Ligustrum lucidum Leaf Extract-Assisted Green Synthesis of Silver Nanoparticles and Nano-Adsorbents Having Potential in Ultrasound-Assisted Adsorptive Removal of Methylene Blue Dye from Wastewater and Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Collection and Drying
2.3. Ultrasound-Assisted Extraction (UAE) of Leaf Extract
2.4. Green Synthesis of AgNPs and AgCS Composites
2.5. Characterization
2.6. Dye Degradation Studies
2.7. Isotherm Analysis
2.8. Assessment of the Antimicrobial Activity of AgNPs and AgCS
3. Results and Discussion
3.1. Formation of AgNPs and AgCS
3.2. Characterization of Synthesized AgNPs and AgCS
3.2.1. Ultraviolet–Visible Analysis
3.2.2. Fourier Transform Infrared (FTIR) Analysis
3.2.3. X-ray Diffraction Analysis (XRD)
3.2.4. Scanning Electron Microscopy and EDX
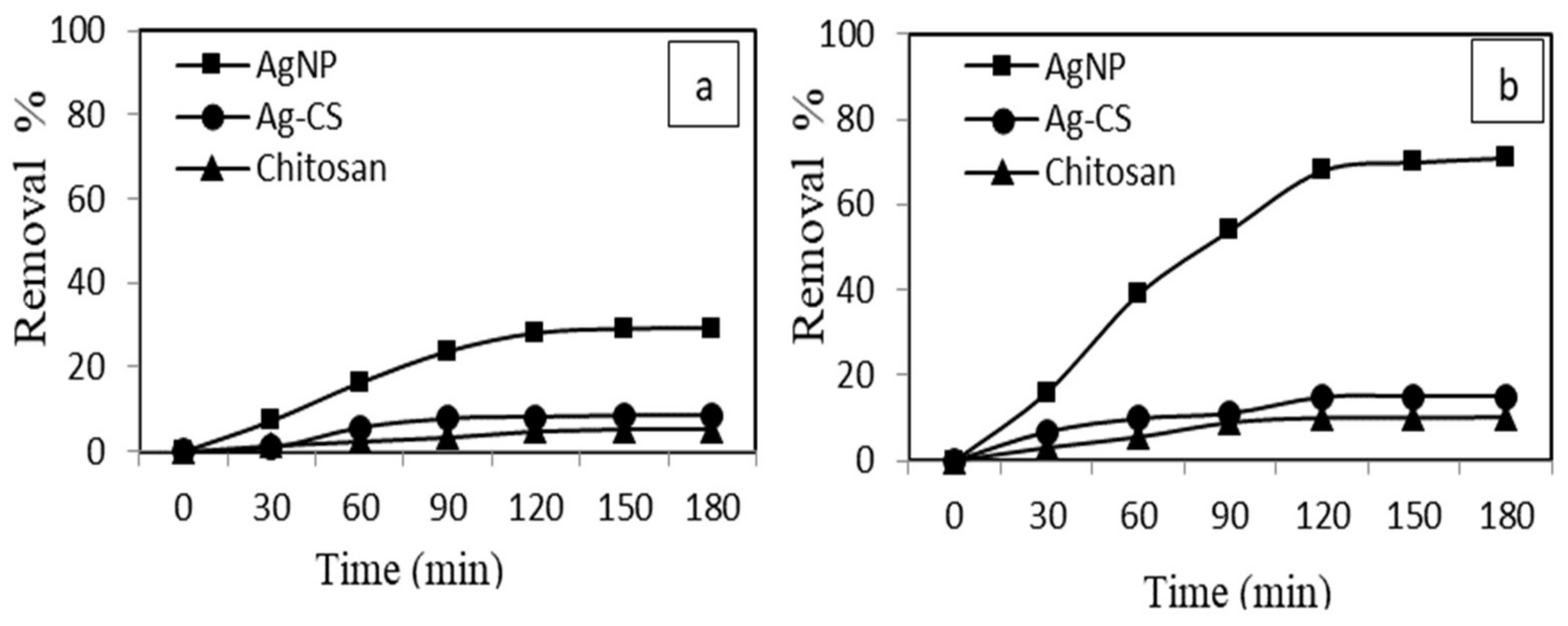
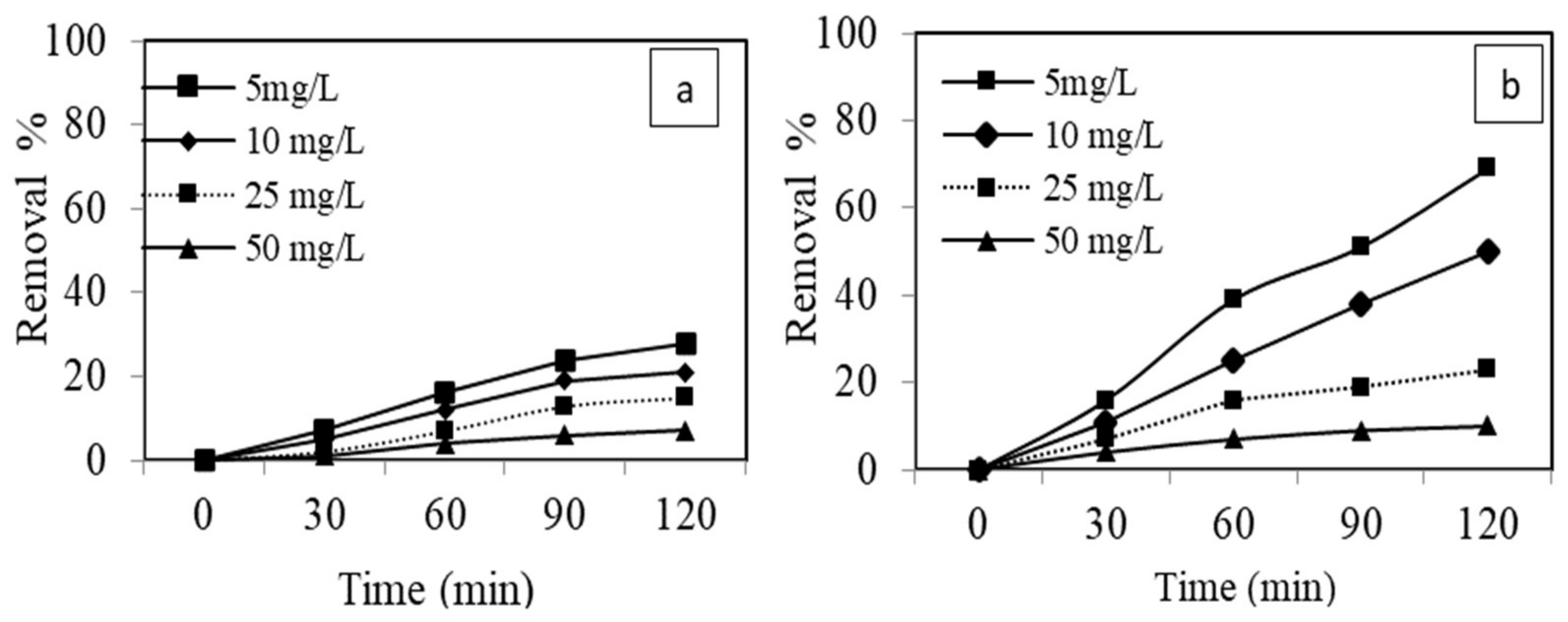
3.3. Adsorptive and Ultrasound-Assisted Adsorptive Performance of AgNPs and AgCS
3.3.1. Effect of pH
3.3.2. Effect of Contact Time
3.3.3. Effect of Initial Dye Concentration
3.4. Isotherms
3.5. Antimicrobial Performance Assessment of AgNPs and AgCS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pirillo, S.; Einschlag, F.S.G.; Ferreira, M.L.; Rueda, E.H. Eriochrome Blue Black R and Fluorescein degradation by hydrogen peroxide oxidation with horseradish peroxidase and hematin as biocatalysts. J. Mol. Catal. B Enzym. 2010, 66, 63–71. [Google Scholar] [CrossRef]
- Bedoui, A.; Ahmadi, M.; Bensalah, N.; Gadri, A. Comparative study of Eriochrome black T treatment by BDD-anodic oxidation and Fenton process. Chem. Eng. J. 2009, 146, 98–104. [Google Scholar] [CrossRef]
- Biczak, R.; Pawłowska, B.; Bałczewski, P.; Rychter, P. The role of the anion in the toxicity of imidazolium ionic liquids. J. Hazard. Mater. 2014, 274, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Yaagoob, I.Y.; Mazumder, M.A.; Al-Muallem, H.A. Fast removal of methylene blue and Hg (II) from aqueous solution using a novel super-adsorbent containing residues of glycine and maleic acid. J. Hazard. Mater. 2019, 369, 642–654. [Google Scholar] [CrossRef] [PubMed]
- El Gaini, L.; Lakraimi, M.; Sebbar, E.; Meghea, A.; Bakasse, M. Removal of indigo carmine dye from water to Mg–Al–CO3-calcined layered double hydroxides. J. Hazard. Mater. 2009, 161, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Aksakal, O.; Ucun, H. Equilibrium, kinetic and thermodynamic studies of the biosorption of textile dye (Reactive Red 195) onto Pinus sylvestris L. J. Hazard. Mater. 2010, 181, 666–672. [Google Scholar] [CrossRef]
- Yang, S.-T.; Chen, S.; Chang, Y.; Cao, A.; Liu, Y.; Wang, H. Removal of methylene blue from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2011, 359, 24–29. [Google Scholar] [CrossRef]
- Malakootian, M.; Mansoorian, H.J.; Hosseini, A.; Khanjani, N. Evaluating the efficacy of alumina/carbon nanotube hybrid adsorbents in removing Azo Reactive Red 198 and Blue 19 dyes from aqueous solutions. Process Saf. Environ. Prot. 2015, 96, 125–137. [Google Scholar] [CrossRef]
- Sultan, M.; Javeed, A.; Uroos, M.; Imran, M.; Jubeen, F.; Nouren, S.; Saleem, N.; Bibi, I.; Masood, R.; Ahmed, W. Linear and crosslinked Polyurethanes based catalysts for reduction of methylene blue. J. Hazard. Mater. 2018, 344, 210–219. [Google Scholar] [CrossRef]
- Mezohegyi, G.; van der Zee, F.P.; Font, J.; Fortuny, A.; Fabregat, A. Towards advanced aqueous dye removal processes: A short review on the versatile role of activated carbon. J. Environ. Manag. 2012, 102, 148–164. [Google Scholar] [CrossRef]
- Kant, S.; Pathania, D.; Singh, P.; Dhiman, P.; Kumar, A. Removal of malachite green and methylene blue by Fe0. 01Ni0. 01Zn0. 98O/polyacrylamide nanocomposite using coupled adsorption and photocatalysis. Appl. Catal. B Environ. 2014, 147, 340–352. [Google Scholar] [CrossRef]
- Huang, X.; Bo, X.; Zhao, Y.; Gao, B.; Wang, Y.; Sun, S.; Yue, Q.; Li, Q. Effects of compound bioflocculant on coagulation performance and floc properties for dye removal. Bioresour. Technol. 2014, 165, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, M.; Jiang, R.; Chen, J.; Huang, Q.; Wen, Y.; Tian, J.; Zhou, N.; Zhang, X.; Wei, Y. Water-dispersible fluorescent nanodiamonds for biological imaging prepared by thiol-ene click chemistry. J. Taiwan Inst. Chem. Eng. 2019, 95, 424–431. [Google Scholar] [CrossRef]
- Steinfeld, B.; Scott, J.; Vilander, G.; Marx, L.; Quirk, M.; Lindberg, J.; Koerner, K. The role of lean process improvement in implementation of evidence-based practices in behavioral health care. J. Behav. Health Serv. Res. 2015, 42, 504–518. [Google Scholar] [CrossRef]
- Ghaedi, M.; Shojaeipour, E.; Ghaedi, A.; Sahraei, R. Isotherm and kinetics study of malachite green adsorption onto copper nanowires loaded on activated carbon: Artificial neural network modeling and genetic algorithm optimization. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 142, 135–149. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Taher, M.M.; Bajpai, A.K.; Heibati, B.; Tyagi, I.; Asif, M.; Agarwal, S.; Gupta, V.K. Removal of noxious Cr (VI) ions using single-walled carbon nanotubes and multi-walled carbon nanotubes. Chem. Eng. J. 2015, 279, 344–352. [Google Scholar] [CrossRef]
- Somma, S.; Reverchon, E.; Baldino, L. Water Purification of Classical and Emerging Organic Pollutants: An Extensive Review. Chem. Eng. 2021, 5, 47. [Google Scholar] [CrossRef]
- Lefebvre, L.; Agusti, G.; Bouzeggane, A.; Edouard, D. Adsorption of dye with carbon media supported on polyurethane open cell foam. Catal. Today 2018, 301, 98–103. [Google Scholar] [CrossRef]
- Mohseni, B.A.; Eslami, A.; Kazemian, H.; Zarrabi, M.; Al-Musawi, T.J. A high density 3-aminopropyltriethoxysilane grafted pumice-derived silica aerogel as an efficient adsorbent for ibuprofen: Characterization and optimization of the adsorption data using response surface methodology. Environ. Technol. Innov. 2020, 18, 100642. [Google Scholar] [CrossRef]
- Nayan, R.S.; Mousumi, D.; Pijush, K.C. 6-Chitin and chitosan-based blends and composites. In Woodhead Publishing Series in Composites Science and Engineering, Biodegradable Polymers, Blends and Composites; Mavinkere Rangappa, S., Parameswaranpillai, J., Siengchin, S., Ramesh, M., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 123–203. ISBN 9780128237915. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Siafaka, P.I.; Pavlidou, E.G.; Chrissafis, K.J.; Bikiaris, D.N. Synthesis and adsorption application of succinyl-grafted chitosan for the simultaneous removal of zinc and cationic dye from binary hazardous mixtures. Chem. Eng. J. 2015, 259, 438–448. [Google Scholar] [CrossRef]
- Ruiz-Mercado, G.J.; Gonzalez, M.A.; Smith, R.L. Expanding GREENSCOPE beyond the gate: A green chemistry and life cycle perspective. Clean Technol. Environ. Policy 2014, 16, 703–717. [Google Scholar] [CrossRef]
- Ngah, W.W.; Teong, L.C.; Hanafiah, M.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Mi, F.L.; Sung, H.W.; Shyu, S.S. Drug release from chitosan–alginate complex beads reinforced by a naturally occurring cross-linking agent. Carbohydr. Polym. 2002, 48, 61–72. [Google Scholar] [CrossRef]
- Pijush, K.C.; Nayan, R.S. MOF and derived materials as aerogels: Structure, property, and performance relations. Coord. Chem. Rev. 2021, 446, 214125. [Google Scholar] [CrossRef]
- Asfaram, A.; Ghaedi, M.; Hajati, S.; Goudarzi, A.; Bazrafshan, A.A. Simultaneous ultrasound-assisted ternary adsorption of dyes onto copper-doped zinc sulfide nanoparticles loaded on activated carbon: Optimization by response surface methodology. Spectrochim. Acta A Mol. Biomol. Spectros. 2015, 145, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, M.; Ghaedi, M.; Dashtian, K.; Hajati, S.; Bazrafshan, A. Sonochemical assisted hydrothermal synthesis of ZnO: Cr nanoparticles loaded activated carbon for simultaneous ultrasound-assisted adsorption of ternary toxic organic dye: Derivative spectrophotometric, optimization, kinetic and isotherm study. Ultrason. Sonochem. 2016, 32, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Al-Musawi, T.J.; Kamani, H.; Bazrafshan, E.; Panahi, A.H.; Silva, M.F.; Abi, G. Optimization the effects of physicochemical parameters on the degradation of cephalexin in sono-Fenton reactor by using box-Behnken response surface methodology. Catal. Lett. 2019, 149, 1186–1196. [Google Scholar] [CrossRef]
- Babushkina, E.A.; Belokopytova, L.V.; Grachev, A.M.; Meko, D.M.; Vaganov, E.A. Variation of the hydrological regime of Bele-Shira closed basin in Southern Siberia and its reflection in the radial growth of Larix sibirica. Reg. Environ. Change 2017, 17, 1725–1737. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Liu, L.; Fang, Z.; Li, J.; Guo, J.; Wei, J. Formation and characterization of silver nanoparticles in aqueous solution via ultrasonic irradiation. Ultrason Sonochem. 2014, 21, 542–548. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, S.; Jiang, S.; Wang, J.; Wei, J.; Xu, S.; Gao, S.; Liu, H.; Qiu, H.; Li, Z. Growth graphene on silver–copper nanoparticles by chemical vapor deposition for high-performance surface-enhanced raman scattering. Appl. Surf. Sci. 2015, 353, 63–70. [Google Scholar] [CrossRef]
- Pathrose, B.; Nampoori, V.; Radhakrishnan, P.; Sahira, H.; Mujeeb, A. Effect of femtosecond laser ablated silver nanoparticles in the thermo-optic properties of basic fuchsin dye. Optik 2016, 127, 3684–3687. [Google Scholar] [CrossRef]
- Fisenko, S.P.; Khodyko, J.A. Low pressure evaporative cooling of micron-sized droplets of solutions and its novel applications. Int. J. Heat Mass Transf. 2009, 52, 3842–3849. [Google Scholar] [CrossRef]
- Hassabo, A.G.; Nada, A.A.; Ibrahim, H.M.; Abou-Zeid, N.Y. Impregnation of silver nanoparticles into polysaccharide substrates and their properties. Carbohydr. Polym. 2015, 122, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Raj, K.P.; Sadayandi, K. Effect of temperature on structural, optical and photoluminescence studies on ZnO nanoparticles synthesized by the standard co-precipitation method. Phys. Rev. B Condens. Matter. 2016, 487, 1–7. [Google Scholar] [CrossRef]
- Zahoranová, T.; Mori, T.; Yan, P.; Ševčíková, K.; Václavů, M.; Matolín, V.; Nehasil, V. Study of the character of gold nanoparticles deposited onto sputtered cerium oxide layers by deposition-precipitation method: Influence of the preparation parameters. Vacuum 2015, 114, 86–92. [Google Scholar] [CrossRef]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Ramani, R.; Srinivas, V.; Sastry, M. Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology 2003, 14, 824. [Google Scholar] [CrossRef]
- Ivanova, T.; Harizanova, A.; Koutzarova, T.; Vertruyen, B. Optical and structural characterization of TiO2 films doped with silver nanoparticles obtained by sol–gel method. Opt. Mater. 2013, 36, 207–213. [Google Scholar] [CrossRef]
- Roy, P.; Das, B.; Mohanty, A.; Mohapatra, S. Green synthesis of silver nanoparticles using Azadirachta indica leaf extract and its antimicrobial study. Applied Nanoscience 2017, 7, 843–850. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi J. Biol. Sci. 2017, 24, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Pan, M. RETRACTED: The Highly Efficient Adsorption of Pb (II) on Graphene Oxides: A Process Combined by Batch Experiments and Modeling Techniques; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Swamy, M.K.; Sudipta, K.; Jayanta, K.; Balasubramanya, S. The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from Leptadenia reticulata leaf extract. Appl. Nanosci. 2015, 5, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Rani, P.; Kumar, V.; Singh, P.P.; Matharu, A.S.; Zhang, W.; Kim, K.-H.; Singh, J.; Rawat, M. Highly stable AgNPs prepared via a novel green approach for catalytic and photocatalytic removal of biological and non-biological pollutants. Environ. Int. 2020, 143, 105924. [Google Scholar] [CrossRef] [PubMed]
- Franta, A.K.; Lenters, T.R.; Mounce, D.; Neradilek, B.; Matsen III, F.A. The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J. Shoulder Elb. Surg. 2007, 16, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Tarno, H.; Qi, H.; Endoh, R.; Kobayashi, M.; Goto, H.; Futai, K. Types of frass produced by the ambrosia beetle Platypus quercivorus during gallery construction, and host suitability of five tree species for the beetle. J. For. Res. 2011, 16, 68–75. [Google Scholar] [CrossRef]
- Veisi, H.; Azizi, S.; Mohammadi, P. Green synthesis of the silver nanoparticles mediated by Thymbra spicata extract and its application as a heterogeneous and recyclable nanocatalyst for catalytic reduction of a variety of dyes in water. J. Clean. Prod. 2018, 170, 1536–1543. [Google Scholar] [CrossRef]
- Al-Senani, G.M.; Al-Kadhi, N. The synthesis and effect of silver nanoparticles on the adsorption of Cu2+ from aqueous solutions. Appl. Sci. 2020, 10, 4840. [Google Scholar] [CrossRef]
- Mahiuddin, M.; Saha, P.; Ochiai, B. Green synthesis and catalytic activity of silver nanoparticles based on Piper chaba stem extracts. Nanomaterials 2020, 10, 1777. [Google Scholar] [CrossRef]
- Al-Zaban, M.I.; Mahmoud, M.A.; AlHarbi, M.A. Catalytic degradation of methylene blue using silver nanoparticles synthesized by honey. Saudi J. Biol. Sci. 2021, 28, 2007–2013. [Google Scholar] [CrossRef]
- Nagalakshmi, C.; Vindhya, V.D.K.; Mukesh, K.K.; Chitti, B.N.; Swambabu, V. Composites of cellulose nanofibers and silver nanoparticles for malachite green dye removal from water. Carbohydr. Polym. Technol. Appl. 2021, 2, 100098. [Google Scholar] [CrossRef]
- Singh, J.; Dhaliwal, A. Effective Removal of Methylene Blue Dye Using Silver Nanoparticles Containing Grafted Polymer of Guar Gum/Acrylic Acid as Novel Adsorbent. J. Polym. Environ. 2021, 29, 71–88. [Google Scholar] [CrossRef]
- Atta, A.M.; Moustafa, Y.M.; Al-Lohedan, H.A.; Ezzat, A.O.; Hashem, A.I. Methylene blue catalytic degradation using silver and magnetite nanoparticles functionalized with a poly (ionic liquid) based on quaternized dialkylethanolamine with 2-acrylamido-2-methylpropane sulfonate-co-vinylpyrrolidone. ACS Omega 2020, 5, 2829–2842. [Google Scholar] [CrossRef] [Green Version]
- Moldovan, B.; Sincari, V.; Perde-Schrepler, M.; David, L. Biosynthesis of silver nanoparticles using Ligustrum ovalifolium fruits and their cytotoxic effects. Nanomaterials 2018, 8, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashif, G.; Hee, C.Y.; Jo, I.H. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. J. Agric. Food Chem. 2009, 57, 4988–4994. [Google Scholar]
- Zia, M.; Gul, S.; Akhtar, J.; Ul Haq, I.; Abbasi, B.H.; Hussain, A.; Naz, S.; Chaudhary, M.F. Green synthesis of silver nanoparticles from grape and tomato juices and evaluation of biological activities. IET Nanobiotechnology 2017, 11, 193–199. [Google Scholar] [CrossRef]
- Hamza, W.; Dammak, N.; Hadjltaief, H.B.; Eloussaief, M.; Benzina, M. Sono-assisted adsorption of cristal violet dye onto tunisian smectite clay: Characterization, kinetics and adsorption isotherms. Ecotoxicol. Environ. Saf. 2018, 163, 365–371. [Google Scholar] [CrossRef]
- Shah, J.A.; Butt, T.A.; Mirza, C.R.; Shaikh, A.J.; Khan, M.S.; Arshad, M.; Riaz, N.; Haroon, H.; Gardazi, S.M.H.; Yaqoob, K. Phosphoric acid activated carbon from Melia azedarach waste sawdust for adsorptive removal of reactive orange 16: Equilibrium modelling and thermodynamic analysis. Molecules 2020, 25, 2118. [Google Scholar] [CrossRef]
- Edet, U.A.; Ifelebuegu, A.O. Kinetics, isotherms, and thermodynamic modeling of the adsorption of phosphates from model wastewater using recycled brick waste. Processes 2020, 8, 665. [Google Scholar] [CrossRef]
- Haq, S.; Rehman, W.; Waseem, M.; Shah, A.; Khan, A.R.; Rehman, M.U.; Ahmad, P.; Khan, B.; Ali, G. Green synthesis and characterization of tin dioxide nanoparticles for photocatalytic and antimicrobial studies. Mater. Res. Express 2020, 7, 025012. [Google Scholar] [CrossRef]
- Femila, E.; Srimathi, R.; Deivasigamani, C. Removal of malachite green using silver nanoparticles via adsorption and catalytic degradation. Int. J. Pharm. Pharm. Sci. 2014, 6, 579–583. [Google Scholar]
- Khalil, M. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Zhao, Y.-X.; Gao, Y.-J.; Gao, F.-P.; Fan, Y.-S.; Li, X.-J.; Duan, Z.-Y.; Wang, H. Anti-bacterial and in vivo tumor treatment by reactive oxygen species generated by magnetic nanoparticles. J. Mater. Chem. B 2013, 1, 5100–5107. [Google Scholar] [CrossRef]
- Vanaja, M.; Gnanajobitha, G.; Paulkumar, K.; Rajeshkumar, S.; Malarkodi, C.; Annadurai, G. Phytosynthesis of silver nanoparticles by Cissus quadrangularis: Influence of physicochemical factors. J. Nanostructure Chem. 2013, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ajitha, B.; Reddy, Y.A.K.; Reddy, P.S. Biosynthesis of silver nanoparticles using Plectranthus amboinicus leaf extract and its antimicrobial activity. Spectrochim Acta A Mol. Biomol. Spectrosc. 2014, 128, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, A.E. The pharmacological importance of Anethum graveolens–A review. Int. J. Pharm. Pharm. Sci. 2014, 6, 11–13. [Google Scholar]
- Ashajyothi, C.; Harish, K.H.; Dubey, N.; Chandrakanth, R.K. Antibiofilm activity of biogenic copper and zinc oxide nanoparticles-antimicrobials collegiate against multiple drug resistant bacteria: A nanoscale approach. J. Nanostructure Chem. 2016, 6, 329–341. [Google Scholar] [CrossRef] [Green Version]
- Jemal, K.; Sandeep, B.; Pola, S. Synthesis, characterization, and evaluation of the antibacterial activity of Allophylus serratus leaf and leaf derived callus extracts mediated silver nanoparticles. J. Nanomater. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Anigol, L.B.; Charantimath, J.S.; Gurubasavaraj, P.M. Effect of concentration and pH on the size of silver nanoparticles synthesized by green chemistry. Org. Med. Chem. Int. J. 2017, 3, 1–5. [Google Scholar] [CrossRef]
- Alishavandi, Z.; Mosallanejad, N.; Shabani, R. Silvernano particle loaded on activated carbon as novel adsorbent for the removal of acid yellow 199 dye. J. Chem. Health Risks 2013, 3, 53–68. [Google Scholar]
- Maleki, A.; Mahvi, A.; Mesdaghinia, A.; Naddafi, K. Degradation and toxicity reduction of phenol by ultrasound waves. Bull. Chem. Soc. Ethiop. 2007, 21, 33–38. [Google Scholar] [CrossRef]
- Sarkar, S.; Sarkar, M. Ultrasound assisted batch operation for the adsorption of hexavalent chromium onto engineered nanobiocomposite. Heliyon 2019, 5, e01491. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, S.K.; Hota, G. Surface functionalization of GO with MgO/MgFe2O4 binary oxides: A novel magnetic nanoadsorbent for removal of fluoride ions. J. Environ. Chem. Eng. 2018, 6, 2918–2931. [Google Scholar] [CrossRef]
- Milenković, D.; Bojić, A.L.; Veljković, V. Ultrasound-assisted adsorption of 4-dodecylbenzene sulfonate from aqueous solutions by corn cob activated carbon. Ultrason. Sonochemistry 2013, 20, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, M.; Kanmani, M.; Debnath, A.; Saha, B. Sono-assisted rapid adsorption of anionic dye onto magnetic CaFe2O4/MnFe2O4 nanocomposite from aqua matrix. Powder Technol. 2019, 354, 496–504. [Google Scholar] [CrossRef]
- Gol, S.; Pena, R.N.; Rothschild, M.F.; Tor, M.; Estany, J. A polymorphism in the fatty acid desaturase-2 gene is associated with the arachidonic acid metabolism in pigs. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayem, S.; Sultana, N.; Haque, M.; Miah, B.; Hasan, M.; Islam, T.; Awal, A.; Uddin, J.; Aziz, M.; Ahammad, A. Green synthesis of gold and silver nanoparticles by using amorphophallus paeoniifolius tuber extract and evaluation of their antibacterial activity. Molecules 2020, 25, 4773. [Google Scholar] [CrossRef]
- Al-Otibi, F.; Al-Ahaidib, R.A.; Alharbi, R.I.; Al-Otaibi, R.M.; Albasher, G. Antimicrobial Potential of Biosynthesized Silver Nanoparticles by Aaronsohnia factorovskyi Extract. Molecules 2021, 26, 130. [Google Scholar] [CrossRef] [PubMed]
- Elfaig, H.; Elsayed, W.; Ahmed, H. Preparation and Characterization of Chitosan/Silver Nano-composite and its Application on Nile Water as Antibacterial Materials. MRS Adv. 2020, 5, 1331–1338. [Google Scholar] [CrossRef]
- Ansari, M.; Khan, H.; Khan, A. Evaluation of antibacterial activity of silver nanoparticles against MSSA and MSRA on isolates from skin infections. J. Biol. Med. 2011, 3, 141–146. Available online: https://www.researchgate.net/publication/279670209.
- Alghuthaymi, M.A.; Abd-Elsalam, K.A.; Shami, A.; Said-Galive, E.; Shtykova, E.V.; Naumkin, A.V. Silver/Chitosan nanocomposites: Preparation and characterization and their fungicidal activity against dairy cattle toxicosis Penicillium expansum. J. Fungi 2020, 6, 51. [Google Scholar] [CrossRef] [Green Version]
- Chandran, S.; Chandhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloevera plant extract. Biotechnol. Prog. 2006, 22, 577. [Google Scholar] [CrossRef]
- Dakal, T.; Kumar, A.; Majumdar, R.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [Green Version]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]

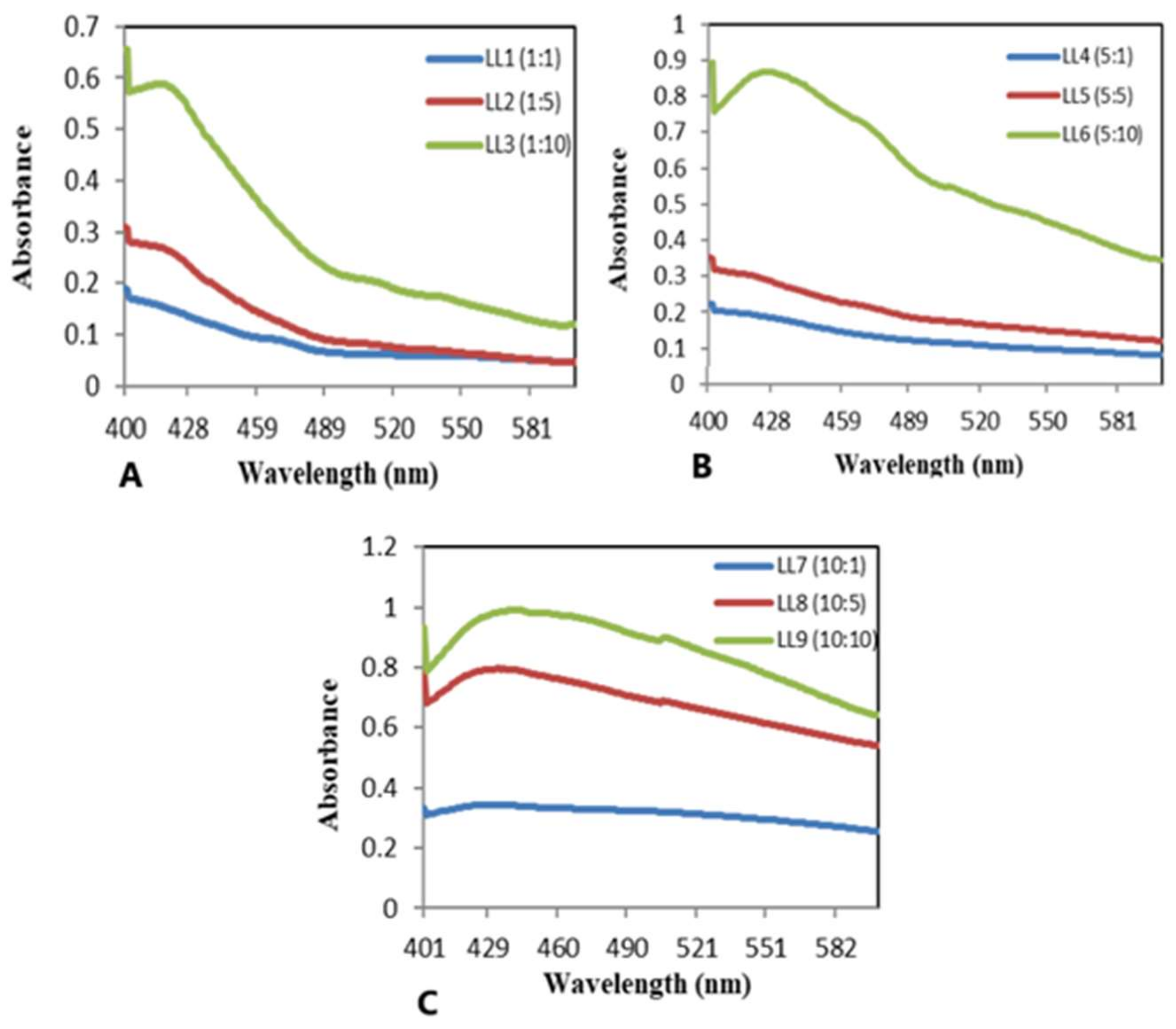


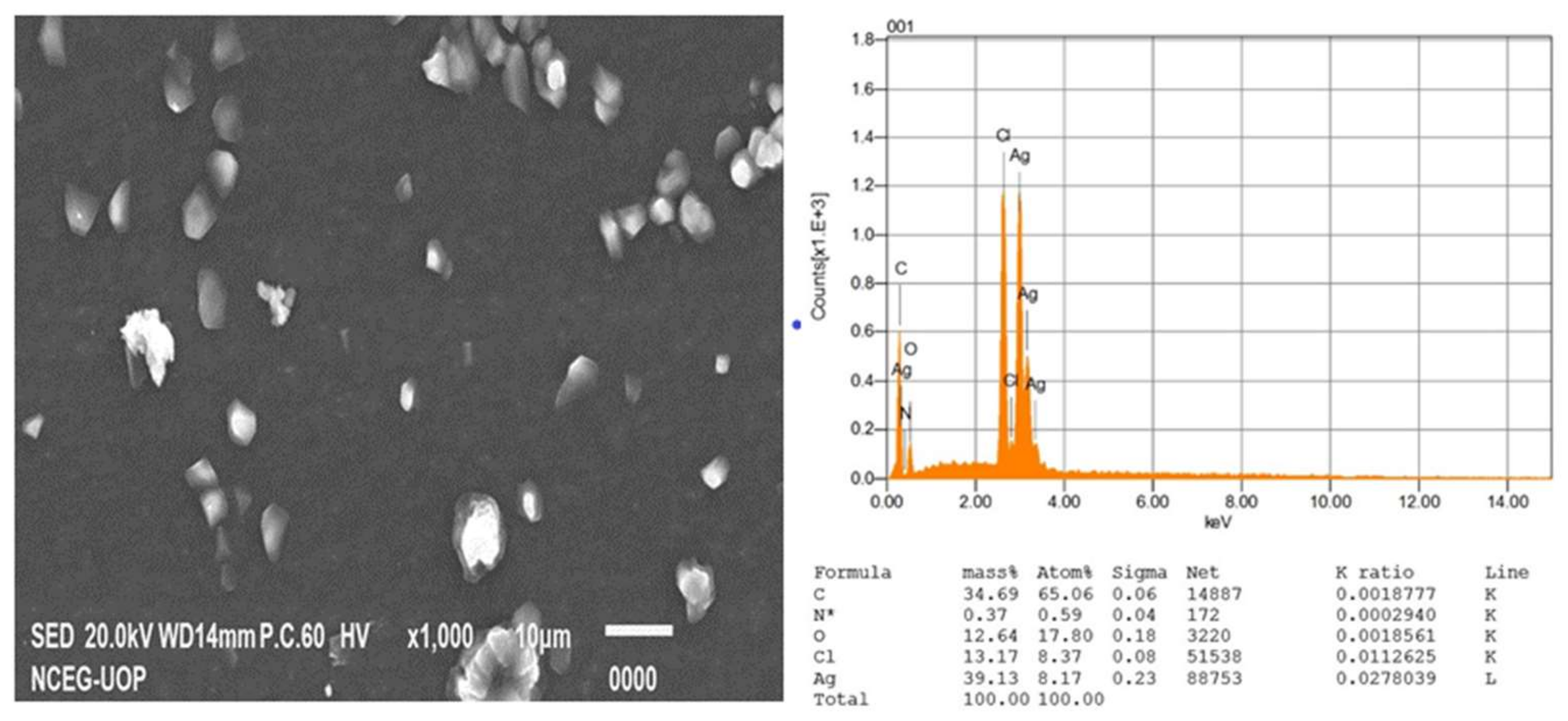
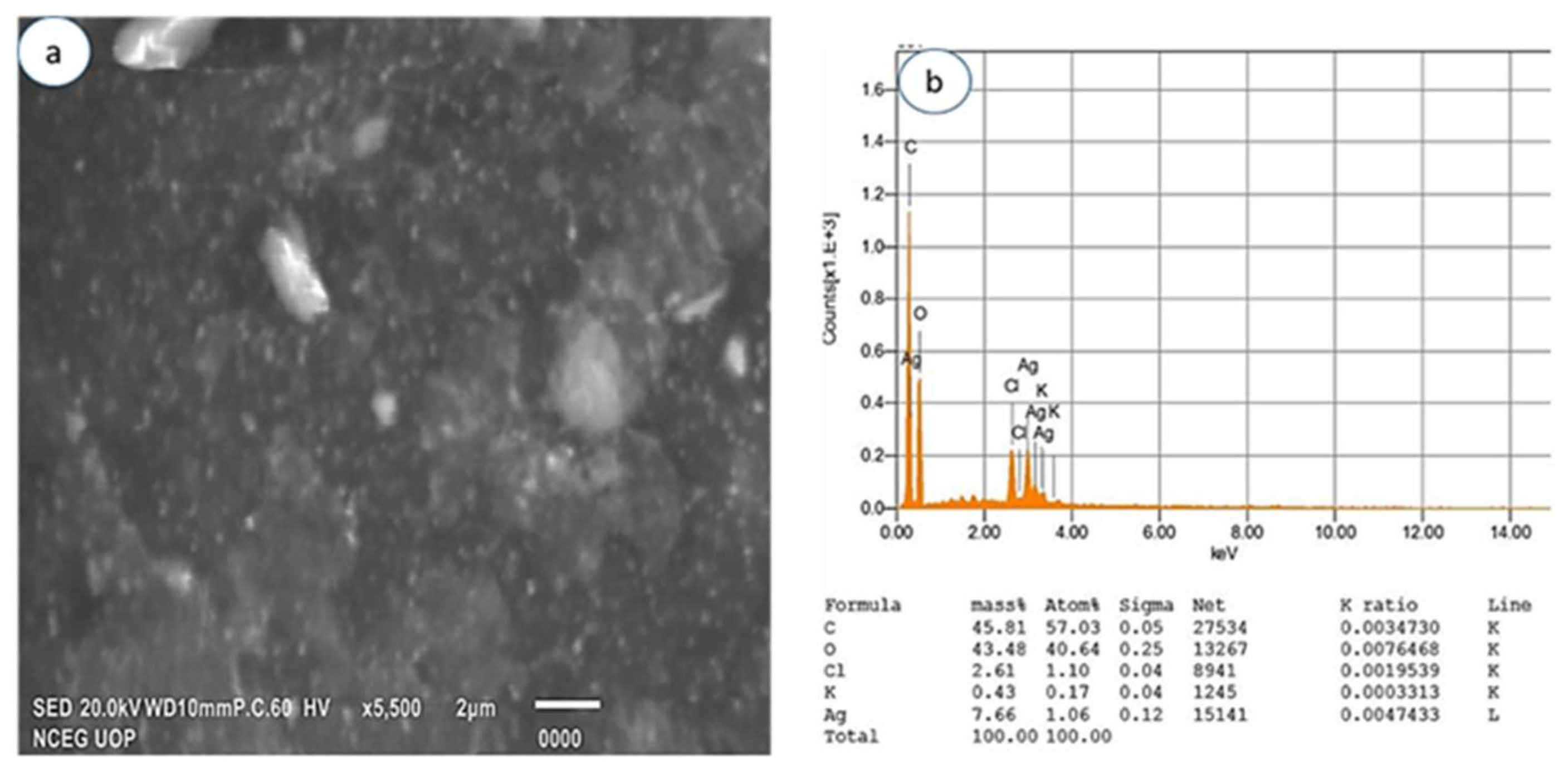
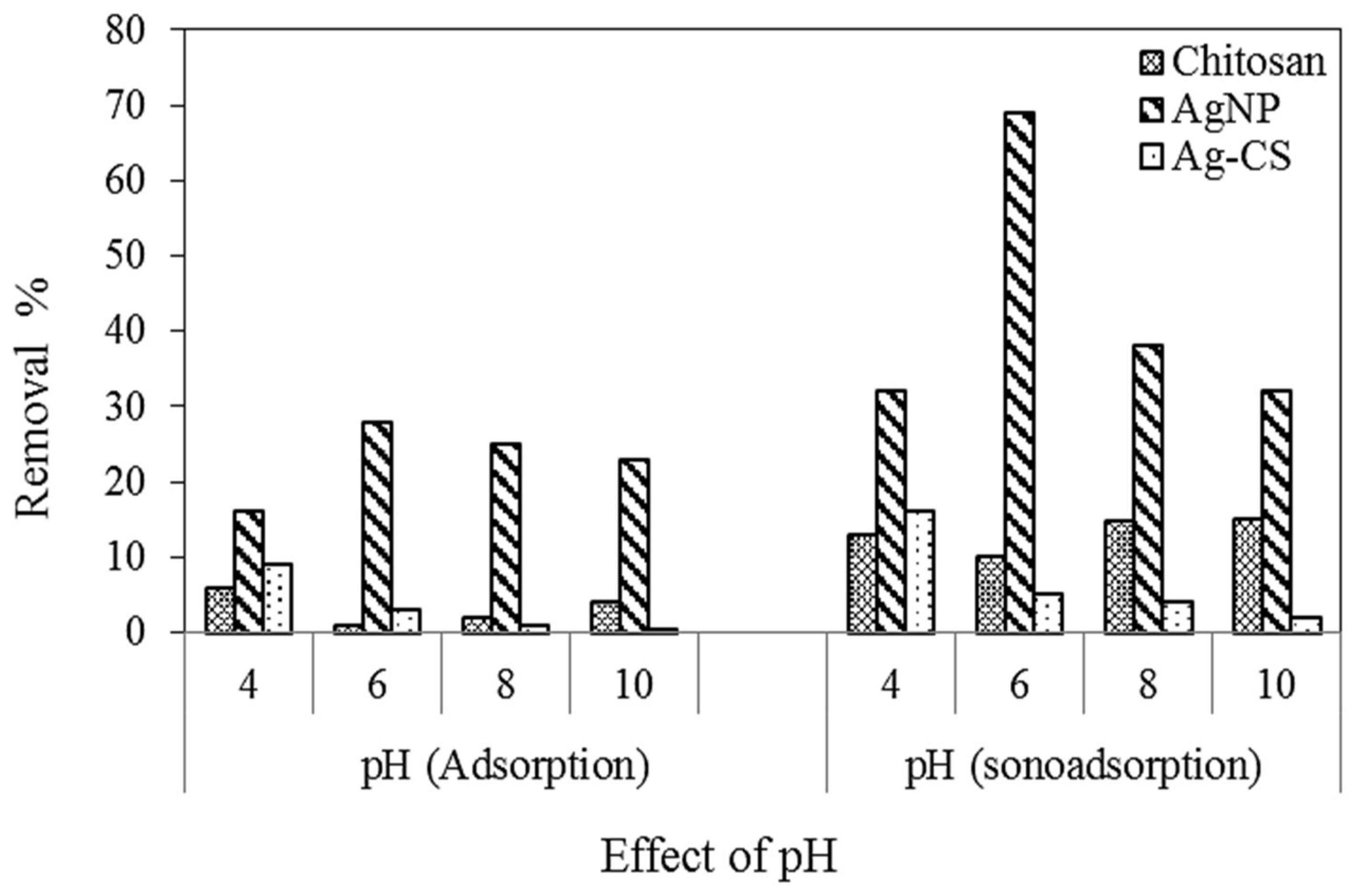
| Sample Number | Code | AgNO3 (mM) in 50 mL | Extract Concentration (mg/mL) in 50 mL |
|---|---|---|---|
| 1 | LL1 | 1 | 1 |
| 2 | LL2 | 1 | 5 |
| 3 | LL3 | 1 | 10 |
| 4 | LL4 | 5 | 1 |
| 5 | LL5 | 5 | 5 |
| 6 | LL6 | 5 | 10 |
| 7 | LL7 | 10 | 1 |
| 8 | LL8 | 10 | 5 |
| 9 | LL9 | 10 | 10 |
| Isotherm Models | Parameters | Adsorption | Sono-Adsorption |
|---|---|---|---|
| Langmuir | qexp | 40 | 60 |
| qm | 49.26 | 50.51 | |
| RL | 0.16 | 0.01 | |
| KL | 0.11 | 2.20 | |
| R2 | 0.9782 | 0.9926 | |
| Freundlich | kF | 7.65 | 15.75 |
| n | 2.11 | 8.83 | |
| R2 | 0.9116 | 0.5566 |
| Samples | Zone of Inhibition (mm) | ||
|---|---|---|---|
| Antibacterial | Antifungal | ||
| MRSA | SA | Ca | |
| M1 | 0.33 ± 0.47 | 0.33 ± 0.47 | 0.33 ± 0.47 |
| M2 | 7.33 ± 0.47 | 21.00 ± 0.82 | 8.00 ± 0.82 |
| M3 | 8.67 ± 1.25 | 14.67 ± 0.47 | 9.00 ± 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultan, M.; Siddique, M.; Khan, R.; Fallatah, A.M.; Fatima, N.; Shahzadi, I.; Waheed, U.; Bilal, M.; Ali, A.; Abbasi, A.M. Ligustrum lucidum Leaf Extract-Assisted Green Synthesis of Silver Nanoparticles and Nano-Adsorbents Having Potential in Ultrasound-Assisted Adsorptive Removal of Methylene Blue Dye from Wastewater and Antimicrobial Activity. Materials 2022, 15, 1637. https://doi.org/10.3390/ma15051637
Sultan M, Siddique M, Khan R, Fallatah AM, Fatima N, Shahzadi I, Waheed U, Bilal M, Ali A, Abbasi AM. Ligustrum lucidum Leaf Extract-Assisted Green Synthesis of Silver Nanoparticles and Nano-Adsorbents Having Potential in Ultrasound-Assisted Adsorptive Removal of Methylene Blue Dye from Wastewater and Antimicrobial Activity. Materials. 2022; 15(5):1637. https://doi.org/10.3390/ma15051637
Chicago/Turabian StyleSultan, Mujaddad, Maria Siddique, Romana Khan, Ahmed M. Fallatah, Nighat Fatima, Irum Shahzadi, Ummara Waheed, Muhammad Bilal, Asmat Ali, and Arshad Mehmood Abbasi. 2022. "Ligustrum lucidum Leaf Extract-Assisted Green Synthesis of Silver Nanoparticles and Nano-Adsorbents Having Potential in Ultrasound-Assisted Adsorptive Removal of Methylene Blue Dye from Wastewater and Antimicrobial Activity" Materials 15, no. 5: 1637. https://doi.org/10.3390/ma15051637
APA StyleSultan, M., Siddique, M., Khan, R., Fallatah, A. M., Fatima, N., Shahzadi, I., Waheed, U., Bilal, M., Ali, A., & Abbasi, A. M. (2022). Ligustrum lucidum Leaf Extract-Assisted Green Synthesis of Silver Nanoparticles and Nano-Adsorbents Having Potential in Ultrasound-Assisted Adsorptive Removal of Methylene Blue Dye from Wastewater and Antimicrobial Activity. Materials, 15(5), 1637. https://doi.org/10.3390/ma15051637







