Effect of Glass Composition on Luminescence and Structure of CsPbBr3 Quantum Dots in an Amorphous Matrix
Abstract
:1. Introduction
2. Materials and Methods
3. Results
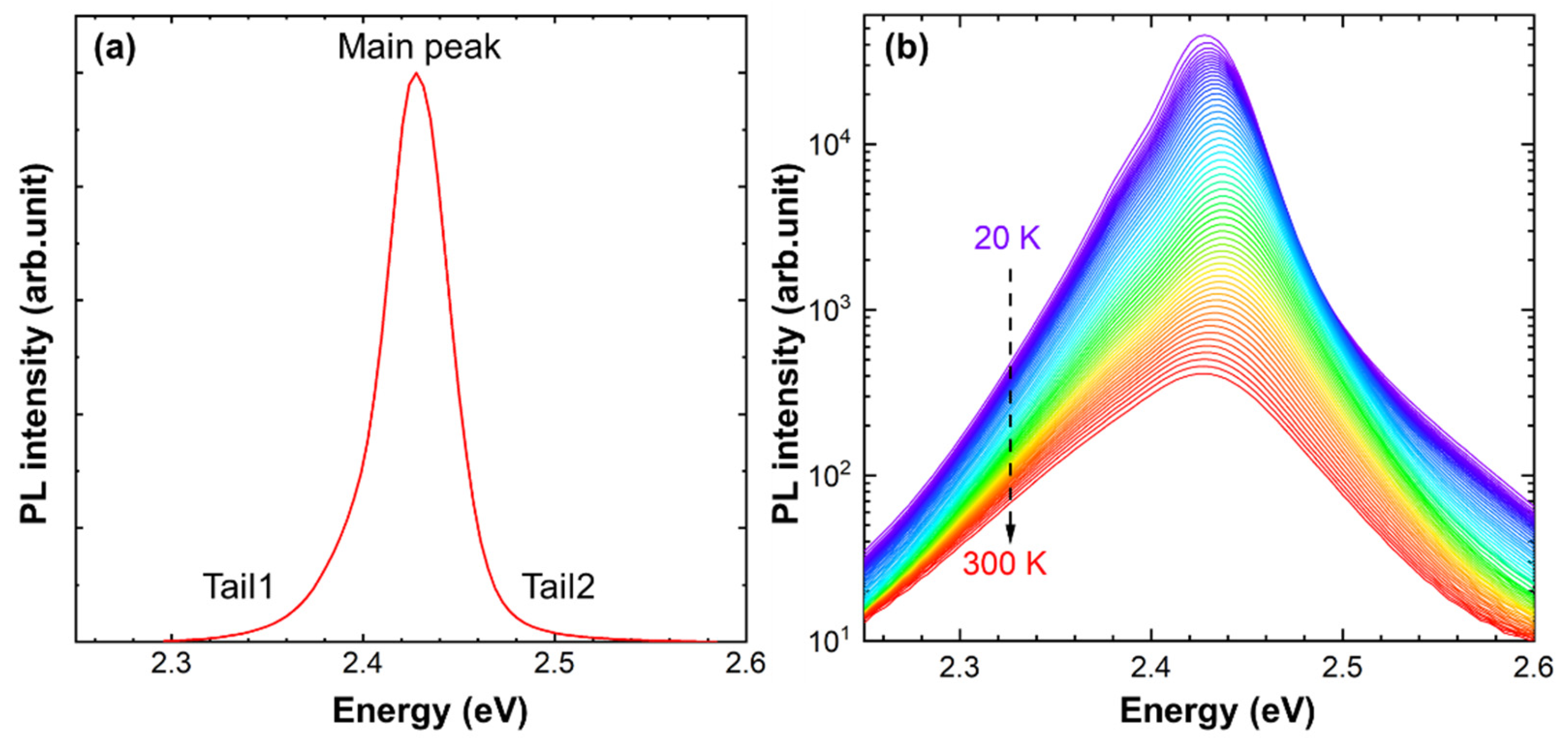
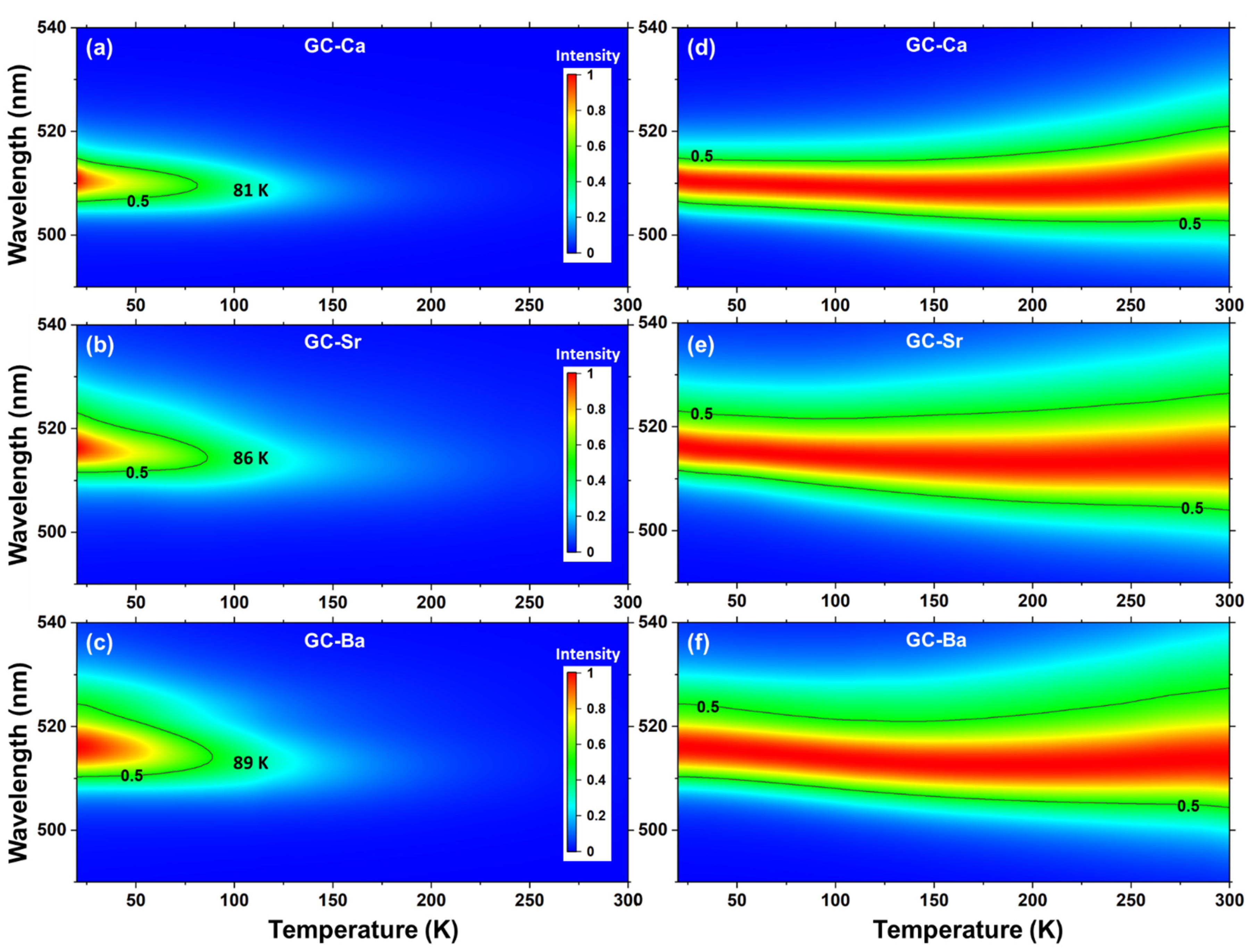
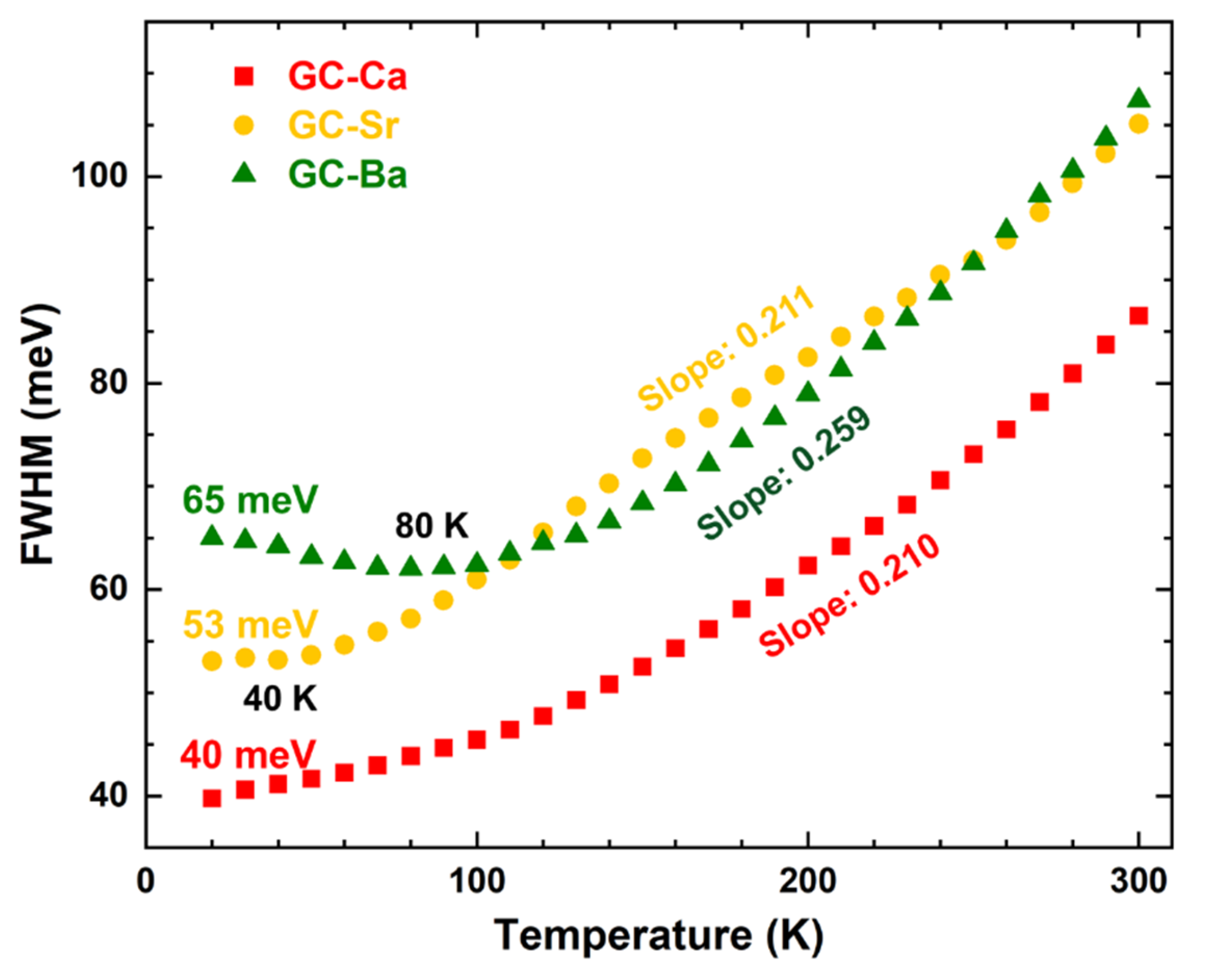
3.1. PL Spectra Evolution with Temperatures of CsPbBr3 QDs in the Glass Matrix
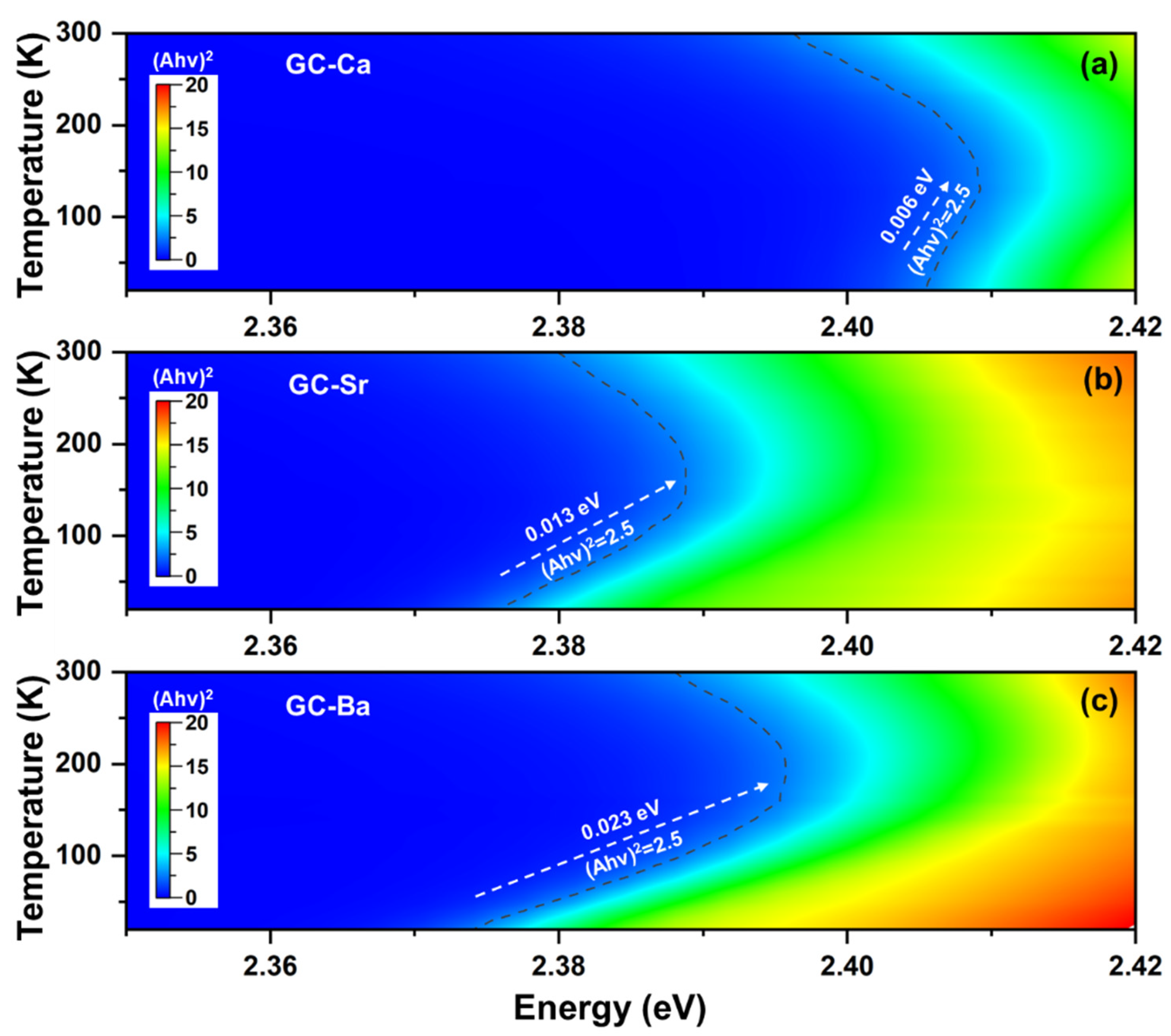
3.2. Absorption Edge Evolution with Temperatures of CsPbBr3 QDs in the Glass Matrix
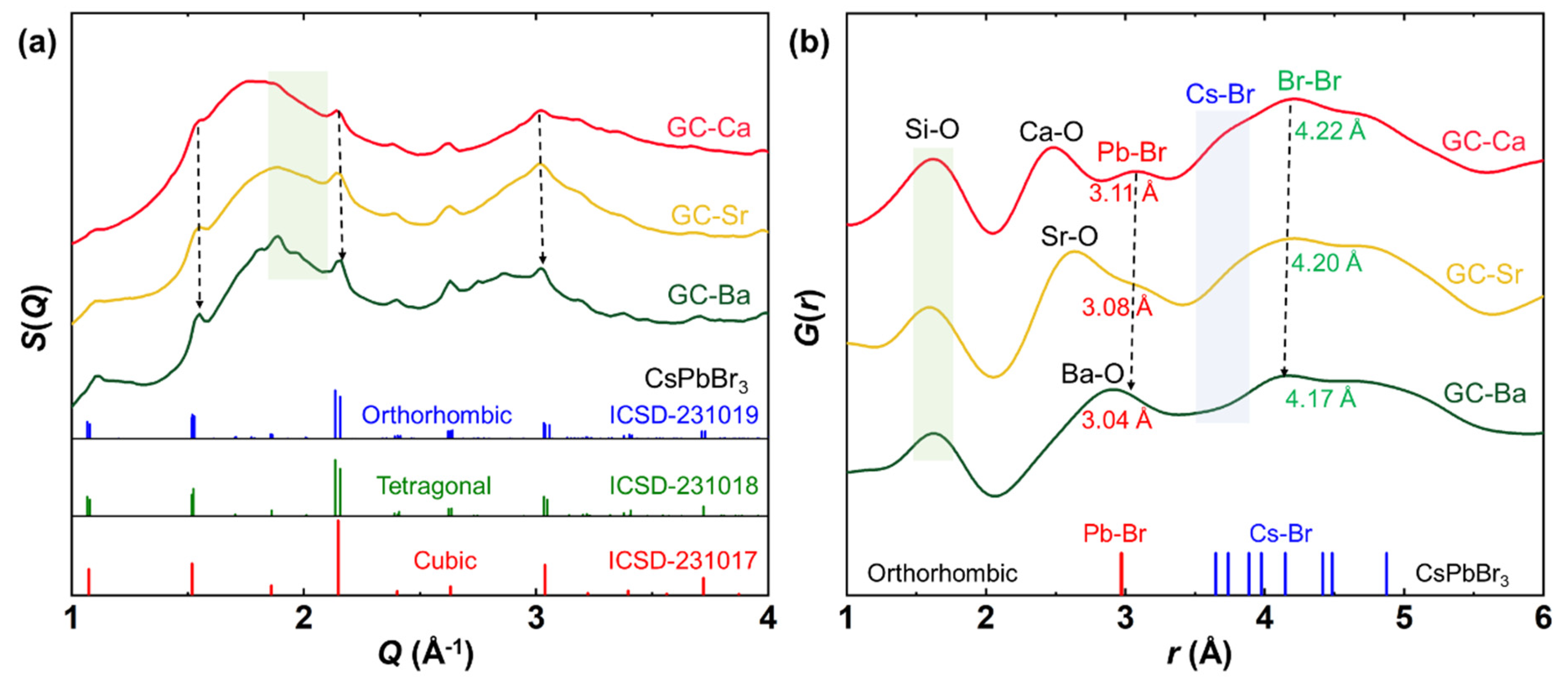
3.3. Crystal and Atomic Pair Structure of CsPbBr3 QDs in the Glass Matrix
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akkerman, Q.A.; Rainò, G.; Kovalenko, M.V.; Manna, L. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals. Nat. Mater. 2018, 17, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Rainò, G.; Becker, M.A.; Bodnarchuk, M.I.; Mahrt, R.F.; Kovalenko, M.V.; Stöferle, T. Superfluorescence from lead halide perovskite quantum dot superlattices. Nature 2018, 563, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.A.; Vaxenburg, R.; Nedelcu, G.; Sercel, P.C.; Shabaev, A.; Mehl, M.J.; Michopoulos, J.G.; Lambrakos, S.G.; Bernstein, N.; Lyons, J.L.; et al. Bright triplet excitons in caesium lead halide perovskites. Nature 2018, 553, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wu, J.; Ou, X.; Huang, B.; Almutlaq, J.; Zhumekenov, A.A.; Guan, X.; Han, S.; Liang, L.; Yi, Z.; et al. All-inorganic perovskite nanocrystal scintillators. Nature 2018, 561, 88–93. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Wang, B.; Zheng, W.L.; Kong, L.; Wan, Q.; Zhang, C.Y.; Li, Z.C.; Cao, X.Y.; Liu, M.M.; Li, L. Ceramic-like stable CsPbBr3 nanocrystals encapsulated in silica derived from molecular sieve templates. Nat. Commun. 2020, 11, 31. [Google Scholar] [CrossRef]
- Yakunin, S.; Protesescu, L.; Krieg, F.; Bodnarchuk, M.I.; Nedelcu, G.; Humer, M.; De Luca, G.; Fiebig, M.; Heiss, W.; Kovalenko, M.V. Low-threshold amplified spontaneous emission and lasing from colloidal nanocrystals of caesium lead halide perovskites. Nat. Commun. 2015, 6, 8056. [Google Scholar] [CrossRef]
- Rathnakumar, S.; Bhaskar, S.; Rai, A.; Saikumar, D.V.V.; Kambhampati, N.S.V.; Sivaramakrishnan, V.; Ramamurthy, S.S. Plasmon-Coupled Silver Nanoparticles for Mobile Phone-Based Attomolar Sensing of Mercury Ions. ACS Appl. Nano Mater. 2021, 4, 8066–8080. [Google Scholar] [CrossRef]
- Bhaskar, S.; Das, P.; Srinivasan, V.; Bhaktha, S.B.N.; Ramamurthy, S.S. Plasmonic-Silver Sorets and Dielectric-Nd2O3 nanorods for Ultrasensitive Photonic Crystal-Coupled Emission. Mater. Res. Bull. 2022, 145, 111558. [Google Scholar] [CrossRef]
- Dutta Choudhury, S.; Badugu, R.; Lakowicz, J.R. Directing Fluorescence with Plasmonic and Photonic Structures. Accounts Chem. Res. 2015, 48, 2171–2180. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.M.; Wan, Q.; Wang, H.M.; Carulli, F.; Sun, X.C.; Zheng, W.L.; Kong, L.; Zhang, Q.; Zhang, C.Y.; Zhang, Q.G.; et al. Suppression of temperature quenching in perovskite nanocrystals for efficient and thermally stable light-emitting diodes. Nat. Photonics 2021, 15, 379–385. [Google Scholar] [CrossRef]
- Wang, Y.H.; Lu, X.J.; Yang, W.G.; Wen, T.; Yang, L.X.; Ren, X.T.; Wang, L.; Lin, Z.S.; Zhao, Y.S. Pressure-Induced Phase Transformation, Reversible Amorphization, and Anomalous Visible Light Response in Organolead Bromide Perovskite. J. Am. Chem. Soc. 2015, 137, 11144–11149. [Google Scholar] [CrossRef]
- Straus, D.B.; Guo, S.; Cava, R.J. Kinetically Stable Single Crystals of Perovskite-Phase CsPbI3. J. Am. Chem. Soc. 2019, 141, 11435–11439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Liu, Z.; Lu, S.; Wang, L.; Feng, X.; Yang, D.; Wang, K.; Xiao, G.; Zhang, L.; Redfern, S.A.T.; et al. Pressure-induced emission of cesium lead halide perovskite nanocrystals. Nat. Commun. 2018, 9, 4506. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Chen, D.; Li, X.; Zhong, J.; Xu, X. In situ crystallization synthesis of CsPbBr3 perovskite quantum dot-embedded glasses with Improved stability for solid-state lighting and random upconverted lasing. ACS Appl. Mater. Inter. 2018, 10, 18918–18926. [Google Scholar] [CrossRef]
- Li, X.Y.; Yang, C.B.; Yu, Y.L.; Li, Z.; Lin, J.D.; Guan, X.F.; Zheng, Z.Q.; Chen, D.Q. Dual-Modal Photon Upconverting and Downshifting Emissions from Ultra-stable CsPbBr3 Perovskite Nanocrystals Triggered by Co-Growth of Tm:NaYbF4 Nanocrystals in Glass. ACS Appl. Mater. Inter. 2020, 12, 18705–18714. [Google Scholar] [CrossRef]
- Lin, J.D.; Lu, Y.X.; Li, X.Y.; Huang, F.; Yang, C.B.; Liu, M.L.; Jiang, N.Z.; Chen, D.Q. Perovskite Quantum Dots Glasses Based Backlit Displays. ACS Energy Lett. 2021, 6, 519–528. [Google Scholar] [CrossRef]
- Sun, K.; Tan, D.; Fang, X.; Xia, X.; Lin, D.; Song, J.; Lin, Y.; Liu, Z.; Gu, M.; Yue, Y.; et al. Three-dimensional direct lithography of stable perovskite nanocrystals in glass. Science 2022, 375, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Guo, Q.; Yang, D.; Xiao, X.; Liu, X.; Xia, Z.; Fan, F.; Qiu, J.; Dong, G. Reversible 3D laser printing of perovskite quantum dots inside a transparent medium. Nat. Photonics 2020, 14, 82–88. [Google Scholar] [CrossRef]
- Huang, X.; Guo, Q.; Kang, S.; Ouyang, T.; Chen, Q.; Liu, X.; Xia, Z.; Yang, Z.; Zhang, Q.; Qiu, J.; et al. Three-Dimensional Laser-Assisted Patterning of Blue-Emissive Metal Halide Perovskite Nanocrystals inside a Glass with Switchable Photoluminescence. ACS Nano 2020, 14, 3150–3158. [Google Scholar] [CrossRef]
- Sun, K.; Tan, D.Z.; Song, J.; Xiang, W.D.; Xu, B.B.; Qiu, J.R. Highly Emissive Deep-Red Perovskite Quantum Dots in Glass: Photoinduced Thermal Engineering and Applications. Adv. Opt. Mater. 2021, 9, 2100094. [Google Scholar] [CrossRef]
- Cheng, Y.; Shen, C.; Shen, L.; Xiang, W.; Lang, X. Tb3+, Eu3+ co-doped CsPbBr3 QDs glass with highly stable and luminous adjustable for white LEDs. ACS Appl. Mater. Inter. 2018, 10, 21434–21444. [Google Scholar] [CrossRef]
- Ding, L.; Liu, S.N.; Zhang, Z.L.; Shao, G.Z.; Xiang, W.D.; Liang, X.J. Stable Zn-doped CsPbBr3 NCs glasses toward an enhanced optical performance for WLED. Ceram. Int. 2019, 45, 22699–22706. [Google Scholar] [CrossRef]
- Liu, J.M.; Liu, S.N.; Chen, Y.; Zhao, Q.L.; Zhao, Y.J.; Xiang, W.D.; Liang, X.J.; Ren, B.D. Sm3+-doped CsPbBr3 NCs glass: A luminescent material for potential use in lighting engineering. Ceram. Int. 2019, 45, 22688–22693. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Shen, L.L.; Zhang, H.L.; Ding, L.; Shao, G.Z.; Liang, X.J.; Xiang, W.D. Novel red-emitting CsPb1-xTixI3 perovskite QDs@glasses with ambient stability for high efficiency white LEDs and plant growth LEDs. Chem. Eng. J. 2019, 378, 122125. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Z.; Zhao, L.; Cao, J.Y.; Yu, X.; Yang, Y.; Yu, S.F.; Qiu, J.B.; Xu, X.H. Long Persistent Luminescence from All-Inorganic Perovskite Nanocrystals. Adv. Opt. Mater. 2020, 8, 2000585. [Google Scholar] [CrossRef]
- Xu, Z.S.; Chen, T.; Zhang, D.D.; Zheng, G.J.; Wang, Z.; Ma, Z.J.; Yan, J.H.; Wang, X.W.; Liu, X.F.; Qiu, J.R. Tuning the optical properties in CsPbBr3 quantum dot-doped glass by modulation of its network topology. J. Mater. Chem. C 2021, 9, 6863–6872. [Google Scholar] [CrossRef]
- Pang, X.L.; Si, S.C.; Xie, L.Q.; Zhang, X.J.; Huang, H.Z.; Liu, S.T.; Xiao, W.X.; Wang, S.R.; Xuan, T.T.; Zhuang, J.L.; et al. Regulating the morphology and luminescence properties of CsPbBr3 perovskite quantum dots through the rigidity of glass network structure. J. Mater. Chem. C 2020, 8, 17374–17382. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, H.; Xie, L.; Xuan, T.; Sun, Y.; Si, S.; Jiang, B.; Chen, W.; Zhuang, J.; Hu, C.; et al. Precipitating CsPbBr3 quantum dots in boro-germanate glass with a dense structure and inert environment toward highly stable and efficient narrow-band green emitters for wide-color-gamut liquid crystal displays. J. Mater. Chem. C 2019, 7, 13139–13148. [Google Scholar] [CrossRef]
- Boora, M.; Malik, S.; Kumar, V.; Bala, M.; Arora, S.; Rohilla, S.; Kumar, A.; Dalal, J. Investigation of structural and impedance spectroscopic properties of borate glasses with high Li+ concentration. Solid State Ion. 2021, 368, 115704. [Google Scholar] [CrossRef]
- Li, W.; Zhao, X.; Liu, C.; Coudert, F.-X. Ab Initio Molecular Dynamics of CdSe Quantum-Dot-Doped Glasses. J. Am. Chem. Soc. 2020, 142, 3905–3912. [Google Scholar] [CrossRef]
- Xia, M.L.; Luo, J.J.; Chen, C.; Liu, H.; Tang, J. Semiconductor Quantum Dots-Embedded Inorganic Glasses: Fabrication, Luminescent Properties, and Potential Applications. Adv. Opt. Mater. 2019, 7, 1900851. [Google Scholar] [CrossRef]
- Tijaria, M.; Sharma, Y.; Kumar, V.; Dahiya, S.; Dalal, J. Effect of Na2O on physical, structural and electrical properties of borate glasses. Mater. Today: Proc. 2021, 45, 3722–3725. [Google Scholar] [CrossRef]
- Li, P.P.; Xie, W.Q.; Mao, W.; Tian, Y.; Huang, F.F.; Xu, S.Q.; Zhang, J.J. Luminescence enhancement of CsPbBr3 quantum dot glasses induced by two unexpected methods: Mechanical and hydration crystallization. J. Mater. Chem. C 2020, 8, 473–480. [Google Scholar] [CrossRef]
- Xiang, X.Q.; Lin, H.; Li, R.F.; Cheng, Y.; Huang, Q.M.; Xu, J.; Wang, C.Y.; Chen, X.Y.; Wang, Y.S. Stress-induced CsPbBr3 nanocrystallization on glass surface: Unexpected mechanoluminescence and applications. Nano Res. 2019, 12, 1049–1054. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhang, R.L.; Yue, Y.; Yan, S.S.; Zhang, L.Y.; Chen, D.P. Room temperature synthesis of CsPbX3 (X = Cl, Br, I) perovskite quantum dots by water-induced surface crystallization of glass. J. Alloy. Compd. 2020, 818, 152872. [Google Scholar] [CrossRef]
- Cottingham, P.; Brutchey, R.L. Depressed Phase Transitions and Thermally Persistent Local Distortions in CsPbBr3 Quantum Dots. Chem. Mat. 2018, 30, 6711–6716. [Google Scholar] [CrossRef]
- Bertolotti, F.; Protesescu, L.; Kovalenko, M.V.; Yakunin, S.; Cervellino, A.; Billinge, S.J.L.; Terban, M.W.; Pedersen, J.S.; Masciocchi, N.; Guagliardi, A. Coherent Nanotwins and Dynamic Disorder in Cesium Lead Halide Perovskite Nanocrystals. ACS Nano 2017, 11, 3819–3831. [Google Scholar] [CrossRef]
- Lao, X.Z.; Zhou, W.; Bao, Y.T.; Wang, X.R.; Yang, Z.; Wang, M.Q.; Xu, S.J. Photoluminescence signatures of thermal expansion, electron-phonon coupling and phase transitions in cesium lead bromide perovskite nanosheets. Nanoscale 2020, 12, 7315–7320. [Google Scholar] [CrossRef]
- Tominaka, S.; Yamada, H.; Hiroi, S.; Kawaguchi, S.I.; Ohara, K. Lepidocrocite-Type Titanate Formation from Isostructural Prestructures under Hydrothermal Reactions: Observation by Synchrotron X-ray Total Scattering Analyses. ACS Omega 2018, 3, 8874–8881. [Google Scholar] [CrossRef]
- Falsini, N.; Roini, G.; Ristori, A.; Calisi, N.; Biccari, F.; Vinattieri, A. Analysis of the Urbach tail in cesium lead halide perovskites. J. Appl. Phys. 2022, 131, 010902. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Wolf, C.; Lee, T.-W. Exciton and lattice dynamics in low-temperature processable CsPbBr3 thin-films. Mater. Today Energy 2018, 7, 199–207. [Google Scholar] [CrossRef]
- Popov, A.I.; Kotomin, E.A.; Maier, J. Analysis of self-trapped hole mobility in alkali halides and metal halides. Solid State Ion. 2017, 302, 3–6. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, C.; Li, X.; Li, Y.; Qiu, S.; Shen, W.; Cai, B.; Sun, Z.; Yang, D.; Deng, Z.; et al. In Situ Passivation of PbBr64− Octahedra toward Blue Luminescent CsPbBr3 Nanoplatelets with Near 100% Absolute Quantum Yield. ACS Energy Lett. 2018, 3, 2030–2037. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, R.; Ueda, J.; Shinozaki, K.; Tanabe, S. Effect of Glass Composition on Luminescence and Structure of CsPbBr3 Quantum Dots in an Amorphous Matrix. Materials 2022, 15, 1678. https://doi.org/10.3390/ma15051678
Zheng R, Ueda J, Shinozaki K, Tanabe S. Effect of Glass Composition on Luminescence and Structure of CsPbBr3 Quantum Dots in an Amorphous Matrix. Materials. 2022; 15(5):1678. https://doi.org/10.3390/ma15051678
Chicago/Turabian StyleZheng, Ruilin, Jumpei Ueda, Kenji Shinozaki, and Setsuhisa Tanabe. 2022. "Effect of Glass Composition on Luminescence and Structure of CsPbBr3 Quantum Dots in an Amorphous Matrix" Materials 15, no. 5: 1678. https://doi.org/10.3390/ma15051678
APA StyleZheng, R., Ueda, J., Shinozaki, K., & Tanabe, S. (2022). Effect of Glass Composition on Luminescence and Structure of CsPbBr3 Quantum Dots in an Amorphous Matrix. Materials, 15(5), 1678. https://doi.org/10.3390/ma15051678






