Self-Supported Fibrous Sn/SnO2@C Nanocomposite as Superior Anode Material for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Chemical/Physical Characterization
2.3. Electrochemical Characterization
3. Results and Discussion
3.1. Chemical/Physical Properties
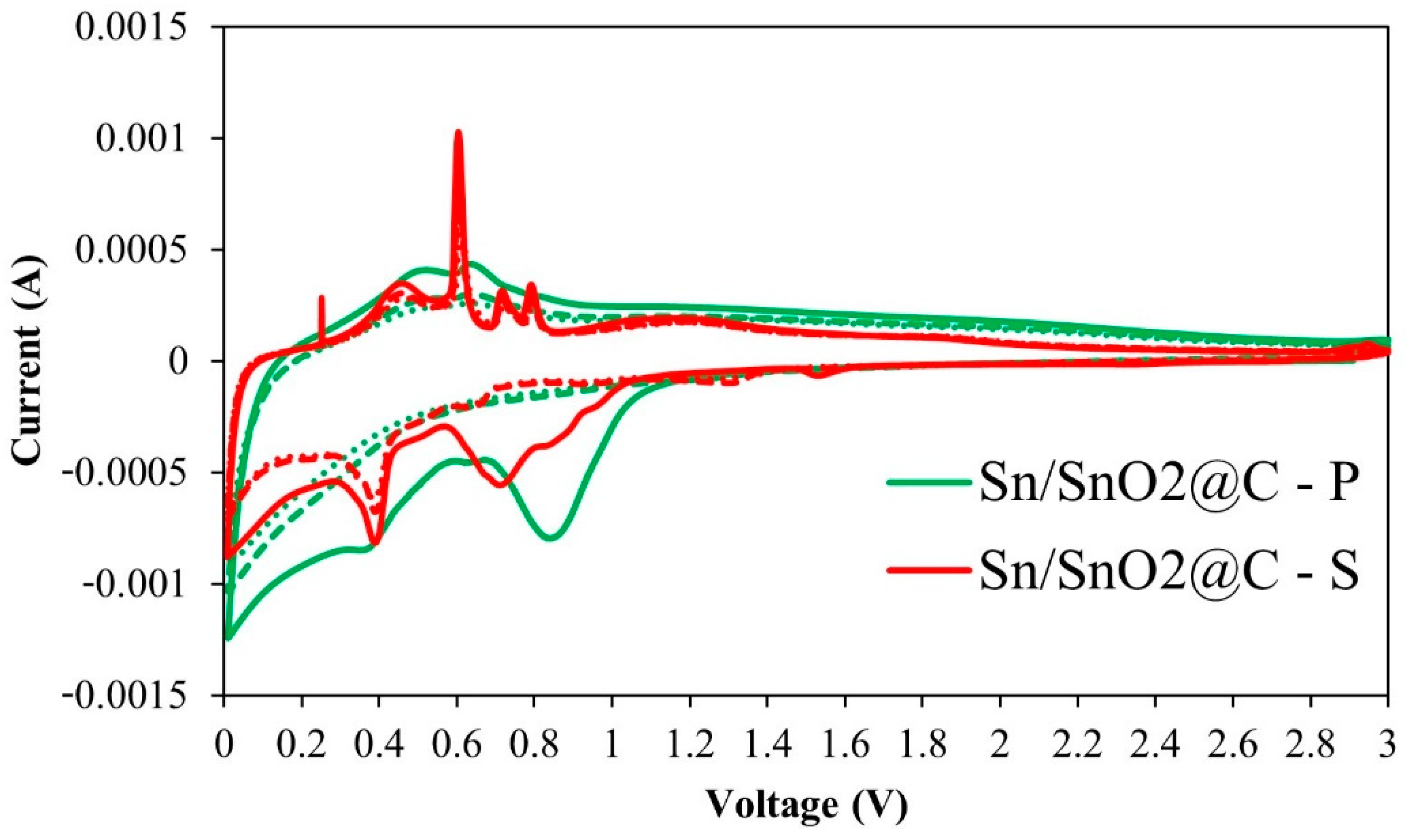
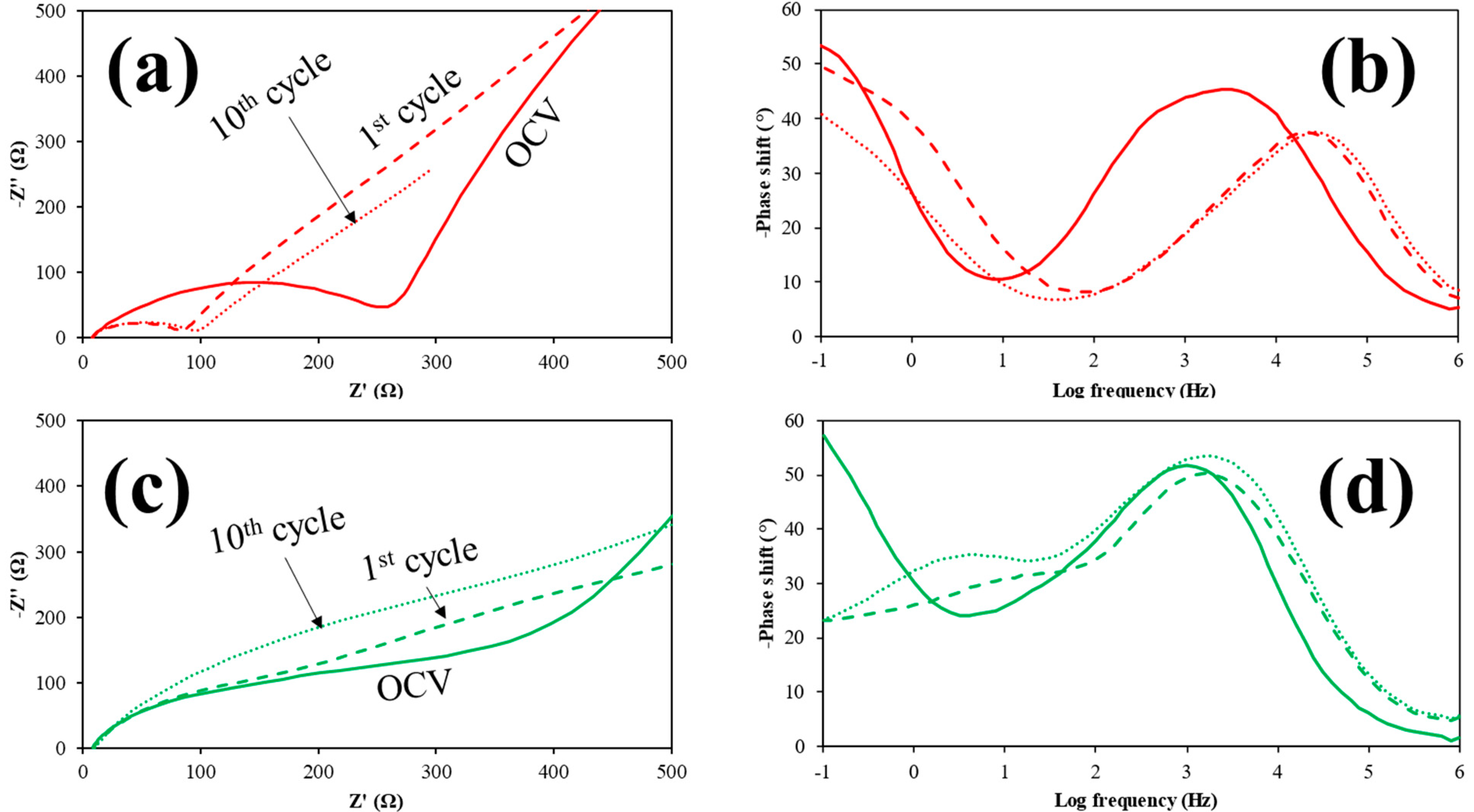
3.2. Electrochemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future and hybridized technologies. J. Mater. Chem. A. 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Roy, P.; Srivastava, S.K. Nanostructured Anode Materials for Lithium Ion Batteries. J. Mater. Chem. A. 2015, 3, 2454–2484. [Google Scholar] [CrossRef]
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A5019–A5025. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Kobayashi, T. Advanced Materials for Clean Energy; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Chung, D.D.L. Review: Electrical applications of carbon materials. J. Mater. Sci. 2004, 39, 2645–2661. [Google Scholar] [CrossRef]
- Bresser, D.; Passerini, S.; Scrosati, B. Leveraging valuable synergies by combining alloying and conversion for lithium-ion anodes. Energy Environ. Sci. 2016, 9, 3348–3367. [Google Scholar] [CrossRef] [Green Version]
- Huggins, R.A. Lithium alloy negative electrodes. J. Power Sources 1999, 81, 13–19. [Google Scholar] [CrossRef]
- Todd, A.D.W.; Ferguson, P.P.; Fleischauer, M.D.; Dahn, J.R. Tin-based materials as negative electrodes for Li-ion batteries: Combinatorial approaches and mechanical methods. Int. J. Energy Res. 2010, 34, 535–555. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Luo, Z.; Zhang, C.; Yu, X.; Zuo, Y.; Zhang, T.; Tang, P.; Arbiol, J.; Llorca, J.; et al. Compositionally tuned NixSn alloys as anode materials for lithium-ion and sodium-ion batteries with a high pseudocapacitive contribution. Electrochim. Acta 2019, 304, 246–254. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, H.; Jin, D.; Zhu, H. Hydrothermal synthesis of SnO nanoflakes as anode materials for lithium-ion batteries. Inorg. Mater. 2007, 43, 1307–1312. [Google Scholar] [CrossRef]
- Du, G.; Zhong, C.; Zhang, P.; Guo, Z.; Chen, Z.; Liu, H. Tin dioxide/carbon nanotube composites with high uniform SnO2 loading as anode materials for lithium ion batteries. Electrochim. Acta 2010, 55, 2582–2586. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, K.Y.; Kim, W.S.; Yoon, S.; Hong, S.H.; Kang, K.; Kim, M. Unveiling origin of additional capacity of SnO2 anode in lithium-ion batteries by realistic ex situ TEM analysis. Nano Energy 2016, 19, 234–245. [Google Scholar] [CrossRef]
- Wang, X.L.; Feygenson, M.; Aronson, M.C.; Han, W.Q. Sn/SnOx Core−Shell Nanospheres: Synthesis, Anode Performance in Li Ion Batteries, and Superconductivity. J. Phys. Chem. C 2010, 114, 14697–14703. [Google Scholar] [CrossRef]
- Ferraresi, G.; Villevieille, C.; Czekaj, I.; Horisberger, M.; Novák, P.; El Kazzi, M. SnO2 model electrode cycled in Li-ion battery reveals the formation of Li2SnO3 and Li8SnO6 phases through conversion reactions. ACS Appl. Mater. Interfaces 2018, 10, 8712–8720. [Google Scholar] [CrossRef] [PubMed]
- Mirolo, M.; Wu, X.; Vaz, C.A.; Novák, P.; El Kazzi, M. Unveiling the Complex Redox Reactions of SnO2 in Li-Ion batteries using operando X-ray photoelectron spectroscopy and in situ X-ray absorption spectroscopy. ACS Appl. Mater. Interfaces 2021, 13, 2547–2557. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Chen, D.; Waller, G.; Ouyang, Y.; Chen, Y.; Zhao, B.; Rainwater, B.; Yang, C.; Zhu, M.; Liu, M. Dramatically enhanced reversibility of Li2O in SnO2-based electrodes: The effect of nanostructure on high initial reversible capacity. Energy Environ. Sci. 2016, 9, 595–603. [Google Scholar] [CrossRef]
- Xie, Y.; Zhu, F. Electrochemical capacitance performance of polyaniline/tin oxide nanorod array for supercapacitor. J. Solid State Electrochem. 2017, 21, 1675–1685. [Google Scholar] [CrossRef]
- Wei, W.; Du, P.; Liu, D.; Wang, H.; Liu, P. Facile mass production of nanoporous SnO2 nanosheets as anode materials for high performance lithium-ion batteries. J. Colloid Interface Sci. 2017, 503, 205–213. [Google Scholar] [CrossRef]
- Miao, C.; Liu, M.; He, Y.-B.; Qin, X.; Tang, L.; Huang, B.; Li, R.; Li, B.; Kang, F. Monodispersed SnO2 nanospheres embedded in framework of graphene and porous carbon as anode for lithium ion batteries. Energy Storage Mater. 2016, 3, 98–105. [Google Scholar] [CrossRef]
- Park, I.J.; Park, S.; Kim, D.H.; Jeong, H.; Lee, S. SnO2 nanowires decorated with forsythia-like TiO2 for photoenergy conversion. Mater. Lett. 2017, 202, 48–51. [Google Scholar] [CrossRef]
- Li, L.; Yin, X.; Liu, S.; Wang, Y.; Chen, L.; Wang, T. Electrospun porous SnO2 nanotubes as high capacity anode materials for lithium ion batteries. Electrochem. Commun. 2010, 12, 1383–1386. [Google Scholar] [CrossRef]
- Song, L.; Yang, S.; Wei, W.; Qu, P.; Xu, M.; Liu, Y. Hierarchical SnO2 nanoflowers assembled by atomic thickness nanosheets as anode material for lithium ion battery. Sci. Bull. 2015, 60, 892–895. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhao, C.; Shen, M.; Pan, Z.; Liu, X. SnO2 nanoparticles encapsulated by curved graphite layers as anode materials for Li-ion batteries with high performances. J. Alloys Compd. 2016, 683, 191–197. [Google Scholar] [CrossRef]
- Chojnacka, A.; Molenda, M.; Bakierska, M.; Dziembaj, R. Electrochemical performance of Sn/SnO2 nanoparticles encapsulated in carbon matrix derived from plant polysaccharides. ECS Trans. 2015, 64, 165–171. [Google Scholar] [CrossRef]
- Deng, Y.; Fang, C.; Chen, G. The developments of SnO2/graphene nanocomposites as anode materials for high performance lithium ion batteries: A review. J. Power Sources 2016, 304, 81–101. [Google Scholar] [CrossRef]
- Li, Y.; Lu, X.; Wang, H.; Xie, C.; Yang, G.; Niu, C. Growth of ultrafine SnO2 nanoparticles within multiwall carbon nanotube networks: Non-solution synthesis and excellent electrochemical properties as anodes for lithium ion batteries. Electrochim. Acta 2015, 178, 778–785. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun nanofibers: New concepts, materials, and applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef]
- Maroni, F.; Bruni, P.; Suzuki, N.; Aihara, Y.; Croce, F. Electrospun tin-carbon nanocomposite as anode material for all solid state lithium-ion batteries. J. Solid State Electrochem. 2019, 23, 1697–1703. [Google Scholar] [CrossRef]
- Meligrana, G.; Ferrari, S.; Lucherini, L.; Celè, J.; Colò, F.; Brugger, J.; Ricciardi, C.; Ruffo, R.; Gerbaldi, C. Na3V2(PO4)3-supported electrospun carbon nanofiber nonwoven fabric as self-standing Na-ion cell cathode. ChemElectroChem 2020, 7, 1652–1659. [Google Scholar] [CrossRef]
- Li, H.; Bai, Y.; Wu, F.; Li, Y.; Wu, C. Budding willow branches shaped Na3V2 (PO4)3/C nanofibers synthesized via an electrospinning technique and used as cathode material for sodium ion batteries. J. Power Sources 2015, 273, 784–792. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, F.; Fan, L.Z. Self-standing Na-storage anode of Fe2 O3 nanodots encapsulated in porous N-doped carbon nanofibers with ultra-high cyclic stability. Nano Res. 2018, 11, 4026–4037. [Google Scholar] [CrossRef]
- Yu, S.; Liu, Z.; Tempel, H.; Kungl, H.; Eichel, R.A. Self-standing nasicon-type electrodes with high mass loading for fast-cycling all-phosphate sodium-ion batteries. J. Mater. Chem. A 2018, 6, 18304–18317. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Dhakate, S.R.; Gurunathan, P.; Ramesha, K. High rate capability and cyclic stability of hierarchically porous Tin oxide (IV)–carbon nanofibers as anode in lithium ion batteries. Appl. Nanosci. 2017, 7, 449–462. [Google Scholar] [CrossRef]
- Aravindan, V.; Sundaramurthy, J.; Kumar, E.N.; Kumar, P.S.; Ling, W.C.; von Hagen, R.; Mathur, S.; Ramakrishna, S.; Madhavi, S. Does carbon coating really improves the electrochemical performance of electrospun SnO2 anodes? Electrochim. Acta 2014, 121, 109–115. [Google Scholar] [CrossRef]
- Meschini, I.; Nobili, F.; Mancini, M.; Marassi, R.; Tossici, R.; Savoini, A.; Focarete, M.L.; Croce, F. High-performance Sn@ carbon nanocomposite anode for lithium batteries. J. Power Sources 2013, 226, 241–248. [Google Scholar] [CrossRef]
- Li, J.; Zou, P.; Wang, R.; Yang, C. Electrospinning Sn@ C nanofibers for high-performance flexible lithium ion battery anodes. IOP Conf. Ser. Earth Environ. Sci. 2019, 300, 042021. [Google Scholar] [CrossRef]
- Yu, Y.; Gu, L.; Wang, C.; Dhanabalan, A.; Van Aken, P.A.; Maier, J. Encapsulation of Sn@ carbon nanoparticles in bamboo-like hollow carbon nanofibers as an anode material in lithium-based batteries. Angew. Chem. 2009, 121, 6607–6611. [Google Scholar] [CrossRef]
- Shen, Z.; Hu, Y.; Chen, Y.; Zhang, X.; Wang, K.; Chen, R. Tin nanoparticle-loaded porous carbon nanofiber composite anodes for high current lithium-ion batteries. J. Power Sources 2015, 278, 660–667. [Google Scholar] [CrossRef]
- Xia, X.; Wang, X.; Zhou, H.; Niu, X.; Xue, L.; Zhang, X.; Wei, Q. The effects of electrospinning parameters on coaxial Sn/C nanofibers: Morphology and lithium storage performance. Electrochim. Acta 2014, 121, 345–351. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Q.; Teng, D.; Yang, X.; Ryu, S. Reticular Sn nanoparticle-dispersed PAN-based carbon nanofibers for anode material in rechargeable lithium-ion batteries. Electrochem. Commun. 2010, 12, 1187–1190. [Google Scholar] [CrossRef]
- Yu, Y.; Gu, L.; Zhu, C.; Van Aken, P.A.; Maier, J. Tin nanoparticles encapsulated in porous multichannel carbon microtubes: Preparation by single-nozzle electrospinning and application as anode material for high-performance Li-based batteries. J. Am. Chem. Soc. 2009, 131, 15984–15985. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, B.; Wang, X.; Park, J.; Dou, S.; Ahn, H.; Kim, K. Sn/graphene nanocomposite with 3D architecture for enhanced reversible lithium storage in lithium ion batteries. J. Mater. Chem. 2009, 19, 8378–8384. [Google Scholar] [CrossRef]
- Seo, S.-D.; Lee, G.-H.; Lim, A.-H.; Min, K.-M.; Kim, J.-C.; Shim, H.-W.; Park, K.-S.; Kim, D.-W. Direct assembly of tin–MWCNT 3D-networked anode for rechargeable lithium ion batteries. RSC Adv. 2012, 2, 3315–3320. [Google Scholar] [CrossRef]
- Wang, W.; Li, P.; Fu, Y.; Ma, X. The preparation of double-void-space SnO2/carbon composite as high-capacity anode materials for lithium-ion batteries. J. Power Sources 2013, 238, 464–468. [Google Scholar] [CrossRef]
- Hameed, N.; Sharp, J.; Nunna, S.; Creighton, C.; Magniez, K.; Jyotishkumar, P.; Salim, N.V.; Fox, B. Structural transformation of polyacrylonitrile fibers during stabilization and low temperature carbonization. Polym. Degrad. Stab. 2016, 128, 39–45. [Google Scholar] [CrossRef]
- Dahn, J.R.; Zheng, T.; Liu, Y.; Xue, J.S. Mechanisms for lithium insertion in carbonaceous materials. Science 1995, 270, 590–593. [Google Scholar] [CrossRef]
- Miao, R.; Zhu, J.; Kang, S.; Yang, J.; Wang, J.; Fu, J.; Li, M.; Shi, C. In-situ mechanochemical synthesis of sub-micro Si/Sn@ SiOx-C composite as high-rate anode material for lithium-ion batteries. Electrochim. Acta 2021, 384, 138413. [Google Scholar] [CrossRef]
- Soin, N.; Roy, S.S.; Ray, S.C.; McLaughlin, J.A. Excitation energy dependence of Raman bands in multiwalled carbon nanotubes. J. Raman Spectrosc. 2010, 41, 1227–1233. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef] [Green Version]
- Schuepfer, D.B.; Badaczewski, F.; Guerra-Castro, J.M.; Hofmann, D.M.; Heiliger, C.; Smarsly, B.; Klar, P.J. Assessing the structural properties of graphitic and non-graphitic carbons by Raman spectroscopy. Carbon 2020, 161, 359–372. [Google Scholar] [CrossRef]
- Muñoz-Márquez, M.Á.; Saurel, D.; Gómez-Cámer, J.L.; Casas-Cabanas, M.; Castillo-Martínez, E.; Rojo, T. Na-Ion Batteries for large scale applications: A review on anode materials and solid electrolyte interphase formation. Adv. Energy Mater. 2017, 7, 1700463. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef] [Green Version]
- Spada, D.; Quinzeni, I.; Bini, M. Orthorhombic and monoclinic modifications of FeNb11O29, as promising anode materials for lithium batteries: Relationships between pseudocapacitive behaviour and structure. Electrochim. Acta 2019, 296, 938–944. [Google Scholar] [CrossRef]
- He, Z.K.; Kamali, A.R.; Wang, Z.R.; Sun, Q.; Shi, Z.; Wang, D. Rapid preparation and characterization of oxygen-deficient SnO2 nanobelts with enhanced Li diffusion kinetics. J. Electroanal. Chem. 2020, 871, 114276. [Google Scholar] [CrossRef]
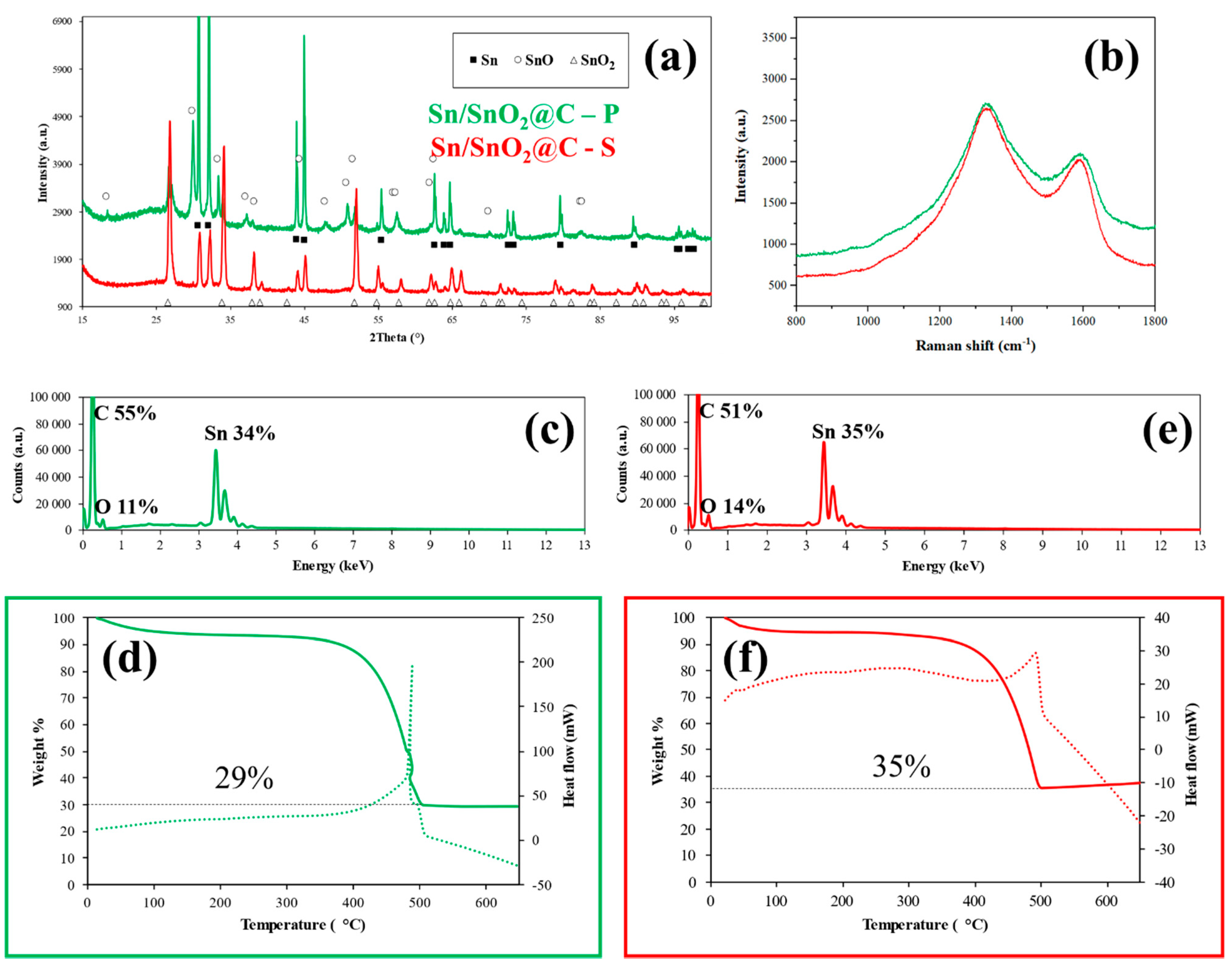
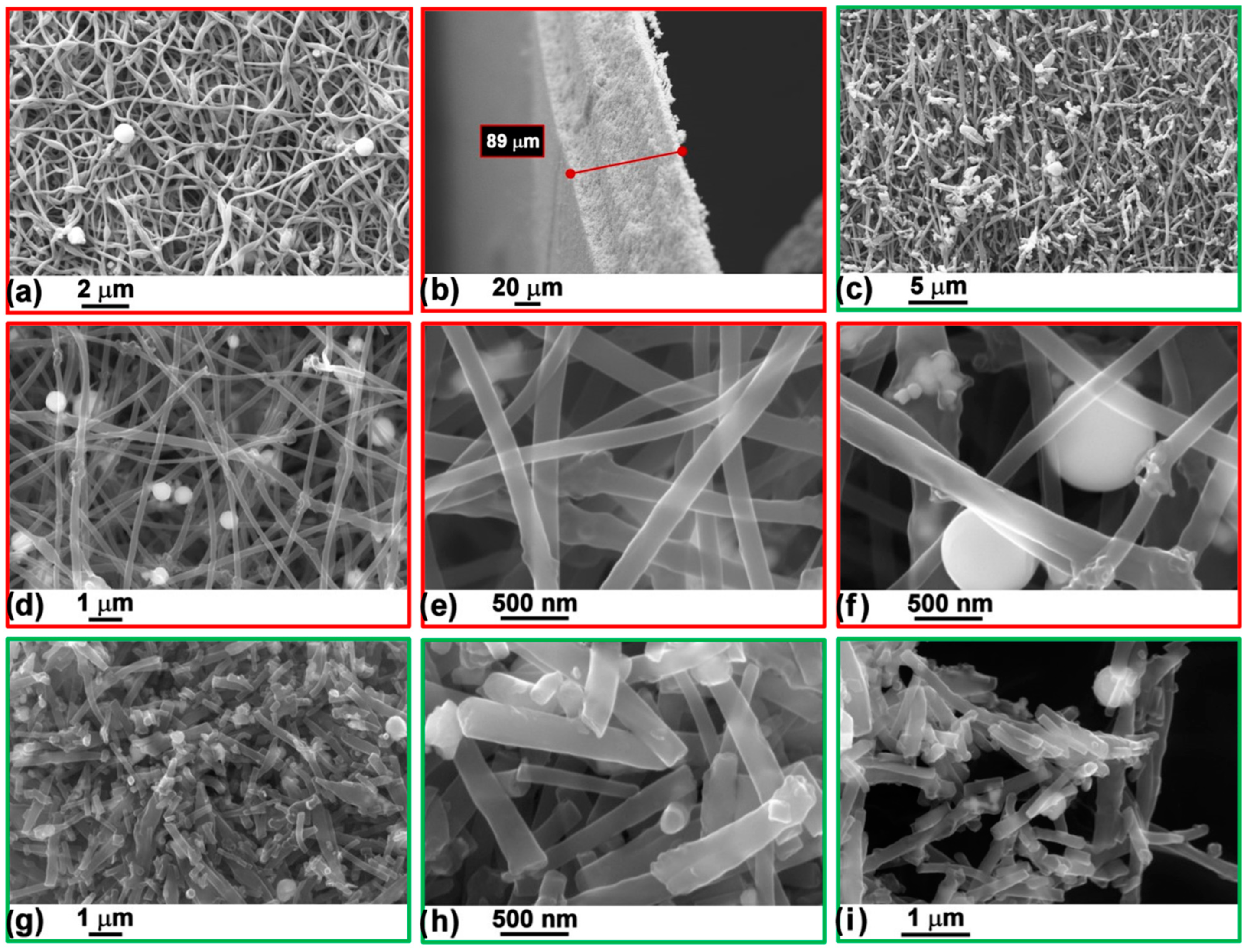


| Sn (wt.%) | Cry. Size (nm) | SnO (wt.%) | Cry. Size (nm) | SnO2 (wt.%) | Cry. Size (nm) | Rwp/GOF | |
|---|---|---|---|---|---|---|---|
| Sn/SnO2@C–P | 62.28 (46) | 120.9 (12) | 12.29 (26) | 33.53 (65) | 25.43 (23) | 45.9 (19) | 5.45/1.39 |
| Sn/SnO2@C–S | 21.80 (19) | 44.37 (86) | - | - | 78.20 (19) | 40.50 (37) | 9.57/1.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spada, D.; Bruni, P.; Ferrari, S.; Albini, B.; Galinetto, P.; Berbenni, V.; Girella, A.; Milanese, C.; Bini, M. Self-Supported Fibrous Sn/SnO2@C Nanocomposite as Superior Anode Material for Lithium-Ion Batteries. Materials 2022, 15, 919. https://doi.org/10.3390/ma15030919
Spada D, Bruni P, Ferrari S, Albini B, Galinetto P, Berbenni V, Girella A, Milanese C, Bini M. Self-Supported Fibrous Sn/SnO2@C Nanocomposite as Superior Anode Material for Lithium-Ion Batteries. Materials. 2022; 15(3):919. https://doi.org/10.3390/ma15030919
Chicago/Turabian StyleSpada, Daniele, Pantaleone Bruni, Stefania Ferrari, Benedetta Albini, Pietro Galinetto, Vittorio Berbenni, Alessandro Girella, Chiara Milanese, and Marcella Bini. 2022. "Self-Supported Fibrous Sn/SnO2@C Nanocomposite as Superior Anode Material for Lithium-Ion Batteries" Materials 15, no. 3: 919. https://doi.org/10.3390/ma15030919
APA StyleSpada, D., Bruni, P., Ferrari, S., Albini, B., Galinetto, P., Berbenni, V., Girella, A., Milanese, C., & Bini, M. (2022). Self-Supported Fibrous Sn/SnO2@C Nanocomposite as Superior Anode Material for Lithium-Ion Batteries. Materials, 15(3), 919. https://doi.org/10.3390/ma15030919










