3.1. Selection of Leaching Conditions
Investigations aimed at the selection of optimal leaching conditions were carried out on a sample of sludge No. 3 (
Table 1).
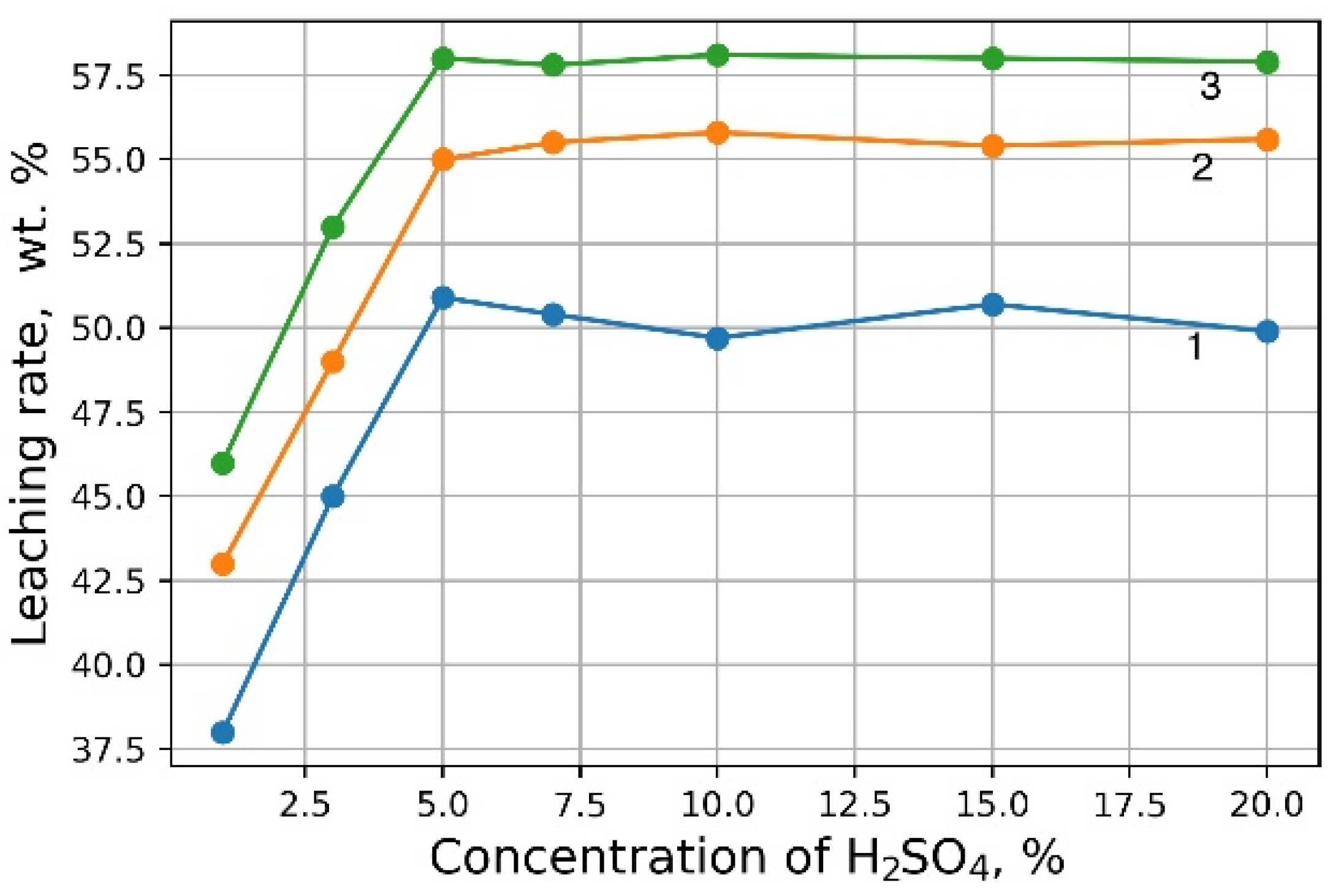
Figure 1 demonstrates the outcomes of H
2SO
4 concentration on the recovery rate of V
2O
5 into solution from the initial sludge at different temperatures and S/L = 1/5 (g/mL). The maximal recovery rate of V
2O
5 into solution (58%) is achieved at the leaching temperature of 80 °C and concentration of 5% of H
2SO
4 solution, without heating, the recovery rate of V
2O
5 is ≈50%. The leaching process at the temperature of 80 °C has such a disadvantage, as the need for constant monitoring of the level S/L due to significant evaporation of the leaching solution. As can be seen from
Figure 1, heating has a small effect on the recovery rate of V
2O
5, thus it was decided to conduct the experiments without heating at 5% H
2SO
4 solution, while the concentration of V
2O
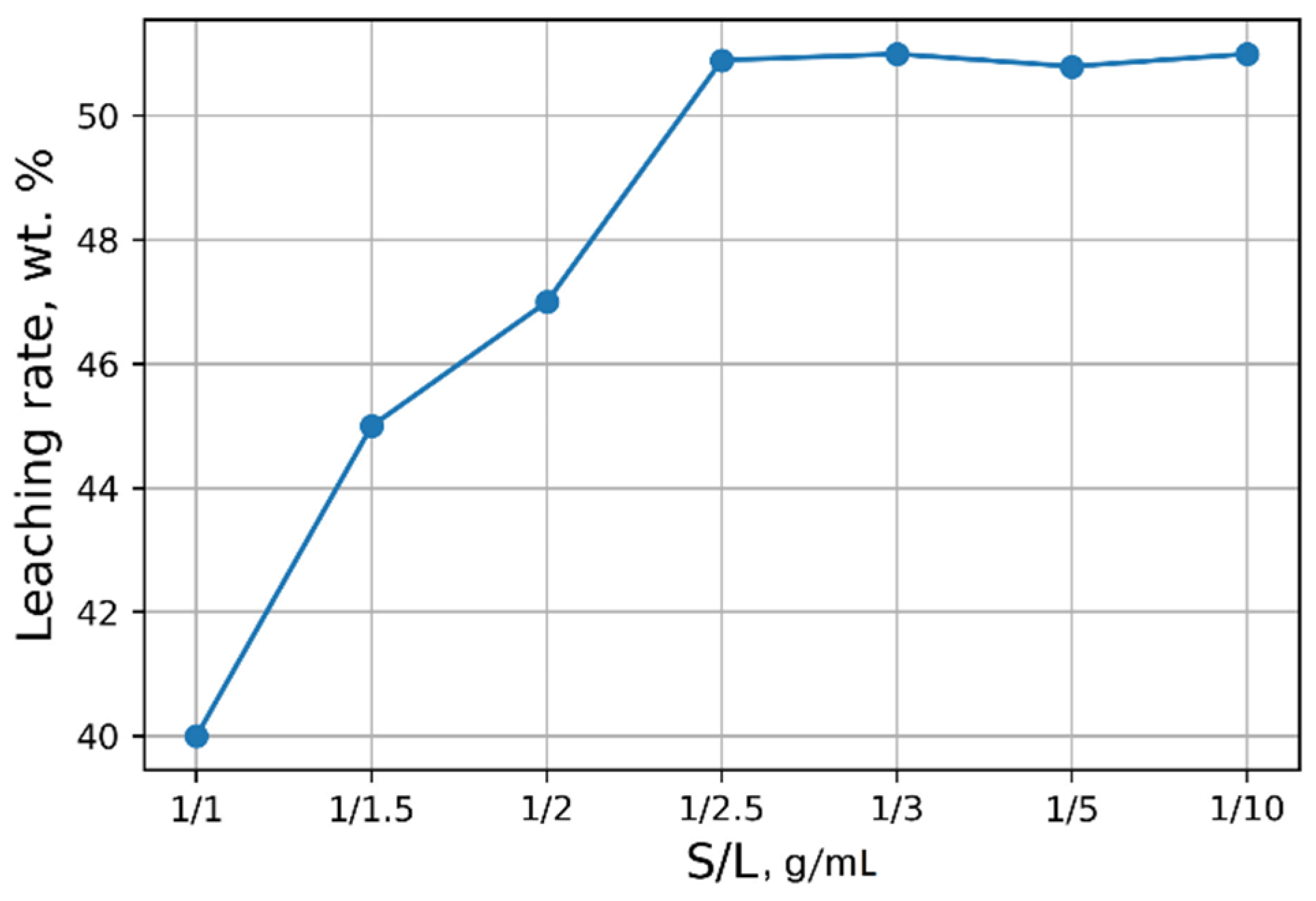
5 in the solution was ≈3 g/L. The optimal S/L ratio was found to be 1/2.5 (g/mL) (
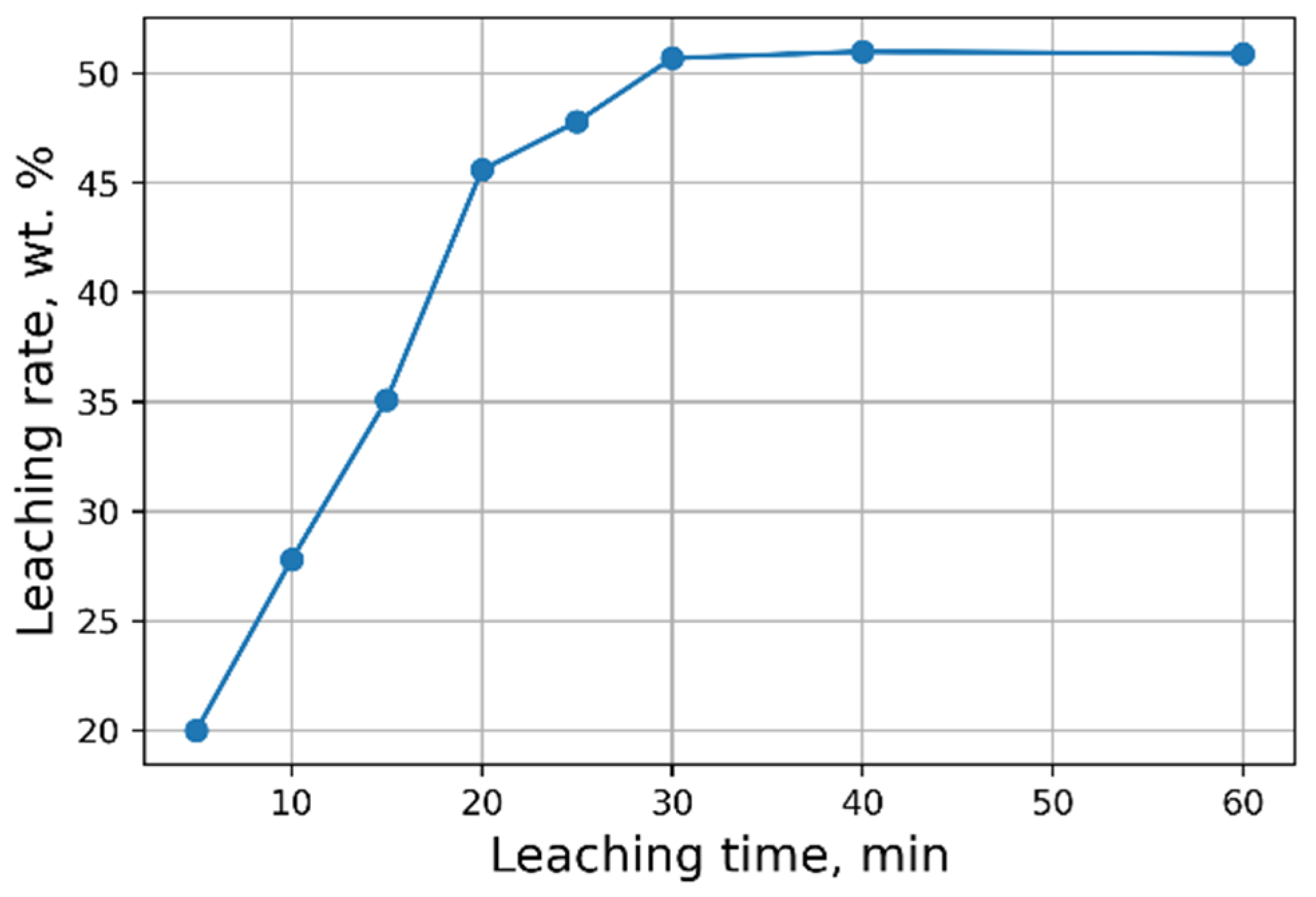
Figure 2). The maximum concentration of vanadium in the solution is reached 30 min after the start of the process (
Figure 3).
The optimal conditions for two-stage counterflow leaching of roasted sludge with 1 wt% CaCO
3 additive were selected in [
33]: H
2SO
4 concentration of the solution is 5–7%, the leaching time is 20 min at each stage of the process; S/L = 1/2.5. These data were used in this work for obtaining vanadium-concentrated solutions with its further precipitation by brucite and NH
4Cl.
3.2. Reducing Leaching
For studies of vanadium leaching with its further reduction by FeSO
4·7H
2O, sludges with V
2O
5 content of 2.25 wt% were used (
Table 1, sample No. 1).
The test sample contains 0.94 wt% V
2O
5a.s., i.e., this amount of V
2O
5 can be converted into a solution by leaching with a 7% H
2SO
4 solution [
33]. Vanadium can be represented in the acid-soluble part in the form of salts: orthovanadates (Me
3VO
4), pyrovanadates (Me
4V
2O
7) and metavanadates (MeVO
3), where Me is a monovalent metal ion. Since the sludge under study is a product of vanadium converter slag processing roasted with limestone, the most possible acid-soluble phases in the sludge are Ca
3(VO
4)
2, Ca
2V
2O
7, Ca(VO
3)
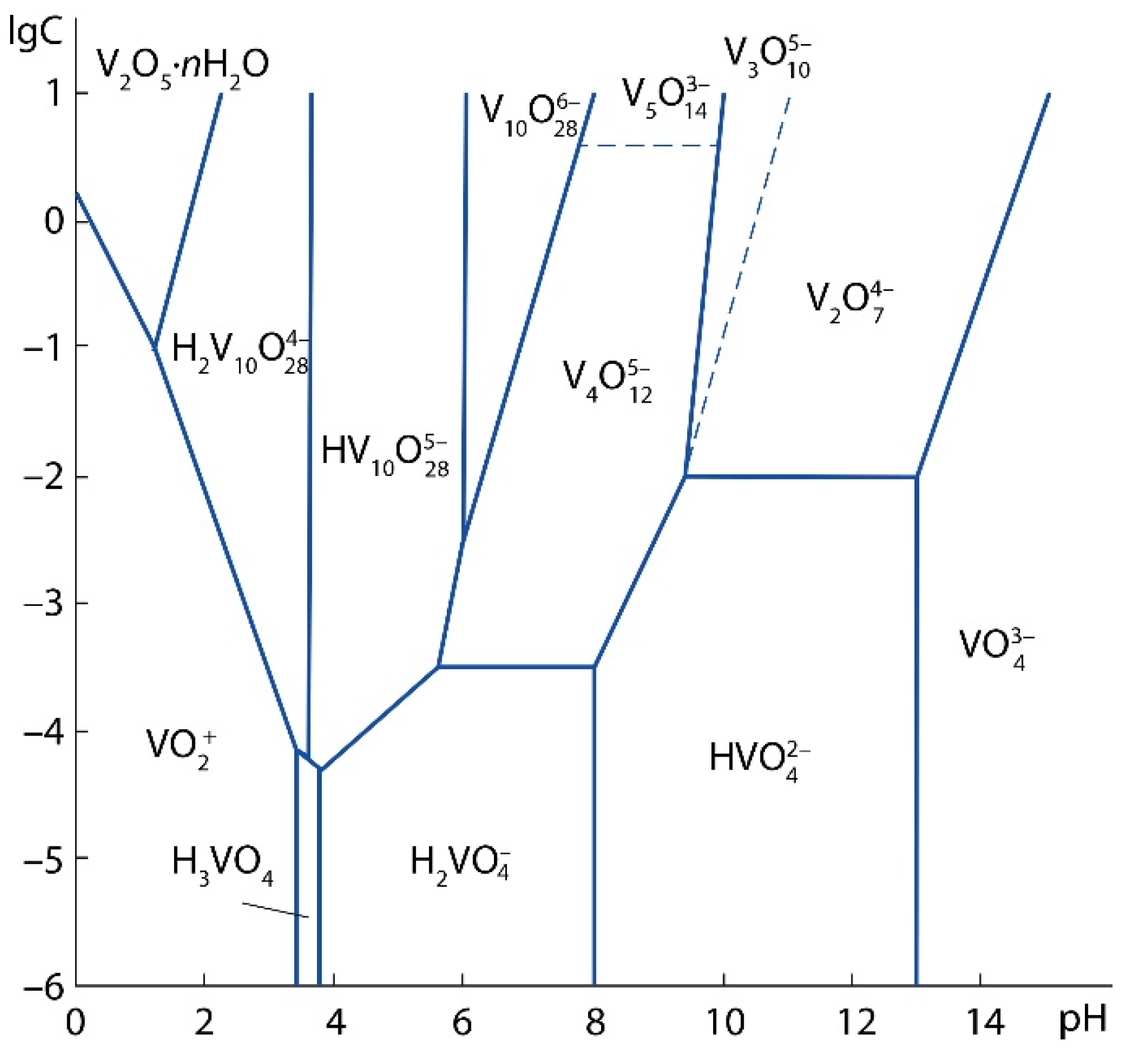
2. As a result of the treatment of calcium vanadates with a solution of sulfuric acid, various vanadium ions can be formed depending on the pH of the solution and the concentration of vanadium, (
Figure 4) [
38].
When vanadium is leached with a 5% H
2SO
4 solution, at which the pH of the resulting solution was 0.6–0.8, and the vanadium concentration in the solution after leaching was ≈ 3 g/L, the formation of VO
2+ ions is probable by the following reaction:
For example, the reaction could occur as followed:
In acidic medium at pH = 2–3 VO
2+ is reduced to VO
2+ by iron sulphate with the following reactions:
The leaching solution acquired a blue color after the addition of FeSO4·7H2O crystals indicating the predominance of V4+ in solution.
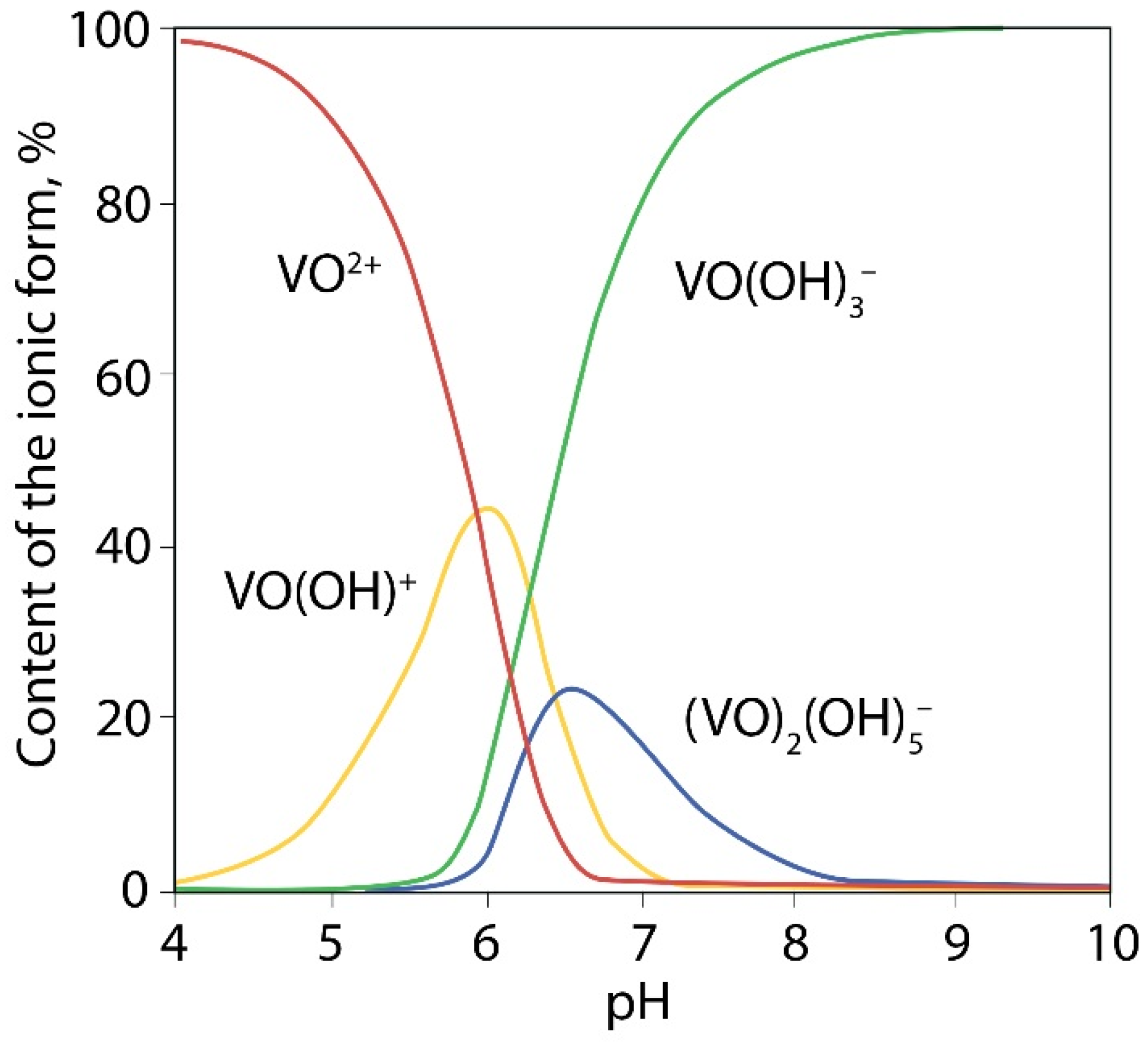
It is known that VO
2+ ions in aqueous solutions are existing mainly in the form of [VO(H
2O)
5]
2+ when pH < 3.5 and as [VO(OH)]
+ at higher pH values (
Figure 5) [
40]. At pH > 4 precipitation of VO(OH)
2 occurs by reaction:
After precipitation by brucite and roasting of the residuum, a light brown concentrate was obtained. As one can see in
Table 2, it was possible to obtain a concentrate with V
2O
5 content of ~22%. This concentrate contains a significant content of impurities, including phosphorus which is a harmful impurity for ferrous metallurgy. Thus, this concentrate requires further processing before obtaining vanadium alloys.
Investigations on reduction by metallic iron were carried out using sludge with V
2O
5 content of 2.78 wt%. (
Table 1, sample No. 2). Optimizing the process, we added iron for vanadium reduction during the leaching process and heated the pulp to 80 °C immediately after iron powder addition. As the concentrate obtained by reduction with iron sulfate contains a significant amount of phosphorus, the pH value was increased to ≈ 1.6. Reduction of V
5+ passing into solution by metallic iron can proceed by the following reactions:
In this process, solutions with V
2O
5 content of ≈6.1 g/L were obtained, while the recovery rate of V
2O
5 into solution was 36.5 wt%. The final concentrate after roasting in the muffle furnace contained 26.5 wt% of V
2O
5. Thus, metallic iron as a reducing agent allowed to increase V
2O
5 content slightly and to reduce phosphorus content in the final concentrate (see
Table 2).
Methods of one-stage reduction leaching with followed precipitation by brucite are ineffective for processing sludge due to the low vanadium content and high content of harmful impurities in the resulting concentrates as shown by investigations.
The method of reducing leaching with of FeSO
4·7H
2O additive was investigated for the recovery of vanadium from solutions with a concentration of ~20 g/L obtained under optimal conditions from roasted sludge with 1% CaCO
3 additive by two-stage counterflow leaching. The process was carried out as previous studies with FeSO
4·7H
2O additive, and as a result, a concentrate with content V
2O
5 of 53.6 wt% was obtained (
Table 3). This concentrate is characterized by a significant content of impurities, however, due to the high vanadium content, it may be suitable for smelting vanadium ligatures [
40].
Table 4 presents the technological parameters and consumption parameters of the considered processes of reducing leaching and subsequent precipitation in terms of 1 g of the resulting concentrate.
3.3. V2O5 Precipitation by Ammonium Salt
Investigations of vanadium concentrate production by precipitation with ammonium salt NH
4Cl from solutions with a content of V
2O
5 > 10 g/L were carried out using initial sludge (
Table 1, Sample No. 3) and roasted sludge with 1% CaCO
3 additive.
A final solution with a concentration of 15 g/L V
2O
5 and pH = 0.65 was obtained from the initial sludge by three-stage counterflow leaching with 5% H
2SO
4 solution at the first stage. It is known that vanadium precipitates from an acidic solution at pH = 1.8–3 and at pH = 4–8 [
12]. At pH = 1.8–3, vanadium can precipitate in the form of ammonium hexavanadate by reaction:
As a result of reaction with NH
4Cl ammonium metavanadate is formed from alkaline solutions:
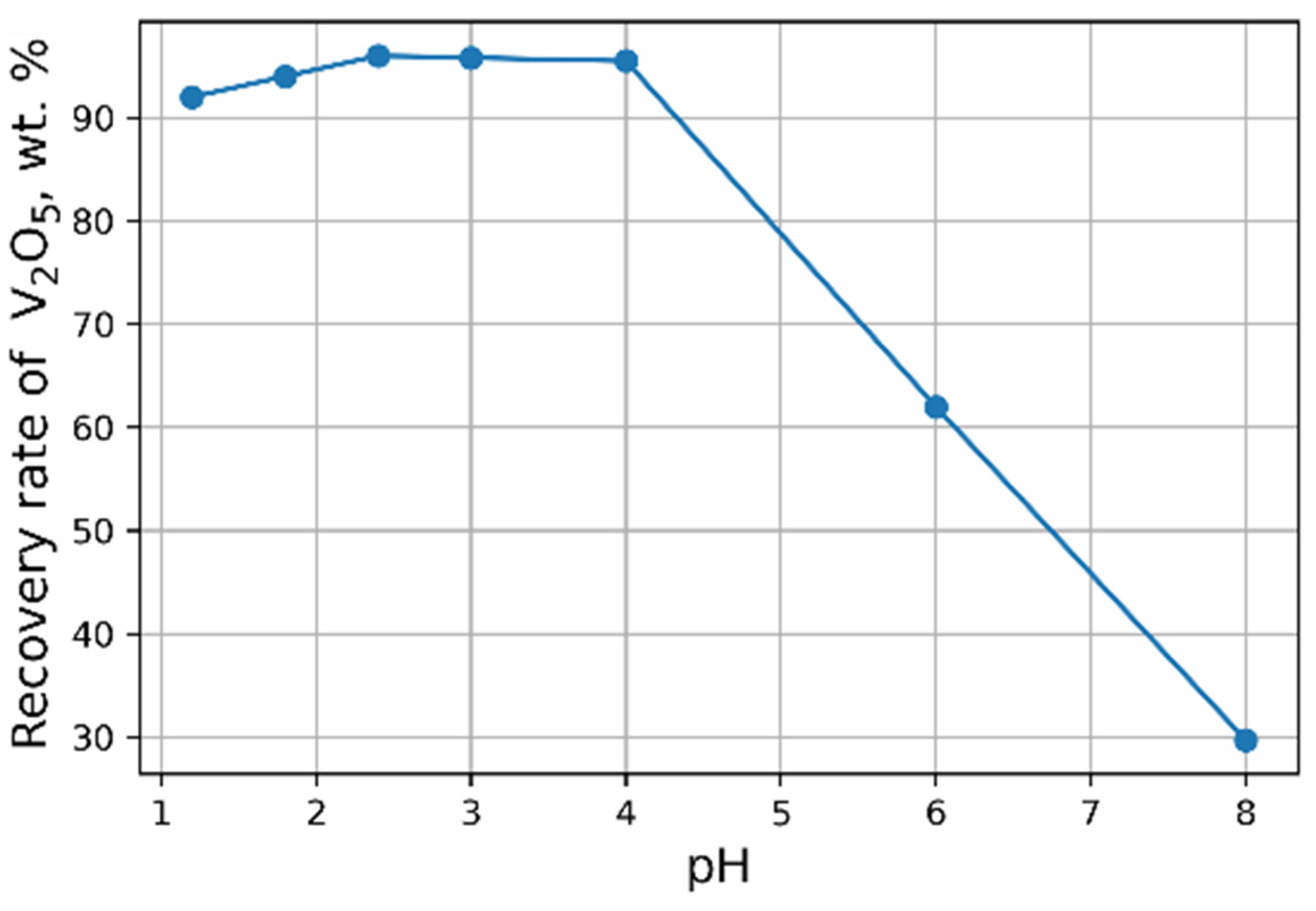
The influence of pH on the recovery rate of V
2O
5 into the concentrate is shown in
Figure 6. Tests at pH < 3 were carried out heating solutions to 95 °C. The maximal recovery rate of 96 % of V
2O
5 into the concentrate from the solution is achieved at pH = 2.4. An increase in NH
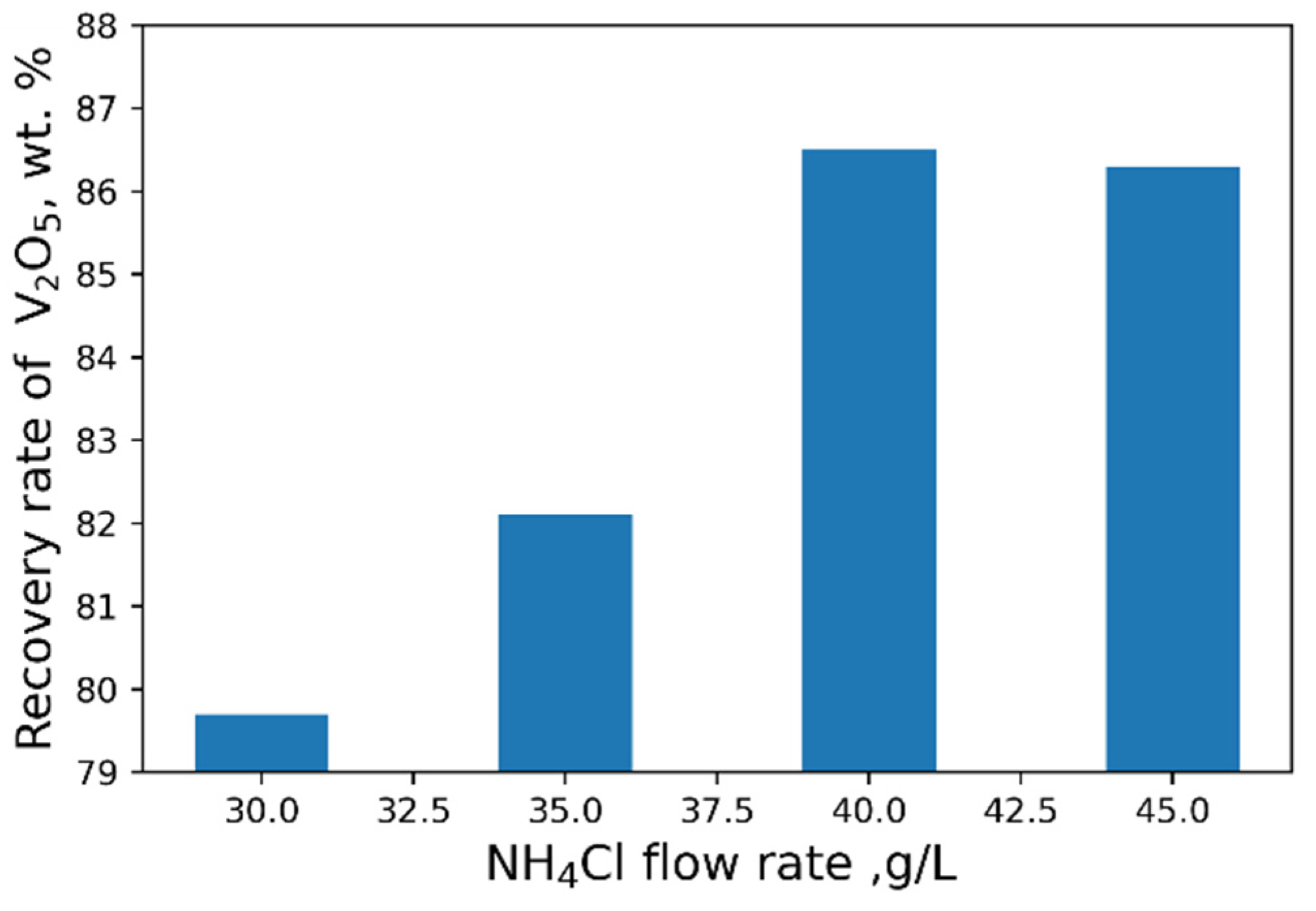
4Cl flow rate does not practically affect the yield of V
2O
5 (
Figure 7).
Compositions of concentrates at different pH values are presented in
Table 5. With an increasing pH, the recovery rate of V
2O
5 into the concentrate decreases, and an increasing amount of impurities is also observed.
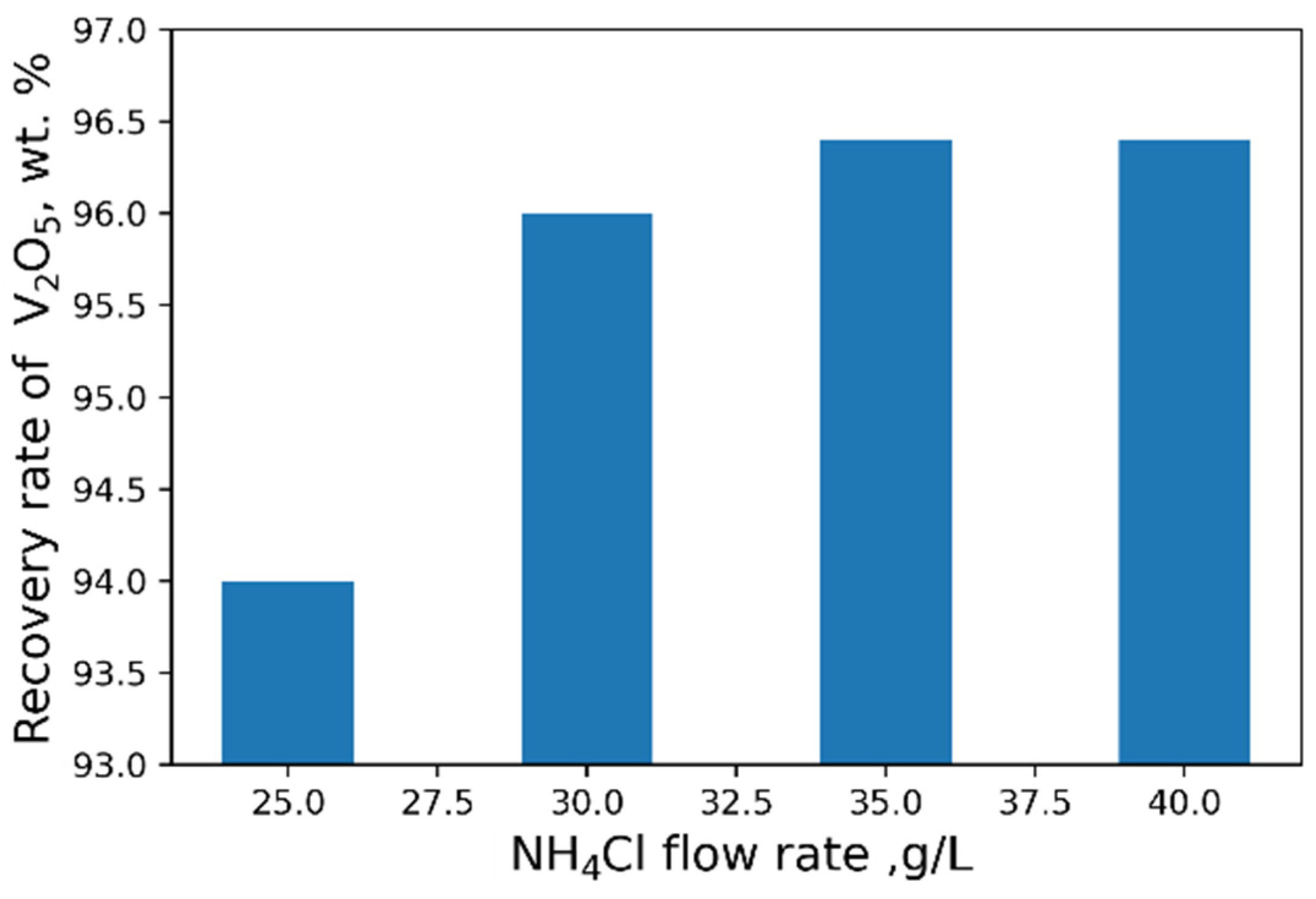
Let us consider the possibility of V2O5 precipitation from a solution with a concentration of V2O5 23 g/L by ammonium salt obtained after two-stage counterflow leaching of roasted sludge with 1% CaCO3 additive.
As a result of our studies, the following optimal parameters were selected: pH = 2.4, 40 g/L NH
4Cl flow rate (
Figure 8 and
Figure 9). At optimal conditions, we obtained the concentrate with V
2O
5 content of 93.6 wt% (
Table 6). However, higher purity vanadium pentoxide requires additional stages of concentrate processing.
Reducing the content of impurities in concentrates obtained by precipitation from solutions with V
2O
5 concentration of 15 and 23 g/L at pH = 2.4 (
Table 5 and
Table 6) was conducted on their washing by repulpation. Washing was carried out with 1% NH
4Cl solution at S/L = 1/10 at a temperature of 95 °C. In this process the removal of soluble sulfates of manganese, iron, titanium, and alkali metals takes place. Due to the low solubility of vanadium compounds in ammonium chloride solution, the losses of V
2O
5 into washing solutions were less than 0.5%. Washing of the concentrate obtained from the initial sludge allowed to increase V
2O
5 content to 84.94 wt% (
Table 7), which is slightly lower than the requirements of the standard (≥90 wt%). Additionally, the concentrate has an increased phosphorus content unsuitable for smelting high-vanadium alloys, such as FeV60, FeV80. The concentrate obtained from the roasted sludge meeting to the grade of VNO-2 (
Table 7). To obtain a cleaner vanadium pentoxide (pure, chemically pure), additional stages of washing from impurities will be required. The parameters of the considered processes are presented in
Table 8.
Parameters of the analyzed processes for obtaining vanadium concentrates from sludges are presented in
Table 9. The most effective technology includes preliminary oxidation roasting of the sludge, two-stage leaching of vanadium from the roasted sludge with a sulfuric acid solution and its further precipitation by hydrolysis or ammonium salts.