Improving the Thermomechanical Properties of Poly(lactic acid) via Reduced Graphene Oxide and Bioderived Poly(decamethylene 2,5-furandicarboxylate)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
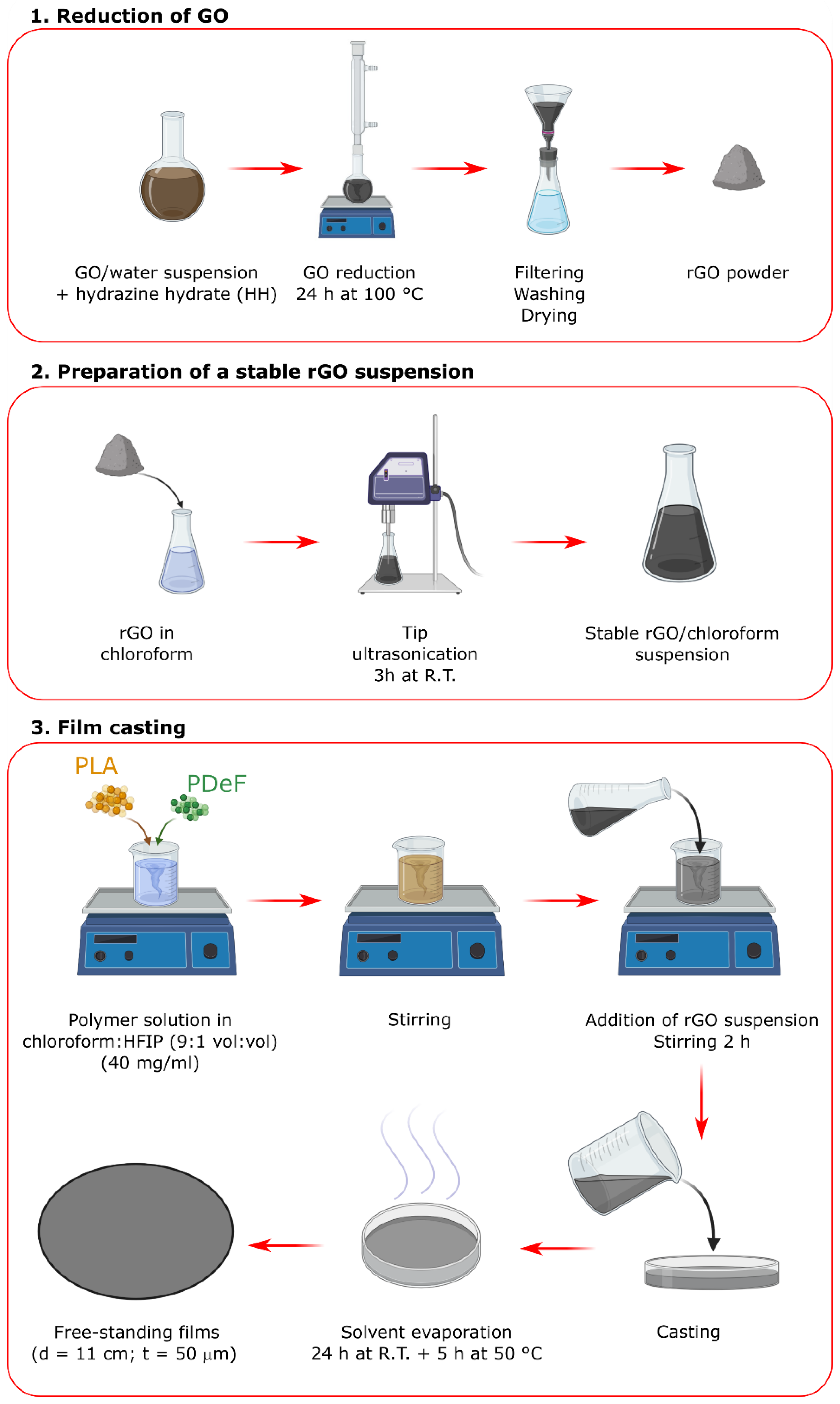
2.2. Sample Preparation
2.3. Characterization
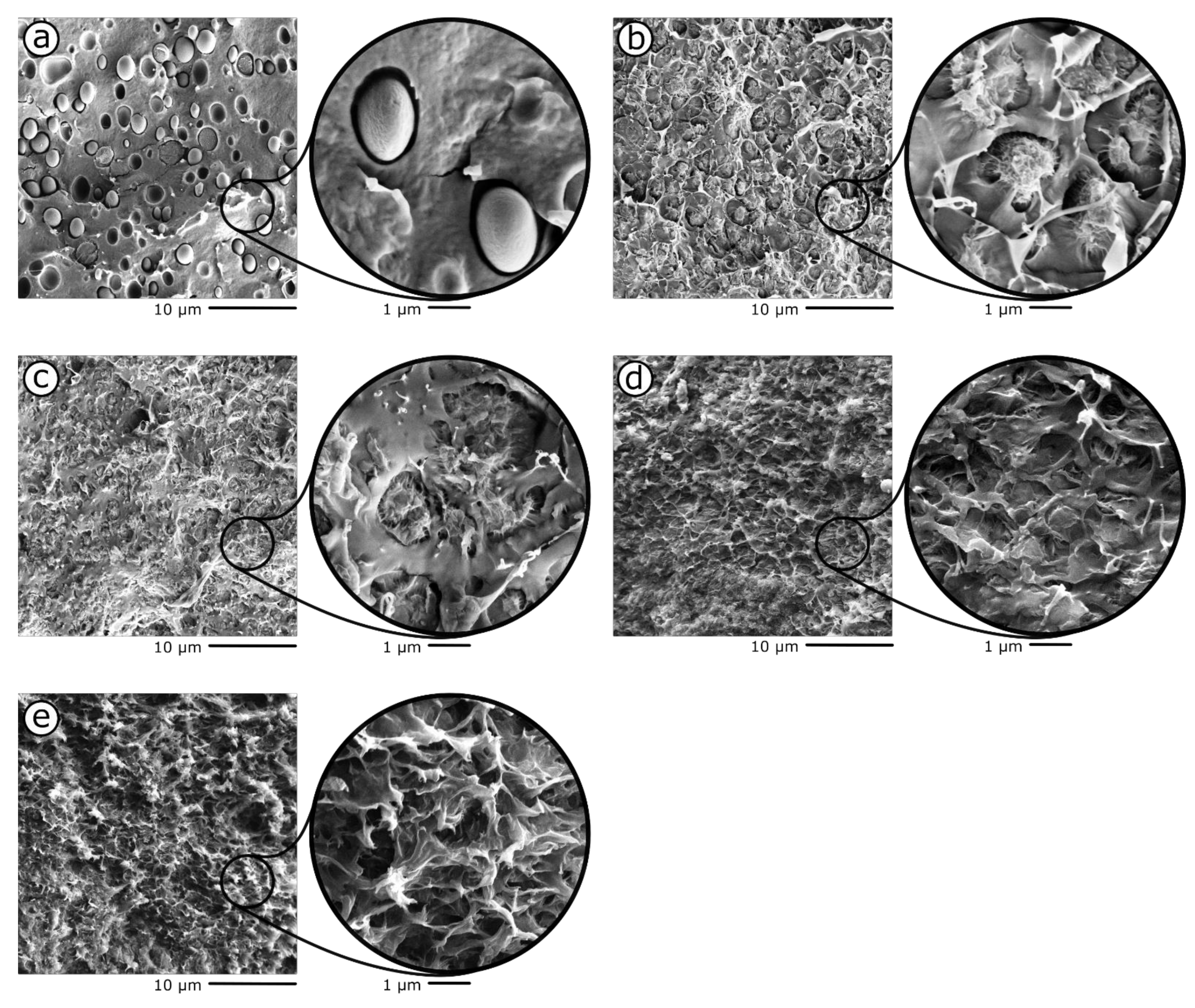
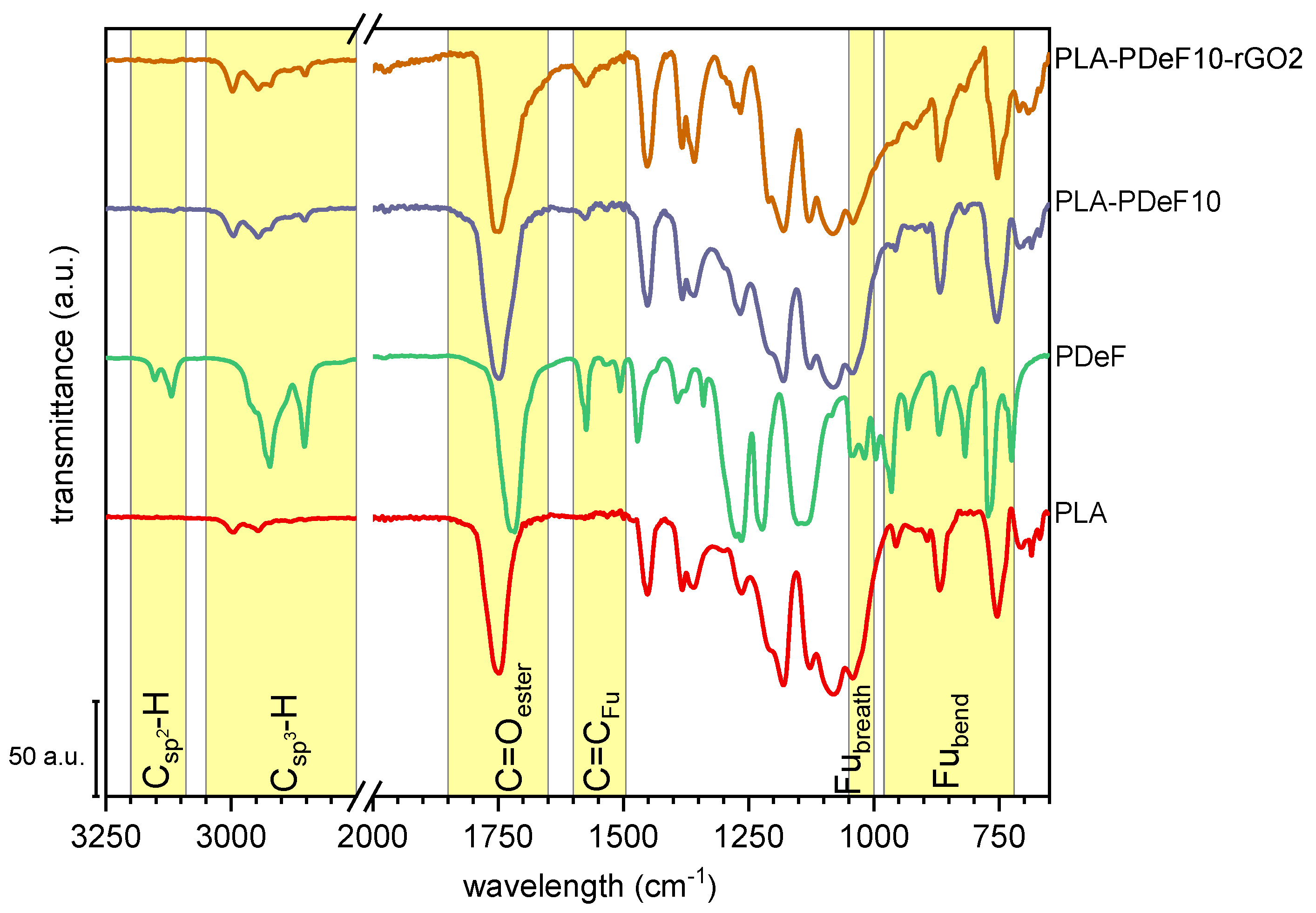
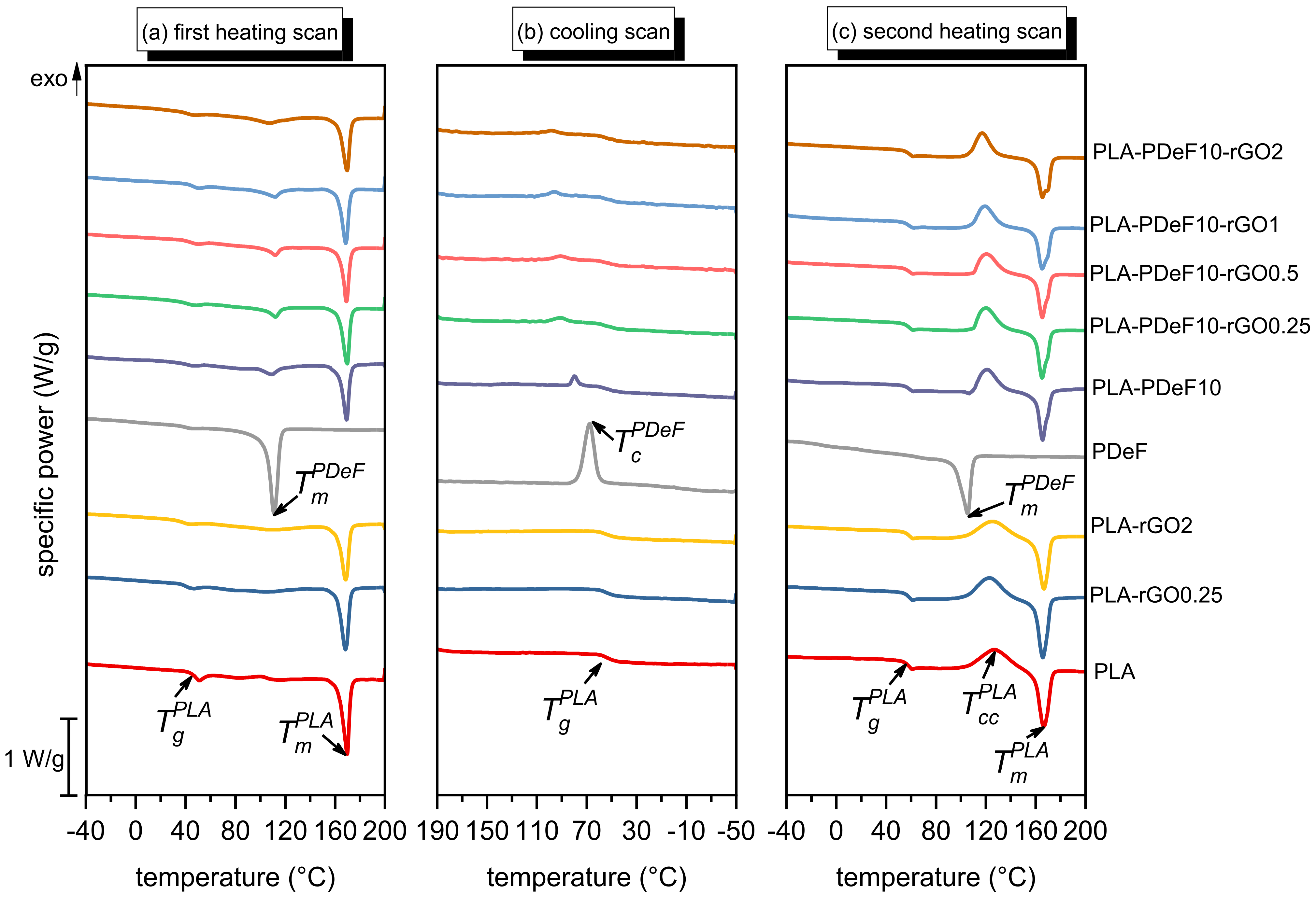
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lasprilla, A.J.; Martinez, G.A.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.; Whiteside, S.; Thomas, R.; Dharman, M.; Hughes, J.; Kim, Y.T. The effect of solvent mixture on the properties of solvent cast polylactic acid (PLA) film. J. Appl. Polym. Sci. 2012, 124, 3577–3582. [Google Scholar] [CrossRef]
- Dorigato, A.; Sebastiani, M.; Pegoretti, A.; Fambri, L. Effect of silica nanoparticles on the mechanical performances of poly(lactic acid). J. Polym. Environ. 2012, 20, 713–725. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Redhwi, H.H.; Al-Arfaj, A.A.; Achilias, D.S. Chemical Recycling of PET in the Presence of the Bio-Based Polymers, PLA, PHB and PEF: A Review. Sustainability 2021, 13, 10528. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties-From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Paszkiewicz, S.; Irska, I.; Piesowicz, E. Environmentally Friendly Polymer Blends Based on Post-Consumer Glycol-Modified Poly(Ethylene Terephthalate) (PET-G) Foils and Poly(Ethylene 2,5-Furanoate) (PEF): Preparation and Characterization. Materials 2020, 13, 2673. [Google Scholar] [CrossRef] [PubMed]
- Bera, M.; Saha, U.; Bhardwaj, A.; Maji, P.K. Reduced graphene oxide (RGO)-induced compatibilization and reinforcement of poly(vinylidene fluoride) (PVDF)-thermoplastic polyurethane (TPU) binary polymer blend. J. Appl. Polym. Sci. 2019, 136, 47010. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Chen, H.; Pyda, M.; Cebe, P. Non-isothermal crystallization of PET/PLA blends. Thermochim. Acta 2009, 492, 61–66. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chueh, J.-Y.; Tseng, H.; Huang, H.-M.; Lee, S.-Y. Preparation and characterization of biodegradable PLA polymeric blends. Biomaterials 2003, 24, 1167–1173. [Google Scholar] [CrossRef]
- Wang, D.; Lu, X.; Qu, J. Role of In situ thermal-reduced graphene oxide on the morphology and properties of biodegradable poly(Lactic acid)/poly(butylene succinate) blends. Polym. Compos. 2018, 39, 3057–3065. [Google Scholar] [CrossRef]
- Sousa, A.F.; Patrício, R.; Terzopoulou, Z.; Bikiaris, D.N.; Stern, T.; Wenger, J.; Loos, K.; Lotti, N.; Siracusa, V.; Szymczyk, A.; et al. Recommendations for replacing PET on packaging, fiber, and film materials with biobased counterparts. Green Chem. 2021, 23, 8795–8820. [Google Scholar] [CrossRef]
- Sousa, A.F.; Silvestre, A.J.D. Plastics from renewable sources as green and sustainable alternatives. Curr. Opin. Green Sustain. Chem. 2022, 33, 100557. [Google Scholar] [CrossRef]
- Pandey, S.; Dumont, M.-J.; Orsat, V.; Rodrigue, D. Biobased 2,5-furandicarboxylic acid (FDCA) and its emerging copolyesters’ properties for packaging applications. Eur. Polym. J. 2021, 160, 110778. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Smyrnioti, D.; Nikolaidis, G.N.; Tsitsimaka, I.; Christodoulou, E.; Bikiaris, D.N.; Charitopoulou, M.A.; Achilias, D.S.; Kapnisti, M.; Papageorgiou, G.Z. Sustainable Plastics from Biomass: Blends of Polyesters Based on 2,5-Furandicarboxylic Acid. Polymers 2020, 12, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terzopoulou, Z.; Papadopoulos, L.; Zamboulis, A.; Papageorgiou, D.G.; Papageorgiou, G.Z.; Bikiaris, D.N. Tuning the Properties of Furandicarboxylic Acid-Based Polyesters with Copolymerization: A Review. Polymers 2020, 12, 1209. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Guigo, N.; Tsanaktsis, V.; Exarhopoulos, S.; Bikiaris, D.N.; Sbirrazzuoli, N.; Papageorgiou, G.Z. Fast Crystallization and Melting Behavior of a Long-Spaced Aliphatic Furandicarboxylate Biobased Polyester, Poly(dodecylene 2,5-furanoate). Ind. Eng. Chem. Res. 2016, 55, 5315–5326. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Kasmi, N.; Siampani, M.; Terzopoulou, Z.N.; Bikiaris, D.N.; Achilias, D.S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Exploring Next-Generation Engineering Bioplastics: Poly(alkylene furanoate)/Poly(alkylene terephthalate) (PAF/PAT) Blends. Polymers 2019, 11, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Zhang, C.; Yang, F.; Weng, Y. Gas barrier properties of furan-based polyester films analyzed experimentally and by molecular simulations. Polymer 2021, 233, 124200. [Google Scholar] [CrossRef]
- Forestier, E.; Combeaud, C.; Guigo, N.; Corvec, G.; Pradille, C.; Sbirrazzuoli, N.; Billon, N. Comparative Analysis of the Mechanical Behaviour of PEF and PET Uniaxial Stretching Based on the Time/Temperature Superposition Principle. Polymers 2021, 13, 3295. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Tsanaktsis, V.; Papageorgiou, D.G.; Chrissafis, K.; Exarhopoulos, S.; Bikiaris, D.N. Furan-based polyesters from renewable resources: Crystallization and thermal degradation behavior of poly(hexamethylene 2,5-furan-dicarboxylate). Eur. Polym. J. 2015, 67, 383–396. [Google Scholar] [CrossRef]
- Xie, H.; Wu, L.; Li, B.-G.; Dubois, P. Biobased Poly(ethylene-co-hexamethylene 2,5-furandicarboxylate) (PEHF) Copolyesters with Superior Tensile Properties. Ind. Eng. Chem. Res. 2018, 57, 13094–13102. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, Z.; Qiu, Z. Synthesis and properties of poly(hexamethylene 2,5-furandicarboxylate-co-adipate) copolyesters. Eur. Polym. J. 2021, 161, 110860. [Google Scholar] [CrossRef]
- Fredi, G.; Dorigato, A.; Bortolotti, M.; Pegoretti, A.; Bikiaris, D.N. Mechanical and Functional Properties of Novel Biobased Poly(decylene-2,5-furanoate)/Carbon Nanotubes Nanocomposite Films. Polymers 2020, 12, 2459. [Google Scholar] [CrossRef]
- Rigotti, D.; Soccio, M.; Dorigato, A.; Gazzano, M.; Siracusa, V.; Fredi, G.; Lotti, N. Novel biobased polylactic acid/poly(pentamethylene 2,5-furanoate) blends for sustainable food packaging. ACS Sustain. Chem. Eng. 2021, 9, 13742–13750. [Google Scholar] [CrossRef]
- Fredi, G.; Rigotti, D.; Bikiaris, D.N.; Dorigato, A. Tuning thermo-mechanical properties of poly(lactic acid) films through blending with bioderived poly(alkylene furanoate)s with different alkyl chain length for sustainable packaging. Polymer 2021, 218, 123527. [Google Scholar] [CrossRef]
- Fredi, G.; Jafari, M.K.; Dorigato, A.; Bikiaris, D.N.; Checchetto, R.; Favaro, M.; Brusa, R.S.; Pegoretti, A. Multifunctionality of reduced graphene oxide in bioderived polylactide/poly(dodecylene furanoate) nanocomposite films. Molecules 2021, 26, 2398. [Google Scholar] [CrossRef]
- Ginzburg, V.V. Influence of Nanoparticles on Miscibility of Polymer Blends. A Simple Theory. Macromolecules 2005, 38, 2362–2367. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Bousmina, M. Compatibilization Efficiency of Organoclay in an Immiscible Polycarbonate/Poly(methyl methacrylate) Blend. Macromol. Rapid Commun. 2005, 26, 450–455. [Google Scholar] [CrossRef]
- Thomas, S.; Shanks, R.; Chandrasekharakurup, S. Design and Applications of Nanostructured Polymer Blends and Nanocomposite Systems; Elsevier: Oxford, UK, 2016. [Google Scholar]
- Siddiqui, T.A.J.; Ghule, B.G.; Shaikh, S.; Shinde, P.V.; Gunturu, K.C.; Zubaidha, P.K.; Yun, J.M.; O’Dwyer, C.; Mane, R.S.; Kim, K.H. Metal-free heterogeneous and mesoporous biogenic graphene-oxide nanoparticle-catalyzed synthesis of bioactive benzylpyrazolyl coumarin derivatives. RSC Adv. 2018, 8, 17373–17379. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Y.; Yu, H.Y.; Yang, L.; Abdalkarim, S.Y.H.; Chen, W.L. Enhancing long-term biodegradability and UV-shielding performances of transparent polylactic acid nanocomposite films by adding cellulose nanocrystal-zinc oxide hybrids. Int. J. Biol. Macromol. 2019, 141, 893–905. [Google Scholar] [CrossRef]
- Silva, V.; Fernandes-Junior, W.S.; Rocha, D.P.; Stefano, J.S.; Munoz, R.A.A.; Bonacin, J.A.; Janegitz, B.C. 3D-printed reduced graphene oxide/polylactic acid electrodes: A new prototyped platform for sensing and biosensing applications. Biosens. Bioelectron. 2020, 170, 112684. [Google Scholar] [CrossRef]
- Samak, N.A.; Selim, M.S.; Hao, Z.; Xing, J. Immobilized arginine/tryptophan-rich cyclic dodecapeptide on reduced graphene oxide anchored with manganese dioxide for microbial biofilm eradication. J. Hazard. Mater. 2022, 426, 128035. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.Q.; Sun, D.W.; Huang, J.H. Synergistic poly(lactic acid) photoreforming and H2 generation over ternary NixCo1-xP/reduced graphene oxide/g-C3N4 composite. Chemosphere 2022, 286, 131905. [Google Scholar] [CrossRef] [PubMed]
- Tsanaktsis, V.; Bikiaris, D.N.; Guigo, N.; Exarhopoulos, S.; Papageorgiou, D.G.; Sbirrazzuoli, N.; Papageorgiou, G.Z. Synthesis, properties and thermal behavior of poly(decylene-2,5-furanoate): A biobased polyester from 2,5-furan dicarboxylic acid. RSC Adv. 2015, 5, 74592–74604. [Google Scholar] [CrossRef]
- Davachi, S.M.; Kaffashi, B. Preparation and Characterization of Poly L-Lactide/Triclosan Nanoparticles for Specific Antibacterial and Medical Applications. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 497–508. [Google Scholar] [CrossRef]
- Perin, D.; Rigotti, D.; Fredi, G.; Papageorgiou, G.Z.; Bikiaris, D.N.; Dorigato, A. Innovative bio-based poly(lactic acid)/poly(alkylene furanoate) fiber blends for sustainable textile applications. J. Polym. Environ. 2021, 29, 3948–3963. [Google Scholar] [CrossRef]
- Perin, D.; Fredi, G.; Rigotti, D.; Lotti, N.; Dorigato, A. Sustainable textile fibers made of bioderived polylactide/poly(pentamethylene 2,5-furanoate) blends. J. Appl. Polym. Sci. 2022, 139, 51740. [Google Scholar] [CrossRef]
- Rigotti, D.; Fredi, G.; Perin, D.; Bikiaris, D.N.; Pegoretti, A.; Dorigato, A. Statistical Modeling and Optimization of the Drawing Process of Bioderived Polylactide/Poly(Dodecylene Furanoate) Wet-Spun Fibers. Polymers 2022, 14, 396. [Google Scholar] [CrossRef]
- Mofokeng, J.P.; Luyt, A.S.; Tábi, T.; Kovács, J. Comparison of injection moulded, natural fibre-reinforced composites with PP and PLA as matrices. J. Thermoplast. Compos. Mater. 2011, 25, 927–948. [Google Scholar] [CrossRef]
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.V.; Podzorova, M.V. Effect of UV Irradiation on the Structural and Dynamic Characteristics of Polylactide and Its Blends with Polyethylene. Russ. J. Phys. Chem. B 2020, 14, 167–175. [Google Scholar] [CrossRef]
- Sucinda, E.F.; Abdul Majid, M.S.; Ridzuan, M.J.M.; Cheng, E.M.; Alshahrani, H.A.; Mamat, N. Development and characterisation of packaging film from Napier cellulose nanowhisker reinforced polylactic acid (PLA) bionanocomposites. Int. J. Biol. Macromol. 2021, 187, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wu, L.; Li, B.-G.; Dubois, P. Poly(ethylene 2,5-furandicarboxylate-mb-poly(tetramethylene glycol)) multiblock copolymers: From high tough thermoplastics to elastomers. Polymer 2018, 155, 89–98. [Google Scholar] [CrossRef]
- Sanusi, O.M.; Papadopoulos, L.; Klonos, P.A.; Terzopoulou, Z.; Hocine, N.A.; Benelfellah, A.; Papageorgiou, G.Z.; Kyritsis, A.; Bikiaris, D.N. Calorimetric and Dielectric Study of Renewable Poly(hexylene 2,5-furan-dicarboxylate)-Based Nanocomposites In Situ Filled with Small Amounts of Graphene Platelets and Silica Nanoparticles. Polymers 2020, 12, 1239. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.; Sousa, A.F.; Andrade, M.; Silva, N.H.C.S.; Freire, C.S.R.; Mendes, A.; Silvestre, A.J.D. Furanoate-Based Nanocomposites: A Case Study Using Poly(Butylene 2,5-Furanoate) and Poly(Butylene 2,5-Furanoate)-co-(Butylene Diglycolate) and Bacterial Cellulose. Polymers 2018, 10, 810. [Google Scholar] [CrossRef] [Green Version]
- Gomes, M.; Gandini, A.; Silvestre, A.J.D.; Reis, B. Synthesis and characterization of poly(2,5-furan dicarboxylate)s based on a variety of diols. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3759–3768. [Google Scholar] [CrossRef]
- Li, F.; Zhang, C.; Weng, Y.; Diao, X.; Zhou, Y.; Song, X. Enhancement of Gas Barrier Properties of Graphene Oxide/Poly (Lactic Acid) Films Using a Solvent-free Method. Materials 2020, 13, 3024. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Li, Q.; Liu, Y.; Hu, G.-H.; Wu, C. Multiple melting behavior of poly (lactic acid) filled with modified carbon black. J. Polym. Sci. Part B: Polym. Phys. 2009, 47, 1971–1980. [Google Scholar] [CrossRef]
- Sanes, J.; Sanchez, C.; Pamies, R.; Aviles, M.D.; Bermudez, M.D. Extrusion of Polymer Nanocomposites with Graphene and Graphene Derivative Nanofillers: An Overview of Recent Developments. Materials 2020, 13, 549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vryonis, O.; Andritsch, T.; Vaughan, A.S.; Lewin, P.L. Effect of surfactant molecular structure on the electrical and thermal performance of epoxy/functionalized-graphene nanocomposites. Polym. Compos. 2020, 41, 2753–2767. [Google Scholar] [CrossRef]

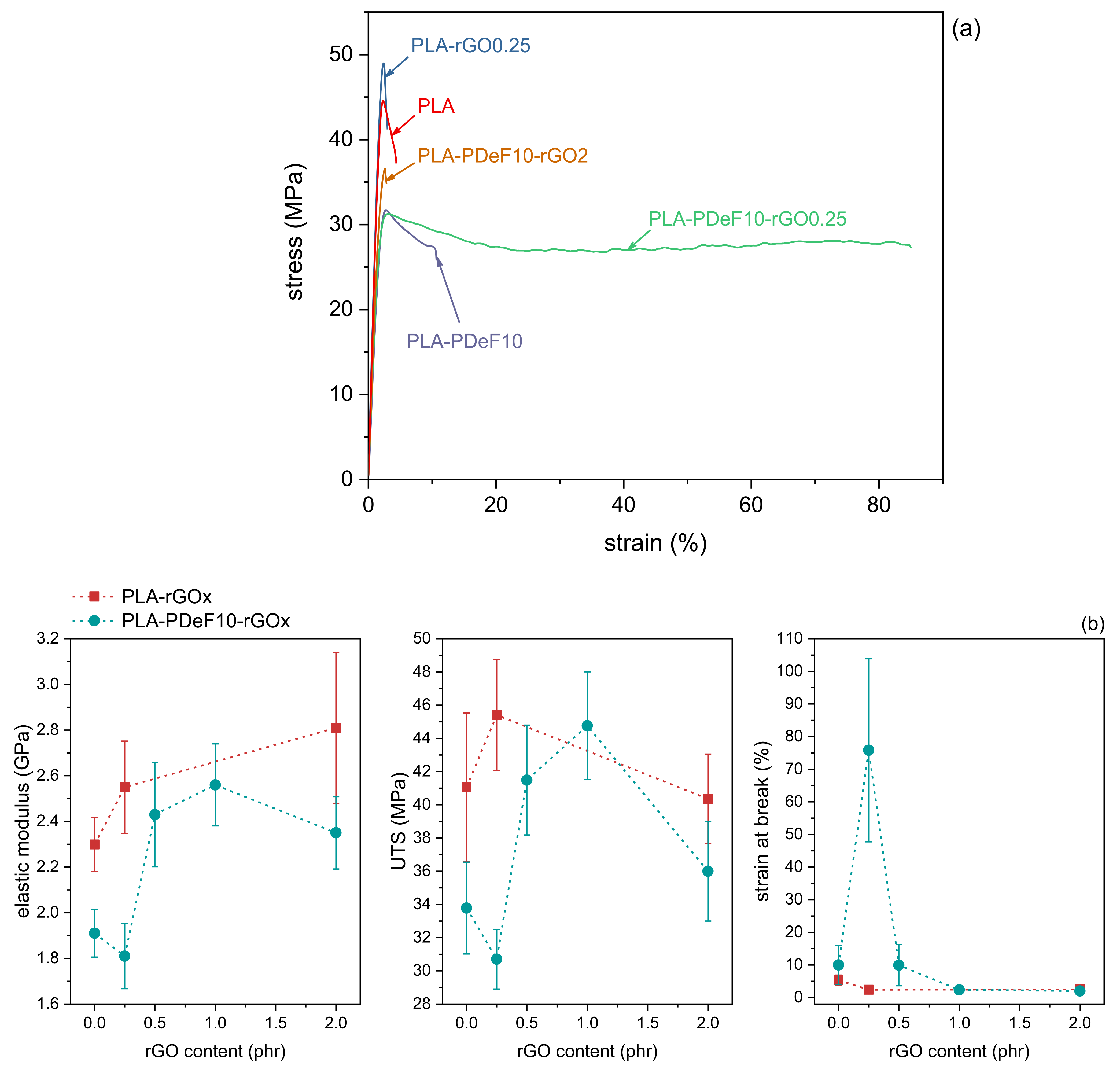
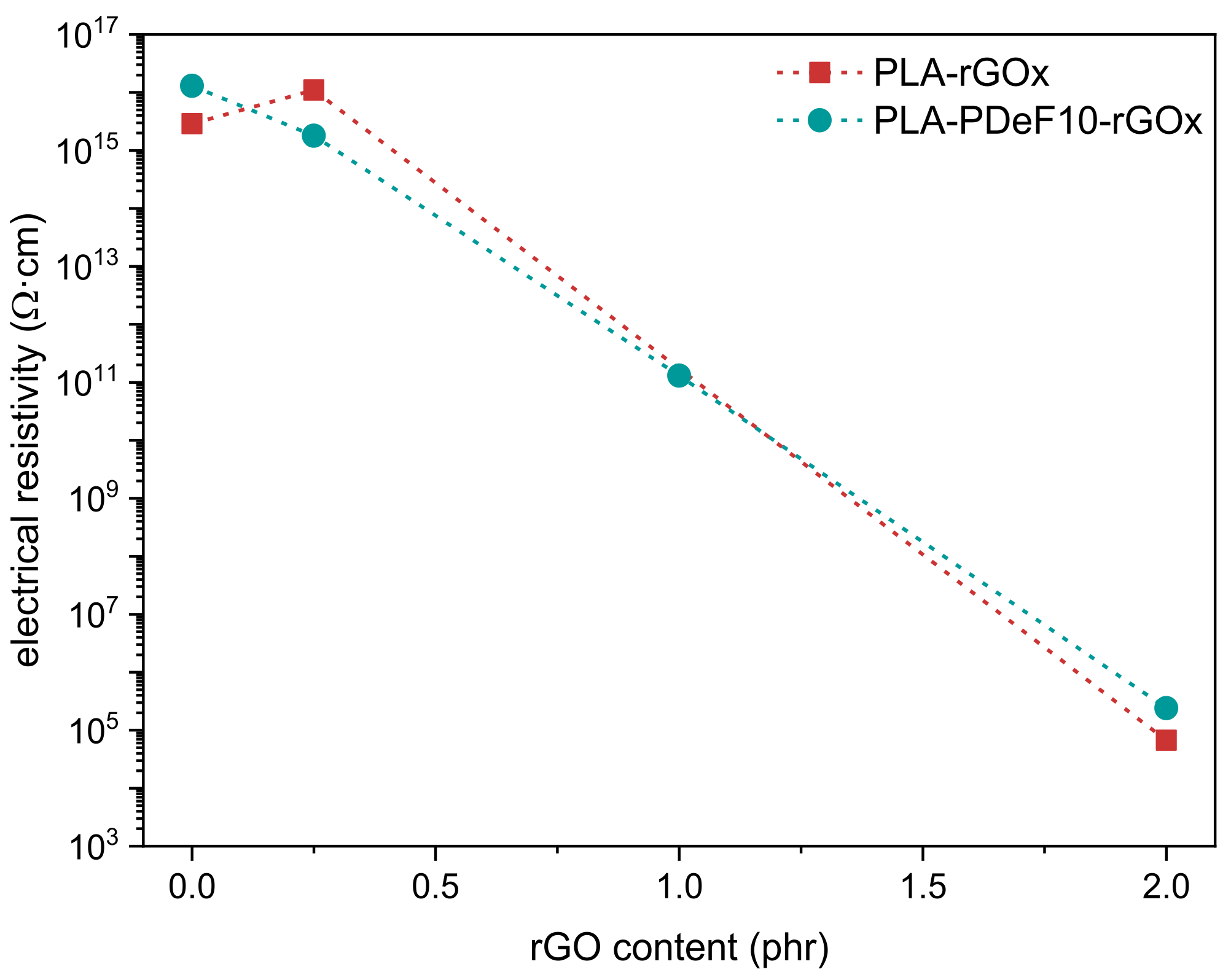
| Sample | PLA (wt%) * | PDeF (wt%) * | rGO (phr) ** |
|---|---|---|---|
| PLA | 100 | 0 | 0 |
| PLA-rGO0.25 | 100 | 0 | 0.25 |
| PLA-rGO2 | 100 | 0 | 2 |
| PLA-PDeF10 | 90 | 10 | 0 |
| PLA-PDeF10-rGO0.25 | 90 | 10 | 0.25 |
| PLA-PDeF10-rGO0.5 | 90 | 10 | 0.5 |
| PLA-PDeF10-rGO1 | 90 | 10 | 1 |
| PLA-PDeF10-rGO2 | 90 | 10 | 2 |
| PLA | PLA-rGO0.25 | PLA-rGO2 | PDeF | PLA-PDeF10 | PLA-PDeF10-rGO0.25 | PLA-PDeF10-rGO0.5 | PLA-PDeF10-rGO1 | PLA-PDeF10-rGO2 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| h1 | (°C) | 40.9 | 40.0 | 39.2 | – | 41.5 | 40.8 | 43.3 | 45.8 | 40.6 |
| (°C) | – | – | – | 110.2 | 109.1 | 111.8 | 111.9 | 111.9 | 106.9 | |
| (J/g) | – | – | – | 78.6 | 7.0 | 8.8 | 6.9 | 10.9 | 10.0 | |
| (°C) | 169.4 | 168.1 | 168.3 | – | 168.4 | 169.2 | 169.8 | 169.0 | 169.0 | |
| (J/g) | 38.7 | 36.1 | 33.1 | – | 29.4 | 30.7 | 27.8 | 28.9 | 30.6 | |
| (%) | 41.3 | 38.6 | 36.0 | – | 34.9 | 36.4 | 33.0 | 34.3 | 36.2 | |
| (%) | – | – | – | 51.4 | 45.8 | 57.7 | 45.3 | 72.0 | 66.7 | |
| c | (°C) | – | – | – | 68.4 | 80.1 | 90.7 | 91.6 | 96.7 | 98.6 |
| (J/g) | – | – | – | 50.1 | 4.2 | 4.0 | 4.9 | 5.8 | 4.7 | |
| h2 | (°C) | 57.4 | 57.9 | 57.9 | – | 58.5 | 58.5 | 58.1 | 57.9 | 58.1 |
| (°C) | – | – | – | 110.4 | – | – | – | – | – | |
| (J/g) | – | – | – | 49.1 | – | – | – | – | – | |
| (°C) | 126.1 | 122.1 | 125.1 | – | 120.9 | 119.9 | 120.2 | 119.1 | 117.1 | |
| (J/g) | 38.0 | 37.7 | 32.7 | – | 32.1 | 30.4 | 25.6 | 29.5 | 26.7 | |
| (°C) | 166.1 | 165.4 | 166.4 | – | 165.5 | 164.5 | 164.6 | 164.8 | 165.1 | |
| (J/g) | 40.2 | 42.6 | 37.7 | – | 34.1 | 32.1 | 28.9 | 30.5 | 31.2 | |
| (%) | 2.3 | 5.2 | 5.4 | – | 2.4 | 2.0 | 3.9 | 1.2 | 5.3 | |
| (%) | – | – | – | 32.1 | – | – | – | – | – |
| Sample | |||
|---|---|---|---|
| PLA | 93.9 | 320.5 | 343.9 |
| PLA-rGO0.25 | 95.6 | 329.5 | 362.5 |
| PLA-rGO2 | 94.5 | 331.4 | 360.9 |
| PLA-PDeF10 | 94.8 | 324.9 | 340.8 |
| PLA-PDeF10-rGO0.25 | 95.1 | 322.6 | 346.0 |
| PLA-PDeF10-rGO0.5 | 95.2 | 315.3 | 352.1 |
| PLA-PDeF10-rGO1 | 93.9 | 320.5 | 343.9 |
| PLA-PDeF10-rGO2 | 92.6 | 315.4 | 356.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fredi, G.; Karimi Jafari, M.; Dorigato, A.; Bikiaris, D.N.; Pegoretti, A. Improving the Thermomechanical Properties of Poly(lactic acid) via Reduced Graphene Oxide and Bioderived Poly(decamethylene 2,5-furandicarboxylate). Materials 2022, 15, 1316. https://doi.org/10.3390/ma15041316
Fredi G, Karimi Jafari M, Dorigato A, Bikiaris DN, Pegoretti A. Improving the Thermomechanical Properties of Poly(lactic acid) via Reduced Graphene Oxide and Bioderived Poly(decamethylene 2,5-furandicarboxylate). Materials. 2022; 15(4):1316. https://doi.org/10.3390/ma15041316
Chicago/Turabian StyleFredi, Giulia, Mahdi Karimi Jafari, Andrea Dorigato, Dimitrios N. Bikiaris, and Alessandro Pegoretti. 2022. "Improving the Thermomechanical Properties of Poly(lactic acid) via Reduced Graphene Oxide and Bioderived Poly(decamethylene 2,5-furandicarboxylate)" Materials 15, no. 4: 1316. https://doi.org/10.3390/ma15041316
APA StyleFredi, G., Karimi Jafari, M., Dorigato, A., Bikiaris, D. N., & Pegoretti, A. (2022). Improving the Thermomechanical Properties of Poly(lactic acid) via Reduced Graphene Oxide and Bioderived Poly(decamethylene 2,5-furandicarboxylate). Materials, 15(4), 1316. https://doi.org/10.3390/ma15041316










