Microstructure and Phase Evolution Characteristics of the In Situ Synthesis of TiC-Reinforced AZ91D Magnesium Matrix Composites
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
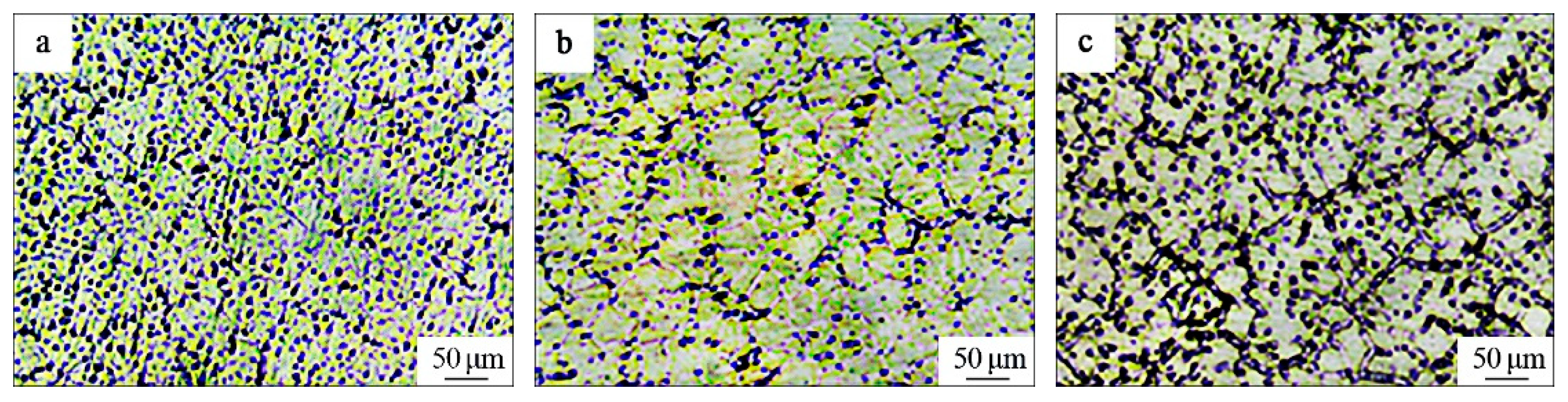
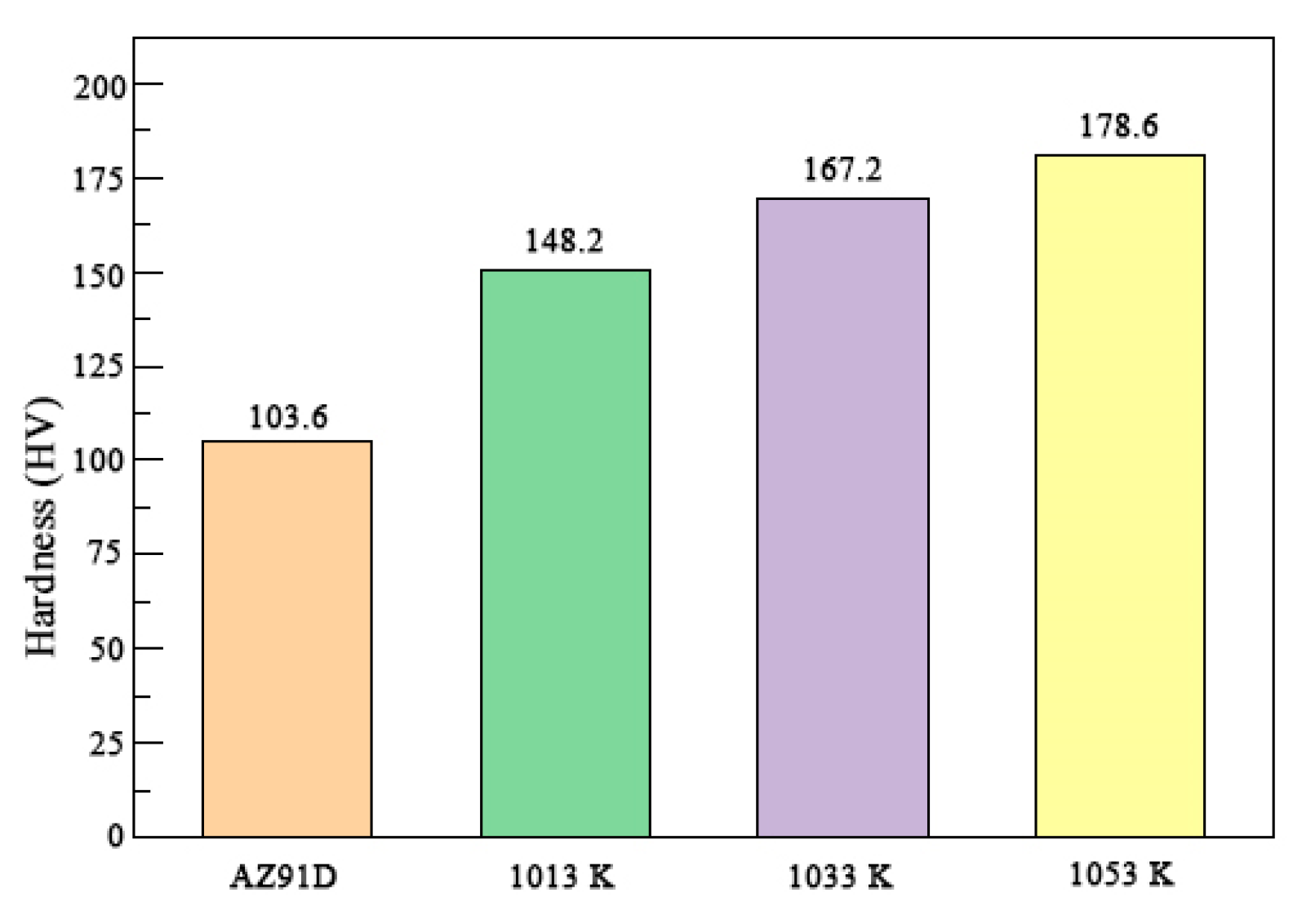
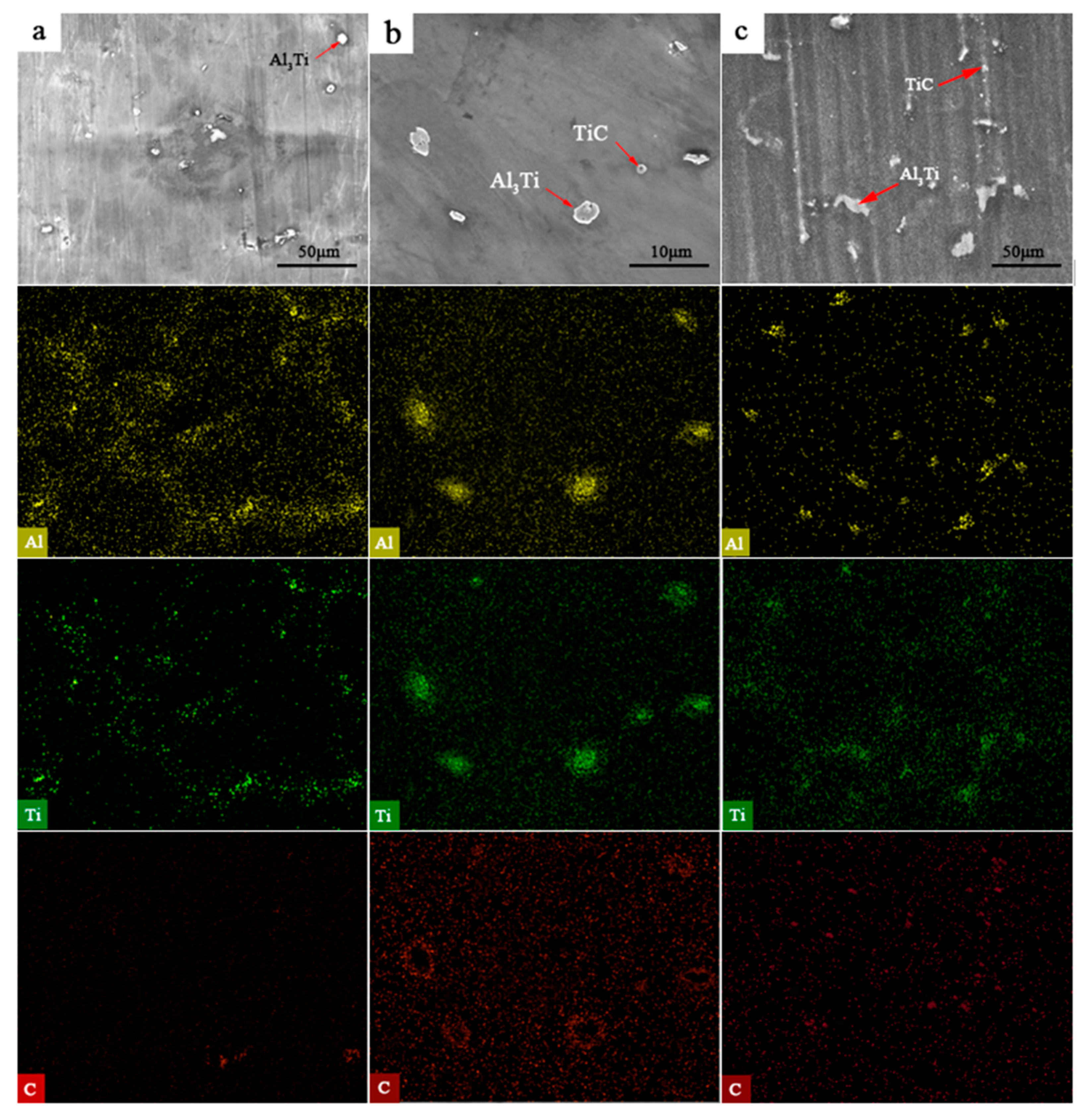
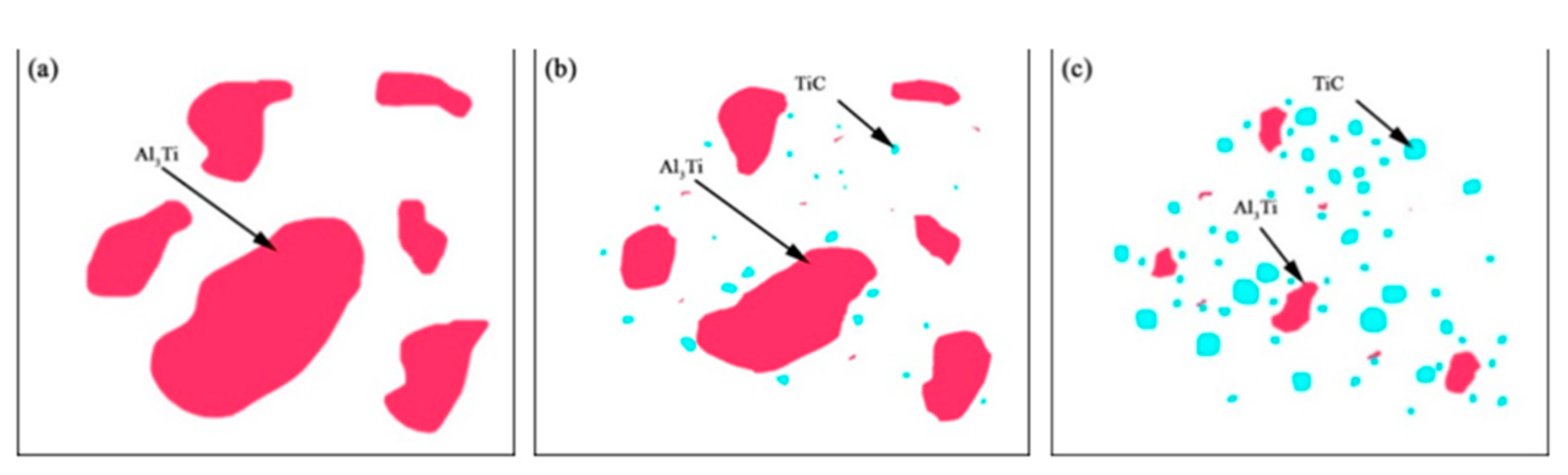
3.1. Microstructure and Hardness of the TiC-Reinforced AZ91D Magnesium Matrix Composites
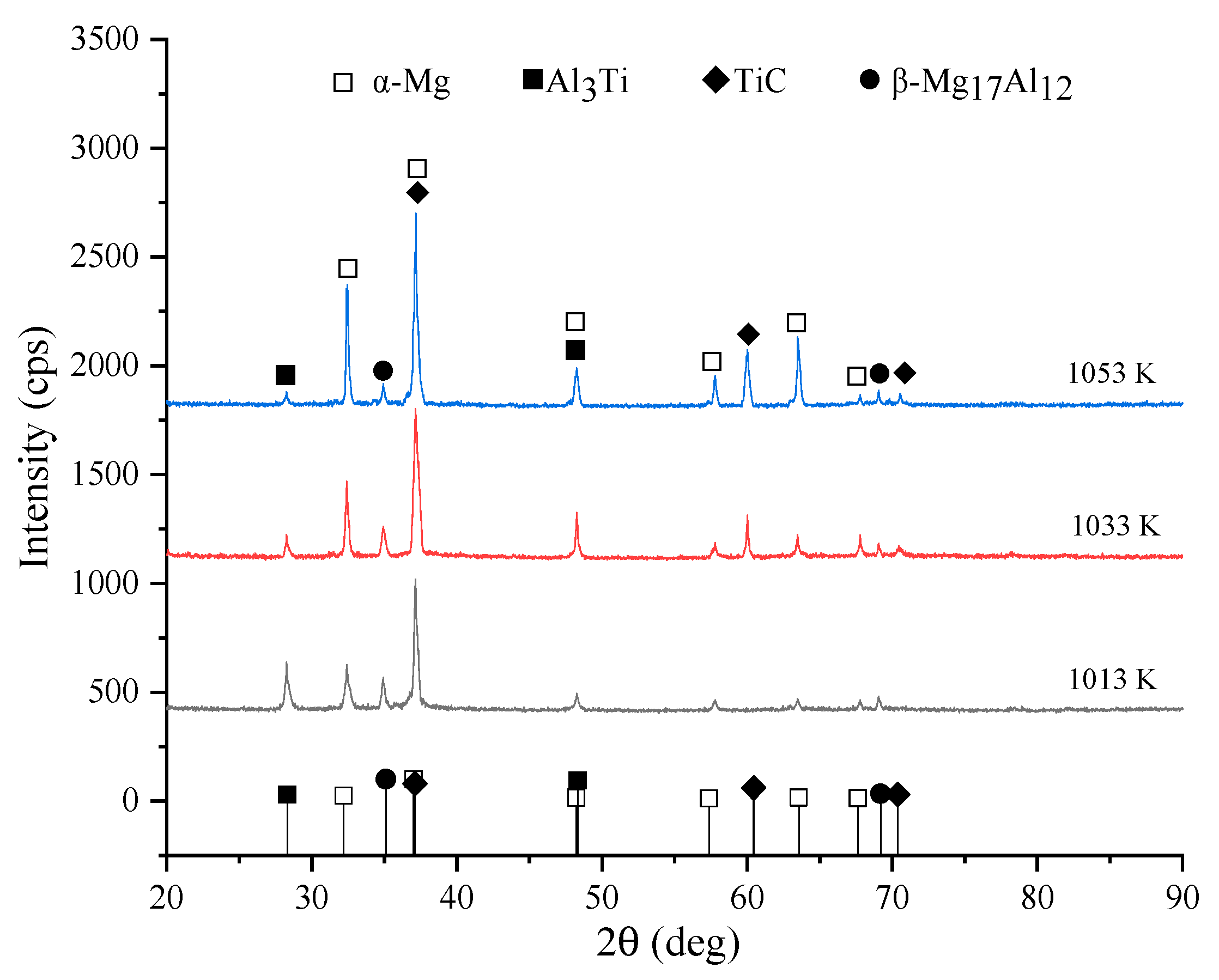
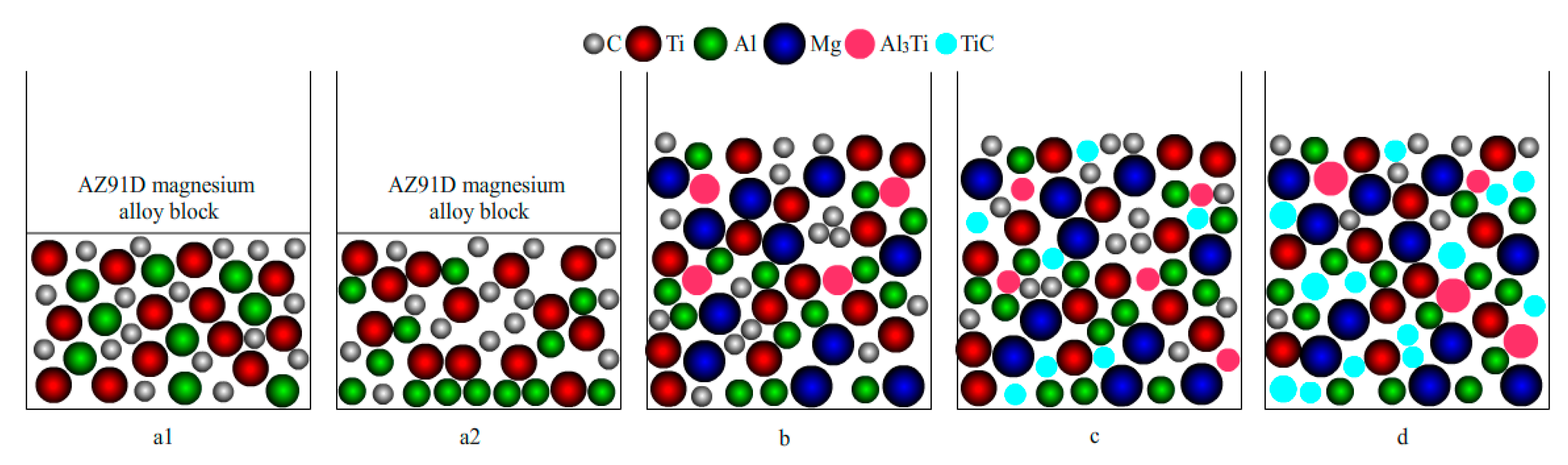
3.2. Formation Mechanism of the In Situ TiC-Reinforced AZ91D Magnesium Alloy
3.2.1. Thermal Analysis of the TiC Formation
3.2.2. Analysis of the TiC Formation Process
4. Conclusions
- (1)
- AZ91D magnesium matrix composites reinforced with TiC were successfully synthesized using an in situ reactive infiltration technique. In the Mg-Al-Ti-C system, the Gibbs free energy change (∆G) values of the three reactions Ti+C→TiC, Al3Ti+C→TiC+3Al, and 3Al+Ti→Al3Ti were negative; thus, these reactions can spontaneously occur according to the theory. However, the ∆G of Al3Ti increased with increasing temperature, and became unstable. The temperature played a critical role in determining the formation of TiC. In the present experiment, the decomposition reaction of Al3Ti started, and the in situ reaction of TiC occurred at 1033 K;
- (2)
- The formation mechanism of TiC in the AZ91D magnesium alloy can be described as follows: When the formation of Al3Ti and TiC as the reaction products is thermodynamically favored, the phase transformation driving force is promoted by the temperature. The Al3Ti reaction between Al and Ti first occurred when the temperature was 1013 K, due to the low energy required for the formation of Al3Ti. At the temperatures of 1033 and 1053 K, the energy provided by the temperature contributed to the formation of TiC via the in situ reaction between Ti and C. Meanwhile, the thermodynamically unfavorable Al3Ti phase decomposed into Al and TiC;
- (3)
- In the AZ91D magnesium alloy melt, the higher the temperature, the easier the in situ TiC particle formation, with the TiC grains becoming coarser. The amount of TiC also increased as the temperature increased. The presence of TiC improved the overall hardness.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Śliwa, R.; Balawender, T.; Hadasik, E.; Kuc, D.; Gontarz, A.; Korbel, A.; Bochniak, W. Metal Forming of Lightweight Magnesium Alloys for Aviation Applications. Arch. Met. Mater. 2017, 62, 1559–1566. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-L.; Yu, G.-K.; Zou, W.-B.; Ji, Y.-S.; Liu, Y.-Z.; Cheng, J.-L. Effect of casting methods on microstructure and mechanical properties of ZM5 space flight magnesium alloy. China Foundry 2018, 15, 418–421. [Google Scholar] [CrossRef] [Green Version]
- Aghion, E.; Bronfin, B.; Eliezer, D. The role of the magnesium industry in protecting the environment. J. Mater. Process. Technol. 2001, 117, 381–385. [Google Scholar] [CrossRef]
- Xu, T.; Yang, Y.; Peng, X.; Song, J.; Pan, F. Overview of advancement and development trend on magnesium alloy. J. Magnes. Alloys 2019, 7, 536–544. [Google Scholar] [CrossRef]
- Liu, H.; Tong, Z.; Yang, Y.; Zhou, W.; Chen, J.; Pan, X.; Ren, X. Preparation of phosphate conversion coating on laser surface textured surface to improve corrosion performance of magnesium alloy. J. Alloy. Compd. 2021, 865, 158701. [Google Scholar] [CrossRef]
- Gu, R.; Shen, J.; Hao, Q.; Wang, J.; Li, D.; Hu, L.; Chen, H. Harnessing superhydrophobic coatings for enhancing the surface corrosion resistance of magnesium alloys. J. Mater. Chem. B 2021, 9, 9893–9899. [Google Scholar] [CrossRef]
- Hou, F.Y.; Mardon, I.; Dong, J.Z.; Goode, C. Innovative Surface Technologies to Create Protective Functional Coatings on Light Metal Alloys. Key Eng. Mater. 2021, 876, 31–38. [Google Scholar] [CrossRef]
- Sun, S.; Deng, N.; Zhang, H.; He, L.; Zhou, H.; Han, B.; Gao, K.; Wang, X. Microstructure and mechanical properties of AZ31 magnesium alloy reinforced with novel sub-micron vanadium particles by powder metallurgy. J. Mater. Res. Technol. 2021, 15, 1789–1800. [Google Scholar] [CrossRef]
- Watanabe, H.; Mukai, T.; Mabuchi, M.; Higashi, K. Superplastic deformation mechanism in powder metallurgy magnesium alloys and composites. Acta Mater. 2001, 49, 2027–2037. [Google Scholar] [CrossRef]
- Knapek, M.; Zemková, M.; Greš, A.; Jablonská, E.; Lukáč, F.; Král, R.; Bohlen, J.; Minárik, P. Corrosion and mechanical properties of a novel biomedical WN43 magnesium alloy prepared by spark plasma sintering. J. Magnes. Alloy. 2021, 9, 853–865. [Google Scholar] [CrossRef]
- Cui, Z.Q.; Zhang, Y.K.; Cheng, Y.L.; Gong, D.Q.; Wang, W.X. Microstructure, mechanical, corrosion properties and cytotoxicity of beta-calcium polyphosphate reinforced ZK61 magnesium alloy composite by spark plasma sintering. Mater. Sci. Eng. C 2019, 99, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Rogal, L.; Garzel, G. Semi-solid state mixing of Mg-Zn-RE alloys—Microstructure and mechanical properties-Science Direct. J. Mater. Process. Technol. 2019, 264, 352–365. [Google Scholar] [CrossRef]
- Xia, M.X.; Zheng, H.X.; Yuan, S.; Li, J.G. Recrystallization of preformed AZ91D magnesium alloys in the semisolid state. Mater. Des. 2005, 26, 343–349. [Google Scholar]
- Liu, J.; Song, D.; Zhang, L.; Yang, X.; Zhu, X.; Sun, W.; Chen, F. Microstructure and compressive properties of solution heat-treated magnesium-Mg2Si in-situ composite foams after complex modification. J. Mater. Res. Technol. 2021, 15, 3673–3682. [Google Scholar] [CrossRef]
- Asselli, A.; Leiva, D.; Huot, J.; Kawasaki, M.; Langdon, T.; Botta, W. Effects of equal-channel angular pressing and accumulative roll-bonding on hydrogen storage properties of a commercial ZK60 magnesium alloy. Int. J. Hydrogen Energy 2015, 40, 16971–16976. [Google Scholar] [CrossRef]
- Banerjee, S.; Poria, S.; Sutradhar, G.; Sahoo, P. Dry sliding tribological behavior of AZ31-WC nano-composites. J. Magnes. Alloys 2019, 7, 315–327. [Google Scholar] [CrossRef]
- Falcon-Franco, L.; Bedolla-Becerril, E.; Lemus-Ruiz, J.; Gonzalez-Rodríguez, J.; Guardian, R.; Rosales, I. Wear performance of TiC as reinforcement of a magnesium alloy matrix composite. Compos. Part B Eng. 2011, 42, 275–279. [Google Scholar] [CrossRef]
- Ali, A.N.; Huang, S.J. Experimental investigations of effects of SiC contents and severe plastic deformation on the micro-structure and mechanical properties of SiCp/AZ61 magnesium metal matrix composites. J. Mater. Process. Technol. 2019, 272, 28–39. [Google Scholar]
- Meher, A.; Mahapatra, M.M.; Samal, P.; Vundavilli, P.R. Study on effect of TiB2 reinforcement on the microstructural and mechanical properties of magnesium RZ5 alloy based metal matrix composites. J. Magnes. Alloy. 2020, 8, 780–792. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, L. Processing of B4C Particulate-reinforced Magnesium-matrix Composites by Metal-assisted Melt Infiltration Technique. J. Mater. Sci. Technol. 2014, 30, 661–665. [Google Scholar] [CrossRef]
- Akbari, E.; Ghassemi Kakroudi, M.; Shahedifar, V.; Ghiasi, H. The influence of different SiC amounts on the microstructure, densification, and mechanical properties of hot-pressed Al2O3-SiC composites. Int. J. Appl. Ceram. Technol. 2020, 17, 491–500. [Google Scholar] [CrossRef]
- Lei, T.; Ouyang, C.; Tang, W.; Li, L.-F.; Zhou, L.-S. Preparation of MgO coatings on magnesium alloys for corrosion protection. Surf. Coat. Technol. 2010, 204, 3798–3803. [Google Scholar] [CrossRef]
- Sharifitabar, M.; Kashefi, M.; Khorshahian, S. Effect of friction stir processing pass sequence on properties of Mg–ZrSiO4–Al2O3 surface hybrid micro/nano-composites. Mater. Des. 2016, 108, 1–7. [Google Scholar] [CrossRef]
- Kucukosman, R.; Sukuroglu, E.E.; Totik, Y.; Sukuroglu, S. Effects of graphene oxide addition on wear behaviour of composite coatings fabricated by plasma electrolytic oxidation (PEO) on AZ91 magnesium alloy. J. Adhes. Sci. Technol. 2020, 35, 242–255. [Google Scholar] [CrossRef]
- Hua, T.; Ouyang, Y.J.; Xie, Z.H.; Wu, L. One-pot scalable in situ growth of highly corrosion-resistant MgAl-LDH/MBT com-posite coating on magnesium alloy under mild conditions. J. Mater. Sci. Technol. 2021, 92, 225–235. [Google Scholar] [CrossRef]
- Mahene, W.L.; Hilonga, A.; Machunda, R. In-situ synthesis of calcium/magnesium phosphate system for water de-fluoridation: Clay ceramic materials. Mater. Chem. Phys. 2021, 278, 125539. [Google Scholar] [CrossRef]
- Liao, L.H.; Wang, H.W.; Li, X.F.; Zhang, X.Q.; Ma, N.H. Damping capacity associated with precipitation in Mg2Si/Mg–9Al composite materials. Mater. Sci. Technol. 2006, 22, 1223–1226. [Google Scholar] [CrossRef]
- Meenashisundaram, G.K.; Gupta, M. Synthesis and characterization of high performance low volume fraction TiC reinforced Mg nanocomposites targeting biocompatible/structural applications. Mater. Sci. Eng. A 2015, 627, 306–315. [Google Scholar] [CrossRef]
- Narayanasamy, P.; Selvakumar, N.; Balasundar, P. Effect of weight percentage of TiC on their tribological properties of magnesium composites. Mater. Today Proc. 2018, 5, 6570–6578. [Google Scholar] [CrossRef]
- Ferreira, V.; Merchán, M.; Egizabal, P.; de Cortázar, M.G.; Irazustabarrena, A.; López-Sabirón, A.M.; Ferreira, G. Technical and environmental evaluation of a new high-performance material based on magnesium alloy reinforced with submicrometre-sized TiC particles to develop automotive lightweight components and make transport sector more sustainable. J. Mater. Res. Technol. 2019, 8, 2549–2564. [Google Scholar] [CrossRef]
- Jiang, Q.C.; Li, X.L.; Wang, H.Y. Fabrication of TiC particulate reinforced magnesium matrix composites. Scr. Mater. 2003, 48, 713–717. [Google Scholar] [CrossRef]
- Nie, K.B.; Guo, Y.C.; Munroe, P.; Deng, K.K.; Kang, X.K. Microstructure and tensile properties of magnesium matrix nano-composite reinforced by high mass fraction of nano-sized particles including TiC and MgZn2. J. Alloys Compd. 2020, 819, 153348. [Google Scholar] [CrossRef]
- Mehra, D.; Mahapatra, M.M.; Harsha, S.P. Processing of RZ5-10wt% TiC in-situ magnesium matrix composite. J. Magnes. Alloys 2018, 6, 100–105. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, C.; Fan, T.; Zhang, D. In situ synthesis and damping capacities of TiC reinforced magnesium matrix composites. Mater. Sci. Eng. A 2008, 496, 242–246. [Google Scholar] [CrossRef]
- Sahoo, B.N.; Panigrahi, S.K. Effect of in-situ (TiC-TiB2) reinforcement on aging and mechanical behavior of AZ91 magnesium matrix composite. Mater. Charact. 2018, 139, 221–232. [Google Scholar] [CrossRef]
- Sahoo, B.N.; Khan, F.; Babu, S.; Panigrahi, S.K.; Janaki Ram, G.D. Microstructural modification and its effect on strengthening mechanism and yield asymmetry of in-situ TiC-TiB2/AZ91 magnesium matrix composite. Mater. Sci. Eng. A 2018, 724, 269–282. [Google Scholar] [CrossRef]
- Sahoo, B.N.; Panigrahi, S.K. Synthesis, characterization and mechanical properties of in-situ (TiC-TiB2) reinforced magnesium matrix composite. Mater. Des. 2016, 109, 300–313. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Nie, K.B.; Munroe, P.; Deng, K.K.; Guo, Y.C.; Han, J.G. Synergistic effects of hybrid (SiC+TiC) nanoparticles and dynamic precipitates in the design of a high-strength magnesium matrix nanocomposite. Mater. Chem. Phys. 2021, 259, 124048. [Google Scholar] [CrossRef]
- Fan, W.; Hao, H. The influence of gadolinium on Al–Ti–C master alloy and its refining effect on AZ31 magnesium alloy. Int. J. Mater. Res. 2021, 112, 233–240. [Google Scholar] [CrossRef]
- Han, G.; Liu, X.F.; Ding, H.M. Grain refinement of AZ31 magnesium alloy by new Mg-Al-C master alloy. Trans. Nonferrous. Met. Soc. 2009, 19, 1057–1064. [Google Scholar] [CrossRef]
- Zhu, H.G.; Zhang, A.W. Principle of Composite Materials; National Defense Industry Press: Beijing, China, 2013; p. 214. [Google Scholar]
- Tian, M.B.; Liu, D.L. Handbook of Thin Film Science and Technology; Machinery Industry Press: Beijing, China, 1991; p. 735. [Google Scholar]
- Bramfitt, B.L. Metallurgy of Continuous-Annealed Sheet Steel; Metallurgical Society of AIME: New York, NY, USA, 1982; pp. 77–78. [Google Scholar]
- Ye, D.L. Data Manual for Practical Inorganic Thermodynamics; Metallurgical Industry Press: Beijing, China, 1981; pp. 1012–1083. [Google Scholar]
- Akaltun, Y.; Aslan, M.; Yetim, T.; Tçayır; Çelik, A. The effect of wettability on corrosion resistance of oxide films produced by SILAR method on magnesium, aluminum and copper substrates. Surf. Coat. Technol. 2016, 292, 121–131. [Google Scholar] [CrossRef]
- Landry, K.; Kalogeropoulou, S.; Eustathopoulos, N. Wettability of carbon by aluminum and aluminum alloys. Mater. Sci. Eng. A 1998, 254, 99–111. [Google Scholar] [CrossRef]
- Heng, X.M.; Liu, J.; Huang, Z.C. Master alloy refiners in aluminum alloys. Light Metal. 2004, 254, 46–50. [Google Scholar]
- Arnberg, L.; Bäckerud, L.; Klang, H. 1: Production and properties of master alloys of Al–Ti–B type and their ability to grain refine aluminium. Met. Technol. 1982, 9, 1–6. [Google Scholar] [CrossRef]



| Substance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 400 K | 500 K | 600 K | 700 K | 800 K | 900 K | 1000 K | 1100 K | 1200 K | ||
| Al3Ti | −142,256 | 98.522 | 106.164 | 114.887 | 123.772 | 132.466 | 140.835 | 148.838 | 156.473 | 163.754 |
| C | 0 | 6.113 | 6.936 | 7.971 | 9.109 | 10.294 | 11.491 | 12.680 | 13.849 | 14.990 |
| TiC | −184,096 | 25.672 | 28.606 | 32.065 | 35.660 | 39.225 | 42.689 | 46.022 | 49.216 | 52.273 |
| Mg2C3 | 75,312 | 104.314 | 112.033 | 120.994 | 130.219 | 139.305 | 148.090 | 156.513 | 164.559 | 172.239 |
| MgC2 | 87,864 | 56.730 | 61.358 | 66.732 | 72.265 | 77.714 | 82.983 | 88.035 | 92.861 | 97.467 |
| Mg | 0 | 33.672 | 35.586 | 37.775 | 40.019 | 42.232 | 44.383 | 47.217 | 50.027 | 52.616 |
| Al | 0 | 29.301 | 31.192 | 33.355 | 35.570 | 37.756 | 39.888 | 42.724 | 45.679 | 48.382 |
| Ti | 0 | 31.632 | 33.558 | 35.755 | 38.001 | 40.210 | 42.350 | 44.411 | 46.392 | 48.427 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Zhang, Z.; Li, X.; Zhou, L.; Zhang, X.; Chen, M. Microstructure and Phase Evolution Characteristics of the In Situ Synthesis of TiC-Reinforced AZ91D Magnesium Matrix Composites. Materials 2022, 15, 1278. https://doi.org/10.3390/ma15041278
Zhou X, Zhang Z, Li X, Zhou L, Zhang X, Chen M. Microstructure and Phase Evolution Characteristics of the In Situ Synthesis of TiC-Reinforced AZ91D Magnesium Matrix Composites. Materials. 2022; 15(4):1278. https://doi.org/10.3390/ma15041278
Chicago/Turabian StyleZhou, Xinjun, Zhengfu Zhang, Xiulan Li, Liyu Zhou, Xudong Zhang, and Manjiao Chen. 2022. "Microstructure and Phase Evolution Characteristics of the In Situ Synthesis of TiC-Reinforced AZ91D Magnesium Matrix Composites" Materials 15, no. 4: 1278. https://doi.org/10.3390/ma15041278
APA StyleZhou, X., Zhang, Z., Li, X., Zhou, L., Zhang, X., & Chen, M. (2022). Microstructure and Phase Evolution Characteristics of the In Situ Synthesis of TiC-Reinforced AZ91D Magnesium Matrix Composites. Materials, 15(4), 1278. https://doi.org/10.3390/ma15041278






