Grützner, and Lars Helbig. 2022. "Treatment of Infection-Related Non-Unions with Bioactive Glass—A Promising Approach or Just Another Method of Dead Space Management?" Materials 15, no. 5: 1697. https://doi.org/10.3390/ma15051697
APA StyleFreischmidt, H., Armbruster, J., Rothhaas, C., Titze, N., Guehring, T., Nurjadi, D., Sonntag, R., Schmidmaier, G., Grützner, P. A., & Helbig, L. (2022). Treatment of Infection-Related Non-Unions with Bioactive Glass—A Promising Approach or Just Another Method of Dead Space Management? Materials, 15(5), 1697. https://doi.org/10.3390/ma15051697







