Reaction Kinetics and Process Model of the Polyacrylonitrile Fibers Stabilization Process Based on Dielectric Measurements
Abstract
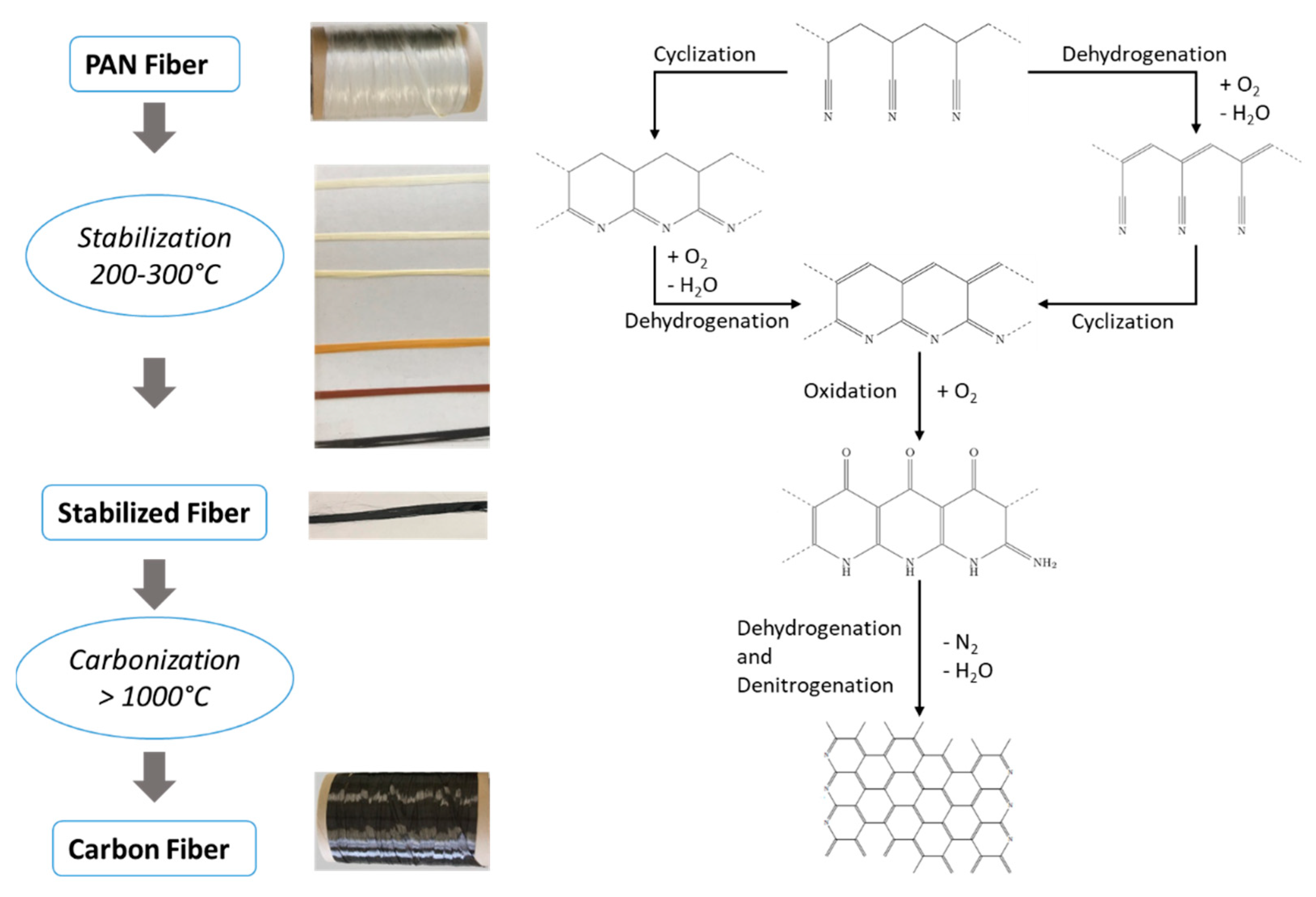
1. Introduction
2. Materials and Methods
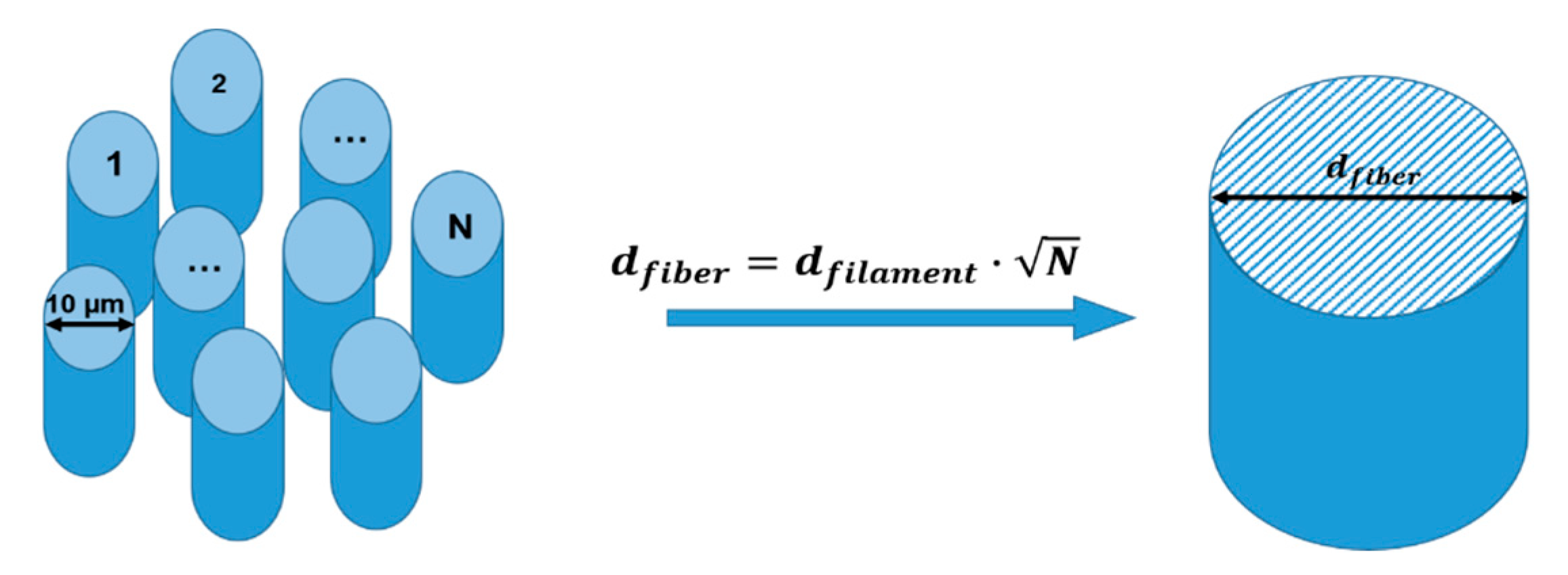
2.1. Materials
2.2. Dielectric Measurements with the Perturbation Method
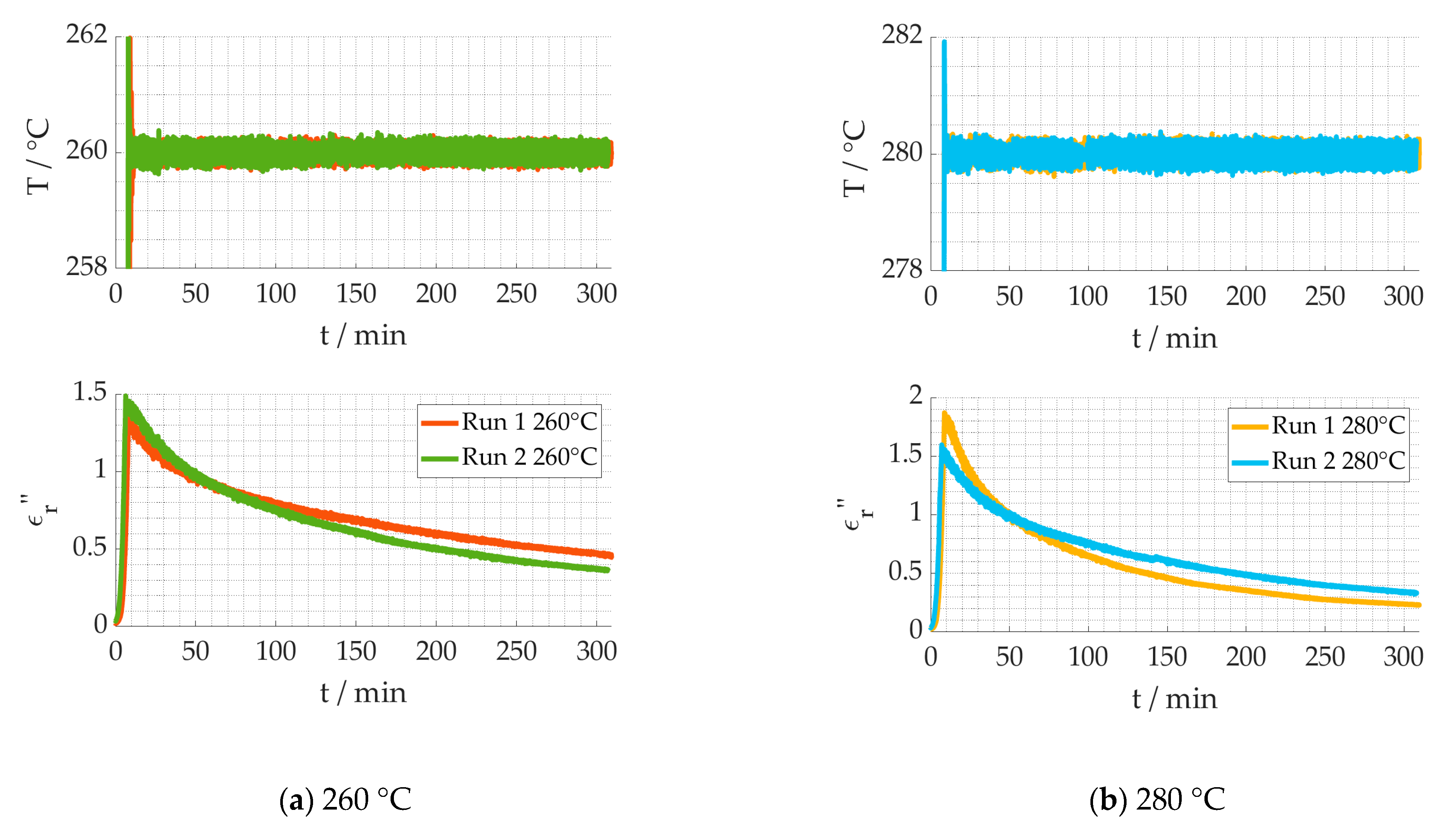
2.3. Dielectric Measurements Error Evaluation
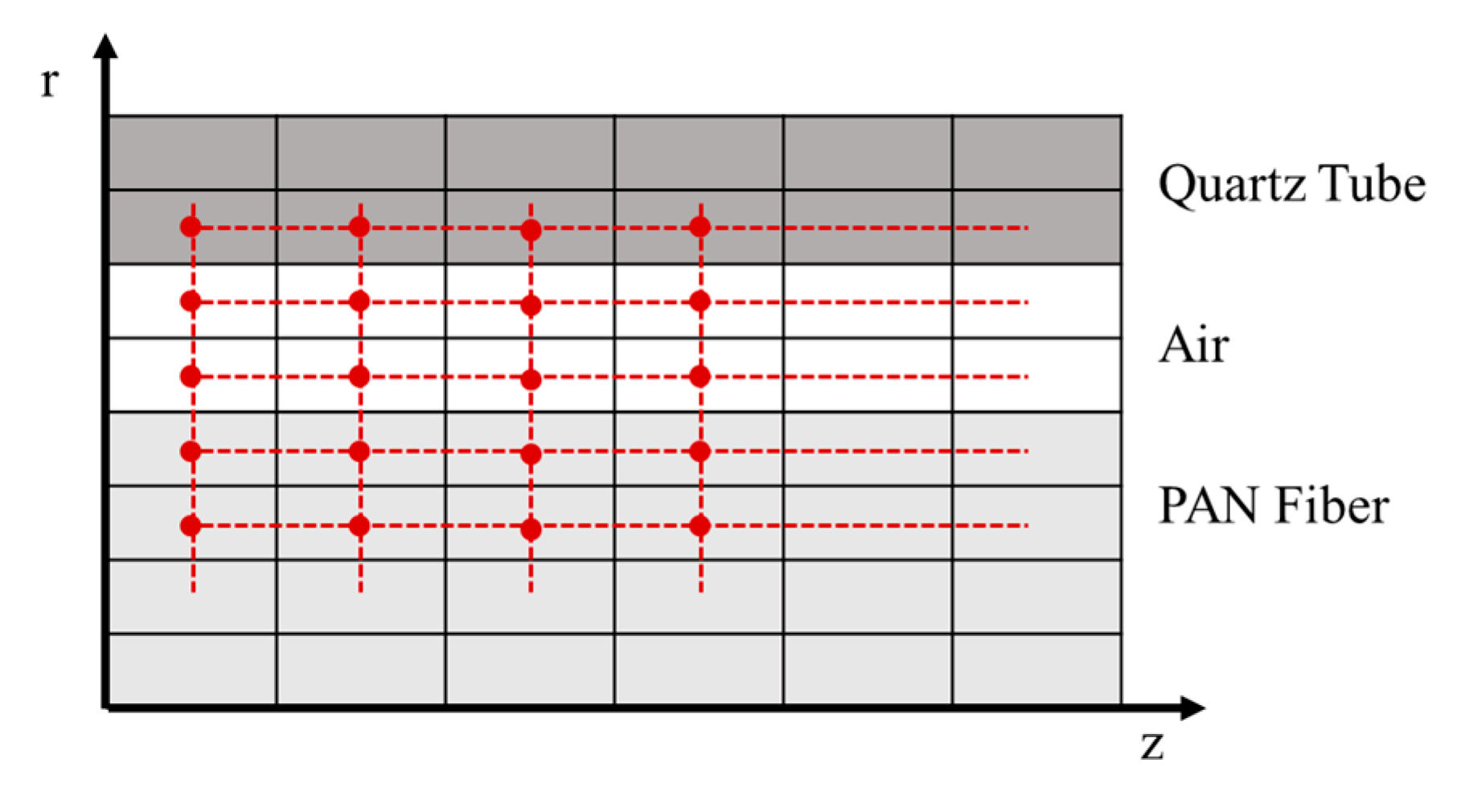
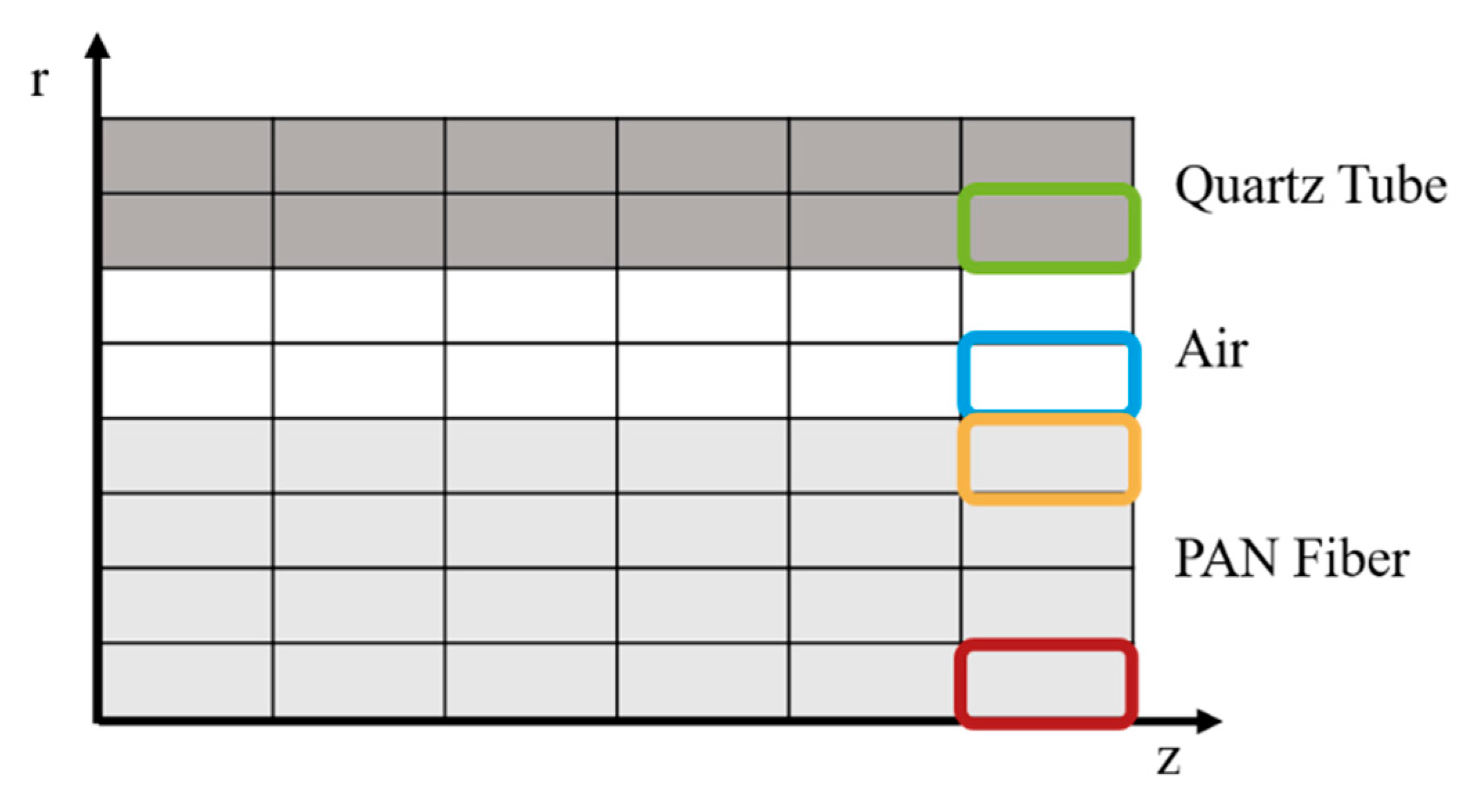
2.4. Thermal Model
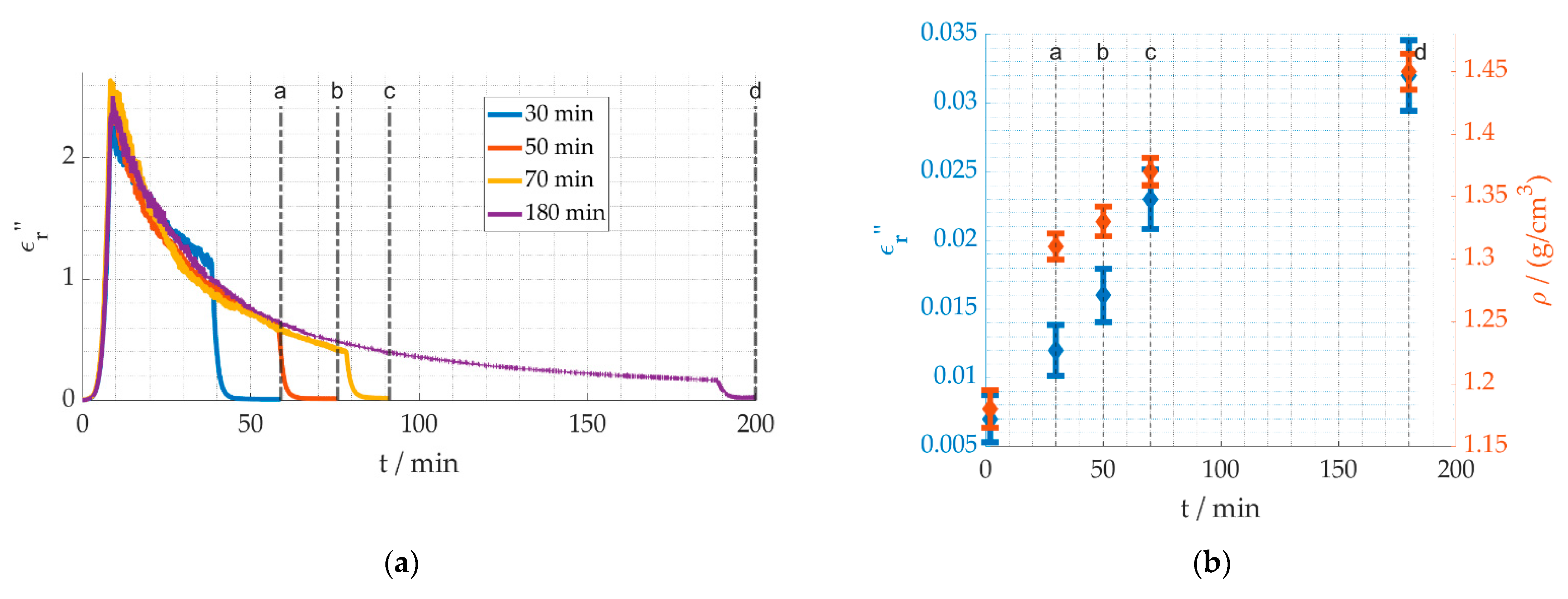
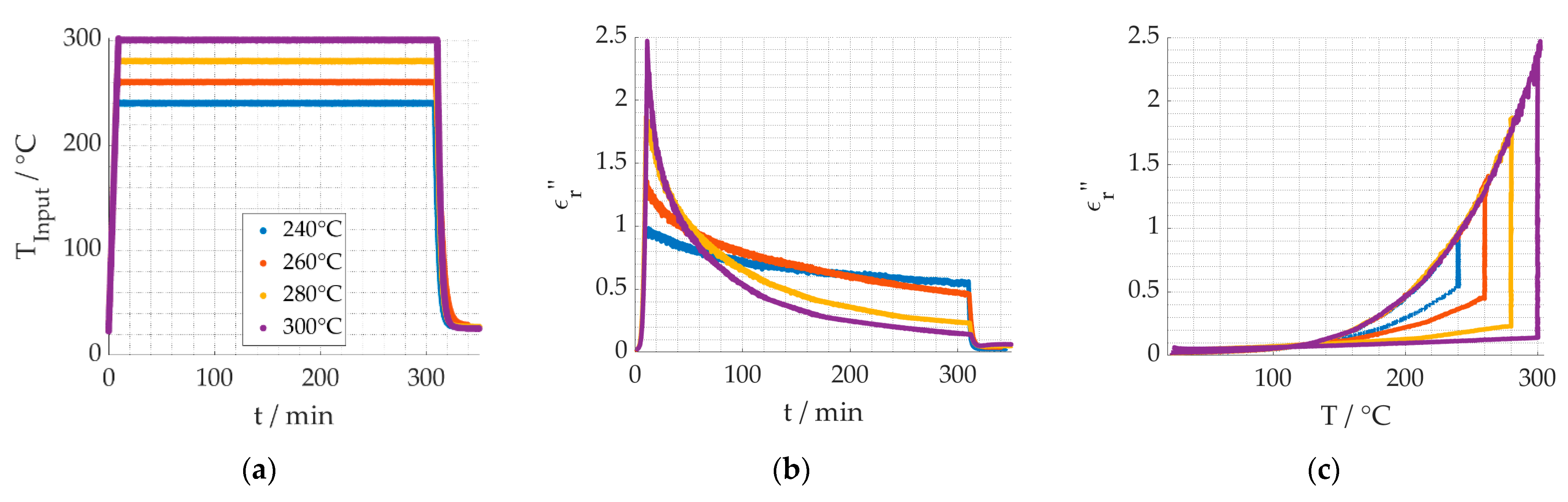
3. Results
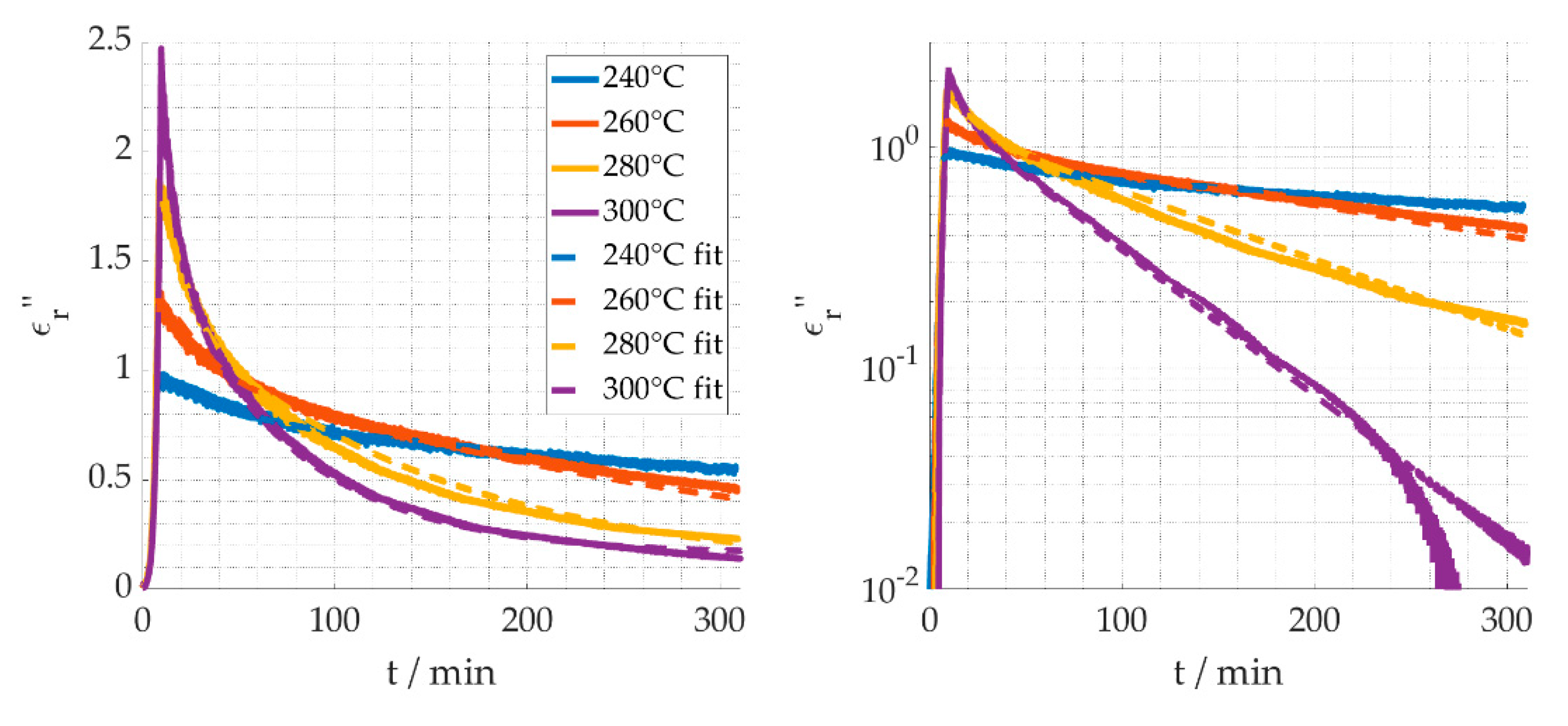
4. Discussion
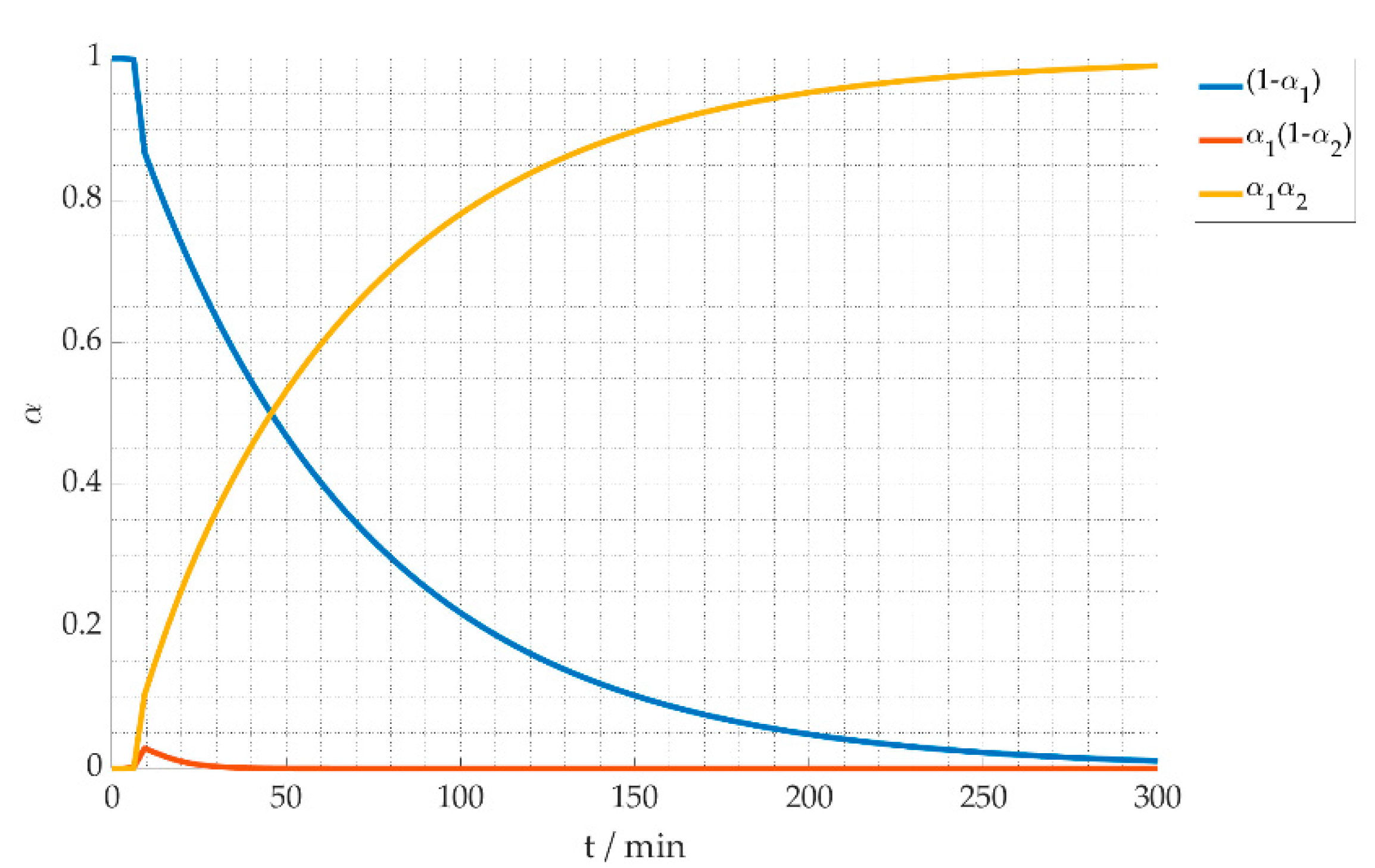
4.1. Reaction Kinetics
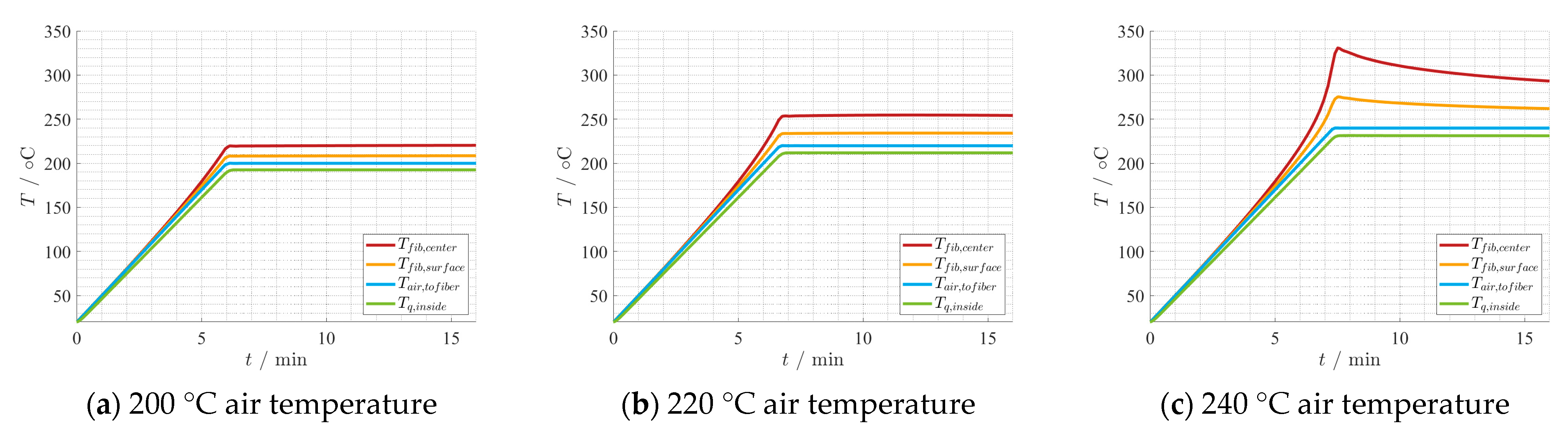
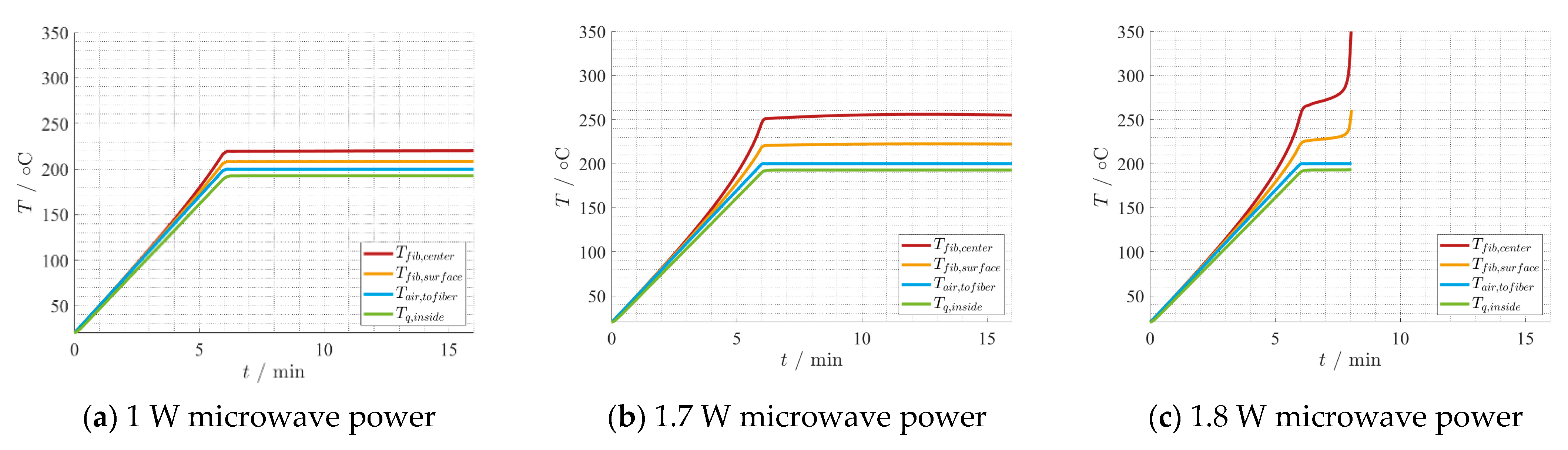
4.2. Thermal Process Model
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, S.-J. Carbon Fibers; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Dragon Plate. Carbon Fiber vs. Aluminum. Available online: https://dragonplate.com/carbon-fiber-vs-aluminum (accessed on 21 October 2021).
- Heine, M. Optimierung der Reaktionsbedingungen von Thermoplastischen Polymerfasern zur Kohlenstoffaser-Herstellung am Beispiel von Polyacrylnitril. PhD. Thesis, Technische Universität Karlsruhe, Karlsruhe, Germany, 1989. [Google Scholar]
- Paulauskas, F.L.; White, T.L. Temperature-dependent Dielectric Measurements of Polyacrylonitrile Fibers during Air Oxidation. In Proceedings of the International SAMPE Symposium and Exhibition, Long Beach, CA, USA, 16–20 May 2004. [Google Scholar]
- Chao, H.-W.; Hsu, H.-C.; Chen, Y.-R.; Chang, T.-H. Characterizing the dielectric properties of carbon fiber at different processing stages. Sci. Rep. 2021, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P. Carbon Fibers and Their Composites; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Wohlmann, B.; Wölki, M.; Hunyar, C.; Emmerich, R.; Kaiser, M.; Graf, M.; Alberts, L.; Nauenburg, K.-D. Stabilization of Polyacrylonitrile Precursor Yarn. EP2475812 B1, 18 July 2012. [Google Scholar]
- Paulauskas, F.; White, T.; Sherman, D. Apparatus and Method for Oxidation and Stabilization of Polymeric Materials. U.S. Patent US7786253 B2, 31 August 2010. [Google Scholar]
- Paulauskas, F.; Naskar, A.; Bonds, T. Advanced Oxidation Method for Producing High-Density Oxidized Polyacrylionitrile Fibers. U.S. Patent US20150252501 A1, 10 September 2015. [Google Scholar]
- Paulauaskas, F.; Bigelow, T.; Meek, T. Diagnostic monitor for carbon fiber processing. U.S. Patent US6375875 B, 23 April 2002. [Google Scholar]
- Paulauskas, F.L.; Carpenter, J.A.; Warren, C.D. Oxidative stabilization of pan fiber precursor. In Automotive Lightweighting Materials, FY 2004 Progress Report; Office of Energy Efficiency and Renewable Energy: Washington, DC, USA, 2004. [Google Scholar]
- Lee, S.-W.; Lee, H.-Y.; Jang, S.-Y.; Jo, S.-M.; Lee, H.-S.; Lee, S. Tensile properties and morphology of carbon fibers stabilized by plasma treatment. Carbon Lett. 2011, 12, 16–20. [Google Scholar] [CrossRef][Green Version]
- Lee, S.-W.; Lee, H.-Y.; Jang, S.-Y.; Jo, S.; Lee, H.-S.; Choe, W.-H.; Lee, S. Efficient preparation of carbon fibers using plasma assisted stabilization. Carbon 2013, 55, 361–365. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, S.; Shen, Z.; Xu, L.; Zhang, L.; Peng, J. Study on the oxidative stabilization of polyacrylonitrile fibers by microwave heating. Polym. Degrad. Stab. 2018, 150, 86–91. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Guo, S.; Xiao, S.; Shen, Z.; Xu, L. Comparison of microwave and conventional heating methods for oxidative stabilization of polyacrylonitrile fibers at different holding time and heating rate. Ceram. Int. 2018, 44, 14377–14385. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, J.; Xu, L.; Guo, S. Study on Structure Evolution and Reaction Mechanism in Microwave Pre-oxidation. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3562–3571. [Google Scholar] [CrossRef]
- Dunham, M.; Edie, D. Model of stabilization for pan-based carbon fiber precursor bundles. Carbon 1992, 30, 435–450. [Google Scholar] [CrossRef]
- Khayyam, H.; Jazar, R.N.; Nunna, S.; Golkarnarenji, G.; Badii, K.; Fakhrhoseini, S.M.; Kumar, S.; Naebe, M. PAN precursor fabrication, applications and thermal stabilization process in carbon fiber production: Experimental and mathematical modelling. Prog. Mater. Sci. 2020, 107, 100575. [Google Scholar] [CrossRef]
- Terra, B.M.; de Andrade, D.A.; de Mesquita, R.N. Characterization of polyacrylonitrile thermal stabilization process for carbon fiber production using intelligent algorithms. Polym. Test. 2021, 100, 107238. [Google Scholar] [CrossRef]
- Yu, M.-J.; Bai, Y.-J.; Wang, C.-G.; Xu, Y.; Guo, P.-Z. A new method for the evaluation of stabilization index of polyacrylonitrile fibers. Mater. Lett. 2007, 61, 2292–2294. [Google Scholar] [CrossRef]
- Chen, L.-F.; Ong, C.; Neo, C.; Varadan, V.; Varadan, V.K. Microwave Electronics: Measurement and Materials Characterization; John Wiley & Sons: West Sussex, UK, 2004. [Google Scholar]
- Ramopoulos, V.; Soldatov, S.; Link, G.; Kayser, T.; Gehringer, M.; Jelonnek, J. Microwave system for in-situ dielectric and calorimetric measurements in a wide temperature range using a TE 111-mode cavity. In Proceedings of the 2015 IEEE MTT-S International Microwave Symposium, Phoenix, AZ, USA, 17–22 May 2015; pp. 1–4. [Google Scholar]
- Dassault Systems. CST Studio Suite 2018. Available online: https://www.3ds.com/de/produkte-und-services/simulia/produkte/cst-studio-suite/ (accessed on 30 November 2021).
- Pozar, D.M. Microwave Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Thermocoupleinfo. Thermocouple Accuracies. Available online: https://www.thermocoupleinfo.com/thermocouple-accuracies.htm (accessed on 30 November 2021).
- JCGM. GUM: Evaluation of Measurement Data—Guide to the Expression of Uncertainty in Measurement; JCGM 100:2008; JCGM: 2010. Available online: https://www.bipm.org/documents/20126/2071204/JCGM_100_2008_E.pdf/cb0ef43f-baa5-11cf-3f85-4dcd86f77bd6 (accessed on 19 December 2021).
- The MathWorks, Inc., Version: Matlab R2019b. Available online: https://de.mathworks.com/products/matlab.html (accessed on 30 November 2021).
- Stephan, P.; Kabelac, S.; Kind, M.; Mewes, D.; Schaber, K.; Wetzel, T. VDI-Wärmeatlas: Fachlicher Träger VDI-Gesellschaft Verfahrenstechnik und Chemieingenieurwesen; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Mehdizadeh, M. Microwave/RF Applicators and Probes: For Material Heating, Sensing, and Plasma Generation; William Andrew: San Diego, CA, USA; Burlington, ON, USA, 2015. [Google Scholar]
- Hofele, J.; Link, G.; Jelonnek, J. Dielectric Measurements of PAN Precursor and Stabilized Fibers. In Proceedings of the 2020 German Microwave Conference (GeMiC), Cottbus, Germany, 9–11 March 2020; pp. 192–195. [Google Scholar]
- SGL Carbon. PANOX Oxidierte PAN-Fasern. Available online: https://www.sglcarbon.com/loesungen/material/panox-oxidierte-pan-fasern/ (accessed on 30 November 2021).
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochimica Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, J.; Liang, J. Correlative study of critical reactions in polyacrylonitrile based carbon fiber precursors during thermal-oxidative stabilization. Polym. Degrad. Stab. 2013, 98, 219–229. [Google Scholar] [CrossRef]
- Badii, K.; Golkarnarenji, G.; Milani, A.S.; Naebe, M.; Khayyam, H. A comprehensive chemical model for the preliminary steps of the thermal stabilization process in a carbon fibre manufacturing line. React. Chem. Eng. 2018, 3, 959–971. [Google Scholar] [CrossRef]
- Gandert, J. Model Set Up for the Stabilization of PAN Fibers- Implementation of the Reaction Kinetics and Analysis of Heat Input and Heat Transfer. Master’s Thesis, Karlsruhe Institute of Technology, Karlsruhe, Germany, 2020. [Google Scholar]




| Parameter | Unit | Value | Confidence Interval | |
|---|---|---|---|---|
| 1/s | 2.44 × 105 | 2.36 × 105 | 2.52 × 105 | |
| kJ/mol | 98.56 | 98.42 | 98.72 | |
| 1/s | 10.2 | 9.5 | 11 | |
| kJ/mol | 39.2 | 38.86 | 39.52 | |
| - | 1083 | 1068 | 1099 | |
| - | 3689 | 3681 | 3696 | |
| - | 2511 | 2115 | 2907 | |
| - | 2572 | 2485 | 2658 | |
| - | 14.5 × 108 | 9.2 × 108 | 19.8 × 108 | |
| - | 13.1 × 103 | 12.9 × 103 | 13.3 × 103 | |
| Fixed Parameter | Value | Unit |
|---|---|---|
| ambient air temperature | 20 | °C |
| air flow | 100 | l/min |
| cavity length | 45 | mm |
| cavity radius | 45 | mm |
| mesh cells in axial direction | 30 | - |
| fiber mesh cells in radial direction | 10 | - |
| air mesh cells in radial direction | 4 | - |
| quartz tube mesh cells in radial direction | 4 | - |
| electrical field strength from CST | 12,000 | V/m |
| heat of reaction for cyclization | −6.53 × 105 | J/kg |
| heat of reaction for dehydration | −4.933 × 106 | J/kg |
| heat of reaction for oxidation | −1.44 × 107 | J/kg |
| effective thermal conductivity of fiber, radial | 0.069 | W/(m·K) |
| effective heat capacity of fiber | 1310 | J/(kg·K) |
| emission coefficient of fiber | 0.95 | - |
| effective density of fiber | 616 | kg/m3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofele, J.; Link, G.; Jelonnek, J. Reaction Kinetics and Process Model of the Polyacrylonitrile Fibers Stabilization Process Based on Dielectric Measurements. Materials 2022, 15, 1222. https://doi.org/10.3390/ma15031222
Hofele J, Link G, Jelonnek J. Reaction Kinetics and Process Model of the Polyacrylonitrile Fibers Stabilization Process Based on Dielectric Measurements. Materials. 2022; 15(3):1222. https://doi.org/10.3390/ma15031222
Chicago/Turabian StyleHofele, Julia, Guido Link, and John Jelonnek. 2022. "Reaction Kinetics and Process Model of the Polyacrylonitrile Fibers Stabilization Process Based on Dielectric Measurements" Materials 15, no. 3: 1222. https://doi.org/10.3390/ma15031222
APA StyleHofele, J., Link, G., & Jelonnek, J. (2022). Reaction Kinetics and Process Model of the Polyacrylonitrile Fibers Stabilization Process Based on Dielectric Measurements. Materials, 15(3), 1222. https://doi.org/10.3390/ma15031222






