Structural, Morphological, Optical and Electrical Characterization of Gahnite Ferroan Nano Composite Derived from Fly Ash Silica and ZnO Mixture
Abstract
:1. Introduction
2. Experimental Section
3. Characterization
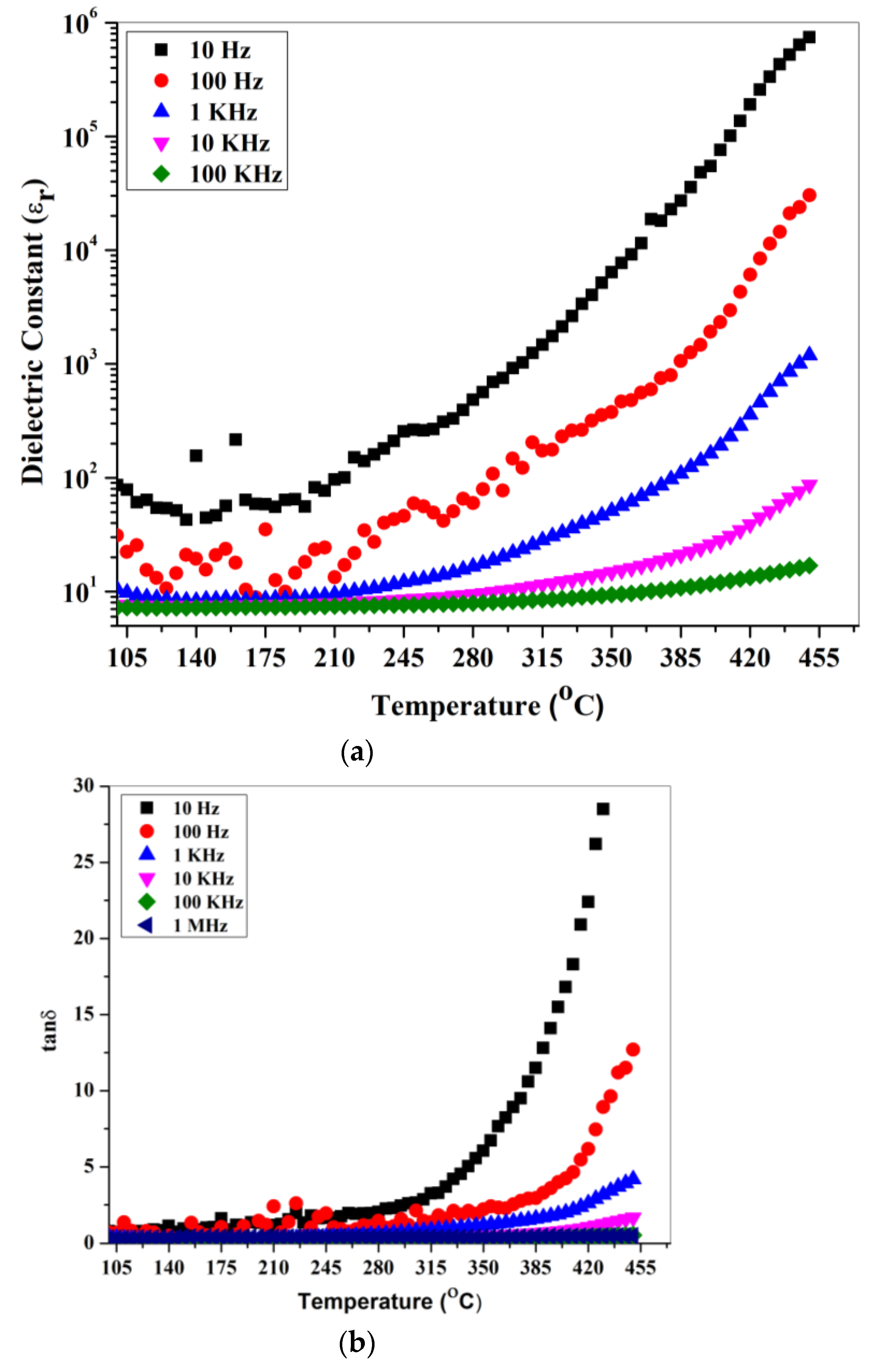
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blaxland, A.B. Occurrence of zinc in granitic biotites. Miner. Depos. 1971, 6, 313–320. [Google Scholar] [CrossRef]
- Tulloch, A.J. Gahnite and columbite in an alkali-feldspar granite from New Zealand. Mineral. Mag. 1981, 44, 275–278. [Google Scholar] [CrossRef]
- Frost, B.R. Ferroan gahnite from quartz-biotite-almandine schist, Wind River Mountains, Wyoming. Am. Mineral. 1973, 58, 831–834. [Google Scholar]
- Sandhaus, D.J.; Craig, J.R. Gahnite in the metamorphosed stratiform massive sulfide deposits of the Mineral District, Virginia, USA. TMPM Tschermaks Mineral. Petrogr. Mitt. 1986, 35, 77–98. [Google Scholar] [CrossRef]
- Gandhi, S.M. On the ferroan gahnite of Mamandur, Madras State, India. Mineral. Mag. 1971, 38, 528–529. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, C.; Sun, W. Fly ash effects: II. The active effect of fly ash. Cem. Concr. Res. 2004, 34, 2057–2060. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- GB1596-88. Fly ash Used in Cement and Concrete; University of New Brunswick: Fredericton, NB, USA, 1988. [Google Scholar]
- Fulekar, M.H. Disposal of Fly Ash—An Environmental Problem. Int. J. Environ. Stud. 1986, 26, 191–215. [Google Scholar] [CrossRef]
- Mohanty, J.K.; Guru, S.R.; Dash, P.; Pradhan, P.K. Fly Ash Management and Condition Monitoring of Ash Pond. Earth Syst. Environ. 2020, 5, 1–13. [Google Scholar] [CrossRef]
- Berry, E.E.; Malhotra, V.M. Flyash for use in Concrete-A Critical Review. J. Am. Concr. Inst. 1980, 77, 59–73. [Google Scholar] [CrossRef]
- Swanepoel, J.C.; Strydom, C.A. Utilisation of fly ash in a geopolymeric material. Appl. Geochem. 2002, 17, 1143–1148. [Google Scholar] [CrossRef]
- Molkere, V.; Aleem, M.I.A.; Arumairaj, P.D. Geopolymer Concrete-A Review. Int. J. Eng. Sci. Emerg. Technol. 2012, 1, 118–122. [Google Scholar]
- Kluczka, J.; Trojanowska, J.; Zołotajkin, M. Utilization of fly ash zeolite for boron removal from aqueous solution. Desalin. Water Treat. 2015, 54, 1839–1849. [Google Scholar] [CrossRef]
- Ghaedi, M.; Mosallanejad, N. Removal of Heavy Metal Ions from Polluted Waters by Using of Low Cost Adsorbents: Review. J. Chem. Heal. Risks 2013, 3, 7–22. [Google Scholar]
- Wouterlood, H.J.; Bowling, K.M.G. Removal and Recovery of Arsenious Oxide from Flue Gases. Environ. Sci. Technol. 1979, 13, 93–97. [Google Scholar] [CrossRef]
- Swami, D.; Buddhi, D. Removal of contaminants from industrial wastewater through various non-conventional technologies: A review. Int. J. Environ. Pollut. 2006, 27, 324–346. [Google Scholar] [CrossRef]
- Lekgoba, T.; Ntuli, F.; Falayi, T. Application of coal fly ash for treatment of wastewater containing a binary mixture of copper and nickel. J. Water Process Eng. 2021, 40, 101822. [Google Scholar] [CrossRef]
- Gorai, S. Utilization of Fly ash for sustainable environment management. J. Mater. Environ. Sci. 2018, 9, 385–393. [Google Scholar]
- Kurajica, S.; Šipušić, J.; Zupancic, M.; Brautović, I.; Albrecht, M. ZnO-Al2O3-SiO2 glass ceramics: Influence of composition on crystal phases, crystallite size and appearance. J. Non. Cryst. Solids 2021, 553. [Google Scholar] [CrossRef]
- Yang, R.; Han, A.; Ye, M.; Chen, X.; Yuan, L. The influence of Mn/N-codoping on the thermal performance of ZnAl2O4as high near-infrared reflective inorganic pigment. J. Alloys Compd. 2017, 696, 1329–1341. [Google Scholar] [CrossRef]
- Pathak, N.; Gupta, S.K.; Sanyal, K.; Kumar, M.; Kadam, R.M.; Natarajan, V. Photoluminescence and EPR studies on Fe3+ doped ZnAl 2O4: An evidence for local site swapping of Fe 3+ and formation of inverse and normal phase. Dalt. Trans. 2014, 43, 9313–9323. [Google Scholar] [CrossRef] [PubMed]
- Ianoş, R.; Muntean, E.; Păcurariu, C.; Lazău, R.; Bandas, C.; Delinescu, G. Combustion synthesis of a blue Co-doped zinc aluminate near-infrared reflective pigment. Dye. Pigment. 2017, 142, 24–31. [Google Scholar] [CrossRef]
- Danchevskaya, M.N.; Ivakin, Y.D.; Torbin, S.N.; Muravieva, G.P.; Ovchinnikova, O.G. Thermovaporous synthesis of complicated oxides. J. Mater. Sci. 2006, 41, 1385–1390. [Google Scholar] [CrossRef]
- Molla, A.R.; Rodrigues, A.M.; Singh, S.P.; Lancelotti, R.F.; Zanotto, E.D.; Rodrigues, A.C.M.; Reza Dousti, M.; de Camargo, A.S.S.; Magon, C.J.; Silva, I.D.A. Crystallization, mechanical, and optical properties of transparent, nanocrystalline gahnite glass-ceramics. J. Am. Ceram. Soc. 2017, 100, 1963–1975. [Google Scholar] [CrossRef]
- Visinescu, D.; Paraschiv, C.; Ianculescu, A.; Jurca, B.; Vasile, B.; Carp, O. The environmentally benign synthesis of nanosized CoxZn1-xAl2O4 blue pigments. Dye. Pigment. 2010, 87, 125–131. [Google Scholar] [CrossRef]
- Sumathi, S.; Kavipriya, A. Structural, optical and photocatalytic activity of cerium doped zinc aluminate. Solid State Sci. 2017, 65, 52–60. [Google Scholar] [CrossRef]
- Ma, C.; Chen, X.Y.; Bao, S.P. Generalized synthesis of 1-D nanoporous aluminates by using a sacrificial template especially evidenced in case of ZnAl2O4:Eu3+ phosphors. Microporous Mesoporous Mater. 2010, 129, 37–41. [Google Scholar] [CrossRef]
- Tsai, M.T.; Chang, Y.S.; Huang, I.B.; Pan, B.Y. Luminescent and structural properties of manganese-doped zinc aluminate spinel nanocrystals. Ceram. Int. 2013, 39, 3691–3697. [Google Scholar] [CrossRef]
- Ravikumar, B.S.; Nagabhushana, H.; Sunitha, D.V.; Sharma, S.C.; Nagabhushana, B.M.; Shivakumara, C. Plant latex mediated green synthesis of ZnAl2O 4:Dy3+ (1-9 mol%) nanophosphor for white light generation. J. Alloys Compd. 2014, 585, 561–571. [Google Scholar] [CrossRef]
- Pattanaik, P.; Kamilla, S.K.; Das, D.P.; Mishra, D.K. Experimental and simulated study of electrical behaviour of ZnO film deposited on Al substrate for device applications. J. Mater. Sci. Mater. Electron. 2014, 25, 3062–3068. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, Y.; Huang, Z.; Kou, Z.; Yuan, X.; Ren, Z.; Zhai, Y.; Du, J.; Zhai, H. Magnetic behaviors of Co1-xZnxFe2O4 nano-particles. J. Appl. Phys. 2015, 117, 17E711. [Google Scholar] [CrossRef]
- Mohanty, B.; Parida, B.N.; Parida, R.K. Structural, dielectric and magnetic behavior of BST modified rare earth ortho-ferrite LaFeO3. Ceram. Int. 2020, 46, 16502–16509. [Google Scholar] [CrossRef]
- Feteira, A. Negative temperature coefficient resistance (NTCR) ceramic thermistors: An industrial perspective. J. Am. Ceram. Soc. 2009, 92, 967–983. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, Q.; Rhodes, S.; Fang, J.; An, L. SiCNO–GO composites with the negative temperature coefficient of resistance for high-temperature sensor applications. J. Am. Ceram. Soc. 2017, 100, 592–601. [Google Scholar] [CrossRef]
- Neella, N.; Gaddam, V.; Rajanna, K.; Nayak, M.M. Negative temperature coefficient behavior of graphene-silver nanocomposite films for temperature sensor applications. In Proceedings of the 2016 IEEE 11th Annual International Conference on Nano/Micro Engineered and Molecular Systems, NEMS 2016, Sendai, Japan, 17–20 April 2016; Institute of Electrical and Electronics Engineers Inc.. pp. 329–332. [Google Scholar]

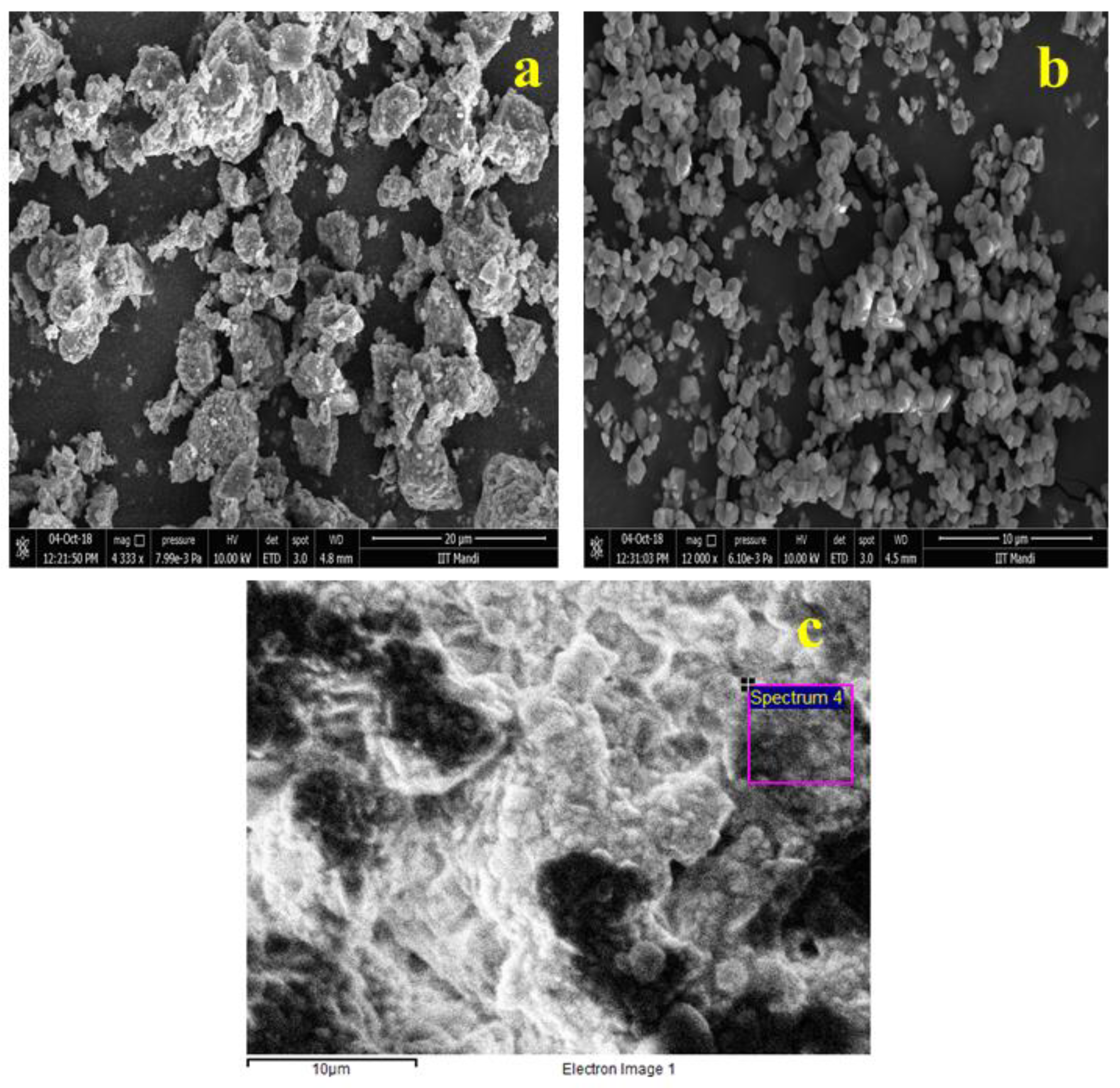


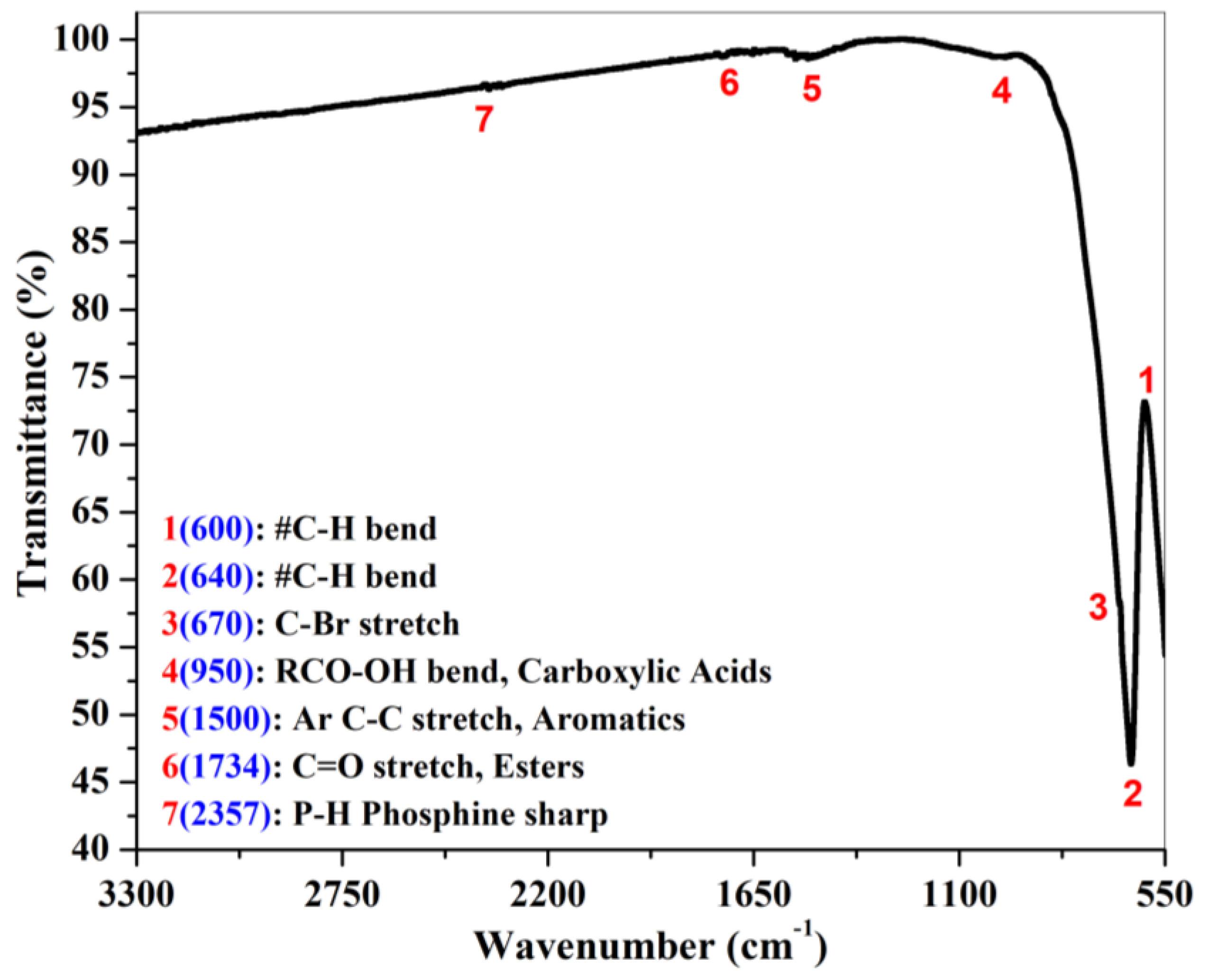
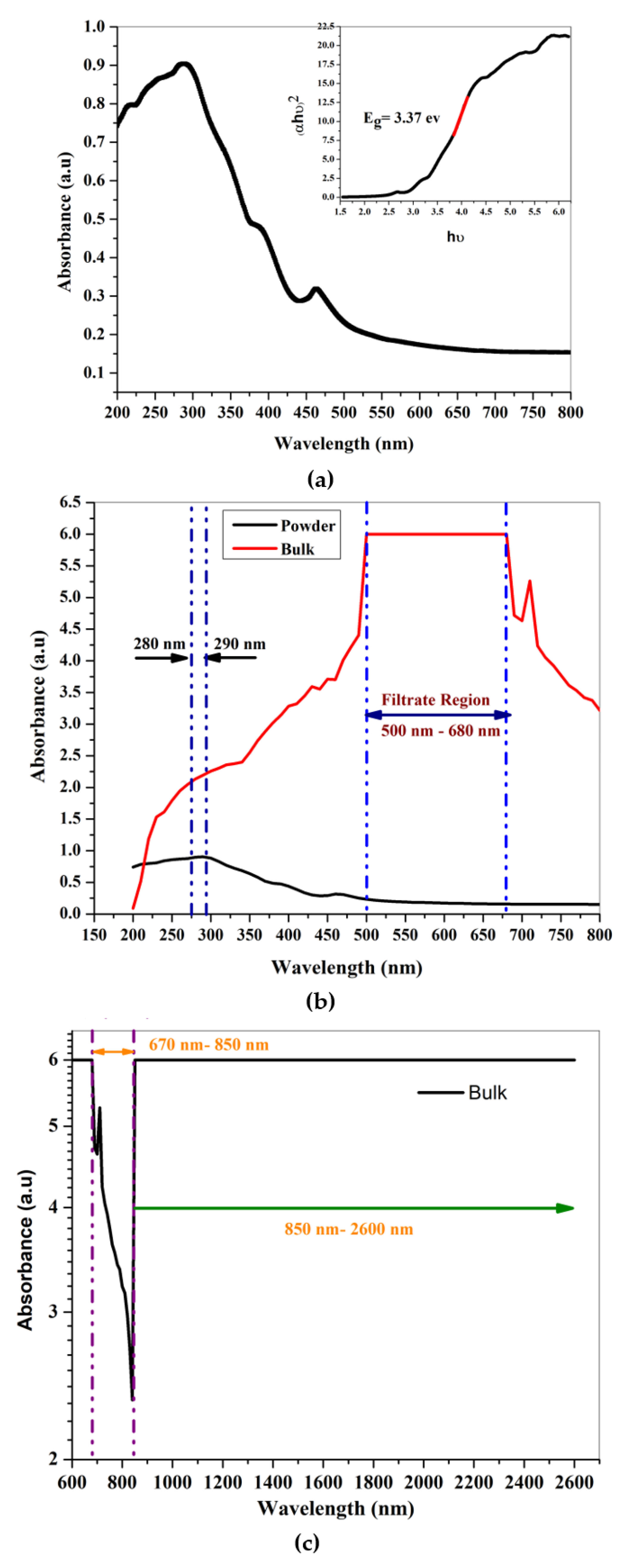

| Compound | Quantification (wt%) |
|---|---|
| SiO2 | 57.13 |
| Al2O3 | 34.24 |
| CaO | 2.84 |
| MgO | 0.91 |
| Fe2O3 | 2.78 |
| K2O | 0.65 |
| TiO2 | 0.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panda, S.S.; Tripathy, H.P.; Pattanaik, P.; Mishra, D.K.; Kamilla, S.K.; Khandual, A.; Holderbaum, W.; Sherwood, R.; Hawkins, G.; Masakapalli, S.K. Structural, Morphological, Optical and Electrical Characterization of Gahnite Ferroan Nano Composite Derived from Fly Ash Silica and ZnO Mixture. Materials 2022, 15, 1388. https://doi.org/10.3390/ma15041388
Panda SS, Tripathy HP, Pattanaik P, Mishra DK, Kamilla SK, Khandual A, Holderbaum W, Sherwood R, Hawkins G, Masakapalli SK. Structural, Morphological, Optical and Electrical Characterization of Gahnite Ferroan Nano Composite Derived from Fly Ash Silica and ZnO Mixture. Materials. 2022; 15(4):1388. https://doi.org/10.3390/ma15041388
Chicago/Turabian StylePanda, Sushree Saraswati, Hara Prasada Tripathy, Priyabrata Pattanaik, Dilip Kumar Mishra, Sushanta Kumar Kamilla, Asimananda Khandual, William Holderbaum, Richard Sherwood, Gary Hawkins, and Shyam Kumar Masakapalli. 2022. "Structural, Morphological, Optical and Electrical Characterization of Gahnite Ferroan Nano Composite Derived from Fly Ash Silica and ZnO Mixture" Materials 15, no. 4: 1388. https://doi.org/10.3390/ma15041388
APA StylePanda, S. S., Tripathy, H. P., Pattanaik, P., Mishra, D. K., Kamilla, S. K., Khandual, A., Holderbaum, W., Sherwood, R., Hawkins, G., & Masakapalli, S. K. (2022). Structural, Morphological, Optical and Electrical Characterization of Gahnite Ferroan Nano Composite Derived from Fly Ash Silica and ZnO Mixture. Materials, 15(4), 1388. https://doi.org/10.3390/ma15041388