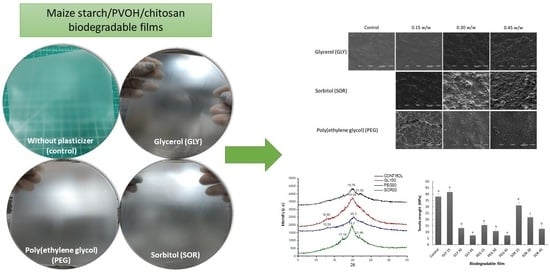
Effect of Plasticizer Content on Mechanical and Water Vapor Permeability of Maize Starch/PVOH/Chitosan Composite Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Filmogenic Solutions
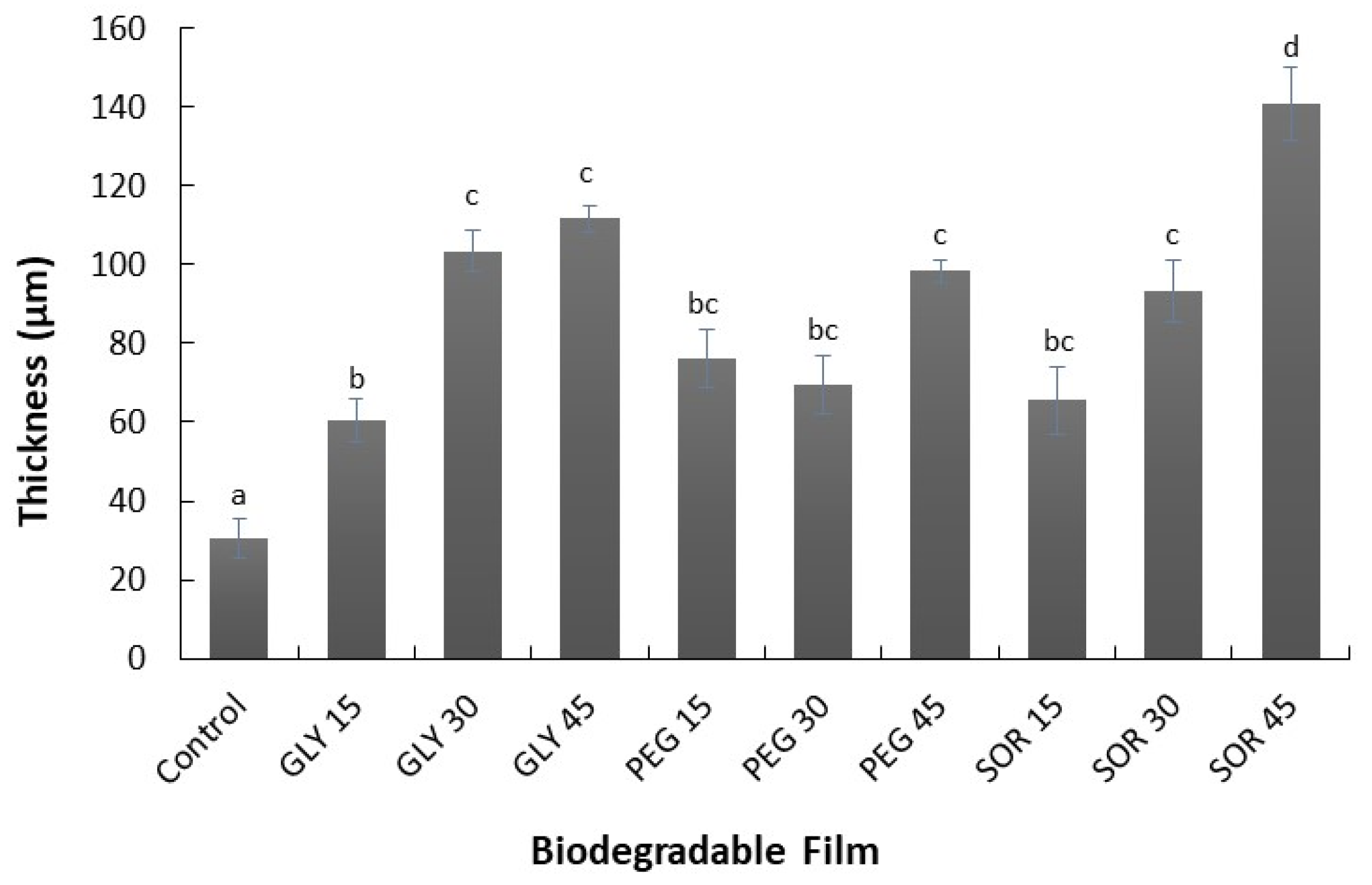
2.3. Thickness
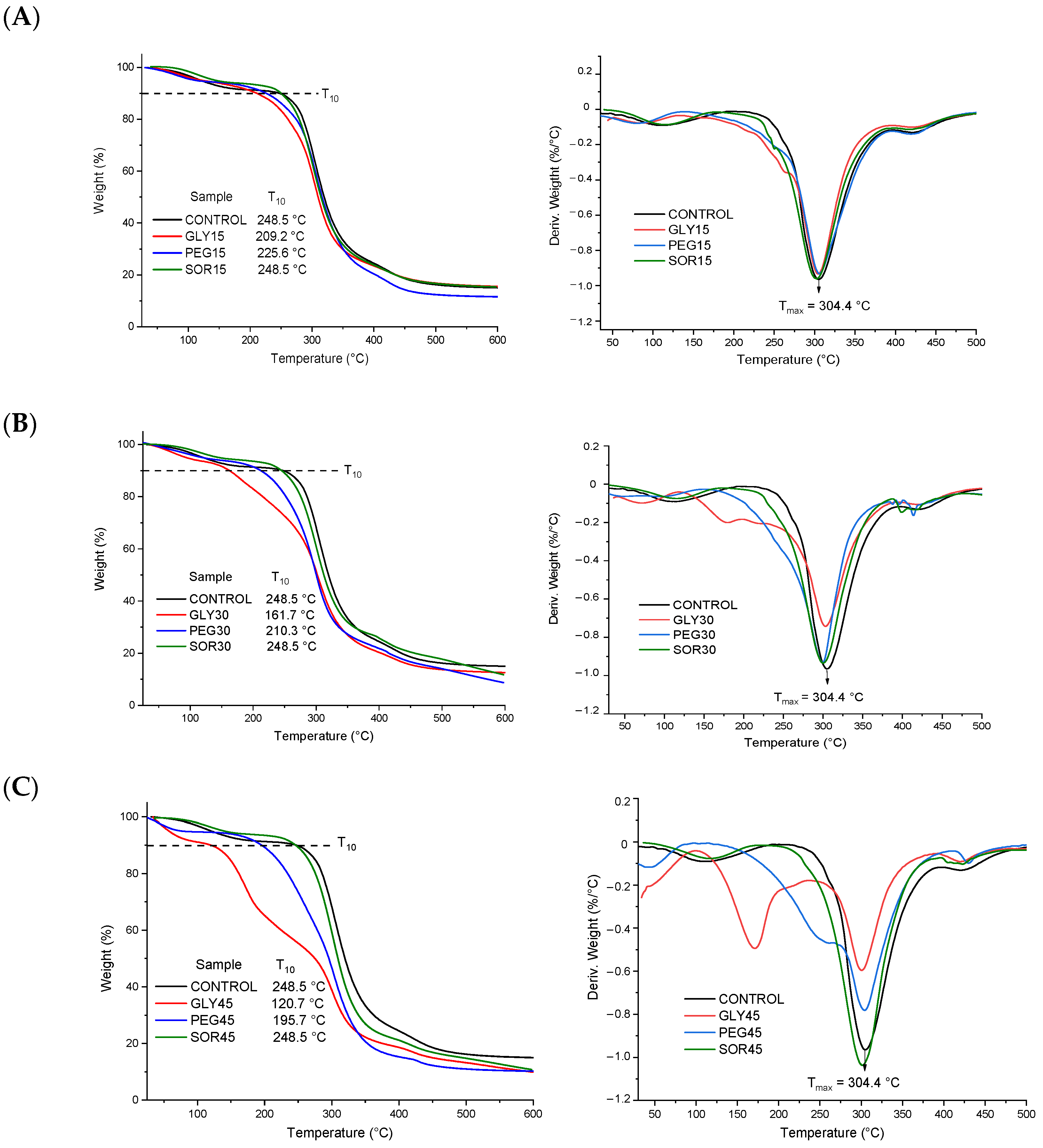
2.4. Thermogravimetric Analysis
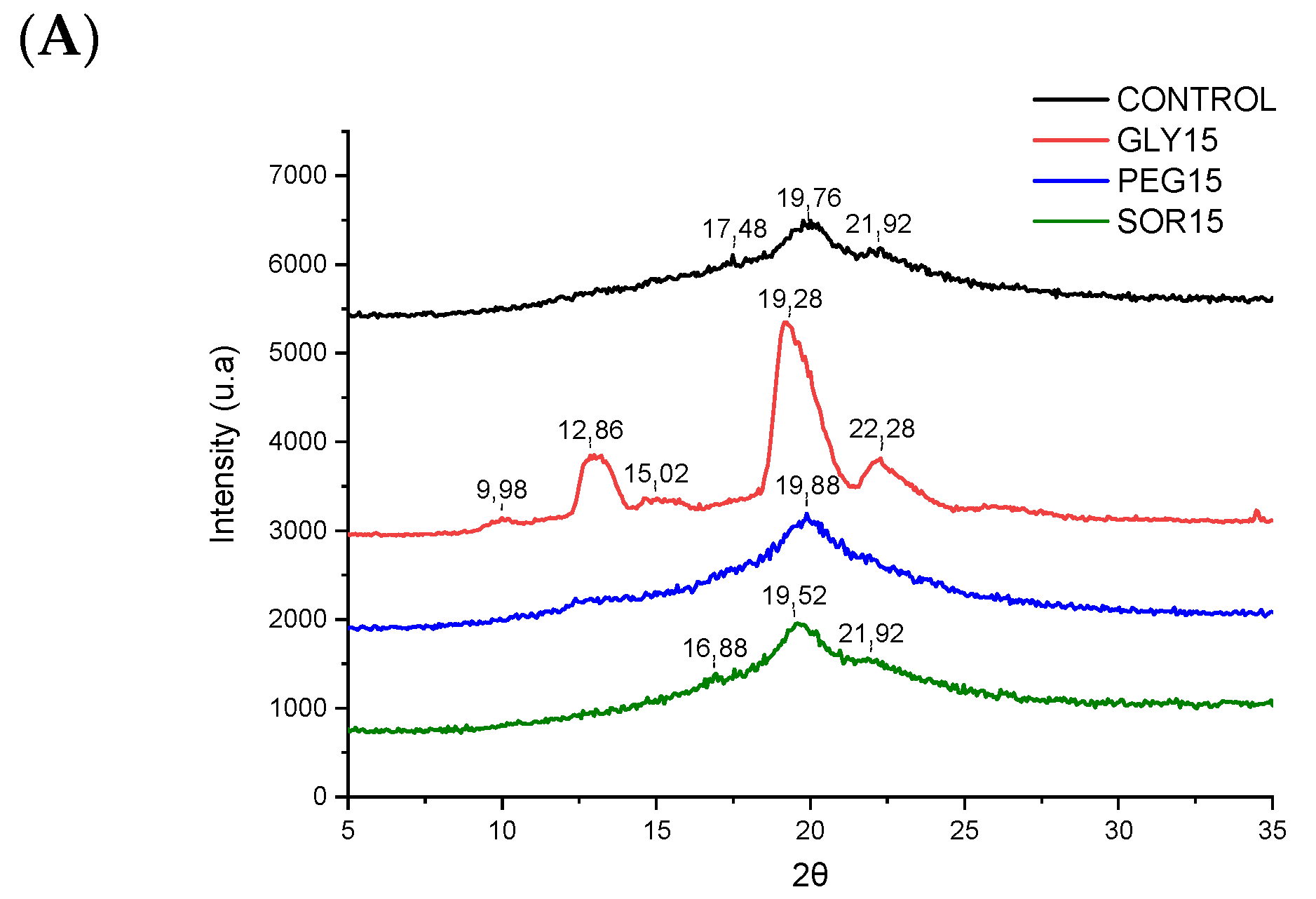
2.5. X-ray Diffraction (XRD) Analysis
2.6. Morphology by SEM
2.7. Mechanical Properties
2.8. Water Vapor Permeability (WVP)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Thickness
3.2. Thermogravimetric Analysis (TGA)
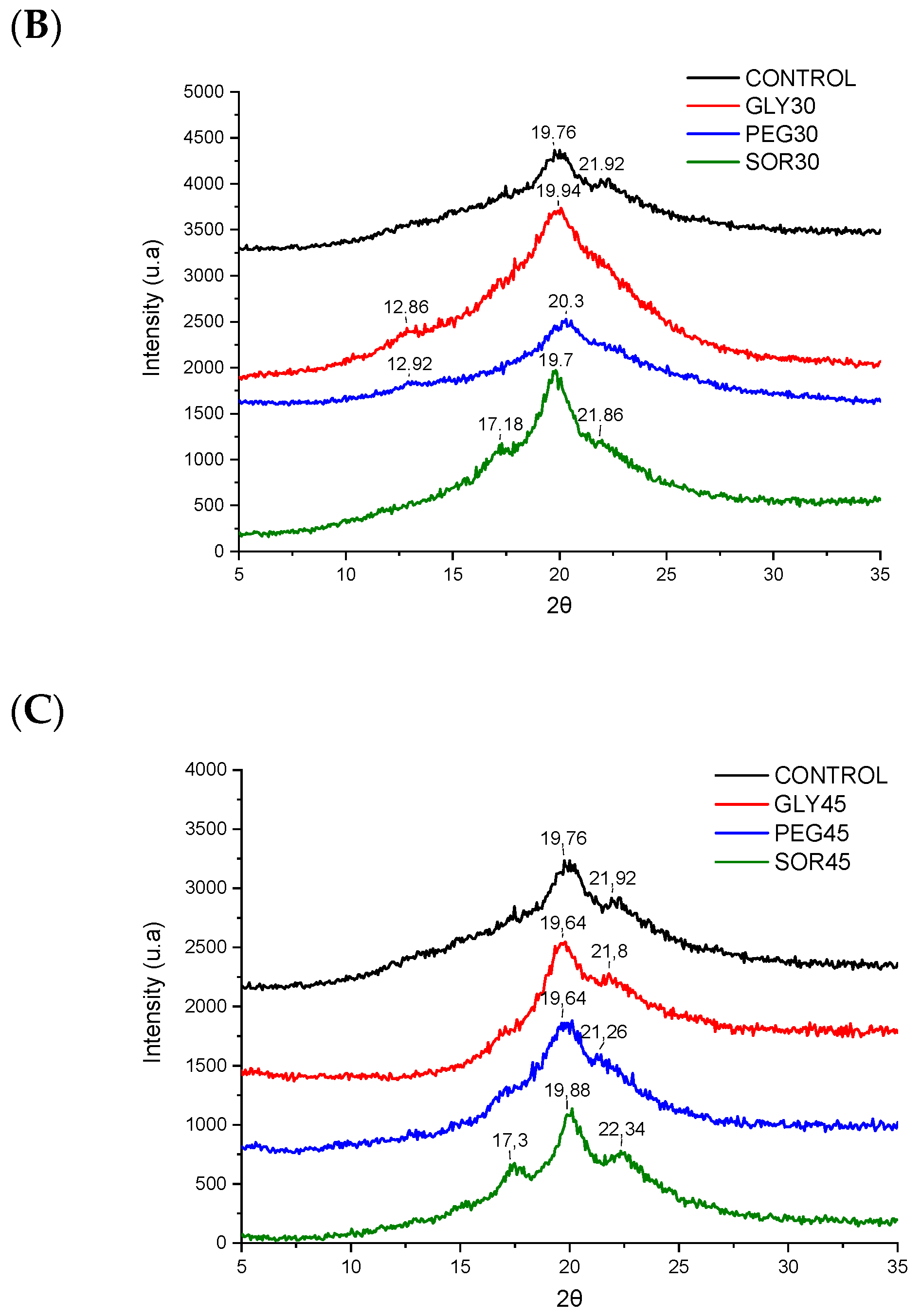
3.3. X-ray Diffraction (XRD)
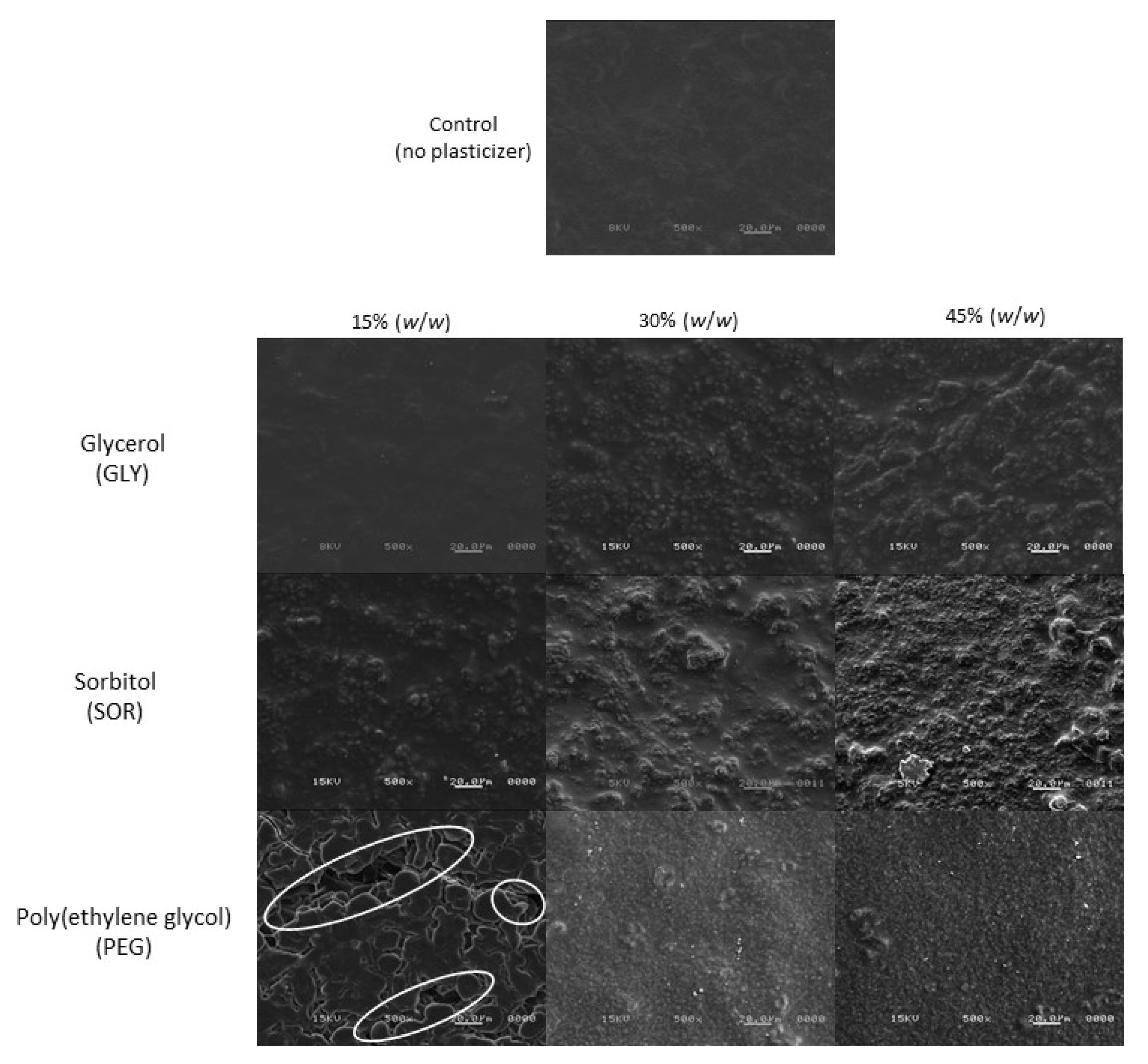
3.4. Morphology by SEM
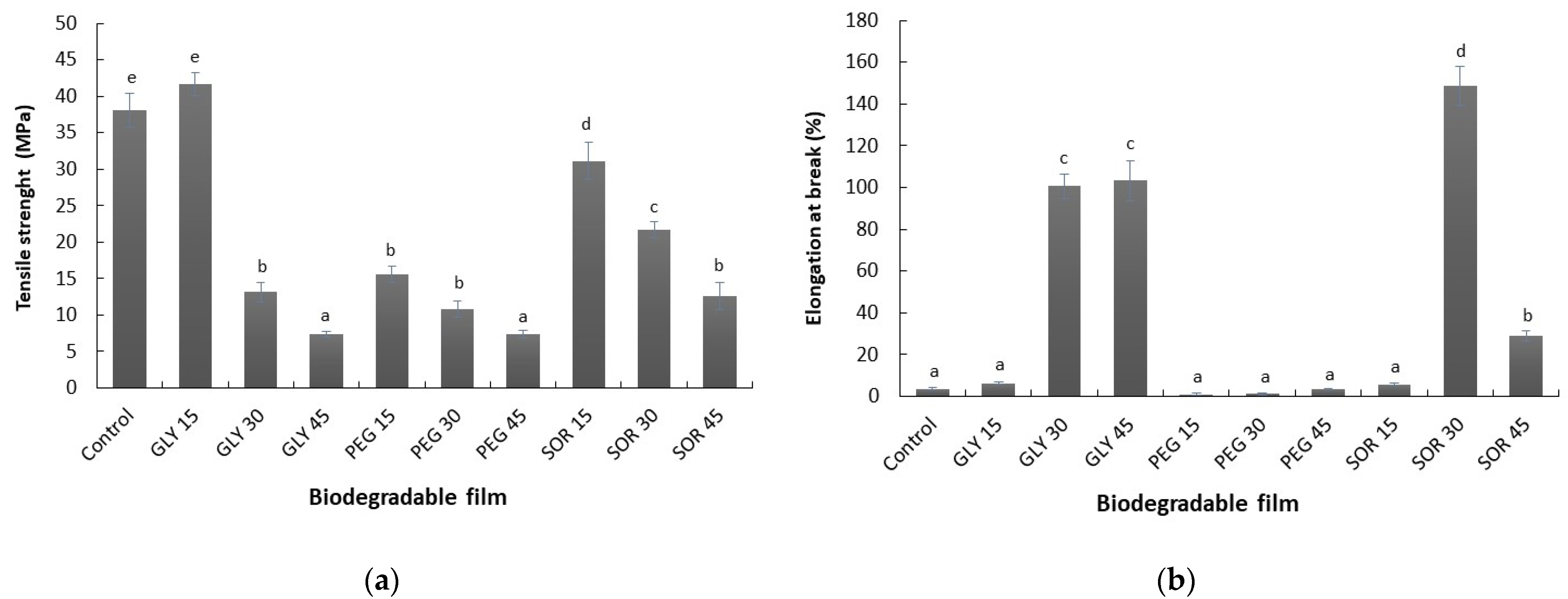
3.5. Mechanical Behavior
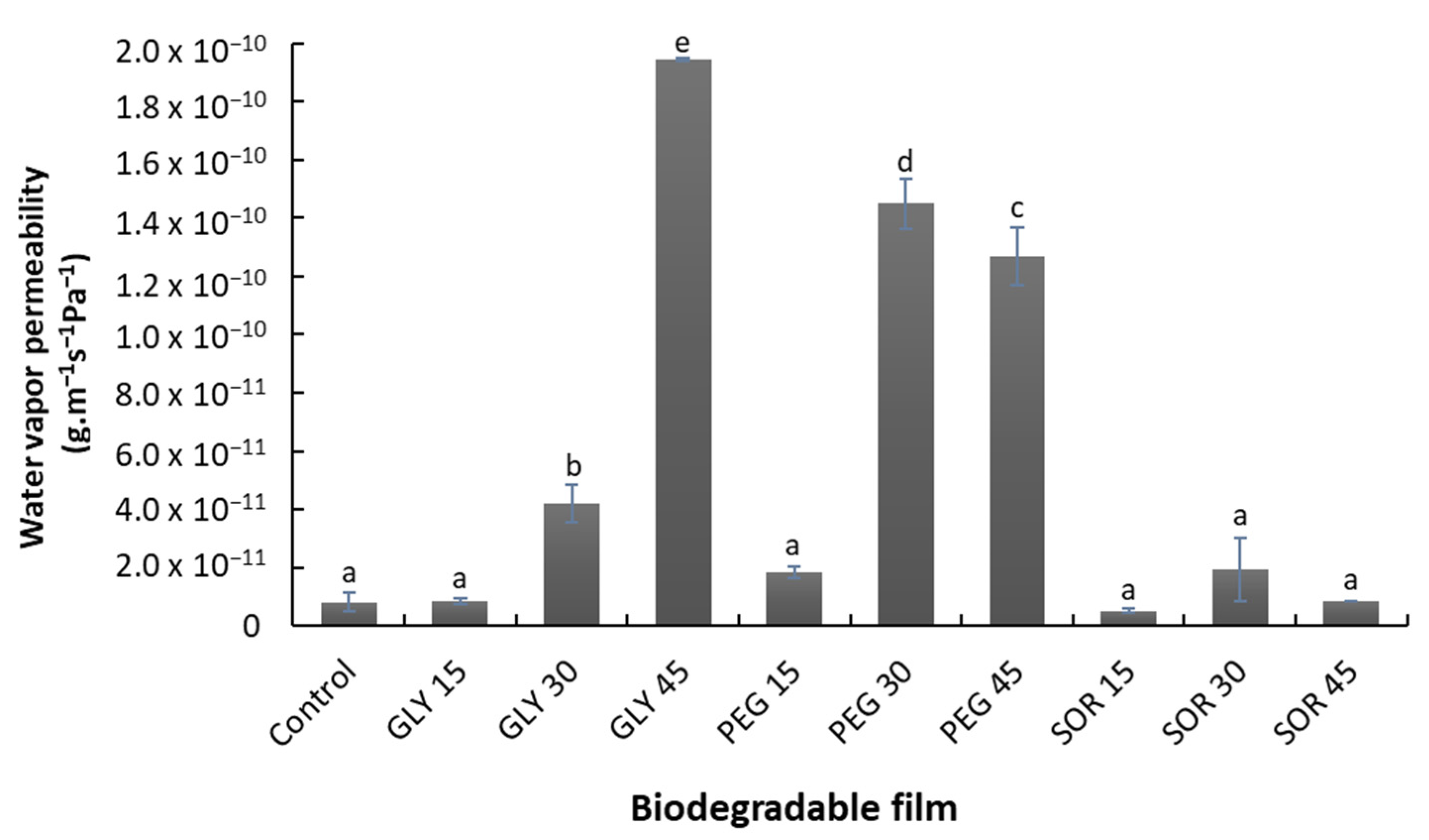
3.6. Gas Permeability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ONU. Plásticos de un solo USO: Una Hoja de Ruta Para la Sostenibilidad; Programa de las Naciones Unidas para el Medio Ambiente: Nairobi, Kenia, 2018. [Google Scholar]
- Ruggeri, E.; Farè, S.; De Nardo, L.; Marelli, B. Edible Biopolymers for Food Preservation. In Sustainable Food Packaging Technology; Athanassiou, A., Ed.; WILEY-VCH GmbH: Weinheim, Germany, 2021; pp. 57–105. [Google Scholar] [CrossRef]
- Luo, Q.; Hossen, M.A.; Zeng, Y.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Gelatin-based composite films and their application in food packaging: A review. J. Food Eng. 2022, 313, 110762. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Gómez-Aldapa, C.A.; Velazquez, G.; Gutierrez, M.C.; Rangel-Vargas, E.; Castro-Rosas, J.; Aguirre-Loredo, R.Y. Effect of polyvinyl alcohol on the physicochemical properties of biodegradable starch films. Mater. Chem. Phys. 2020, 239, 122027. [Google Scholar] [CrossRef]
- Calambas, H.L.; Fonseca, A.; Adames, D.; Aguirre-Loredo, Y.; Caicedo, C. Physical-Mechanical Behavior and Water-Barrier Properties of Biopolymers-Clay Nanocomposites. Molecules 2021, 26, 6734. [Google Scholar] [CrossRef]
- Dangaran, K.; Tomasula, P.M.; Qi, P. Structure and Function of Protein-Based Edible Films and Coatings. In Edible Films and Coatings for Food Applications; Huber, K.C., Embuscado, M.E., Eds.; Springer: New York, NY, USA, 2009; pp. 25–56. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Plasticizers in edible films and coatings. In Innovations in Food Packaging; Han, J.H., Ed.; Academic Press: London, UK, 2005; pp. 403–433. [Google Scholar] [CrossRef]
- Schmeling, N.; Konietzny, R.; Sieffert, D.; Rölling, P.; Staudt, C. Functionalized copolyimide membranes for the separation of gaseous and liquid mixtures. Beilstein J. Org. Chem. 2010, 6, 789–800. [Google Scholar] [CrossRef]
- Höfer, R.; Hinrichs, K. Additives for the Manufacture and Processing of Polymers. In Polymers—Opportunities and Risks II: Sustainability, Product Design and Processing; Eyerer, P., Weller, M., Hübner, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 97–145. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Plasticizer effect on mechanical properties of β-lactoglobulin films. J. Food Eng. 2001, 50, 149–155. [Google Scholar] [CrossRef]
- Razavi, S.M.A.; Mohammad Amini, A.; Zahedi, Y. Characterisation of a new biodegradable edible film based on sage seed gum: Influence of plasticiser type and concentration. Food Hydrocoll. 2015, 43, 290–298. [Google Scholar] [CrossRef]
- Jiménez-Regalado, E.J.; Caicedo, C.; Fonseca-García, A.; Rivera-Vallejo, C.C.; Aguirre-Loredo, R.Y. Preparation and Physicochemical Properties of Modified Corn Starch—Chitosan Biodegradable Films. Polymers 2021, 13, 4431. [Google Scholar] [CrossRef]
- Fonseca-García, A.; Caicedo, C.; Jiménez-Regalado, E.J.; Morales, G.; Aguirre-Loredo, R.Y. Effects of Poloxamer Content and Storage Time of Biodegradable Starch-Chitosan Films on Its Thermal, Structural, Mechanical, and Morphological Properties. Polymers 2021, 13, 2341. [Google Scholar] [CrossRef]
- ASTM. E96. Standard Test Methods for Water Vapor Transmission of Materials; ASTM International: West Conshohocken, PA. USA, 2002. [Google Scholar]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of plasticizer type and concentration on physical properties of biodegradable films based on sugar palm (arenga pinnata) starch for food packaging. J. Food Sci. Technol. 2016, 53, 326–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abera, G.; Woldeyes, B.; Demash, H.D.; Miyake, G. The effect of plasticizers on thermoplastic starch films developed from the indigenous Ethiopian tuber crop Anchote (Coccinia abyssinica) starch. Int. J. Biol. Macromol. 2020, 155, 581–587. [Google Scholar] [CrossRef]
- Caicedo, C.; Aguirre Loredo, R.Y.; Fonseca García, A.; Ossa, O.H.; Vázquez Arce, A.; Calambás Pulgarin, H.L.; Ávila Torres, Y. Rheological, Thermal, Superficial, and Morphological Properties of Thermoplastic Achira Starch Modified with Lactic Acid and Oleic Acid. Molecules 2019, 24, 4433. [Google Scholar] [CrossRef] [Green Version]
- Tiozon, R.J.N.; Bonto, A.P.; Sreenivasulu, N. Enhancing the functional properties of rice starch through biopolymer blending for industrial applications: A review. Int. J. Biol. Macromol. 2021, 192, 100–117. [Google Scholar] [CrossRef]
- Cheetham, N.W.H.; Tao, L. Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohydr. Polym. 1998, 36, 277–284. [Google Scholar] [CrossRef]
- van Soest, J.J.G.; Hulleman, S.H.D.; de Wit, D.; Vliegenthart, J.F.G. Crystallinity in starch bioplastics. Ind. Crops Prod. 1996, 5, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Srichuwong, S.; Sunarti, T.C.; Mishima, T.; Isono, N.; Hisamatsu, M. Starches from different botanical sources I: Contribution of amylopectin fine structure to thermal properties and enzyme digestibility. Carbohydr. Polym. 2005, 60, 529–538. [Google Scholar] [CrossRef]
- Corradini, E.; Carvalho, A.J.F.d.; Curvelo, A.A.d.S.; Agnelli, J.A.M.; Mattoso, L.H.C. Preparation and characterization of thermoplastic starch/zein blends. Mater. Res. 2007, 10, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Popescu, M.-C.; Dogaru, B.-I.; Goanta, M.; Timpu, D. Structural and morphological evaluation of CNC reinforced PVA/Starch biodegradable films. Int. J. Biol. Macromol. 2018, 116, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-García, A.; Jiménez-Regalado, E.J.; Aguirre-Loredo, R.Y. Preparation of a novel biodegradable packaging film based on corn starch-chitosan and poloxamers. Carbohydr. Polym. 2021, 251, 117009. [Google Scholar] [CrossRef] [PubMed]
- Kahvand, F.; Fasihi, M. Plasticizing and anti-plasticizing effects of polyvinyl alcohol in blend with thermoplastic starch. Int. J. Biol. Macromol. 2019, 140, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Martins da Costa, J.C.; Lima Miki, K.S.; da Silva Ramos, A.; Teixeira-Costa, B.E. Development of biodegradable films based on purple yam starch/chitosan for food application. Heliyon 2020, 6, e03718. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.; Nakayama, A.; Aiba, S.-i. Edible films made from hydroxypropyl starch and gelatin and plasticized by polyols and water. Carbohydr. Polym. 1998, 36, 105–119. [Google Scholar] [CrossRef]
- Ibrahim, M.I.J.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M. Physical, thermal, morphological, and tensile properties of cornstarch-based films as affected by different plasticizers. Int. J. Food Prop. 2019, 22, 925–941. [Google Scholar] [CrossRef]
- Ma, X.; Qiao, C.; Wang, X.; Yao, J.; Xu, J. Structural characterization and properties of polyols plasticized chitosan films. Int. J. Biol. Macromol. 2019, 135, 240–245. [Google Scholar] [CrossRef]
- Honary, S.; Golkar, M. Effect of Polymer Grade and Plasticizer Molecular Weights on Viscoelastic Behavior of Coating Solutions. Iran. J. Pharm. Res. 2010, 2, 125–127. [Google Scholar] [CrossRef]
- Gheribi, R.; Puchot, L.; Verge, P.; Jaoued-Grayaa, N.; Mezni, M.; Habibi, Y.; Khwaldia, K. Development of plasticized edible films from Opuntia ficus-indica mucilage: A comparative study of various polyol plasticizers. Carbohydr. Polym. 2018, 190, 204–211. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Tessaro, L.; Ramos, A.P.; Tapia-Blácido, D.R. Which plasticizer is suitable for films based on babassu starch isolated by different methods? Food Hydrocoll. 2019, 89, 143–152. [Google Scholar] [CrossRef]
- Huntrakul, K.; Harnkarnsujarit, N. Effects of plasticizers on water sorption and aging stability of whey protein/carboxy methyl cellulose films. J. Food Eng. 2020, 272, 109809. [Google Scholar] [CrossRef]
- Liang, T.; Wang, L. Preparation and characterization of a novel edible film based on Artemisia sphaerocephala Krasch. gum: Effects of type and concentration of plasticizers. Food Hydrocoll. 2018, 77, 502–508. [Google Scholar] [CrossRef]
- Aguirre-Loredo, R.Y.; Rodríguez-Hernández, A.I.; Morales-Sánchez, E.; Gómez-Aldapa, C.A.; Velazquez, G. Effect of equilibrium moisture content on barrier, mechanical and thermal properties of chitosan films. Food Chem. 2016, 196, 560–566. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Z.; Zhang, L.; Wang, X.; Li, L. Effects of plasticizer type and concentration on rheological, physico-mechanical and structural properties of chitosan/zein film. Int. J. Biol. Macromol. 2020, 143, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Barjas, G.; Nesic, A.; Bravo-Arrepol, G.; Rodríguez-Llamazares, S.; Valdés, O.; Banerjee, A.; Castaño, J.; Delattre, C. Bioactive Pectin-Murta (Ugni molinae T.) Seed Extract Films Reinforced with Chitin Fibers. Molecules 2021, 26, 7477. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caicedo, C.; Díaz-Cruz, C.A.; Jiménez-Regalado, E.J.; Aguirre-Loredo, R.Y. Effect of Plasticizer Content on Mechanical and Water Vapor Permeability of Maize Starch/PVOH/Chitosan Composite Films. Materials 2022, 15, 1274. https://doi.org/10.3390/ma15041274
Caicedo C, Díaz-Cruz CA, Jiménez-Regalado EJ, Aguirre-Loredo RY. Effect of Plasticizer Content on Mechanical and Water Vapor Permeability of Maize Starch/PVOH/Chitosan Composite Films. Materials. 2022; 15(4):1274. https://doi.org/10.3390/ma15041274
Chicago/Turabian StyleCaicedo, Carolina, Claudio Alonso Díaz-Cruz, Enrique Javier Jiménez-Regalado, and Rocio Yaneli Aguirre-Loredo. 2022. "Effect of Plasticizer Content on Mechanical and Water Vapor Permeability of Maize Starch/PVOH/Chitosan Composite Films" Materials 15, no. 4: 1274. https://doi.org/10.3390/ma15041274
APA StyleCaicedo, C., Díaz-Cruz, C. A., Jiménez-Regalado, E. J., & Aguirre-Loredo, R. Y. (2022). Effect of Plasticizer Content on Mechanical and Water Vapor Permeability of Maize Starch/PVOH/Chitosan Composite Films. Materials, 15(4), 1274. https://doi.org/10.3390/ma15041274