Flexible and Stretchable Bioelectronics
Abstract
:1. Introduction
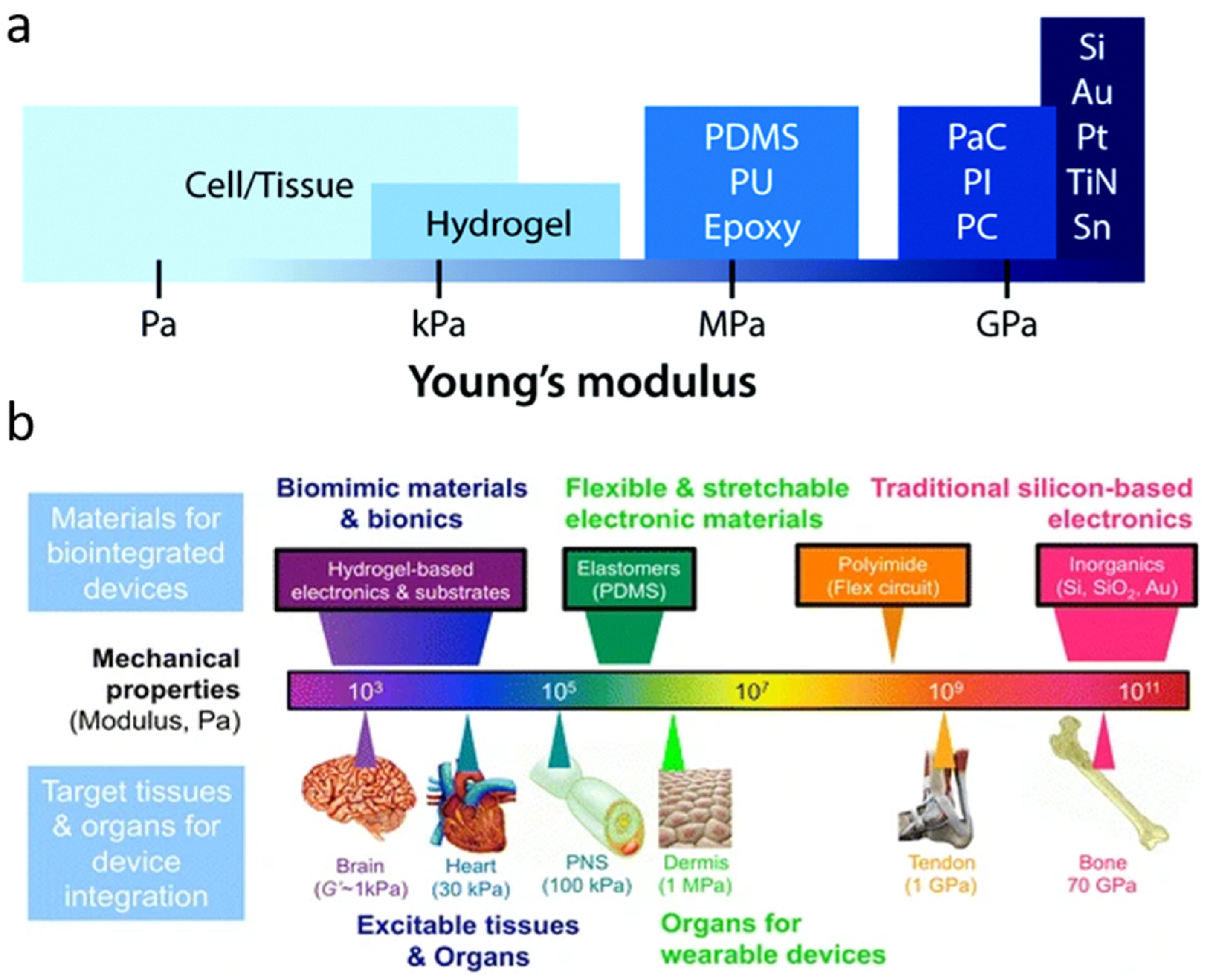
2. Opportunities and Limitations in Bioelectronic Devices
3. Materials
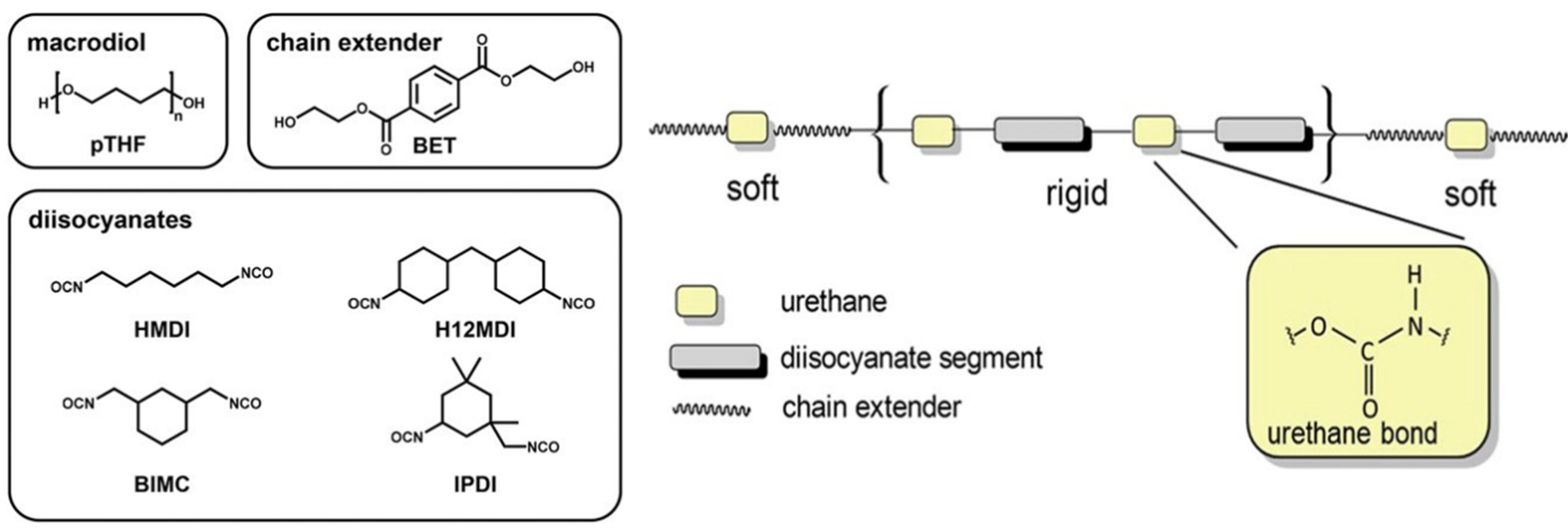
3.1. Polymeric Substrates
3.2. Conductive Materials
3.2.1. Metal Nanowires
3.2.2. Conductive Polymers and Conductive Liquids
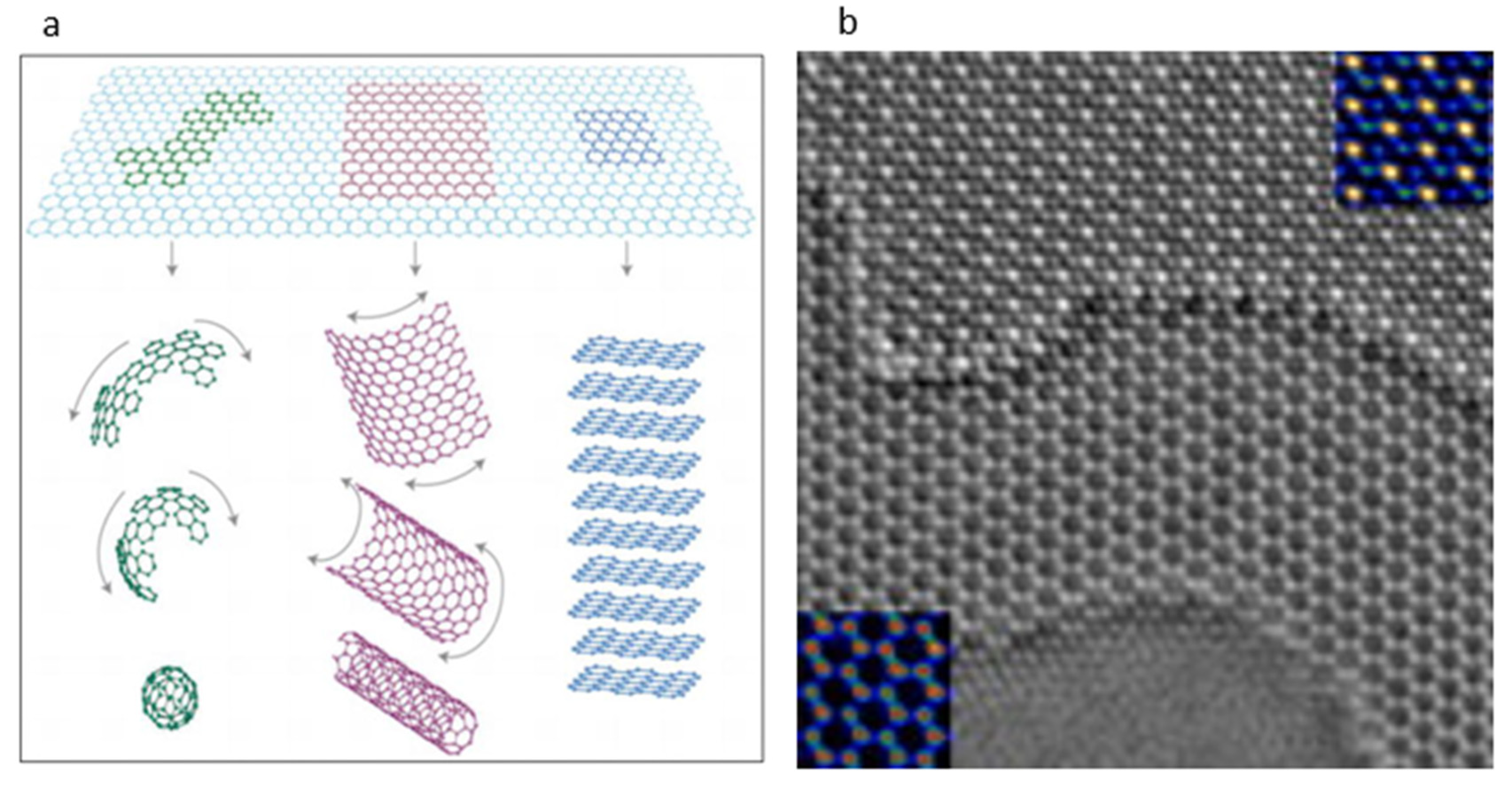
3.3. Carbon-Based Materials
3.3.1. Graphene
3.3.2. Carbon Black
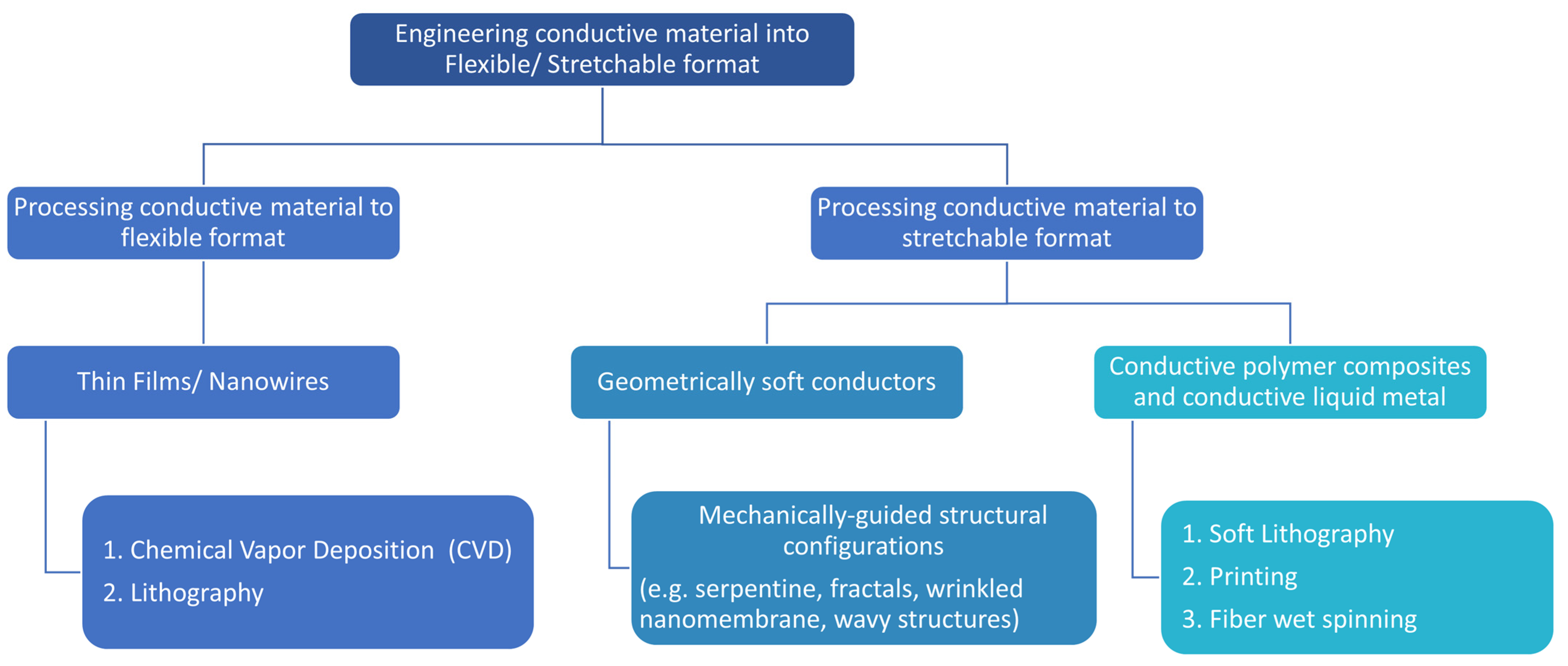
4. Engineering Organic/Inorganic Functional Materials in Stretchable Bioelectronic Devices
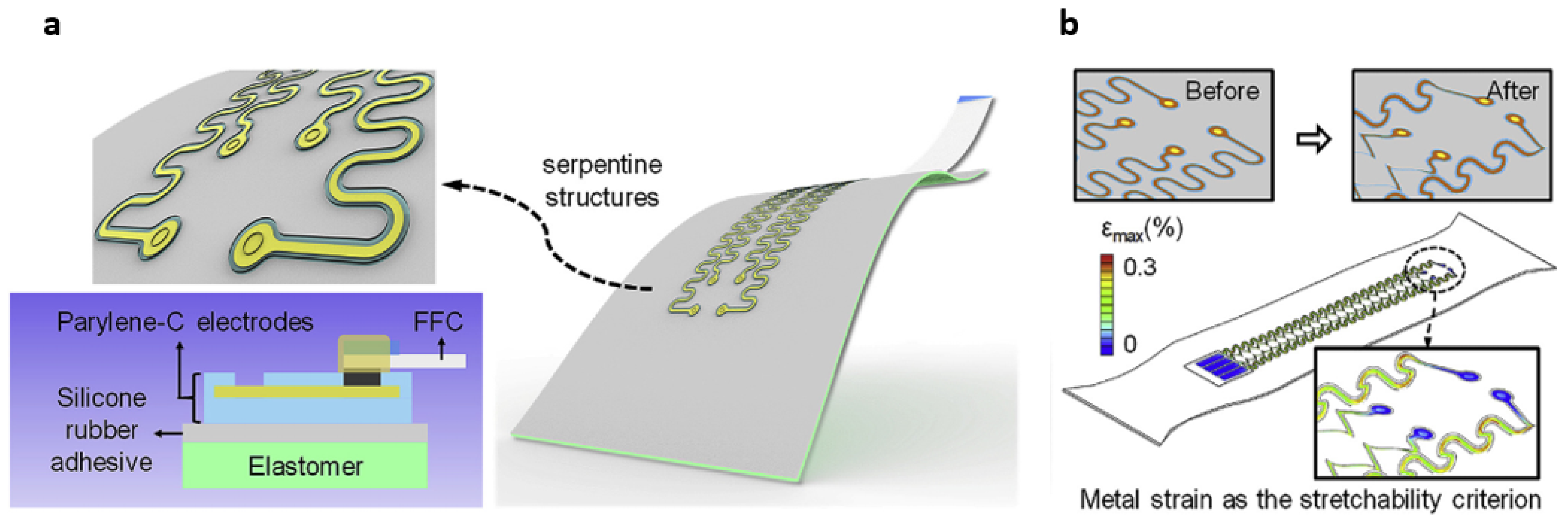
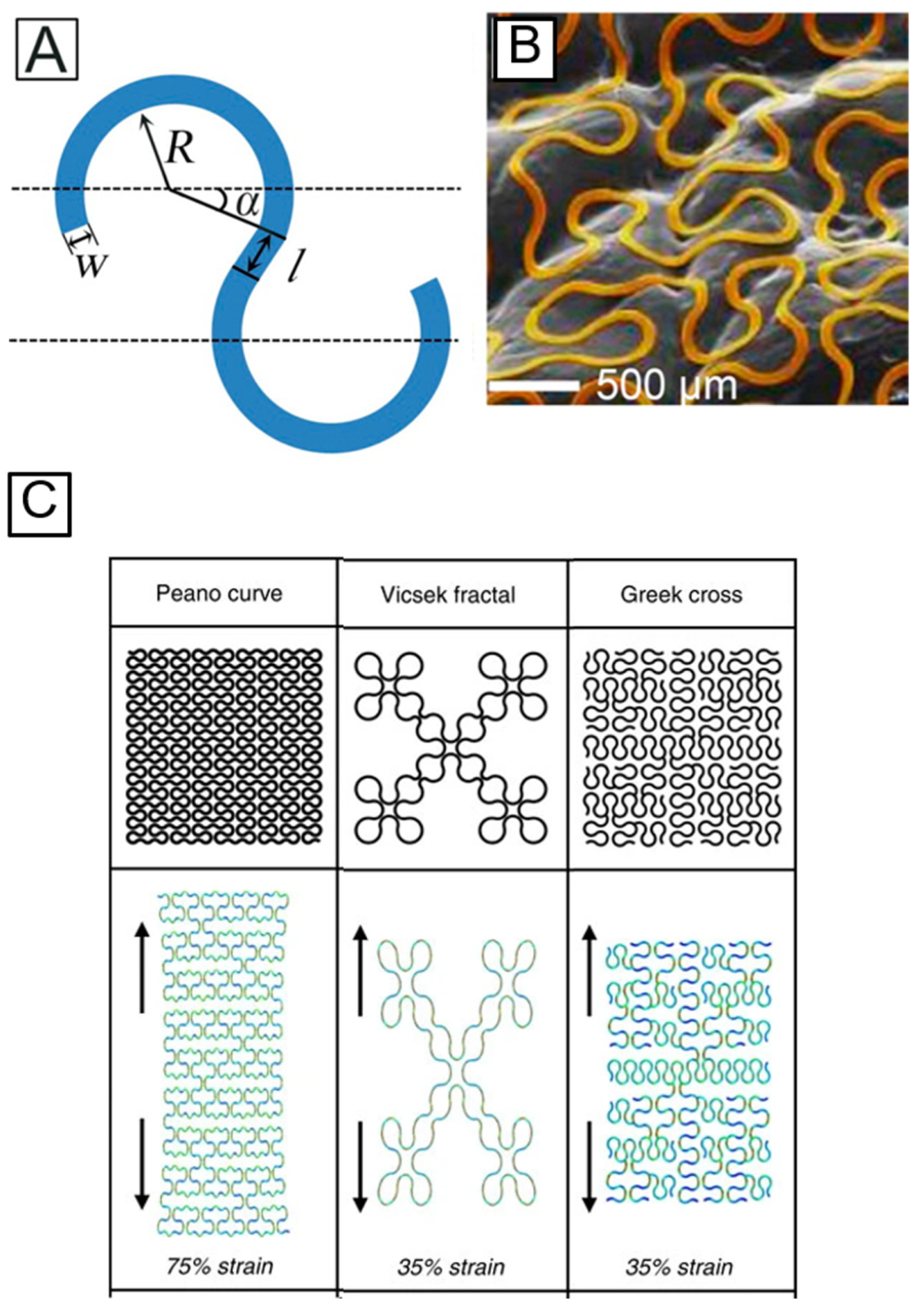
4.1. Structural Configurations to Render Stretchability to Hard Inorganic Materials
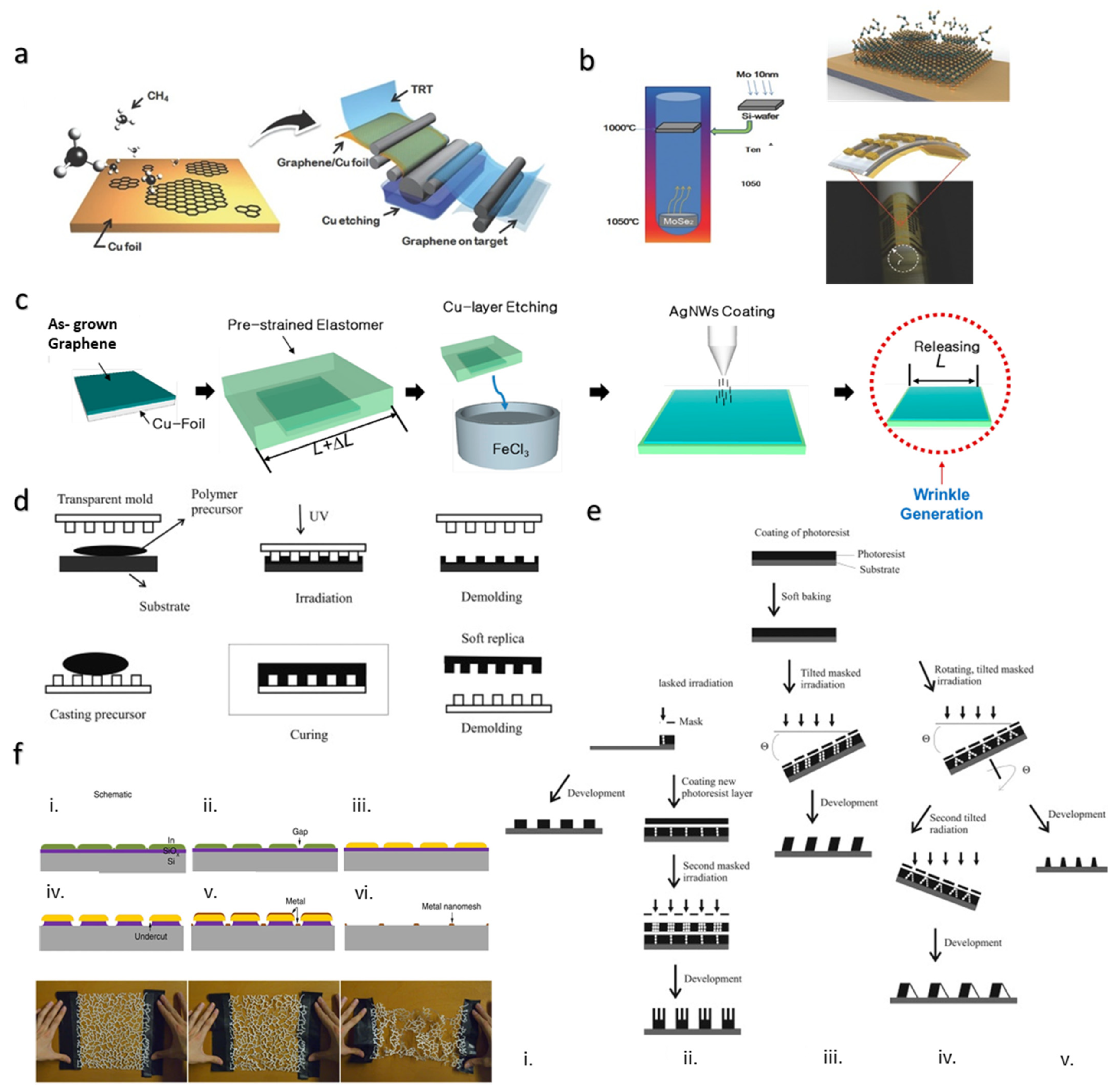
4.2. Chemical Vapor Deposition for Creating Thin Films
4.3. Lithography Method for Creating Thin Films and Nanowires
4.4. Printing for Creating Thin Films of Conductive Liquids
4.5. Wet Spinning Method for Flexible/Stretchable and Conductive Fiber Production

5. Transducers and Communication
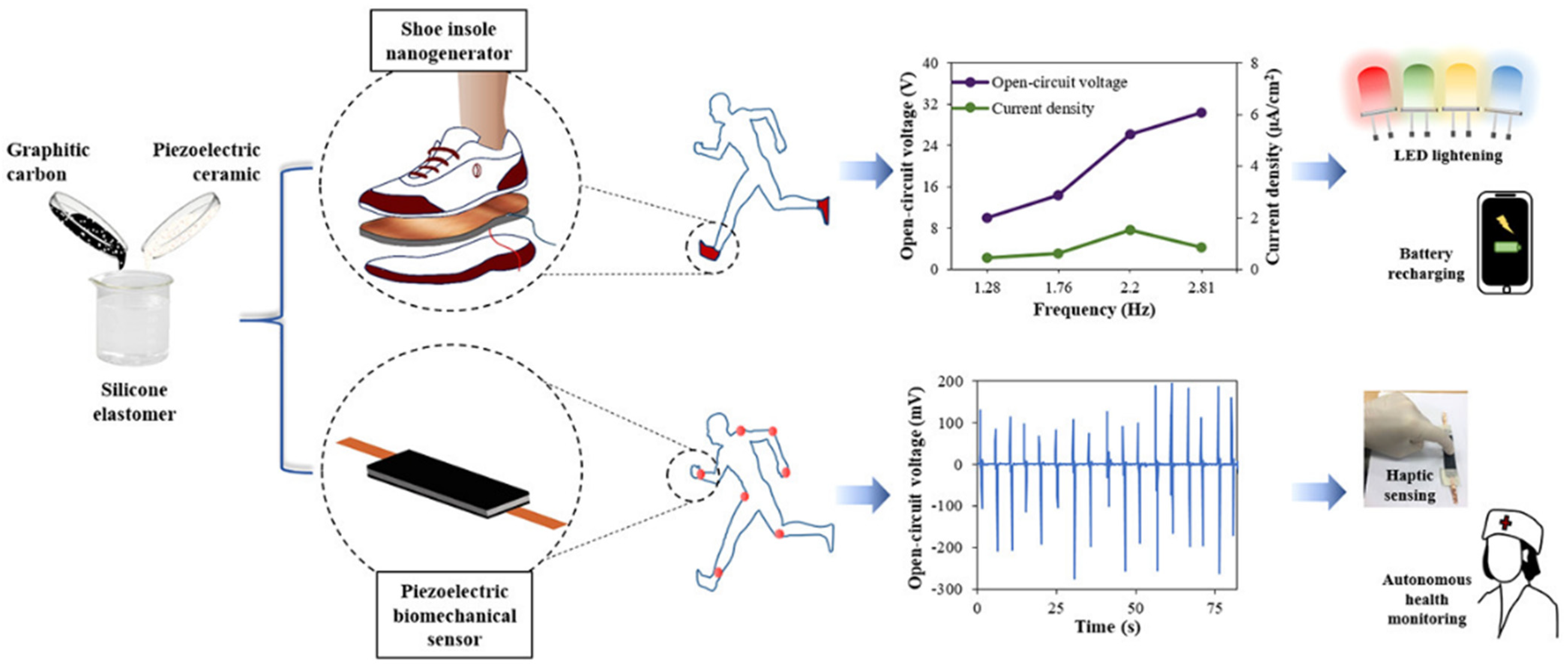
5.1. Piezoelectric Transducers
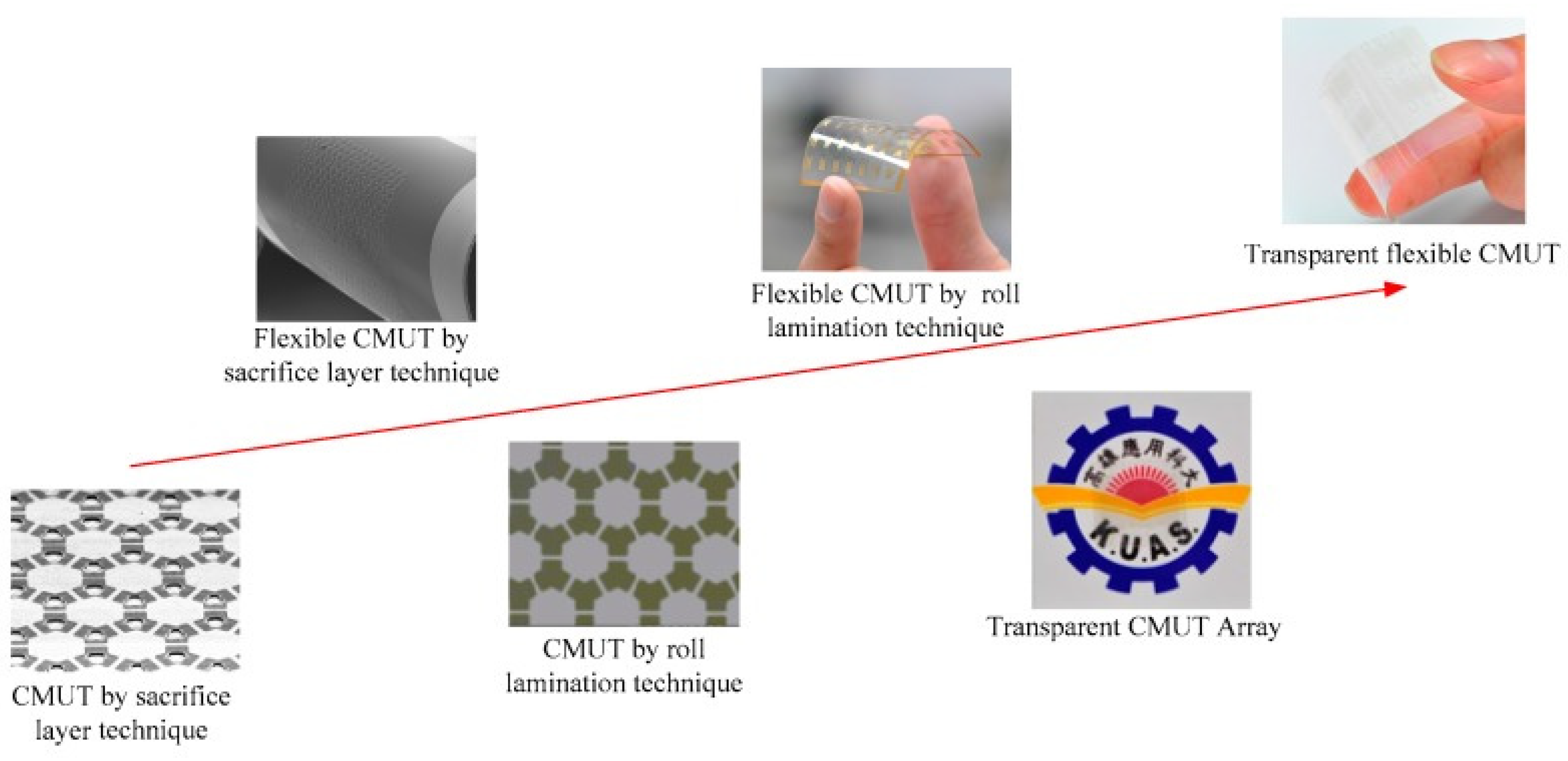
5.2. Ultrasonic Transducers
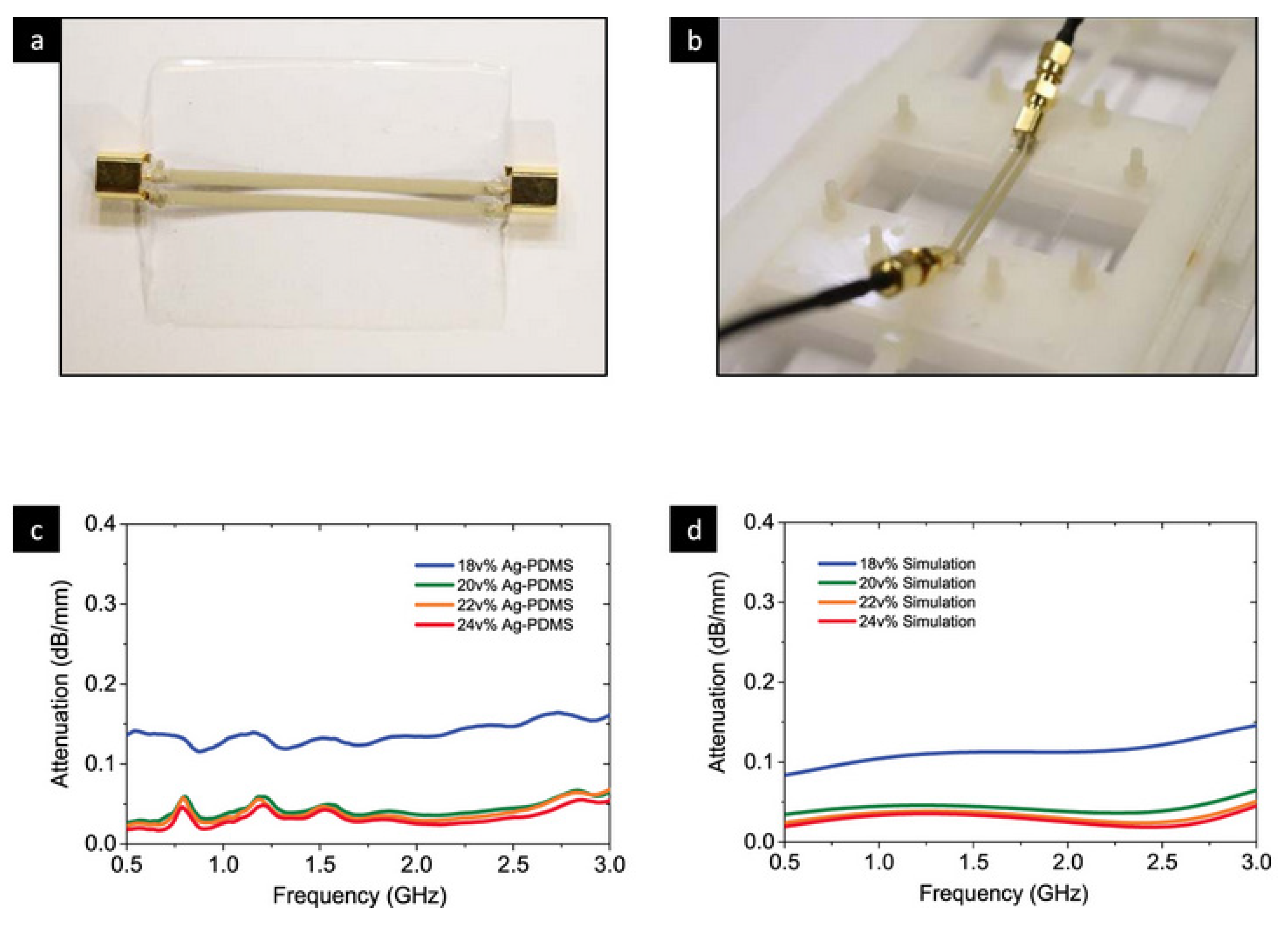
5.3. Wearable Antenna Communication
5.4. Wireless Communication
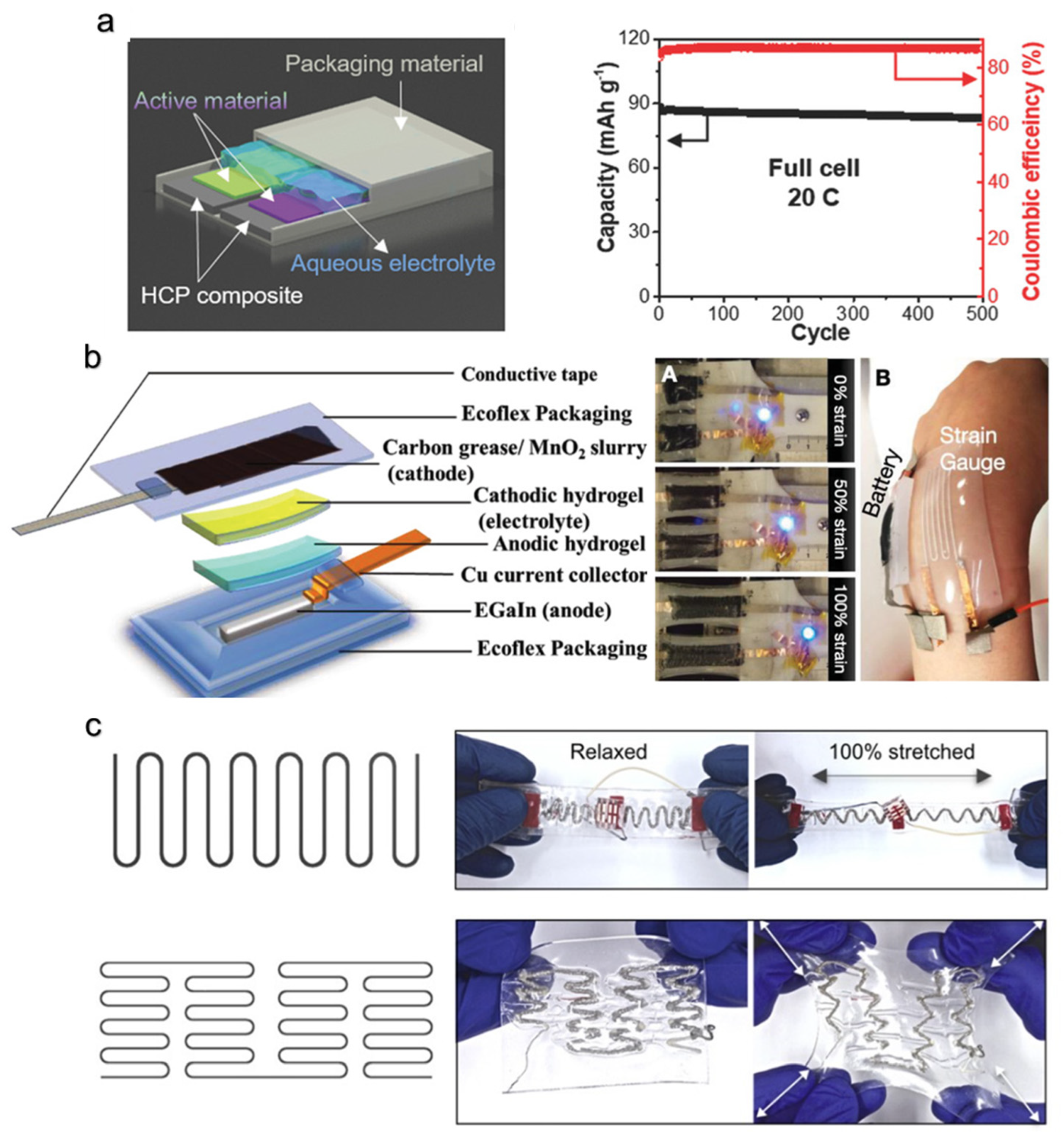
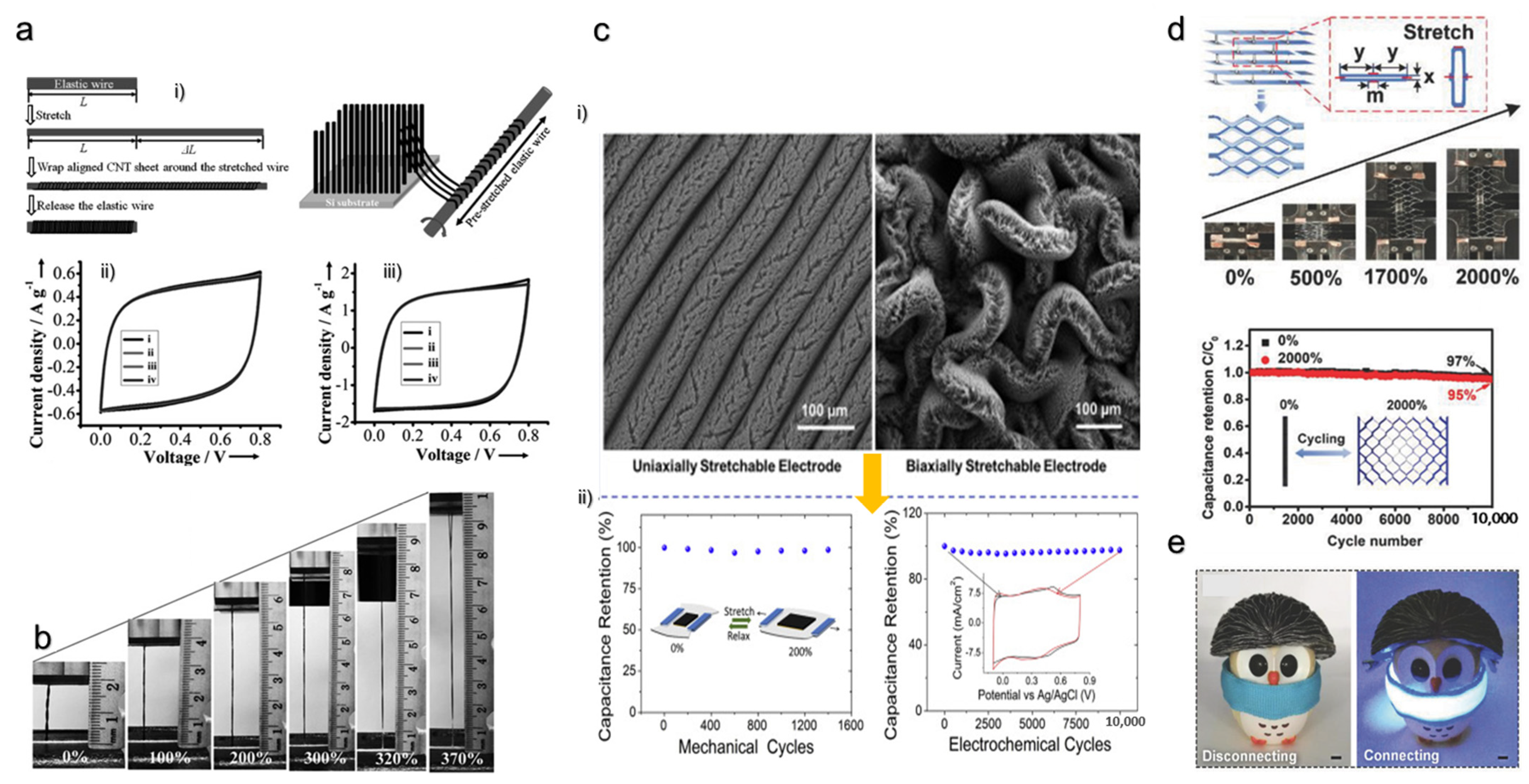
6. Power Sources
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peeples, L. The rise of bioelectric medicine sparks interest among researchers, patients, and industry. Proc. Natl. Acad. Sci. USA. 2019, 116, 24379–24382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Liu, Q. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Someya, T.; Bao, Z.; Malliaras, G.G. The rise of plastic bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tian, B. Inorganic semiconductor biointerfaces. Nat. Rev. Mater. 2018, 3, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Yin, C.; Wang, Q.; Zhang, T.; Chen, S.; Lu, C.; Zhao, K.; Xu, W.; Pan, T.; Gao, M.; et al. Flexible bioelectronics for physiological signals sensing and disease treatment. J. Mater. 2020, 6, 397–413. [Google Scholar] [CrossRef]
- Khan, Y.; Ostfeld, A.E.; Lochner, C.M.; Pierre, A.; Arias, A.C. Monitoring of Vital Signs with Flexible and Wearable Medical Devices. Adv. Mater. 2016, 28, 4373–4395. [Google Scholar] [CrossRef]
- Shi, G.; Lowe, S.E.; Zhao, H. Flexible and stretchable inorganic electronics: Conductive materials, fabrication strategy, and applicable devices. In 2D Materials for Photonic and Optoelectronic Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 199–252. ISBN 9780081026373. [Google Scholar]
- Wu, X.; Peng, H. Polymer-based flexible bioelectronics. Sci. Bull. 2019, 64, 634–640. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.C.; Shim, H.J.; Lee, W.; Koo, J.H.; Kim, D.H. Material-Based Approaches for the Fabrication of Stretchable Electronics. Adv. Mater. 2020, 32, 1902743. [Google Scholar] [CrossRef]
- Bauer, S.; Baier, H.; Baumgartner, C.; Bohlmann, K.; Fauser, S.; Graf, W.; Hillenbrand, B.; Hirsch, M.; Last, C.; Lerche, H.; et al. Transcutaneous Vagus Nerve Stimulation (tVNS) for Treatment of Drug-Resistant Epilepsy: A Randomized, Double-Blind Clinical Trial (cMPsE02). Brain Stimul. 2016, 9, 356–363. [Google Scholar] [CrossRef]
- SetPoint Medical Receives FDA Breakthrough Device Designation for Its Novel Bioelectronic Platform. Available online: https://setpointmedical.com/setpoint-medical-receives-fda-breakthrough-device-designation-for-its-novel-bioelectronic-platform/ (accessed on 6 February 2022).
- Carreno, F.R.; Frazer, A. Vagal Nerve Stimulation for Treatment-Resistant Depression. Neurotherapeutics 2017, 14, 716–727. [Google Scholar] [CrossRef]
- Jonsson, A.; Song, Z.; Nilsson, D.; Meyerson, B.A.; Simon, D.T.; Linderoth, B.; Berggren, M. Therapy using implanted organic bioelectronics. Sci. Adv. 2015, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlov, V.A.; Tracey, K.J. Bioelectronic medicine: Updates, challenges and paths forward. Bioelectron. Med. 2019, 5, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Yang, T.; Zhang, Y.; Qu, G.; Wei, S.; Liu, Z.; Kong, T.; Liu, Y.; Qu, G.; Wei, S.; et al. Ultrastretchable and Wireless Bioelectronics Based on All-Hydrogel Microfluidics. Adv. Mater. 2019, 31, 1902783. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Kim, M.K.; Lee, C.H. Skin-Mountable Biosensors and Therapeutics: A Review. Annu. Rev. Biomed. Eng. 2019, 21, 299–323. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Sharma, S.; Cass, A.E.G.; Antiochia, R. Microneedle-based biosensor for minimally-invasive lactate detection. Biosens. Bioelectron. 2019, 123, 152–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keum, D.H.; Kim, S.K.; Koo, J.; Lee, G.H.; Jeon, C.; Mok, J.W.; Mun, B.H.; Lee, K.J.; Kamrani, E.; Joo, C.K.; et al. Wireless smart contact lens for diabetic diagnosis and therapy. Sci. Adv. 2020, 6, eaba3252. [Google Scholar] [CrossRef] [Green Version]
- Ezeokafor, I.; Upadhya, A.; Shetty, S. Neurosensory Prosthetics: An Integral Neuromodulation Part of Bioelectronic Device. Front. Neurosci. 2021, 15, 1442. [Google Scholar] [CrossRef]
- Koopman, F.A.; Chavan, S.S.; Miljko, S.; Grazio, S.; Sokolovic, S.; Schuurman, P.R.; Mehta, A.D.; Levine, Y.A.; Faltys, M.; Zitnik, R.; et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in Rheumatoid arthritis. Proc. Natl. Acad. Sci. USA. 2016, 113, 8284–8289. [Google Scholar] [CrossRef] [Green Version]
- Vagus Nerve Stimulation (VNS) for Treatment Resistant Depression (TRD)|CMS. Available online: https://www.cms.gov/Medicare/Coverage/Coverage-with-Evidence-Development/VNS (accessed on 5 February 2022).
- Electrical spinal Cord Stimulation Helps Paraplegics Walk. Available online: https://aabme.asme.org/posts/wireless-implant-helps-paraplegics-walk-again (accessed on 6 February 2022).
- Grahn, P.J.; Mallory, G.W.; Khurram, O.U.; Berry, B.M.; Hachmann, J.T.; Bieber, A.J.; Bennet, K.E.; Min, H.K.; Chang, S.Y.; Lee, K.H.; et al. A neurochemical closed-loop controller for deep brain stimulation: Toward individualized smart neuromodulation therapies. Front. Neurosci. 2014, 8, 169. [Google Scholar] [CrossRef] [Green Version]
- Olmos de Koo, L.C.; Gregori, N.Z. Step-by-Step Guide to Retinal Implant Surgery—Retina Today. Available online: https://retinatoday.com/articles/2015-july-aug/step-by-step-guide-to-retinal-implant-surgery (accessed on 5 February 2022).
- Svirsky, M. Cochlear implants and electronic hearing. Phys. Today 2017, 70, 52. [Google Scholar] [CrossRef] [Green Version]
- Milestone Embedded Device Advances Medical Electronics. Available online: https://aabme.asme.org/posts/embedded-device-stimulates-nerve-recovery-then-absorbed-by-body (accessed on 6 February 2022).
- Walker, G.M.; Ramsey, J.M.; Cavin, R.K.; Herr, D.J.C.; Zhirnov, V. A Framework for BIOELECTRONICS Discovery and Innovation. 2009. Available online: https://www.nist.gov/system/files/documents/pml/div683/bioelectronics_report.pdf (accessed on 22 January 2022).
- Boehler, C.; Carli, S.; Fadiga, L.; Stieglitz, T.; Asplund, M. Tutorial: Guidelines for standardized performance tests for electrodes intended for neural interfaces and bioelectronics. Nat. Protoc. 2020, 15, 3557–3578. [Google Scholar] [CrossRef] [PubMed]
- Iota Biosciences—Vanishingly Small Bioelectronics. Available online: https://iota.bio/ (accessed on 5 February 2022).
- Singer, A.; Robinson, J.T. Wireless Power Delivery Techniques for Miniature Implantable Bioelectronics. Adv. Healthc. Mater. 2021, 10, 2100664. [Google Scholar] [CrossRef] [PubMed]
- Durable miniaturized bioelectronics. Nat. Biomed. Eng. 2017, 1, 1. [CrossRef] [Green Version]
- ISO 10993-1:2003. Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing; ISO: Geneva, Switzerland, 2003. [Google Scholar]
- Saliterman, S.S. Biocompatibility, FDA and ISO 10993—[PDF Document]. Available online: https://fdocuments.in/document/biocompatibility-fda-and-iso-10993.html (accessed on 22 January 2022).
- Gray, M.; Meehan, J.; Ward, C.; Langdon, S.P.; Kunkler, I.H.; Murray, A.; Argyle, D. Implantable biosensors and their contribution to the future of precision medicine. Vet. J. 2018, 239, 21–29. [Google Scholar] [CrossRef]
- Ware, T.; Simon, D.; Arreaga-Salas, D.E.; Reeder, J.; Rennaker, R.; Keefer, E.W.; Voit, W. Fabrication of responsive, softening neural interfaces. Adv. Funct. Mater. 2012, 22, 3470–3479. [Google Scholar] [CrossRef]
- Fan, X.; Nie, W.; Tsai, H.; Wang, N.; Huang, H.; Cheng, Y.; Wen, R.; Ma, L.; Yan, F.; Xia, Y. PEDOT:PSS for Flexible and Stretchable Electronics: Modifications, Strategies, and Applications. Adv. Sci. 2019, 6, 1900813. [Google Scholar] [CrossRef] [Green Version]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev 2019, 48, 1642. [Google Scholar] [CrossRef] [Green Version]
- Bettinger, C.J. Recent advances in materials and flexible electronics for peripheral nerve interfaces. Bioelectron. Med. 2018, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Zhang, L.; Yue, S.; Yu, S.; Wei, J.; Ouyang, J. Enhancement in the Mechanical Stretchability of PEDOT:PSS Films by Compounds of Multiple Hydroxyl Groups for Their Application as Transparent Stretchable Conductors. Macromolecules 2021, 54, 1234–1242. [Google Scholar] [CrossRef]
- Tee, B.C.K.; Ouyang, J. Soft Electronically Functional Polymeric Composite Materials for a Flexible and Stretchable Digital Future. Adv. Mater. 2018, 30, 1802560. [Google Scholar] [CrossRef]
- Li, C.Y.; Liao, Y.C. Adhesive Stretchable Printed Conductive Thin Film Patterns on PDMS Surface with an Atmospheric Plasma Treatment. ACS Appl. Mater. Interfaces 2016, 8, 11868–11874. [Google Scholar] [CrossRef]
- Burke, A.; Hasirci, N. Polyurethanes in biomedical applications. In Biomaterials: From Molecules to Engineered Tissues; Springer: Boston, MA, USA, 2004; Volume 553, pp. 83–101. [Google Scholar]
- Ji, B.; Xie, Z.; Hong, W.; Jiang, C.; Guo, Z.; Wang, L.; Wang, X.; Yang, B.; Liu, J. Stretchable Parylene-C electrodes enabled by serpentine structures on arbitrary elastomers by silicone rubber adhesive. J. Mater. 2020, 6, 330–338. [Google Scholar] [CrossRef]
- Lecomte, A.; Degache, A.; Descamps, E.; Dahan, L.; Bergaud, C. Biostability Assessment of Flexible Parylene C-based Implantable Sensor in Wireless Chronic Neural Recording. Procedia Eng. 2016, 168, 189–192. [Google Scholar] [CrossRef]
- De La Oliva, N.; Mueller, M.; Stieglitz, T.; Navarro, X.; del Valle, J. On the use of Parylene C polymer as substrate for peripheral nerve electrodes. Sci. Rep. 2018, 8, 5965. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with designer functionalities-Properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Zhao, W.; Zhang, M.; Qin, H.; Xie, Y. Flexible, stretchable sensors for wearable health monitoring: Sensing mechanisms, materials, fabrication strategies and features. Sensors 2018, 18, 645. [Google Scholar] [CrossRef] [Green Version]
- Kanyanta, V.; Ivankovic, A. Mechanical characterisation of polyurethane elastomer for biomedical applications. J. Mech. Behav. Biomed. Mater. 2010, 3, 51–62. [Google Scholar] [CrossRef]
- Seidler, K.; Ehrmann, K.; Steinbauer, P.; Rohatschek, A.; Andriotis, O.G.; Dworak, C.; Koch, T.; Bergmeister, H.; Grasl, C.; Schima, H.; et al. A structural reconsideration: Linear aliphatic or alicyclic hard segments for biodegradable thermoplastic polyurethanes? J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2214–2224. [Google Scholar] [CrossRef]
- Günther, F.; Lima, E.F.S.; Rossi de Aguiar, K.M.F.; Bearzi, J.R.; Simões, M.B.; Schneider, R.; Bini, R.A.; Ribeiro, S.J.L.; Man, M.W.C.; Rischka, K.; et al. PDMS-urethanesil hybrid multifunctional materials: Combining CO2 use and sol–gel processing. J. Sol-Gel Sci. Technol. 2020, 95, 693–709. [Google Scholar] [CrossRef]
- Shi, C.Y.; Zhang, Q.; Yu, C.Y.; Rao, S.J.; Yang, S.; Tian, H.; Qu, D.H. An Ultrastrong and Highly Stretchable Polyurethane Elastomer Enabled by a Zipper-Like Ring-Sliding Effect. Adv. Mater. 2020, 32, 2000345. [Google Scholar] [CrossRef]
- Ying, W.B.; Wang, G.; Kong, Z.; Yao, C.K.; Wang, Y.; Hu, H.; Li, F.; Chen, C.; Tian, Y.; Zhang, J.; et al. A Biologically Muscle-Inspired Polyurethane with Super-Tough, Thermal Reparable and Self-Healing Capabilities for Stretchable Electronics. Adv. Funct. Mater. 2021, 31, 2009869. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, R.; Liu, Y.; Le, T.; Wong, C.P. Highly conductive, flexible, bio-compatible poly-urethane based isotropic conductive adhesives for flexible electronics. In Proceedings of the 2012 IEEE 62nd Electronic Components and Technology Conference, San Diego, CA, USA, 29 May–1 June 2012; pp. 406–411. [Google Scholar]
- Hong, H.J.; Kwan, S.M.; Lee, D.S.; Kim, S.M.; Kim, Y.H.; Lim, J.S.; Hwang, J.Y.; Jeong, H.S. Highly flexible and stretchable thermally conductive composite film by polyurethane supported 3D networks of boron nitride. Compos. Sci. Technol. 2017, 152, 94–100. [Google Scholar] [CrossRef]
- Huang, W.; Li, J.; Zhao, S.; Han, F.; Zhang, G.; Sun, R.; Wong, C.P. Highly electrically conductive and stretchable copper nanowires-based composite for flexible and printable electronics. Compos. Sci. Technol. 2017, 146, 169–176. [Google Scholar] [CrossRef]
- Szostak, K.M.; Grand, L.; Constandinou, T.G. Neural interfaces for intracortical recording: Requirements, fabrication methods, and characteristics. Front. Neurosci. 2017, 11, 665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehler, C.; Aqrawe, Z.; Asplund, M. Applications of PEDOT in bioelectronic medicine. Bioelectron. Med. 2019, 2, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Hansen, T.S.; West, K.; Hassager, O.; Larsen, N.B. Highly stretchable and conductive polymer material made from poly(3,4-ethylenedioxythiophene) and polyurethane elastomers. Adv. Funct. Mater. 2007, 17, 3069–3073. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, S.; Li, P.; Xia, Y.; Zhang, X.; Du, D.; Isikgor, F.H.; Ouyang, J. Review on application of PEDOTs and PEDOT:PSS in energy conversion and storage devices. J. Mater. Sci. Mater. Electron. 2015, 26, 4438–4462. [Google Scholar] [CrossRef]
- Mohammed, M.G.; Kramer, R. All-Printed Flexible and Stretchable Electronics. Adv. Mater. 2017, 29, 1604965. [Google Scholar] [CrossRef]
- Deshmukh, K.; Basheer Ahamed, M.; Deshmukh, R.R.; Khadheer Pasha, S.K.; Bhagat, P.R.; Chidambaram, K. Biopolymer Composites with High Dielectric Performance: Interface Engineering. In Biopolymer Composites in Electronics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–128. ISBN 9780081009741. [Google Scholar]
- Li, Y.; Abedalwafa, M.A.; Tang, L.; Li, D.; Wang, L. Electrospun nanofibers for sensors. In Electrospinning: Nanofabrication and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 571–601. ISBN 9780323512701. [Google Scholar]
- Song, E.; Choi, J.-W. Conducting Polyaniline Nanowire and Its Applications in Chemiresistive Sensing. Nanomaterials 2013, 3, 498–523. [Google Scholar] [CrossRef]
- Hong, S.Y.; Lee, Y.H.; Park, H.; Jin, S.W.; Jeong, Y.R.; Yun, J.; You, I.; Zi, G.; Ha, J.S. Stretchable Active Matrix Temperature Sensor Array of Polyaniline Nanofibers for Electronic Skin. Adv. Mater. 2016, 28, 930–935. [Google Scholar] [CrossRef]
- Kalita, G.; Tanemura, M. Fundamentals of Chemical Vapor Deposited Graphene and Emerging Applications. In Graphene Materials; IntechOpen: London, UK, 2016; p. 13. [Google Scholar] [CrossRef] [Green Version]
- Duy, L.T.; Seo, H. Eco-friendly, Self-healing, and Stretchable Graphene Hydrogels Functionalized with Diol Oligomer for Wearable Sensing Applications. Sensors Actuators B Chem. 2020, 321, 128507. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Jinschek, J.R.; Yucelen, E.; Calderon, H.A.; Freitag, B. Quantitative atomic 3-D imaging of single/double sheet graphene structure. Carbon 2011, 49, 556–562. [Google Scholar] [CrossRef]
- Amrollahi-Sharifabadi, M.; Koohi, M.K.; Zayerzadeh, E.; Hablolvarid, M.H.; Hassan, J.; Seifalian, A.M. In vivo toxicological evaluation of graphene oxide nanoplatelets for clinical application. Int. J. Nanomed. 2018, 13, 4757–4769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santhiago, M.; Corrêa, C.C.; Bernardes, J.S.; Pereira, M.P.; Oliveira, L.J.M.; Strauss, M.; Bufon, C.C.B. Flexible and Foldable Fully-Printed Carbon Black Conductive Nanostructures on Paper for High-Performance Electronic, Electrochemical, and Wearable Devices. ACS Appl. Mater. Interfaces 2017, 9, 24365–24372. [Google Scholar] [CrossRef]
- Cholleti, E.R.; Stringer, J.; Assadian, M.; Battmann, V.; Bowen, C.; Aw, K. Highly stretchable capacitive sensor with printed carbon black electrodes on barium titanate elastomer composite. Sensors 2019, 19, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Assadian, M.; Ramezani, M.; Aw, K.C. Printing of Soft Stretch Sensor from Carbon Black Composites. Proceedings 2018, 2, 732. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Gao, Q.; Fu, J.; Chen, Q.; Zhu, J.; Sun, Y.; He, Y. Multimaterial 3D Printing of Highly Stretchable Silicone Elastomers. ACS Appl. Mater. Interfaces 2019, 11, 23573–23583. [Google Scholar] [CrossRef]
- Properties: Silicone Rubber. Available online: https://www.azom.com/properties.aspx?ArticleID=920 (accessed on 22 January 2022).
- Johnston, I.D.; McCluskey, D.K.; Tan, C.K.L.; Tracey, M.C. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. J. Micromechanics Microengineering 2014, 24, 035017. [Google Scholar] [CrossRef]
- Pritchard, R.H.; Lava, P.; Debruyne, D.; Terentjev, E.M. Precise determination of the Poisson ratio in soft materials with 2D digital image correlation. Soft Matter 2013, 9, 6037–6045. [Google Scholar] [CrossRef]
- Tharakan, S.A.; Raju, S.; Balasubramaniam, R.; Muthusamy, S. Synthesis and characterization of flexible/stretchable polyimide having long/bulky aryl pendent group and polyimide/silicon dioxide nanocomposites. Polym. Compos. 2019, 40, 832–843. [Google Scholar] [CrossRef]
- Parylene Properties [Compare Parylene to Other Conformal Coatings]. Available online: https://vsiparylene.com/parylene-properties/#chemical-properties (accessed on 22 January 2022).
- Sim, W.; Kim, B.; Choi, B.; Park, J.-O. Theoretical and experimental studies on the parylene diaphragms for microdevices. Microsyst. Technol. 2005, 11, 11–15. [Google Scholar] [CrossRef]
- Kayser, L.V.; Lipomi, D.J. Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS. Adv. Mater. 2019, 31, 1806133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoei, A.R.; Khorrami, M.S. Mechanical properties of graphene oxide: A molecular dynamics study. Fuller. Nanotub. Carbon Nanostructures 2016, 24, 594–603. [Google Scholar] [CrossRef]
- Liu, N.; Chortos, A.; Lei, T.; Jin, L.; Kim, T.R.; Bae, W.-G.; Zhu, C.; Wang, S.; Pfattner, R.; Chen, X.; et al. Ultratransparent and stretchable graphene electrodes. Sci. Adv. 2017, 3, e1700159. [Google Scholar] [CrossRef] [Green Version]
- Cao, G. Atomistic Studies of Mechanical Properties of Graphene. Polymers 2014, 6, 2404. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Tan, Y.; Yuan, Q.; Berlin, S.-V. Recent progress in flexible and wearable bio-electronics based on nanomaterials. Sci. Adv. 2017, 3, e1700159. [Google Scholar] [CrossRef]
- Decataldo, F.; Cramer, T.; Martelli, D.; Gualandi, I.; Korim, W.S.; Yao, S.T.; Tessarolo, M.; Murgia, M.; Scavetta, E.; Amici, R.; et al. Stretchable Low Impedance Electrodes for Bioelectronic Recording from Small Peripheral Nerves. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, Z.; Xiao, J. Stretchable Thin Film Materials: Fabrication, Application, and Mechanics. J. Electron. Packag. Trans. ASME 2016, 138, 020801. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Lin, H.; Yang, H.; Ni, B.; Li, H.; Wang, X.; Zhang, S.; Yang, H.; Ni, B.; Li, H.; et al. Highly Flexible and Stretchable Nanowire Superlattice Fibers Achieved by Spring-Like Structure of Sub-1 nm Nanowires. Adv. Funct. Mater. 2019, 29, 1903477. [Google Scholar] [CrossRef]
- Trung, T.Q.; Lee, N.-E.; Lee, E.N.; Trung, T.Q. Recent Progress on Stretchable Electronic Devices with Intrinsically Stretchable Components. Adv. Mater. 2017, 29, 1603167. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, M.; Xia, K.; Gong, X.; Wang, H.; Yin, Z.; Guan, B.; Zhang, Y. Intrinsically Stretchable and Conductive Textile by a Scalable Process for Elastic Wearable Electronics. ACS Appl. Mater. Interfaces 2017, 9, 13331–13338. [Google Scholar] [CrossRef]
- Fan, J.A.; Yeo, W.-H.; Su, Y.; Hattori, Y.; Lee, W.; Jung, S.-Y.; Zhang, Y.; Liu, Z.; Cheng, H.; Falgout, L.; et al. Fractal design concepts for stretchable electronics. Nat. Commun. 2014, 5, 3266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Shin, M.; Thiyagarajan, K.; Jeong, U. Approaches to Stretchable Polymer Active Channels for Deformable Transistors. Macromolecules 2016, 49, 433–444. [Google Scholar] [CrossRef]
- Jang, H.; Park, Y.J.; Chen, X.; Das, T.; Kim, M.S.; Ahn, J.H. Graphene-Based Flexible and Stretchable Electronics. Adv. Mater. 2016, 28, 4184–4202. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, B.; Zou, X.; Cheng, H.M. Chemical Vapor Deposition Growth and Applications of Two-Dimensional Materials and Their Heterostructures. Chem. Rev. 2018, 118, 6091–6133. [Google Scholar] [CrossRef]
- Rhyee, J.-S.; Kwon, J.; Dak, P.; Kim, J.H.; Kim, S.M.; Park, J.; Hong, Y.K.; Song, W.G.; Omkaram, I.; Alam, M.A.; et al. High-Mobility Transistors Based on Large-Area and Highly Crystalline CVD-Grown MoSe2 Films on Insulating Substrates. Adv. Mater. 2016, 28, 2316–2321. [Google Scholar] [CrossRef]
- Lee, S.K.; Rana, K.; Ahn, J.H. Graphene Films for Flexible Organic and Energy Storage Devices. J. Phys. Chem. Lett. 2013, 4, 831–841. [Google Scholar] [CrossRef]
- Jun, K.W.; Kim, J.N.; Jung, J.Y.; Oh, I.K. Wrinkled graphene-AgNWs hybrid electrodes for smart window. Micromachines 2017, 8, 43. [Google Scholar] [CrossRef] [Green Version]
- Acikgoz, C.; Hempenius, M.A.; Huskens, J.; Vancso, G.J. Polymers in conventional and alternative lithography for the fabrication of nanostructures. Eur. Polym. J. 2011, 47, 2033–2052. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.F.; Sun, T.; Liu, Q.; Suo, Z.; Ren, Z. Highly stretchable and transparent nanomesh electrodes made by grain boundary lithography. Nat. Commun. 2014, 5, 3121. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Fu, S.; Zhang, R.; Boon, E.; Lee, W.; Fisher, F.T.; Yang, E.-H. Graphene—Vertically aligned carbon nanotube hybrid on PDMS as stretchable electrodes. Nanotechnology 2017, 28, 465302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Lv, L.; Zhang, J.; Zhang, S.; Xu, P.; Li, C.; Zhang, Z.; Li, Y.; Xu, Y.; Wang, J. Enhanced Stretchable and Sensitive Strain Sensor via Controlled Strain Distribution. Nanomaterials 2020, 10, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiefenauer, R.F.; Dalgaty, T.; Keplinger, T.; Tian, T.; Shih, C.-J.J.; Vörös, J.; Aramesh, M. Monolayer Graphene Coupled to a Flexible Plasmonic Nanograting for Ultrasensitive Strain Monitoring. Small 2018, 14, 1801187. [Google Scholar] [CrossRef]
- Kim, M.; Kim, C.; Alrowais, H.; Brand, O. Multiscale and Uniform Liquid Metal Thin-Film Patterning Based on Soft Lithography for 3D Heterogeneous Integrated Soft Microsystems: Additive Stamping and Subtractive Reverse Stamping. Adv. Mater. Technol. 2018, 3, 1800061. [Google Scholar] [CrossRef]
- Li, D.; Lai, W.Y.; Zhang, Y.Z.; Huang, W. Printable Transparent Conductive Films for Flexible Electronics. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Albrecht, A.; Bobinger, M.; Salmerón, J.F.; Becherer, M.; Cheng, G.; Lugli, P.; Rivadeneyra, A. Over-stretching tolerant conductors on rubber films by inkjet-printing silver nanoparticles for wearables. Polymers 2018, 10, 1413. [Google Scholar] [CrossRef] [Green Version]
- Shin, W.; Lim, J.; Lee, Y.; Park, S.; Kim, H.J.H.; Cho, H.; Shin, J.; Yoon, Y.; Lee, H.; Kim, H.J.H.; et al. Shear-assisted laser transfer of metal nanoparticle ink to an elastomer substrate. Materials 2018, 10, 2511. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.G.; An, H.S.; Kim, J.Y.; Park, J.U. High-resolution, reconfigurable printing of liquid metals with three-dimensional structures. Sci. Adv. 2019, 5, eaaw2844. [Google Scholar] [CrossRef] [Green Version]
- Molina-Lopez, F.; Gao, T.Z.; Kraft, U.; Zhu, C.; Öhlund, T.; Pfattner, R.; Feig, V.R.; Kim, Y.; Wang, S.; Yun, Y.; et al. Inkjet-printed stretchable and low voltage synaptic transistor array. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Amendola, S.; Palombi, A.; Marrocco, G. Inkjet Printing of Epidermal RFID Antennas by Self-Sintering Conductive Ink. IEEE Trans. Microw. Theory Tech. 2018, 66, 1561–1569. [Google Scholar] [CrossRef]
- Khan, Y.; Pavinatto, F.J.; Lin, M.C.; Liao, A.; Swisher, S.L.; Mann, K.; Subramanian, V.; Maharbiz, M.M.; Arias, A.C. Inkjet-Printed Flexible Gold Electrode Arrays for Bioelectronic Interfaces. Adv. Funct. Mater. 2016, 26, 1004–1013. [Google Scholar] [CrossRef]
- Agarwala, S.; Lee, J.M.; Ng, W.L.; Layani, M.; Yeong, W.Y.; Magdassi, S. A novel 3D bioprinted flexible and biocompatible hydrogel bioelectronic platform. Biosens. Bioelectron. 2018, 102, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Salcedo, E.; Berndt, A.; Choi, D.; Ryu, J.E. Pulsed light sintering of silver nanoparticles for large deformation of printed stretchable electronics. Adv. Compos. Hybrid Mater. 2018, 1, 193–198. [Google Scholar] [CrossRef]
- Liu, S.; Yuen, M.C.; White, E.L.; Boley, J.W.; Deng, B.; Cheng, G.J.; Kramer-Bottiglio, R. Laser Sintering of Liquid Metal Nanoparticles for Scalable Manufacturing of Soft and Flexible Electronics. ACS Appl. Mater. Interfaces 2018, 10, 28232–28241. [Google Scholar] [CrossRef]
- Lopes, P.A.; Paisana, H.; De Almeida, A.T.; Majidi, C.; Tavakoli, M. Hydroprinted Electronics: Ultrathin Stretchable Ag-In-Ga E-Skin for Bioelectronics and Human-Machine Interaction. ACS Appl. Mater. Interfaces 2018, 10, 38760–38768. [Google Scholar] [CrossRef]
- Mo, M.; Chen, C.; Gao, H.; Chen, M.; Li, D. Wet-spinning assembly of cellulose nanofibers reinforced graphene/polypyrrole microfibers for high performance fiber-shaped supercapacitors. Electrochim. Acta 2018, 269, 11–20. [Google Scholar] [CrossRef]
- Yang, Z.; Jia, Y.; Niu, Y.; Zhang, Y.; Zhang, C.; Li, P.; Zhu, M.; Li, Q. One-step wet-spinning assembly of twisting-structured graphene/carbon nanotube fiber supercapacitor. J. Energy Chem. 2020, 51, 434–441. [Google Scholar] [CrossRef]
- He, X.; Zi, Y.; Guo, H.; Zheng, H.; Xi, Y.; Wu, C.; Wang, J.; Zhang, W.; Lu, C.; Wang, Z.L. A Highly Stretchable Fiber-Based Triboelectric Nanogenerator for Self-Powered Wearable Electronics. Adv. Funct. Mater. 2017, 27, 1604378. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, X.; Hoe Chan, K.; Wang, R.; Cao, Z.; Sun, J.; Wei Ho, G. A stretchable fiber nanogenerator for versatile mechanical energy harvesting and self-powered full-range personal healthcare monitoring. Nano Energy 2017, 41, 511–518. [Google Scholar] [CrossRef]
- Cong, H.P.; Ren, X.C.; Wang, P.; Yu, S.H. Wet-spinning assembly of continuous, neat, and macroscopic graphene fibers. Sci. Rep. 2012, 2, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.W.; Kwon, S.N.; Na, S.I. Stretchable and electrically conductive polyurethane- silver/graphene composite fibers prepared by wet-spinning process. Compos. Part B Eng. 2019, 167, 573–581. [Google Scholar] [CrossRef]
- Dong, M.; Xue, Z.; Liu, J.; Yan, M.; Xia, Y.; Wang, B. Preparation of carrageenan fibers with extraction of Chondrus via wet spinning process. Carbohydr. Polym. 2018, 194, 217–224. [Google Scholar] [CrossRef]
- Zhang, J.; Seyedin, S.; Qin, S.; Lynch, P.A.; Wang, Z.; Yang, W.; Wang, X.; Razal, J.M. Fast and scalable wet-spinning of highly conductive PEDOT:PSS fibers enables versatile applications. J. Mater. Chem. A 2019, 7, 6401–6410. [Google Scholar] [CrossRef]
- Tang, Z.; Jia, S.; Wang, F.; Bian, C.; Chen, Y.; Wang, Y.; Li, B. Highly Stretchable Core-Sheath Fibers via Wet-Spinning for Wearable Strain Sensors. ACS Appl. Mater. Interfaces 2018, 10, 6624–6635. [Google Scholar] [CrossRef]
- Seyedin, M.Z.; Razal, J.M.; Innis, P.C.; Wallace, G.G. Strain-responsive polyurethane/PEDOT:PSS elastomeric composite fibers with high electrical conductivity. Adv. Funct. Mater. 2014, 24, 2957–2966. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Wang, R.; Sun, J.; Gao, L. A Stretchable and Highly Sensitive Graphene-Based Fiber for Sensing Tensile Strain, Bending, and Torsion. Adv. Mater. 2015, 27, 7365–7371. [Google Scholar] [CrossRef]
- Pan, J.; Yang, M.; Luo, L.; Xu, A.; Tang, B.; Cheng, D.; Cai, G.; Wang, X. Stretchable and Highly Sensitive Braided Composite Yarn@Polydopamine@Polypyrrole for Wearable Applications. ACS Appl. Mater. Interfaces 2019, 11, 7338–7348. [Google Scholar] [CrossRef]
- Paik, S.; Kim, G.; Chang, S.; Lee, S.; Jin, D.; Jeong, K.Y.; Lee, I.S.; Lee, J.; Moon, H.; Lee, J.; et al. Near-field sub-diffraction photolithography with an elastomeric photomask. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kavand, H.; Van Lintel, H.; Bakhshi Sichani, S.; Bonakdar, S.; Kavand, H.; Koohsorkhi, J.; Renaud, P. Cell-Imprint Surface Modification by Contact Photolithography-Based Approaches: Direct-Cell Photolithography and Optical Soft Lithography Using PDMS Cell Imprints. ACS Appl. Mater. Interfaces 2019, 11, 10559–10566. [Google Scholar] [CrossRef]
- Pinto, V.C.; Sousa, P.J.; Cardoso, V.F.; Minas, G. Optimized SU-8 Processing for Low-Cost Microstructures Fabrication without Cleanroom Facilities. Micromachines 2014, 5, 738–755. [Google Scholar] [CrossRef] [Green Version]
- Xiang, C.; Yang, Y.; Penner, R.M. Cheating the diffraction limit: Electrodeposited nanowires patterned by photolithography. Chem. Commun. 2009, 859–873. [Google Scholar] [CrossRef]
- Del Barrio, J.; Sánchez-Somolinos, C.; Del Barrio, J.; Sánchez-Somolinos, C. Light to Shape the Future: From Photolithography to 4D Printing. Adv. Opt. Mater. 2019, 7, 1900598. [Google Scholar] [CrossRef]
- Kavusi, S.; El Gamal, A. Quantitative Study of High-Dynamic-Range Image Sensor Architectures. In Proceedings Volume 5301, Sensors and Camera Systems for Scientific, Industrial, and Digital Photography Applications V; Blouke, M.M., Sampat, N., Motta, R.J., Eds.; SPIE: Bellingham, WA, USA, 2004. [Google Scholar]
- Ludwig, G. Considerations Underlying the Use of Ultrasound to Detect Gallstones and Foreign Bodies in Tissue; Naval Medical Research Institute: Bethesda, MD, USA, 1949. [Google Scholar]
- Dahiya, R.; Valle, M. Robotic Tactile Sensing Technologies and System; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Pons, J.L.L.; Rocon, E.; Forner-Cordero, A.; Moreno, J. Biomedical instrumentation based on piezoelectric ceramics. J. Eur. Ceram. Soc. 2007, 27, 4191–4194. [Google Scholar] [CrossRef]
- Hennacy, R.; Gunther, R. A piezoelectric crystal method for measuring static and dynamic pressure distributions in the feet. J. Am. Podiatr. Med. Assoc. 1975, 65. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, I.; Khalid, H.R.; Lee, H.-K. Flexible Piezoelectric Transducers for Energy Harvesting and Sensing from Human Kinematics. ACS Appl. Electron. Mater. 2020, 2, 3346–3357. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Javid, F.; Joe, P.; Von Erlach, T.; Bensel, T.; Wei, Z.; Saxton, S.; Cleveland, C.; Booth, L.; McDonnell, S.; et al. Flexible piezoelectric devices for gastrointestinal motility sensing. Nat. Biomed. Eng. 2017, 1, 807–817. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Qiu, Y.; Wu, H.; Qin, W.; Liao, Y.; Yu, Q.; Cheng, H. Stretchable piezoelectric energy harvesters and self-powered sensors for wearable and implantable devices. Biosens. Bioelectron. 2020, 168, 112569. [Google Scholar] [CrossRef]
- Gong, S.; Zhang, B.; Zhang, J.; Lin Wang, Z.; Ren, K.; Gong, S.; Zhang, B.; Zhang, J.; Wang, Z.L.; Ren, K. Biocompatible Poly(lactic acid)-Based Hybrid Piezoelectric and Electret Nanogenerator for Electronic Skin Applications. Adv. Funct. Mater. 2020, 30, 1908724. [Google Scholar] [CrossRef]
- Sun, R.; Carreira, S.C.; Chen, Y.; Xiang, C.; Xu, L.; Zhang, B.; Chen, M.; Farrow, I.; Scarpa, F.; Rossiter, J. Stretchable Piezoelectric Sensing Systems for Self-Powered and Wireless Health Monitoring. Adv. Mater. Technol. 2019, 4, 1900100. [Google Scholar] [CrossRef] [Green Version]
- Ha, T.; Tran, J.; Liu, S.; Jang, H.; Jeong, H.; Mitbander, R.; Huh, H.; Qiu, Y.; Duong, J.; Wang, R.L.; et al. A Chest-Laminated Ultrathin and Stretchable E-Tattoo for the Measurement of Electrocardiogram, Seismocardiogram, and Cardiac Time Intervals. Adv. Sci. 2019, 6, 1900290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinsaat, J.E.Q.; de Wild, T.; Nüesch, F.A.; Damjanovic, D.; Krämer, R.; Schürch, G.; Häfliger, D.; Clemens, F.; Sebastian, T.; Dascalu, M.; et al. Stretchable piezoelectric elastic composites for sensors and energy generators. Compos. Part B Eng. 2020, 198, 108211. [Google Scholar] [CrossRef]
- Guldiken, R.; Onen, O. MEMS ultrasonic transducers for biomedical applications. In MEMS for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Ramanaviciene, A.; Virzonis, D.; Vanagas, G.; Ramanavicius, A. Capacitive micromachined ultrasound transducer (cMUT) for immunosensor design. Analyst 2010, 135, 1531–1534. [Google Scholar] [CrossRef] [PubMed]
- Development of a Novel Transparent Flexible Capacitive Micromachined Ultrasonic Transducer. Sensors 2017, 17, 1443. [CrossRef] [PubMed] [Green Version]
- Wang, C.; Li, X.; Hu, H.; Zhang, L.; Huang, Z.; Lin, M.; Zhang, Z.; Yin, Z.; Huang, B.; Gong, H.; et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2018, 2, 687–695. [Google Scholar] [CrossRef]
- Hu, H.; Zhu, X.; Wang, C.; Zhang, L.; Li, X.; Lee, S.; Huang, Z.; Chen, R.; Chen, Z.; Wang, C.; et al. Stretchable ultrasonic transducer arrays for three-dimensional imaging on complex surfaces. Sci. Adv. 2018, 4, eaar3979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Xi, J.; Huang, W.; Yuen, M.M.F. Stretchable conductive elastomer for wireless wearable communication applications. Sci. Rep. 2017, 7, 10958. [Google Scholar] [CrossRef] [Green Version]
- Smida, A.; Iqbal, A.; Alazemi, A.J.; Waly, M.I.; Ghayoula, R.; Kim, S. Wideband Wearable antenna for Biomedical Telemetry applications. IEEE Access 2020, 8, 15687–15694. [Google Scholar] [CrossRef]
- Mohamadzade, B.; Hashmi, R.M.; Simorangkir, R.B.V.B.; Gharaei, R.; Ur Rehman, S.; Abbasi, Q.H. Recent Advances in Fabrication Methods for Flexible Antennas in Wearable Devices: State of the Art. Sensors 2019, 19, 2312. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Raveendran, V.; Chen, J. Wearable triboelectric nanogenerators for biomechanical energy harvesting. Nano Energy 2020, 77, 105303. [Google Scholar] [CrossRef]
- Park, S.; Heo, S.W.; Lee, W.; Inoue, D.; Jiang, Z.; Yu, K.; Jinno, H.; Hashizume, D.; Sekino, M.; Yokota, T.; et al. Self-powered ultra-flexible electronics via nano-grating-patterned organic photovoltaics. Nature 2018, 561, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Lu, Z.; Cong, S.; Cheng, G.; Ma, P.; Lou, Y.; Ding, J.; Yuan, N.; Rümmeli, M.H.; Zou, G. Ultraflexible and Lightweight Bamboo-Derived Transparent Electrodes for Perovskite Solar Cells. Small 2019, 15, 1902878. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Potje-Kamloth, K.; Haase, F.; Detemple, P.; Völklein, F.; Doll, T. Coin-size coiled-up polymer foil thermoelectric power generator for wearable electronics. Sens. Actuators A Phys. 2006, 132, 325–330. [Google Scholar] [CrossRef]
- Lu, Z.; Layani, M.; Zhao, X.; Tan, L.P.; Sun, T.; Fan, S.; Yan, Q.; Magdassi, S.; Hng, H.H. Fabrication of flexible thermoelectric thin film devices by inkjet printing. Small 2014, 10, 3551–3554. [Google Scholar] [CrossRef] [PubMed]
- Song, W.-J.; Park, J.; Kim, D.H.; Bae, S.; Kwak, M.-J.; Shin, M.; Kim, S.; Choi, S.; Jang, J.-H.; Shin, T.J.; et al. Jabuticaba-Inspired Hybrid Carbon Filler/Polymer Electrode for Use in Highly Stretchable Aqueous Li-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702478. [Google Scholar] [CrossRef]
- Liu, D.; Su, L.; Liao, J.; Reeja-Jayan, B.; Majidi, C. Rechargeable Soft-Matter EGaIn-MnO 2 Battery for Stretchable Electronics. Adv. Energy Mater. 2019, 9, 1902798. [Google Scholar] [CrossRef]
- Zamarayeva, A.M.; Ostfeld, A.E.; Wang, M.; Duey, J.K.; Deckman, I.; Lechêne, B.P.; Davies, G.; Steingart, D.A.; Arias, A.C. Flexible and stretchable power sources for wearable electronics. Sci. Adv. 2017, 3, e1602051. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Cao, C.; Cao, Y.; Han, Q.; Parker, C.B.; Glass, J.T. Robust and High-Performance Electrodes via Crumpled Au-CNT Forests for Stretchable Supercapacitors. Matter 2020, 2, 1307–1323. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Hao, R.; Peng, H.; Dai, L. High-performance, stretchable, wire-shaped supercapacitors. Angew. Chemie-Int. Ed. 2015, 54, 618–622. [Google Scholar] [CrossRef]
- Lv, Z.; Tang, Y.; Zhu, Z.; Wei, J.; Li, W.; Xia, H.; Jiang, Y.; Liu, Z.; Luo, Y.; Ge, X.; et al. Honeycomb-Lantern-Inspired 3D Stretchable Supercapacitors with Enhanced Specific Areal Capacitance. Adv. Mater. 2018, 30, 1805468. [Google Scholar] [CrossRef]
- Joshipura, I.D.; Finn, M.; Tan, S.T.M.; Dickey, M.D.; Lipomi, D.J. Stretchable bioelectronics—Current and future. MRS Bull. 2017, 42, 960–967. [Google Scholar] [CrossRef]
- Nassar, J.M.; Mishra, K.; Lau, K.; Aguirre-Pablo, A.A.; Hussain, M.M. Recyclable Nonfunctionalized Paper-Based Ultralow-Cost Wearable Health Monitoring System. Adv. Mater. Technol. 2017, 2, 1600228. [Google Scholar] [CrossRef]
- Ray, T.R.; Choi, J.; Bandodkar, A.J.; Krishnan, S.; Gutruf, P.; Tian, L.; Ghaffari, R.; Rogers, J.A. Bio-Integrated Wearable Systems: A Comprehensive Review. Chem. Rev. 2019, 119, 5461–5533. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, L.; Wang, H.; Zhang, B.; Wang, X.; Stening, R.Y.Z.; Sheng, X.; Yin, L. Materials Strategies and Device Architectures of Emerging Power Supply Devices for Implantable Bioelectronics. Small 2020, 16, 1902827. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhang, Y.; Liang, Z.; Cao, Y.; Han, Z.; Feng, X. Flexible inorganic bioelectronics. NPJ Flex. Electron. 2020, 4, 1–20. [Google Scholar] [CrossRef] [Green Version]
| Wearable Devices | Implantable Devices |
|---|---|
| Nonirritating to Dermal tissue | Nonirritating, compliant to match surrounding tissue |
| Not cytotoxic, via leachable substances | Not cytotoxic |
| Either non degrading, or degraded substances are safe | Degraded substances are safe |
| Hemocompatibility (Indirect contact) | Hemocompatibility (direct contact) |
| Minimize/eliminate biofouling |
| Materials | Company | Young’s Modulus | Stretchability | Poisson’s Ratio | Applications |
|---|---|---|---|---|---|
| Silicone | Azo Materials | 0.001–0.05 GPa | 1000%–2000% [73] | 0.47–0.49 [74] | Insulation |
| PDMS | Dow’s Sylgard 184 | ~1–3 MPa [75] | ~1000% [47] | ~0.5 [76] | Encapsulation/Nonconductive polymer |
| Polyimide | Dupont | 2.5 GPa | 260% [77] | 0.34 at 23 °C | Neurocortical electrode arrays |
| Parylene-C | - | 2.8 GPa, 3.2 GPa, 4.5 GPa [78,79] | 20–200% [78] | 0.4 [79] | Neural electrode substrate |
| PEDOT-PSS | - | 1–7.5 GPa | ~10% [80] | 0.34 | Conductive tracts |
| Polyurethane elastomer | - | 4.7 MPa, 5.3 MPa, 7.4 MPa [48] | ~1890% [51] | 0.45–0.5 | Vascular grafts, blood bags |
| Graphene | - | ~1 TPa [81] | ~30% [82] | 0.456 ± 0.008 [83] | Logic gates, transistors |
| Fabrication Method | Advantages | Disadvantages |
|---|---|---|
| Chemical vapor deposition (CVD) Application: For creating thin films | Grow thin solid film on the substrate. Grow large-area and high-quality graphene film on metal substrate. | Need a metal (Ni, Cu) substrate to grow graphene layer. Cannot use polymer substrate to grow graphene layer. Challenge of growing large-grain crystal, which is required to enhance the electronic, mechanical, and thermal properties. Alternative: Modified CVD can be used to produce large-grain and highly crystalline film. Crystal growth variation at different areas. Requires transfer of the 2D film from the growth substrate to the target stretchable device substrate to add the stretchability property to the device. Possible contamination and damage during transfer of the film to the target. |
| Plasma-enhanced CVD (PECVD) Application: For creating thin films on polymer substrate. | Potential for direct growth of graphene layer on polymer substrate. Utilizes low processing temperature, providing an opportunity to use polymer as substrate. | Inferior graphene properties. Formation of cracks in the graphene structure may occur if repeatedly strained, leading to an increase in resistance. |
| UV lithography Application: For creating patterns for circuits for microelectronics, sensing, and optoelectronics. Can also be used to create high-aspect-ratio structures and 3D nanostructures such as nanowires (A novel top-down fabrication process for a vertically-stacked silicon-nanowire array). | Fabricate desired pattern ranging from nm to µm size on substrate. Soft and stretchable substrate can be used. | Can pattern only photoresist and may require a photomask for masked lithography [126,127]. Can be expensive if infrastructure such as a mask aligner is needed to develop complicated structures [128]. Resolution of the projection optics is diffraction-limited [126,129]. Challenging to design complex optics. Use of harsh processing conditions such as ion, plasma, acid, or temperature treatment to remove barrier layer, which limits the use of the substrate or photoresist that cannot handle such harsh processing [130]. |
| Electron beam (E-beam) lithography Application: For producing patterns in a photomask | Produce a photomask. High-resolution approach to creating a photomask. | High resolution approach and therefore, time consuming. Not suitable for mass production. |
| Soft lithography Application: Thin film fabrication on soft substrates. | Micro/nano-structures can be fabricated on a soft substrate. Can be used to fabricate a mold. Easy, fast, low-cost, mass reproducible process. Does not require harsh processing chemicals. | Requires a stamp or a mold. |
| Printing Application: For creating thin film circuits of conductive liquids. | Can be used with conductive polymer composites and conductive liquid metals, thereby aiding in the fabrication of flexible and stretchable devices. Fast, accurate, and fully automated non-contact approach. Repeatable and scalable. Capability of achieving electronics with complex patterns without compromising the electrical conductivity of devices. | Requires good choice of conductive material and a solvent with appropriate viscosity, surface tension, and rheology. Requires the sintering process to create a conductive path. |
| Wet spinning method Application: For fabricating stretchable and electrically conductive fibers for wearable electronics (strain sensors, supercapacitors, nanogenerators). | Good technology for wearable electronics as the fibers can withstand mechanical deformations such as bending, folding, twisting, and stretching. A facile and viable strategy to fluidly spin the macroscopic fiber in a continuous way. Ability to control the diameter of the fiber by choosing an appropriate nozzle size of the spinneret. | Shear-thinning spinning ink is required for efficient flow. Requires selection of an appropriate coagulation bath so that the extruded spinning ink is rapidly vulcanized without any breakup. |
| Frequency | Application |
|---|---|
| 401–402 MHz | Medical Implant Communication Services (MICS). |
| 402–405 MHz | |
| 403.5–403.8 MHz | |
| 405–406 MHz | |
| 413–457 MHz | Medradio Micropower Networks (MMNs), transmit and relay data for implanted and body-worn medical devices for diagnostics and therapeutic functions. |
| 2.4 and 5 GHz | Wi-Fi, smart hospital beds, mobile nursing stations. |
| 2.404–2.478 GHz | Bluetooth, indoor navigation for patients, connectivity between the device and the smartphone for heath-data monitoring. |
| 2.36–2.4 GHz | New Medical BAN (MBAN). |
| New Medical BAN (MBAN) | Industrial, Scientific, and Medical (ISM). |
| 3.1–10.76 GHz | Ultra-Wideband (UWB). |
| 57–64 G | The band plans and rules at mmW-60 BAN. |
| 59–66 GHz |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chitrakar, C.; Hedrick, E.; Adegoke, L.; Ecker, M. Flexible and Stretchable Bioelectronics. Materials 2022, 15, 1664. https://doi.org/10.3390/ma15051664
Chitrakar C, Hedrick E, Adegoke L, Ecker M. Flexible and Stretchable Bioelectronics. Materials. 2022; 15(5):1664. https://doi.org/10.3390/ma15051664
Chicago/Turabian StyleChitrakar, Chandani, Eric Hedrick, Lauren Adegoke, and Melanie Ecker. 2022. "Flexible and Stretchable Bioelectronics" Materials 15, no. 5: 1664. https://doi.org/10.3390/ma15051664
APA StyleChitrakar, C., Hedrick, E., Adegoke, L., & Ecker, M. (2022). Flexible and Stretchable Bioelectronics. Materials, 15(5), 1664. https://doi.org/10.3390/ma15051664







