Effect on Microstructure and Mechanical Properties of Microwave-Assisted Sintered H13 Steel Powder with Different Vanadium Contents
Abstract
1. Introduction
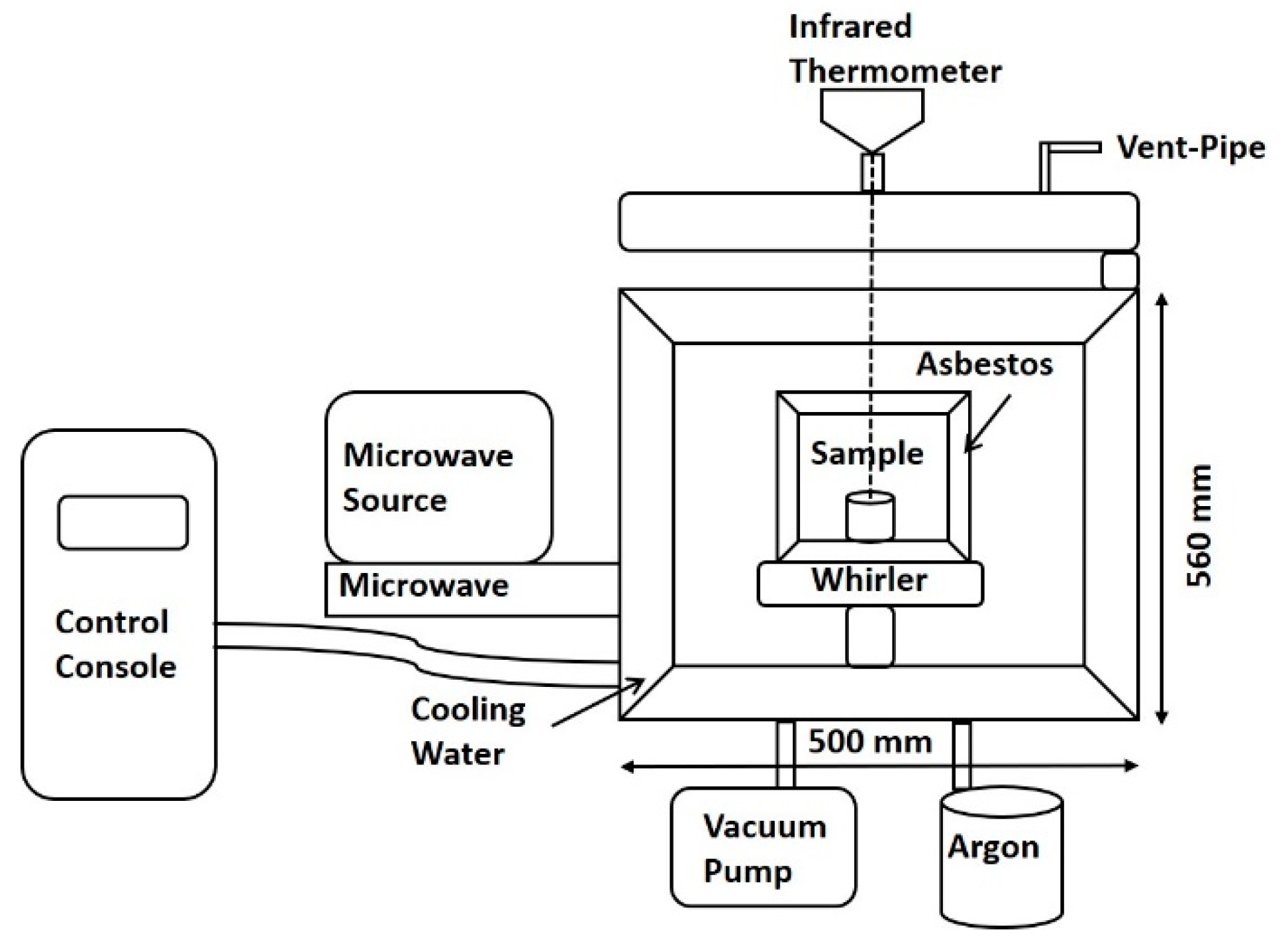
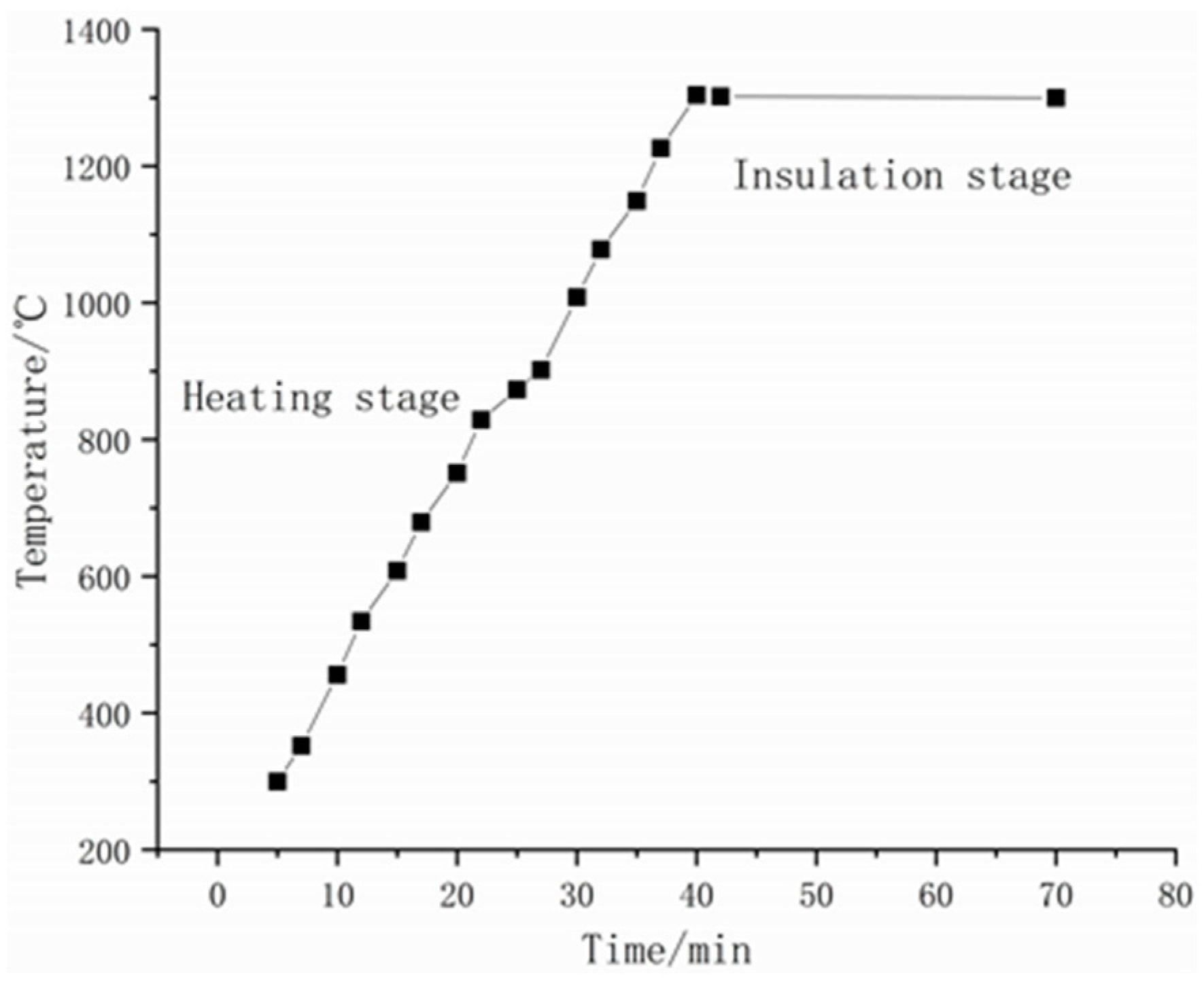
2. Materials and Methods
3. Results
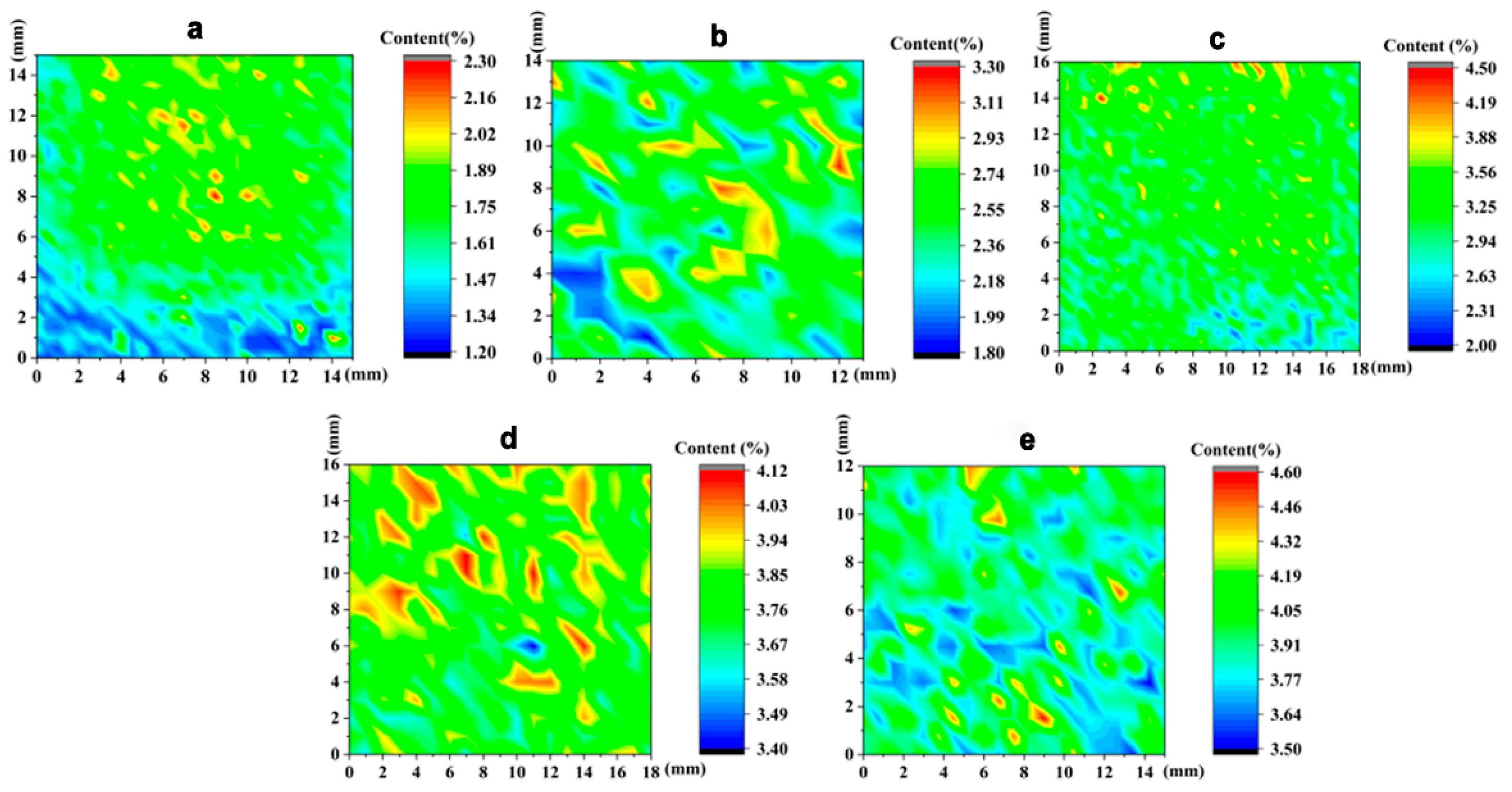
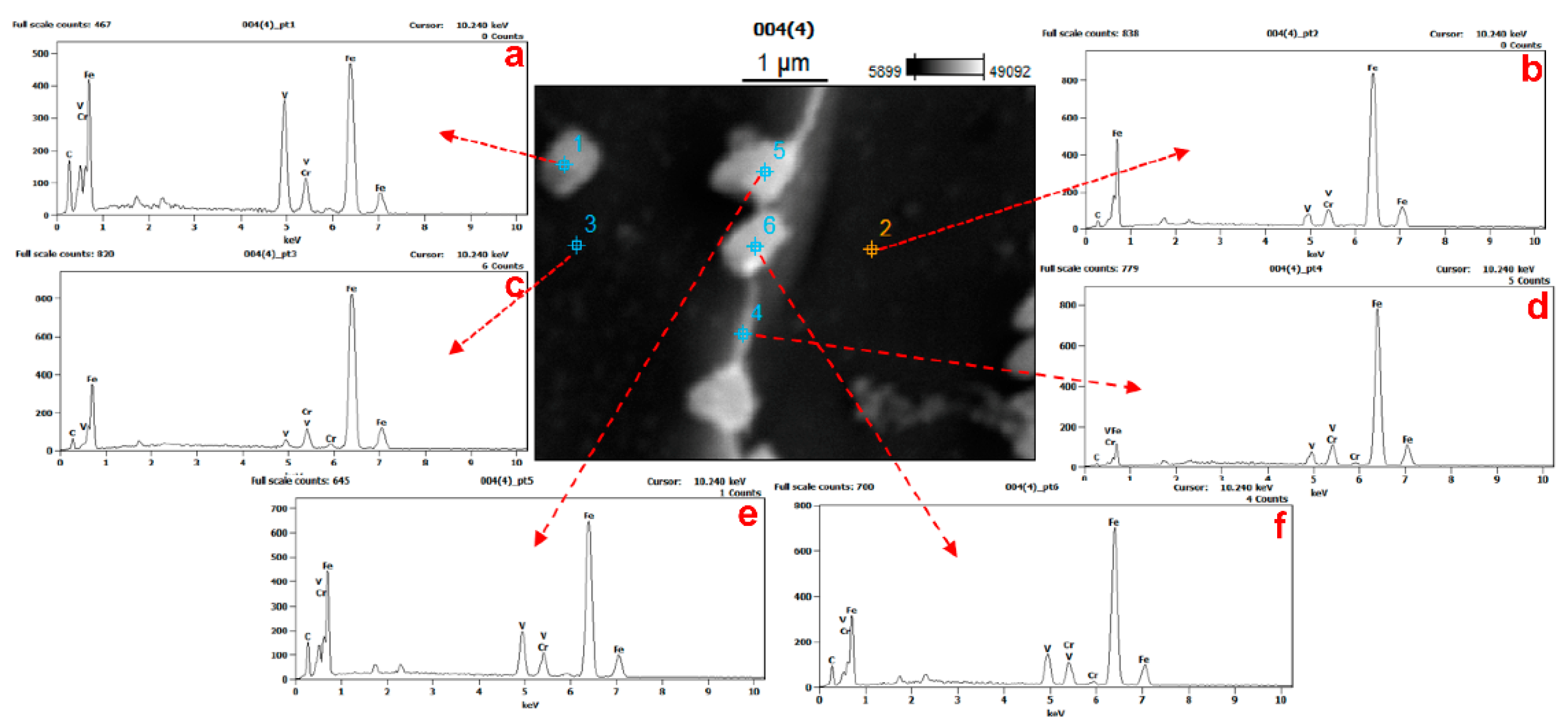
3.1. Distributions of Vanadium in Samples
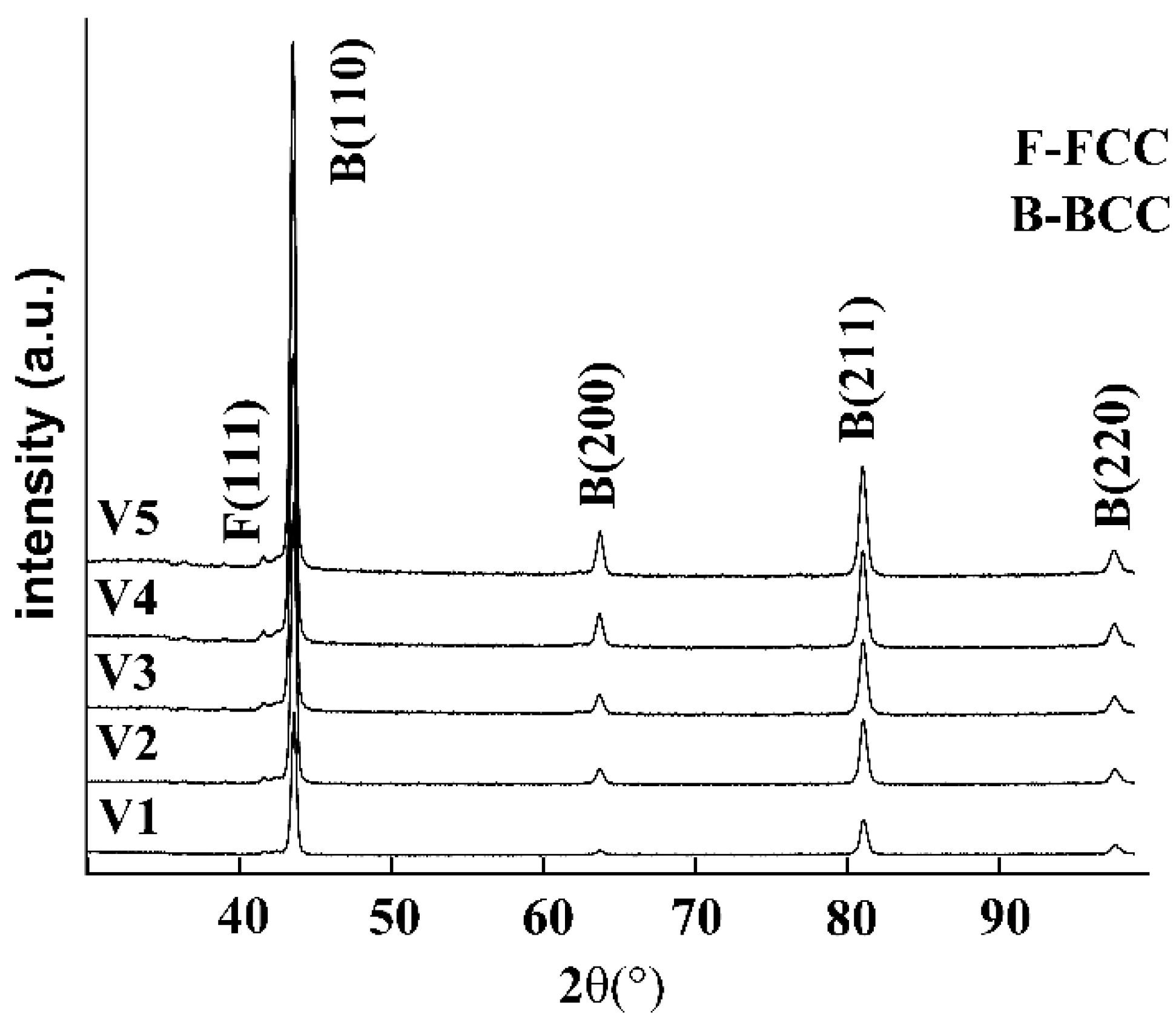
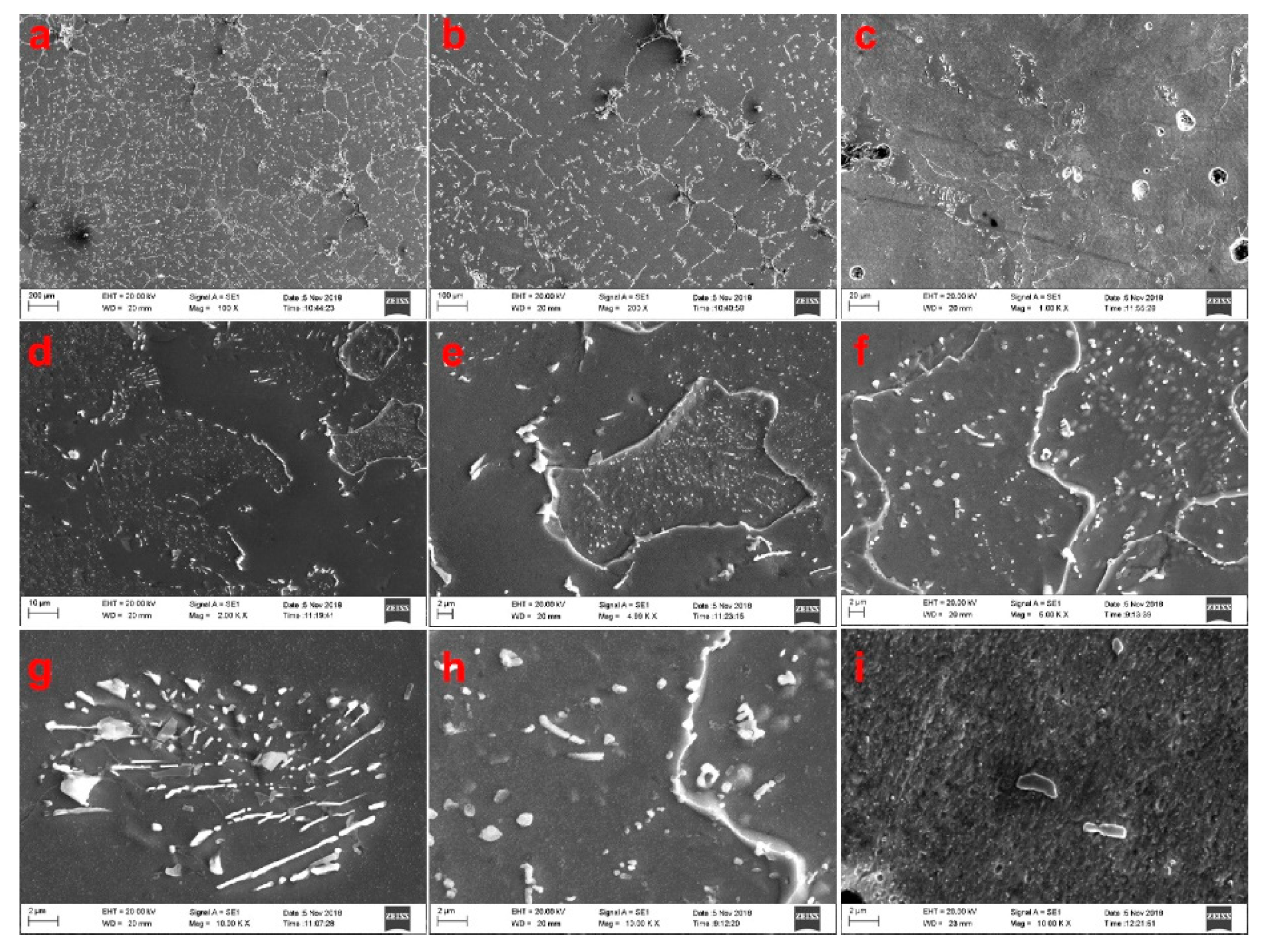
3.2. Effects of Vanadium on Microstructure
3.3. Effects of Vanadium on Hardness
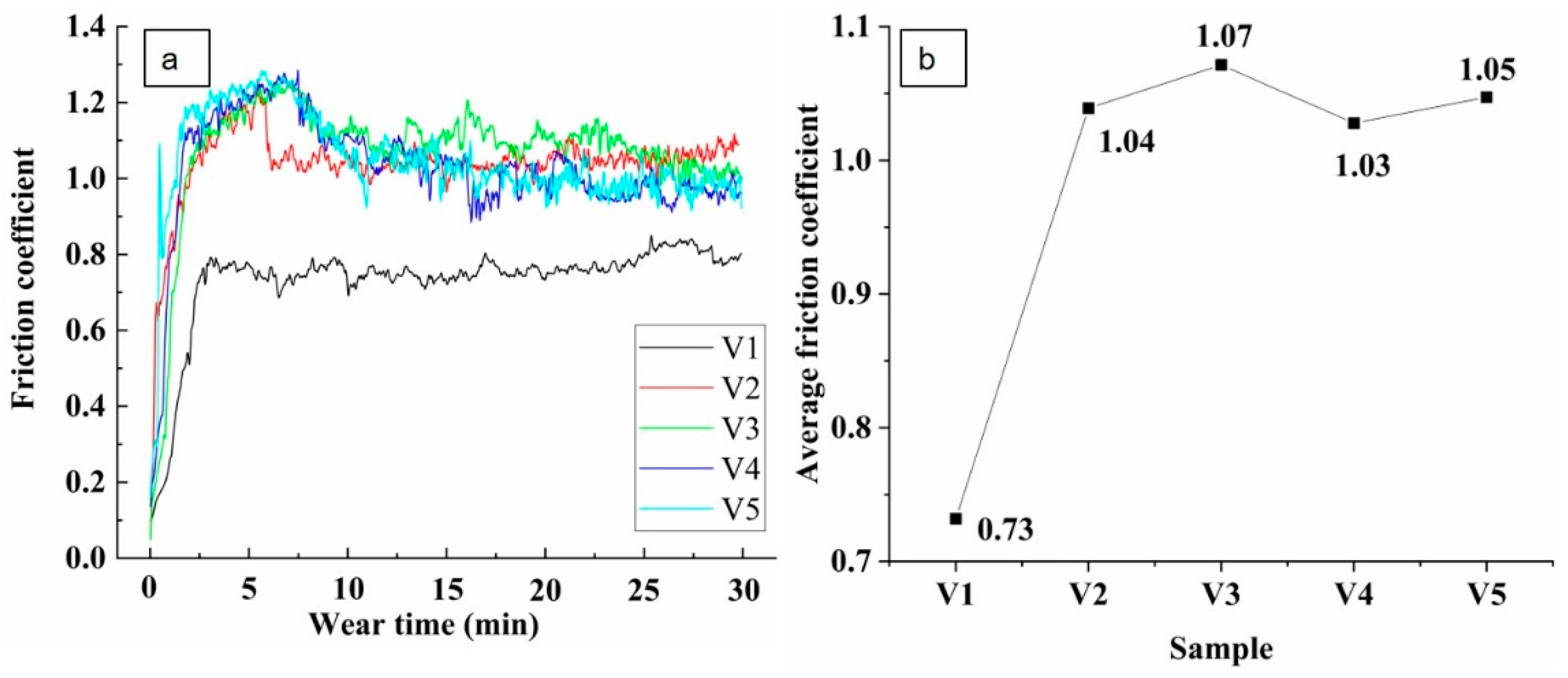
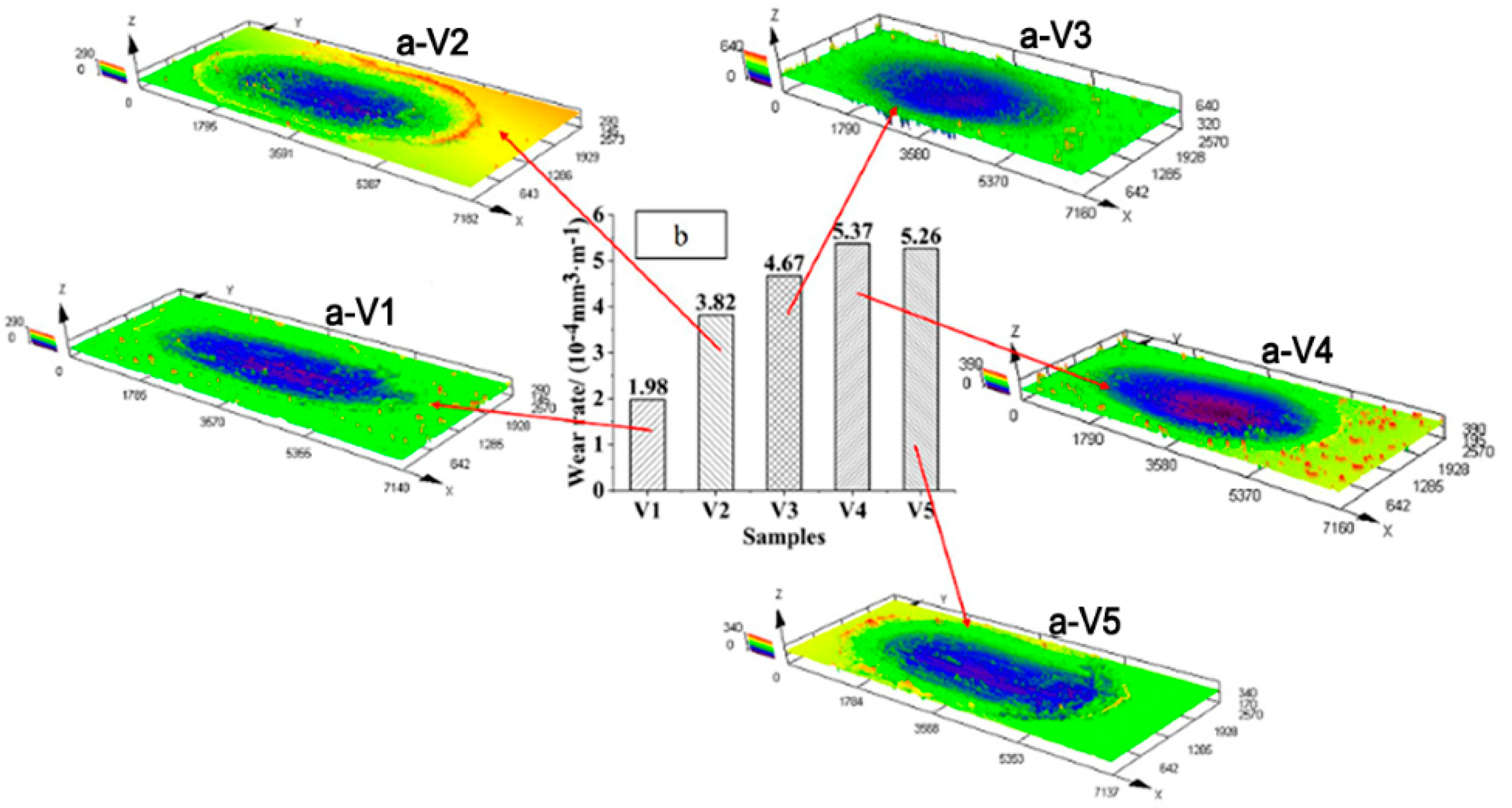
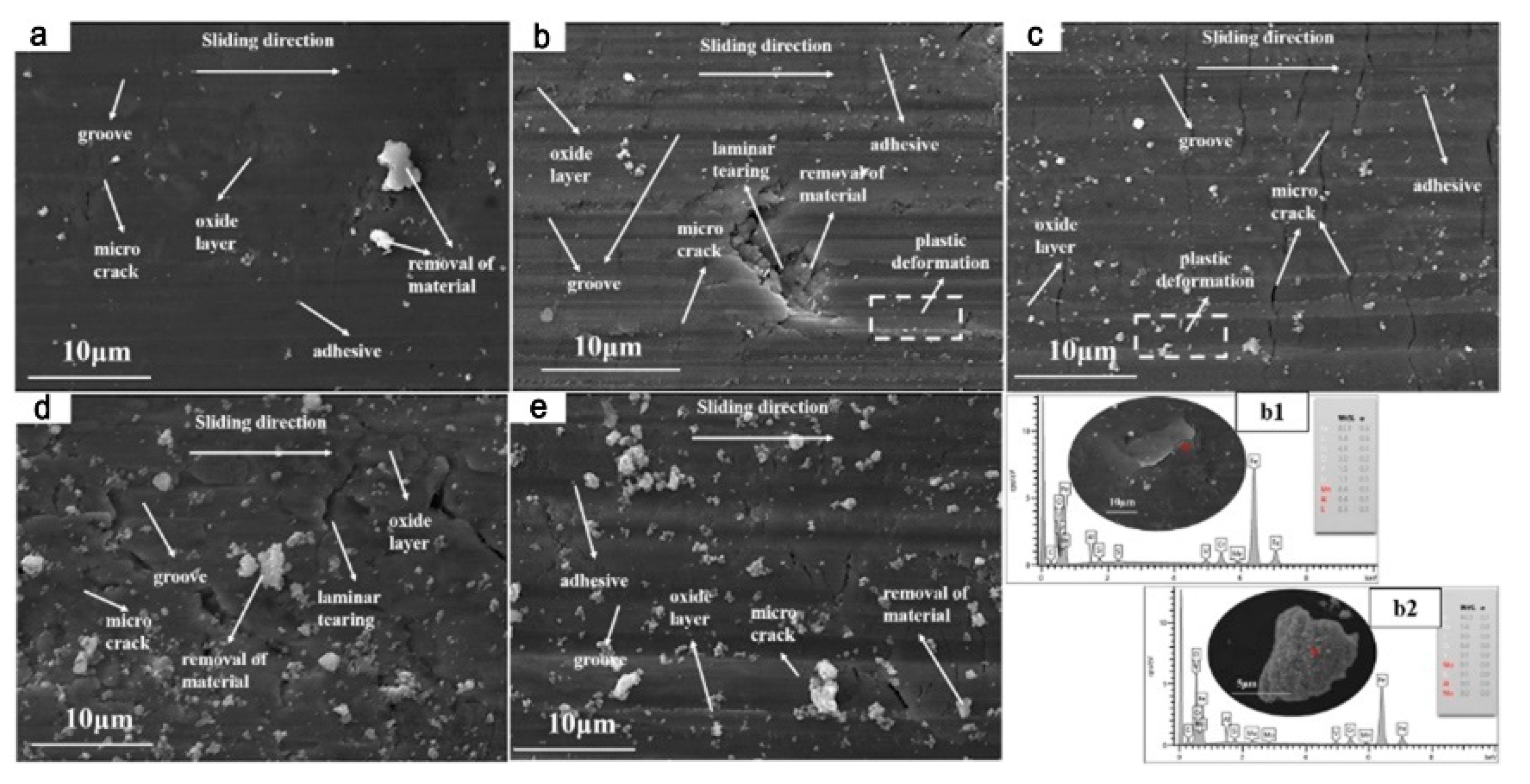
3.4. Effects of Vanadium on Friction and Wear
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yadoji, P.; Peelamedu, R.; Agrawal, D.; Roy, R. Microwave sintering of Ni–Zn ferrites: Comparison with conventional sintering. Mater. Sci. Eng. B 2003, 98, 269–278. [Google Scholar] [CrossRef]
- Ehsan, G.; Hossein, P.A.; Maryam, A.; Hossein, M.A.; Touradj, E. WC-Co Particles Reinforced Aluminum Matrix by Conventional and Microwave Sintering. Mater. Res. 2015, 18, 1197–1202. [Google Scholar]
- Manière, C.; Zahrah, T.; Olevsky, E.A. Inherent heating instability of direct microwave sintering process: Sample analysis for porous 3Y-ZrO2. Scr. Mater. 2017, 128, 49–52. [Google Scholar] [CrossRef]
- Roy, R.; Agrawal, D.; Cheng, J.; Gedevanishvili, S. Full sintering of powdered-metal bodies in a microwave field. Nature 1999, 399, 304. [Google Scholar] [CrossRef]
- Gedevanishvili, S.; Agrawal, D.; Roy, R. Microwave Combustion Synthesis and Sintering of Intermetallics and Alloys. J. Mater. Sci. Lett. 1999, 18, 665–668. [Google Scholar] [CrossRef]
- Walkiewicz, J.W.; Kazonich, G.; Mcgill, S.L. Microwave heating characteristics of selected minerals and compounds. Miner. Metall. Process. 1988, 5, 39–42. [Google Scholar]
- Thomas, P.; Sathapathy, L.N.; Dwarakanath, K.; Varma, B.K. Microwave synthesis and sintering characteristics of CaCu3Ti4O12. Bull. Mater. Sci. 2007, 30, 567–570. [Google Scholar] [CrossRef]
- Kongoli, F. The Sohn International Symposium on Advanced Processing of Metals and Materials. JOM 2006, 58, 95. [Google Scholar] [CrossRef][Green Version]
- Chen, X.; Zhao, L.; Jiang, L.; Wang, H. Microstructures and Properties of Cu-rGO Composites Prepared by Microwave Sintering. Materials 2021, 14, 4899. [Google Scholar] [CrossRef]
- Yang, L.; Wang, L.; Gao, J.; Guo, S.; Ye, X.; Koppala, S.; Hu, T.; Hou, M.; Hu, L. Optimization of Process Parameters for Preparing Metallic Matrix Diamond Tool Bits by Microwave Pressureless Sintering Using Response Surface Methodology. Materials 2018, 11, 2185. [Google Scholar] [CrossRef]
- Ghasali, E.; Fazili, A.; Alizadeh, M.; Shirvanimoghaddam, K.; Ebadzadeh, T. Evaluation of Microstructure and Mechanical Properties of Al-TiC Metal Matrix Composite Prepared by Conventional, Microwave and Spark Plasma Sintering Methods. Materials 2017, 10, 1255. [Google Scholar] [CrossRef] [PubMed]
- Leparoux, S.; Vaucher, S.; Beffort, O. Assessment of Microwave Heating for Sintering of Al/SiC and for in-situ Synthesis of TiC. Adv. Eng. Mater. 2003, 5, 449–453. [Google Scholar] [CrossRef]
- Jian, Z.; Cheng, J.; Yuan, R.; Zhang, Y. Property and technology of WC Co fine grain cemented carbide in microwave sintering. Chin. J. Nonferrous Met. 1999, 30, 567–570. [Google Scholar]
- Pan, Z.; Wang, G.; Cai, L. Research and Application of High Strength Hot Work Die Steel in China. Special Steel 2003, 24, 1–5. [Google Scholar]
- Qian, L.; Ping, L.; Zheng, S.; Lei, W. Effects of Quenching Temperature and Tempeing Temperature on the Thermal Fatigue of 4Cr5MoSiV1 Steel. Hot Work. Technol. 1995, 4, 10–12. [Google Scholar]
- Chen, R.; Wang, Z.; Qi, L.; Zhong, L.; Hu, X. The carbides, tensile properties, and work-hardening behavior of annealed H13 die steels with varied yttrium contents. Mater. Sci. Eng. A 2021, 806, 140856. [Google Scholar] [CrossRef]
- Ivanov, V.V.; Ferguson, W.G.; Paine, I.R. Study of Thermal Fatigue of H13 Die Steel with Various Surface Treatments. Int. J. Mod. Phys. B 2003, 17, 1671–1677. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Z.; Xie, J. Improving strength and ductility of H13 die steel by pre-tempering treatment and its mechanism. Mater. Sci. Eng. A 2019, 752, 101–114. [Google Scholar] [CrossRef]
- Guo, H.F.; Hao, X.; Zhi-Hui, H.E.; Gao, W.H. Effect of Rare Earth La on Mechanical Properties of 45Cr2NiMoVSi Hot Die Steel. Foundry Technol. 2007, 28, 295. [Google Scholar]
- Liu, S. Study on rare-earth doped cemented carbides in China. Int. J. Refract. Met. Hard Mater. 2009, 27, 528–534. [Google Scholar]
- Guan, Q.F.; Jiang, Q.C.; Fang, J.R.; Jiang, H. Microstructures and Thermal Fatigue Behavior of Cr-Ni-Mo Hot Work Die Steel Modified by Rare Earth. Trans. Iron Steel Inst. Jpn. 2003, 43, 784–789. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Jiang, Z.; Geng, X.; Chen, M.; Cui, S. Effect of Rare Earth–Magnesium Alloy on Inclusion Evolution in Industrial Production of Die Steel. Steel Res. Int. 2019, 90, 1900103. [Google Scholar] [CrossRef]
- Ma, X.; An, Z.; Chen, L.; Mao, T.; Wang, J.; Long, H.; Hongyan, X. The effect of rare earth alloying on the hot workability of duplex stainless steel—A study using processing map. Mater. Des. 2015, 86, 848–854. [Google Scholar] [CrossRef]
- Hamidzadeh, M.A.; Meratian, M.; Saatchi, A. Effect of cerium and lanthanum on the microstructure and mechanical properties of AISI D2 tool steel. Mater. Sci. Eng. A 2013, 571, 193–198. [Google Scholar] [CrossRef]
- Yang, S.N.; Li, S.-Z.; Sun, F.; Shan, A.D. Influences of Vanadium and Copper on Microstructure and High-Temperature Mechanical Properties of 11Cr-2W Low Activation Martensite Steel. Mater. Mech. Eng. 2015, 39, 9–13+19. [Google Scholar]
- Liu, K.; Wang, F.; Li, C.; Su, L. Influence of Vanadium on Microstructure and Properties of Medium-chromium White Cast Iron. Chin. J. Eng. 2005, 26, 604–607. [Google Scholar]
- Li, Y.; Yang, Z. The Effects of v on phase transformation of high carbon steel during continuous cooling. Acta Metall. Sin. 2010, 46, 1501–1510. [Google Scholar]
- Ma, Y.H.; Sun, Y.F.; Zhao, J.Y.; Xiao, Z.Y.; Si, G.D. Effects of Vanadium on Microstructures and Properties of CADI. Foundry 2011, 60, 779–783. [Google Scholar]
- Liu, P.; Yang, J.H.; Huang, J.H. Application of Digitized Spectroscopic Analysis Technique in Visible Spectrum Analysis of Vanadium of Ferroalloys. Modern Cast Iron 2008, 2, 82–85. [Google Scholar]
- Chen, X.; Zhao, L.; Li, D.; Jiang, L.; Wang, H. Characteristics of microwave melting H13 steel powder with different tungsten contents. Mater. Lett. 2021, 294, 129803. [Google Scholar] [CrossRef]
- Tian, J.; Hu, Y.; Liu, X.; Yang, Z. Wear performance and mechanisms of H13 steels sliding against different Rock types. Surface Topogr. Metrol. Prop. 2020, 8, 025003. [Google Scholar] [CrossRef]




| Sample | Elementary Composition (%) | ||||
|---|---|---|---|---|---|
| C | Si | Mn | P | S | |
| 0.34 | 1.17 | 0.5 | 0.011 | 0.0073 | |
| H13 | Cr | Ni | Cu | Mo | V |
| 5 | 0.098 | 0.032 | 1.16 | 1 | |
| As | W | ||||
| 0.0026 | <0.1 | ||||
| Location | C | V | Cr | Fe |
|---|---|---|---|---|
| pt1 | 11.17 | 24.86 | 3.92 | 60.05 |
| pt2 | 2.50 | 4.15 | 5.03 | 88.32 |
| pt3 | 3.03 | 1.92 | 5.79 | 89.26 |
| pt4 | 1.29 | 3.70 | 5.39 | 89.62 |
| pt5 | 9.59 | 11.47 | 4.74 | 74.20 |
| pt6 | 6.08 | 8.45 | 5.68 | 79.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhao, L.; Wei, M.; Huang, D.; Jiang, L.; Wang, H. Effect on Microstructure and Mechanical Properties of Microwave-Assisted Sintered H13 Steel Powder with Different Vanadium Contents. Materials 2022, 15, 1273. https://doi.org/10.3390/ma15041273
Chen X, Zhao L, Wei M, Huang D, Jiang L, Wang H. Effect on Microstructure and Mechanical Properties of Microwave-Assisted Sintered H13 Steel Powder with Different Vanadium Contents. Materials. 2022; 15(4):1273. https://doi.org/10.3390/ma15041273
Chicago/Turabian StyleChen, Xuebin, Lei Zhao, Min Wei, Danqi Huang, Liwu Jiang, and Haizhou Wang. 2022. "Effect on Microstructure and Mechanical Properties of Microwave-Assisted Sintered H13 Steel Powder with Different Vanadium Contents" Materials 15, no. 4: 1273. https://doi.org/10.3390/ma15041273
APA StyleChen, X., Zhao, L., Wei, M., Huang, D., Jiang, L., & Wang, H. (2022). Effect on Microstructure and Mechanical Properties of Microwave-Assisted Sintered H13 Steel Powder with Different Vanadium Contents. Materials, 15(4), 1273. https://doi.org/10.3390/ma15041273






