Roughness and SEM Analysis of Manual and Ultrasonic Instrumentation over Different Crown Materials for Dental Implants Restorations
Abstract
1. Introduction
2. Materials and Methods
- All the vestibular surfaces of the first five elements of each material were mechanically treated with a peek insert (ICS-IC1, Mectron, Carasco, Italy) mounted on a MultiPiezo ultrasonic motor (Mectron, Carasco, Italy);
- All the lingual surfaces of the first five elements of each material were mechanically treated with a steel insert (PS, EMS, Nyon, Switzerland) mounted on a Mini Piezon ultrasonic motor (EMS, Nyon, Switzerland);
- All the vestibular surfaces of the second five elements of each material were manually treated with titanium curettes (Wingrove 3-4, PDT, Missoula, MT, USA);
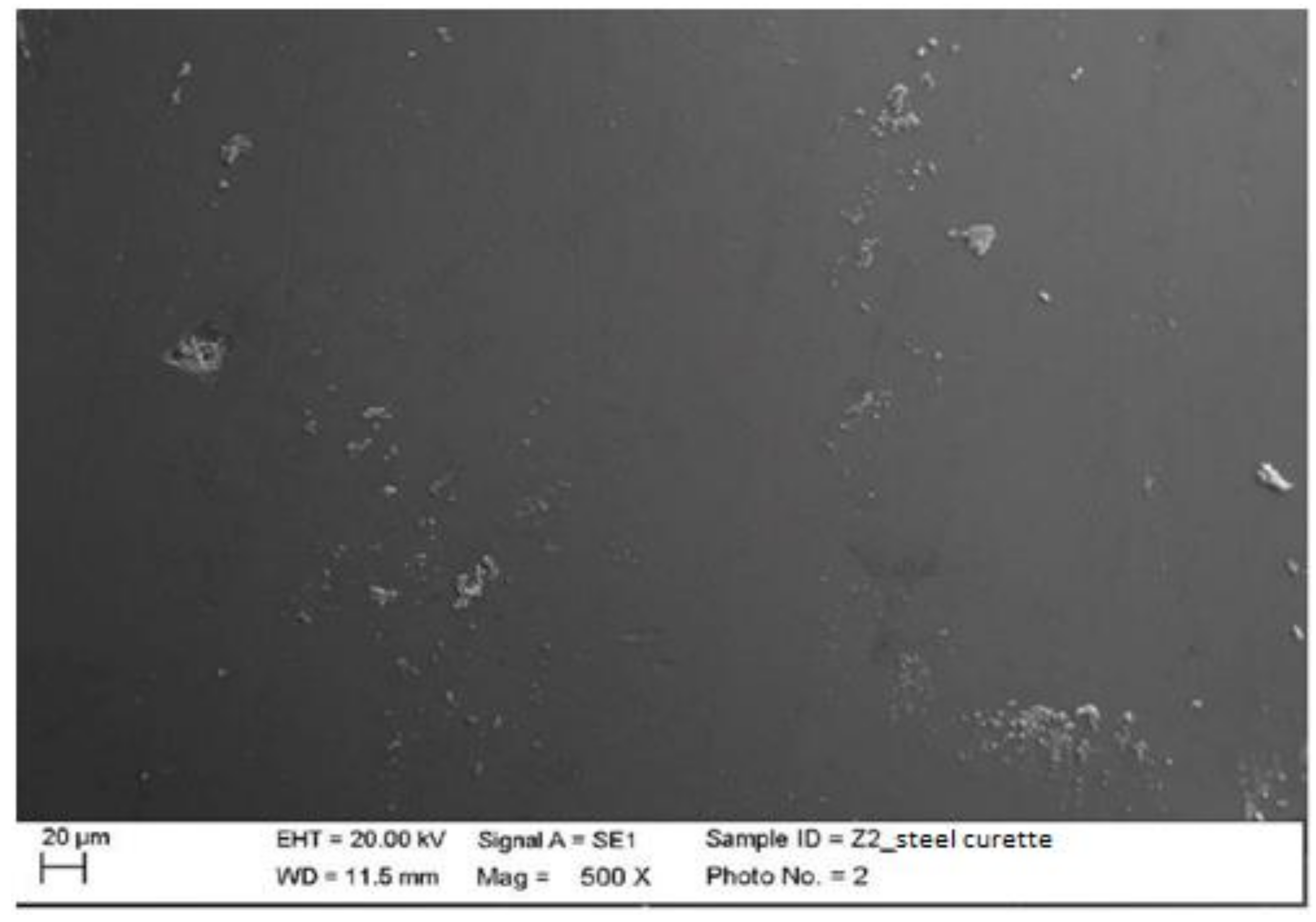
- All the lingual surfaces of the second five elements of each material were manually treated with 11-12 steel curettes (PDT, Missoula, MT, USA).
3. Statistical Analyses
4. Results
- 1.
- Composite
- 2.
- Lithium disilicate
- 3.
- Ceramic
- 4.
- Zirconium
4.1. SEM Analysis after Instrumentation
- 1.
- Composite
- 2.
- Lithium disilicate
- 3.
- Ceramic
- 4.
- Zirconium
4.2. Statistical Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Becker, D.J. Life Expectancy. J. Gen. Int. Med. 2016, 31, 973. [Google Scholar] [CrossRef][Green Version]
- Gibney, J.W. Fixed prosthodontics for the edentulous patient. J. Oral Implantol. 2000, 26, 104–108. [Google Scholar] [CrossRef]
- Morgano, S.M.; Brackett, S.E. Foundation restorations in fixed prosthodontics: Current knowledge and future needs. J. Prosthet. Dent. 1999, 82, 643–657. [Google Scholar] [CrossRef]
- Christensen, G.J. Fixed prosthodontics—State of the art. Aust. Dent. J. 1995, 40, 164–166. [Google Scholar] [CrossRef]
- Pesce, P.; Lagazzo, A.; Barberis, F.; Repetto, L.; Pera, F.; Baldi, D.; Menini, M. Mechanical characterisation of multi vs. uni-directional carbon fiber frameworks for dental implant applications. Mater. Sci. Eng. 2019, 102, 186–191. [Google Scholar] [CrossRef]
- Christensen, G.J. Ongoing changes in fixed prosthodontics. J. Am. Dent. Assoc. 2007, 138, 1257–1259. [Google Scholar] [CrossRef]
- Basnyat, S.K.; Sapkota, B.; Shrestha, S. Oral Hygiene and Gingival Health in Patients with Fixed Prosthodontic Appliances—A Six Month Follow-up. Kathmandu Univ. Med. J. (KUMJ) 2015, 13, 328–332. [Google Scholar] [CrossRef][Green Version]
- Bluma, E.; Vidzis, A.; Zigurs, G. The influence of fixed prostheses on periodontal health. Stomatologija 2016, 18, 112–121. [Google Scholar]
- Menini, M.; Dellepiane, E.; Chvartszaid, D.; Baldi, D.; Schiavetti, I.; Pera, P. Influence of Different Surface Characteristics on Peri-implant Tissue Behavior: A Six-Year Prospective Report. Int. J. Prosthodont. 2015, 28, 389–395. [Google Scholar] [CrossRef]
- Vercellotti, T.; Stacchi, C.; Russo, C.; Rebaudi, A.; Vincenzi, G.; Pratella, U.; Baldi, D.; Mozzati, M.; Monagheddu, C.; Sentineri, R.; et al. Ultrasonic implant site preparation using piezosurgery: A multicenter case series study analyzing 3,579 implants with a 1- to 3-year follow-up. Int. J. Periodontics Restor. Dent. 2014, 34, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Baldi, D.; Izzotti, A.; Bonica, P.; Pera, P.; Pulliero, A. Degenerative periodontal-diseases and oral osteonecrosis: The role of gene-environment interactions. Mutat. Res. 2009, 667, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Schierano, G.; Vercellotti, T.; Modica, F.; Corrias, G.; Russo, C.; Cavagnetto, D.; Baldi, D.; Romano, F.; Carossa, S. A 4-Year Retrospective Radiographic Study of Marginal Bone Loss of 156 Titanium Implants Placed with Ultrasonic Site Preparation. Int. J. Periodontics Restor. Dent. 2019, 39, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Lombardi, T.; Baldi, D.; Bugea, C.; Rapani, A.; Perinetti, G.; Itri, A.; Carpita, D.; Audenino, G.; Bianco, G.; et al. Immediate Loading of Implant-Supported Single Crowns after Conventional and Ultrasonic Implant Site Preparation: A Multicenter Randomized Controlled Clinical Trial. BioMed Res. Int. 2018, 2018, 6817154. [Google Scholar] [CrossRef]
- Baldi, D.; Menini, M.; Colombo, J.; Lertora, E.; Pera, P. Evaluation of a New Ultrasonic Insert for Prosthodontic Preparation. Int. J. Prosthodont. 2017, 30, 496–498. [Google Scholar] [CrossRef][Green Version]
- Baldi, D.; Menini, M.; Pera, F.; Ravera, G.; Pera, P. Sinus floor elevation using osteotomes or piezoelectric surgery. Int. J. Oral Maxillofac. Surg. 2011, 40, 497–503. [Google Scholar] [CrossRef]
- Sinjari, B.; D’Addazio, G.; Bozzi, M.; Celletti, R.; Traini, T.; Mavriqi, L.; Caputi, S. Comparison of a Novel Ultrasonic Scaler Tip vs. Conventional Design on a Titanium Surface. Materials 2018, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Menini, M.; Piccardo, P.; Baldi, D.; Dellepiane, E.; Pera, P. Morphological and chemical characteristics of different titanium surfaces treated by bicarbonate and glycine powder air abrasive systems. Implant Dent. 2015, 24, 47–56. [Google Scholar] [CrossRef]
- Menini, M.; Dellepiane, E.; Baldi, D.; Longobardi, M.G.; Pera, P.; Izzotti, A. Microarray expression in peri-implant tissue next to different titanium implant surfaces predicts clinical outcomes: A split-mouth study. Clin. Oral Implant. Res. 2017, 28, e121–e134. [Google Scholar] [CrossRef]
- Baldi, D.; Longobardi, M.; Cartiglia, C.; La Maestra, S.; Pulliero, A.; Bonica, P.; Micale, R.T.; Menini, M.; Pera, P.; Izzotti, A. Dental implants osteogenic properties evaluated by cDNA microarrays. Implant Dent. 2011, 20, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Baldi, D.; Menini, M.; Pera, F.; Ravera, G.; Pera, P. Plaque accumulation on exposed titanium surfaces and peri-implant tissue behavior. A preliminary 1-year clinical study. Int. J. Prosthodont. 2009, 22, 447–455. [Google Scholar]
- Beldüz Kara, N.; Yilmaz, Y. Assessment of oral hygiene and periodontal health around posterior primary molars after their restoration with various crown types. Int. J. Paediatr. Dent. 2014, 24, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Reeves, J. Periodontal health—Challenges in restorative dentistry. Prim. Dent. J. 2014, 3, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Snyder, N.C. Role of the dental hygienist in the prosthodontic practice. J. Prosthet. Dent. 1984, 52, 885–888. [Google Scholar] [CrossRef]



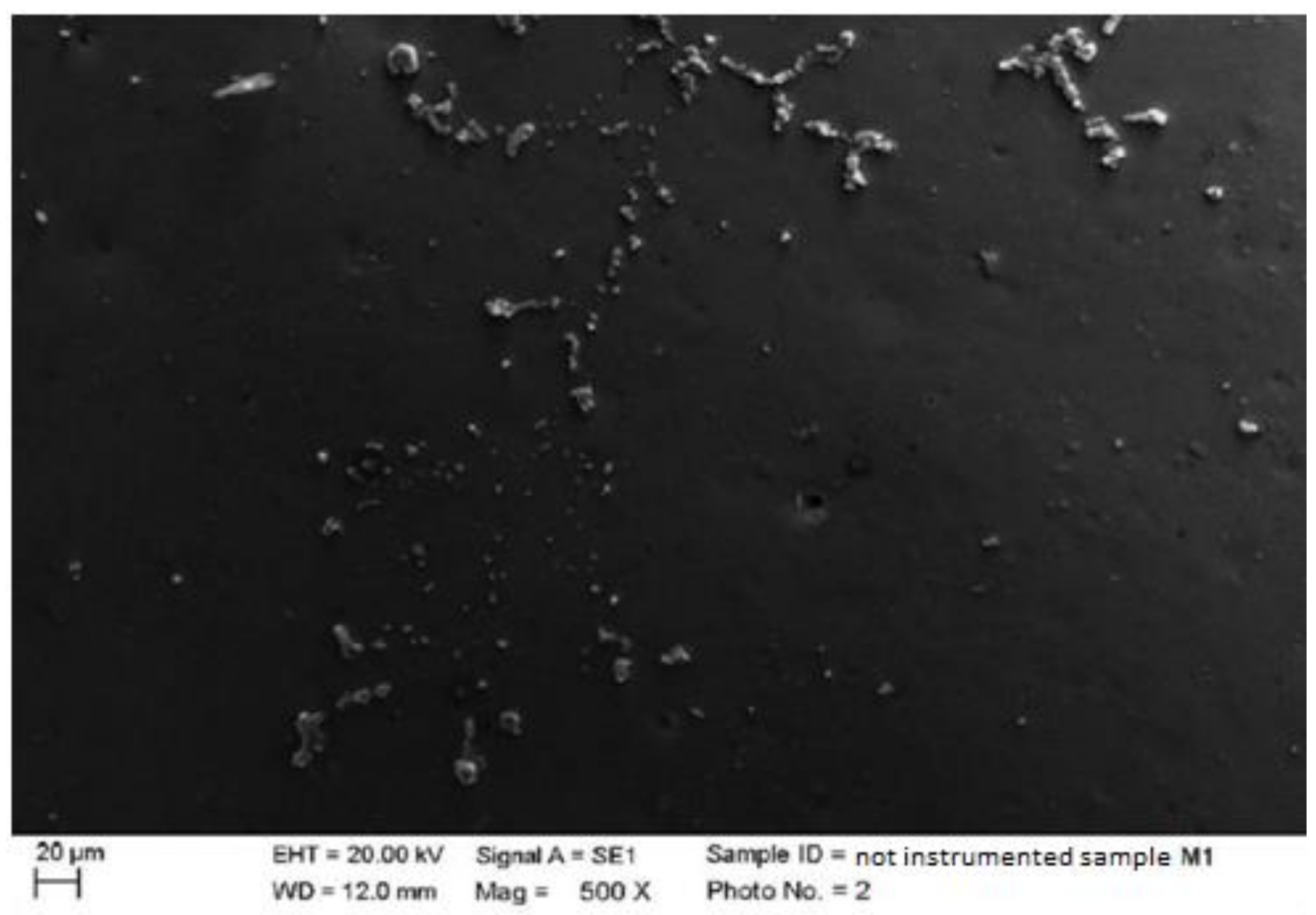
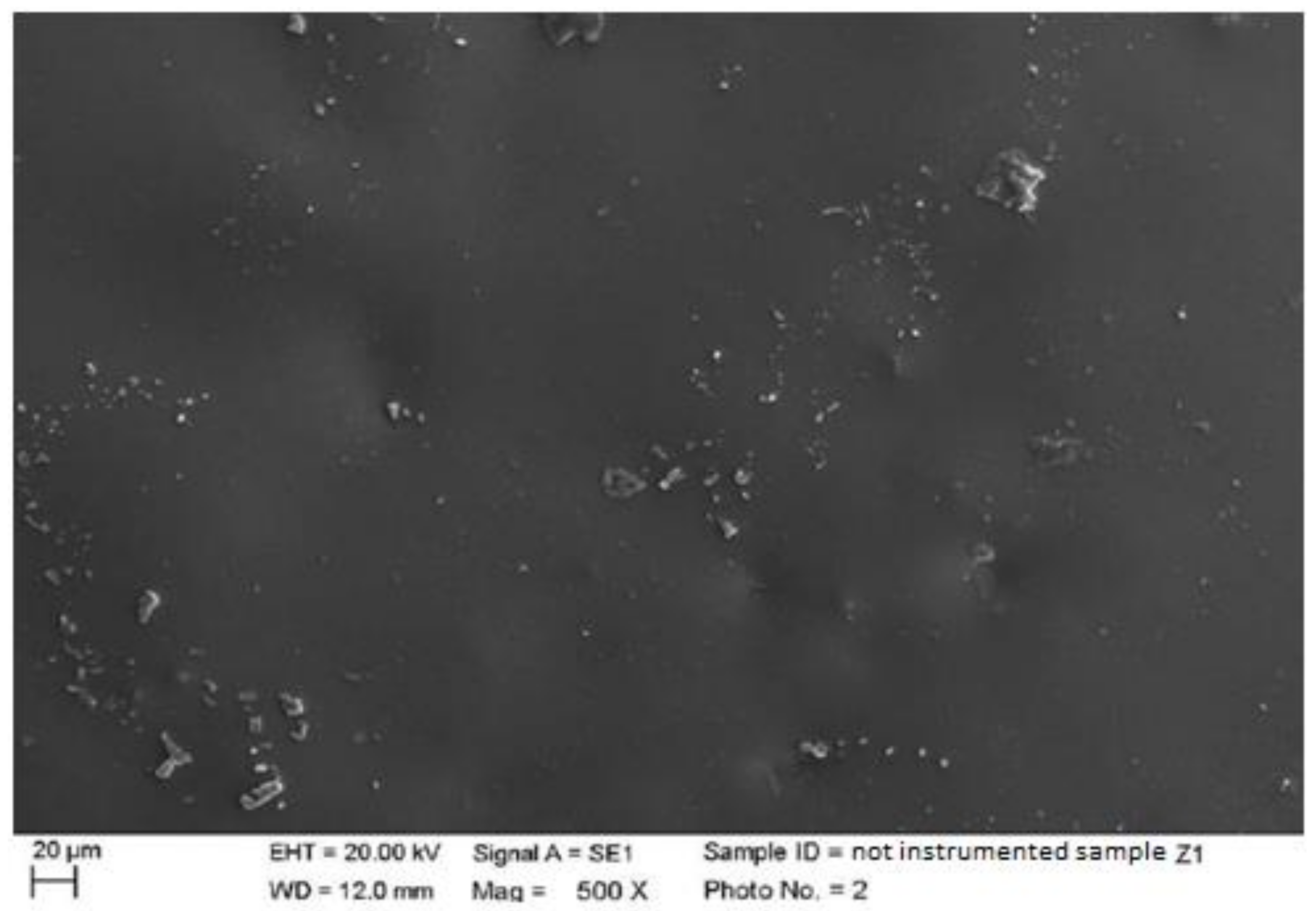
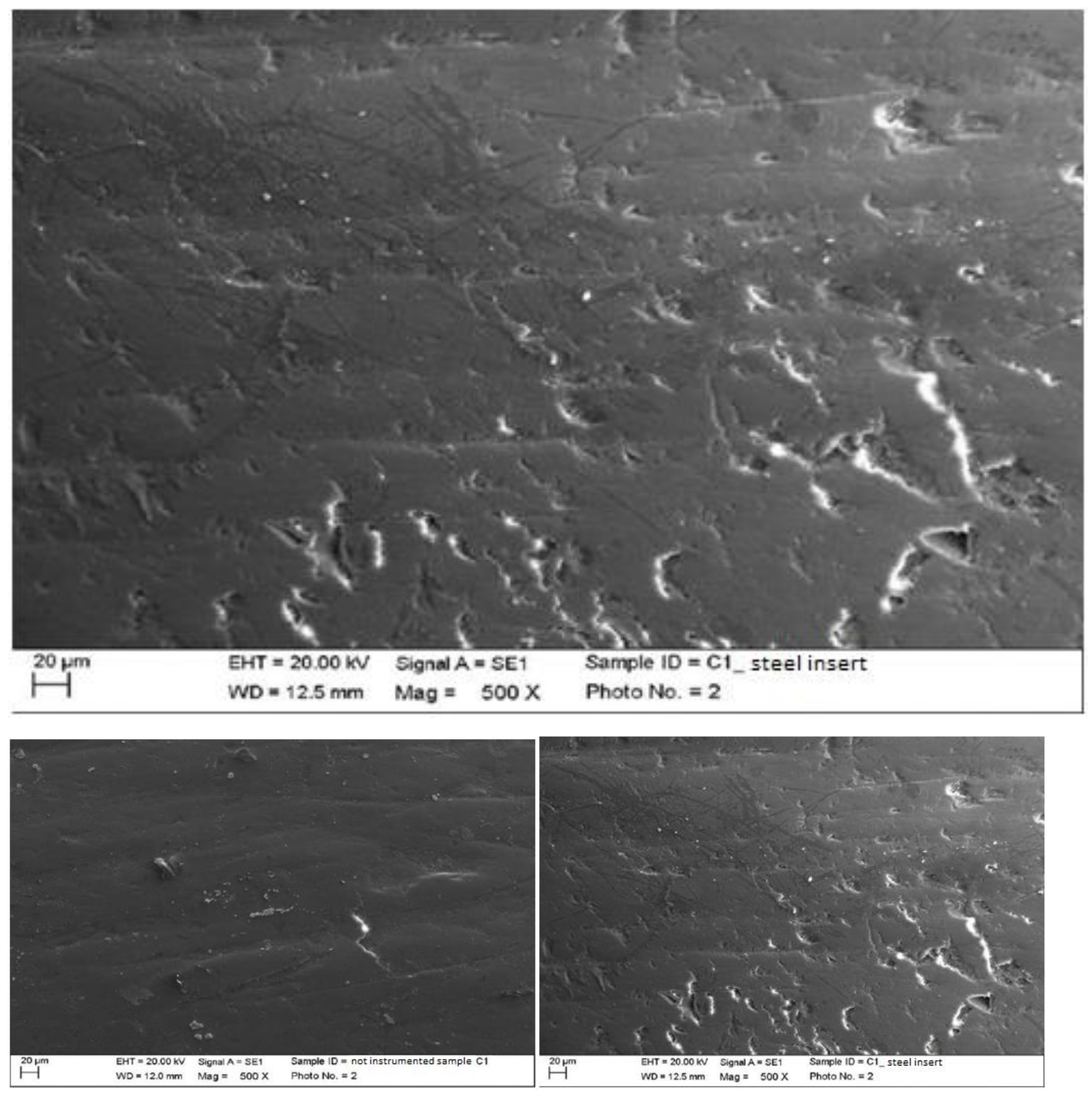

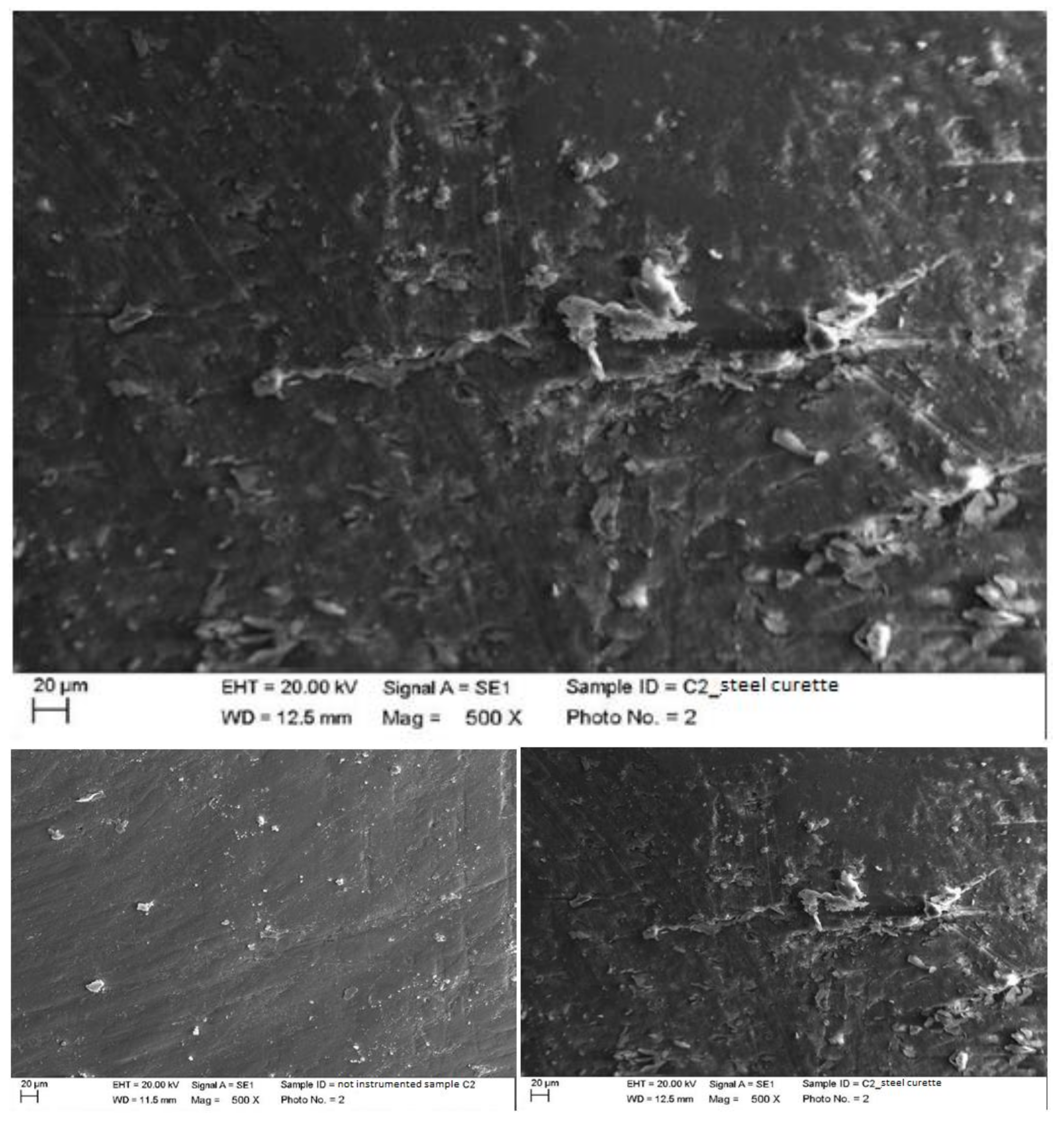
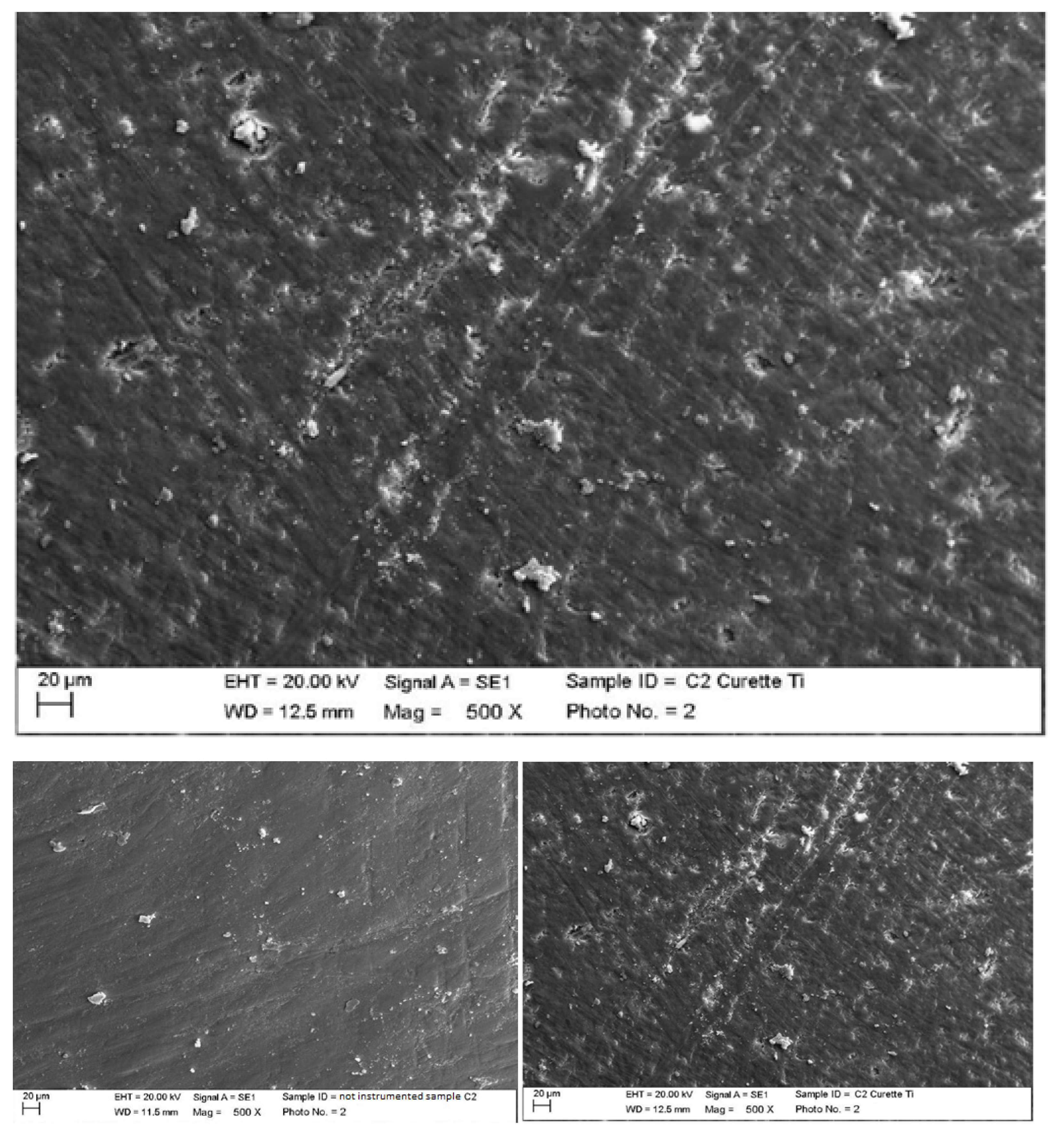




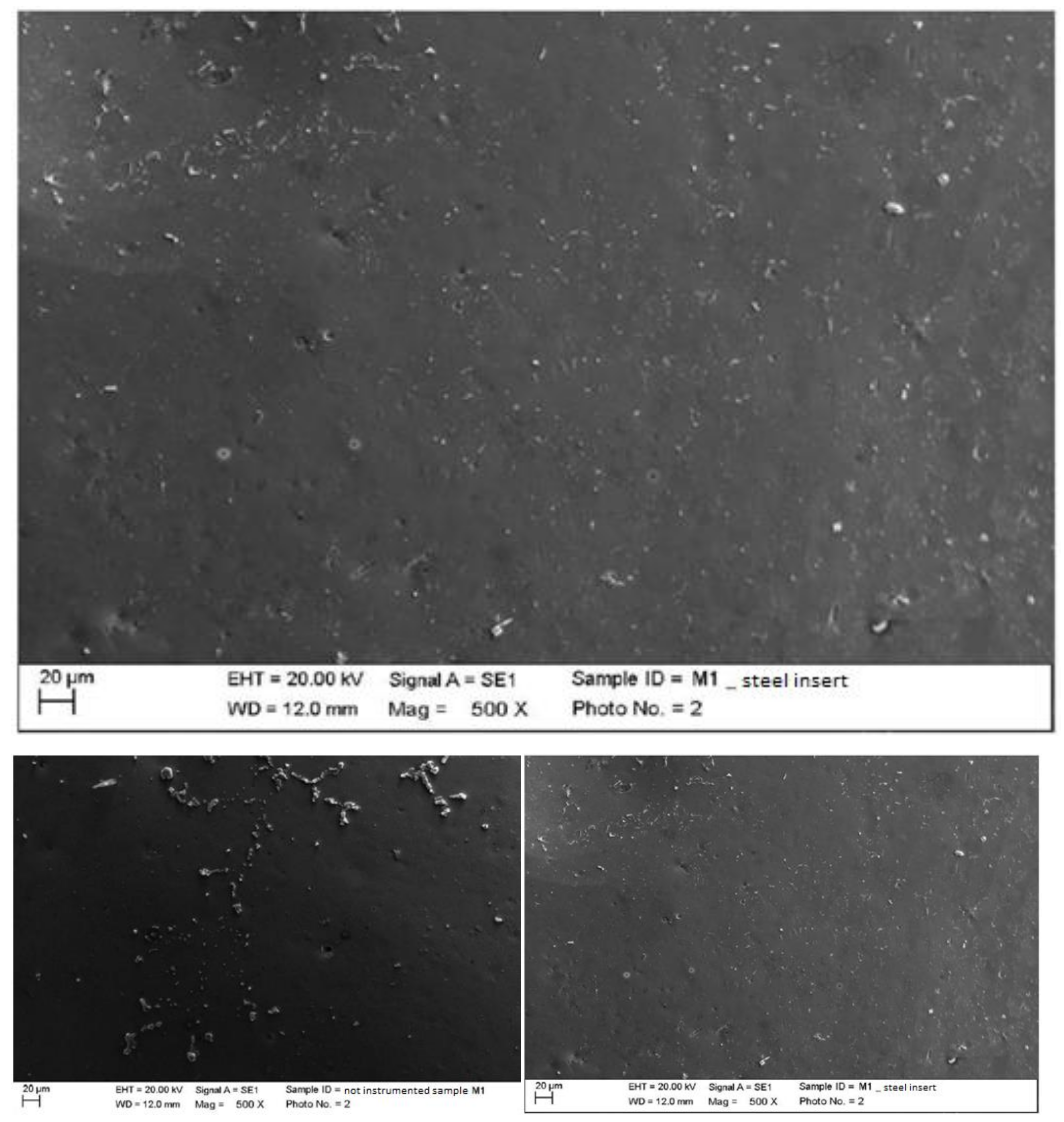


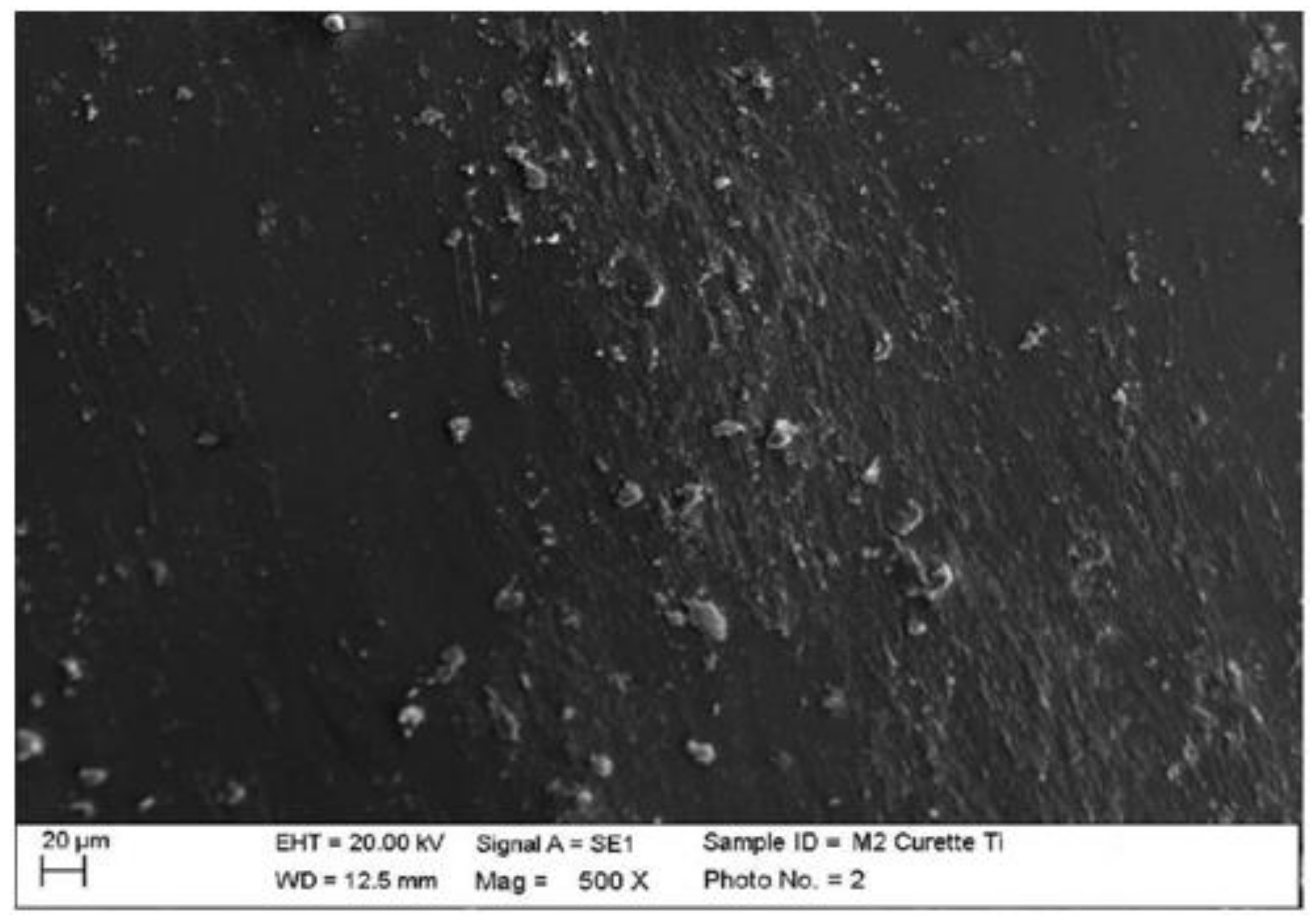

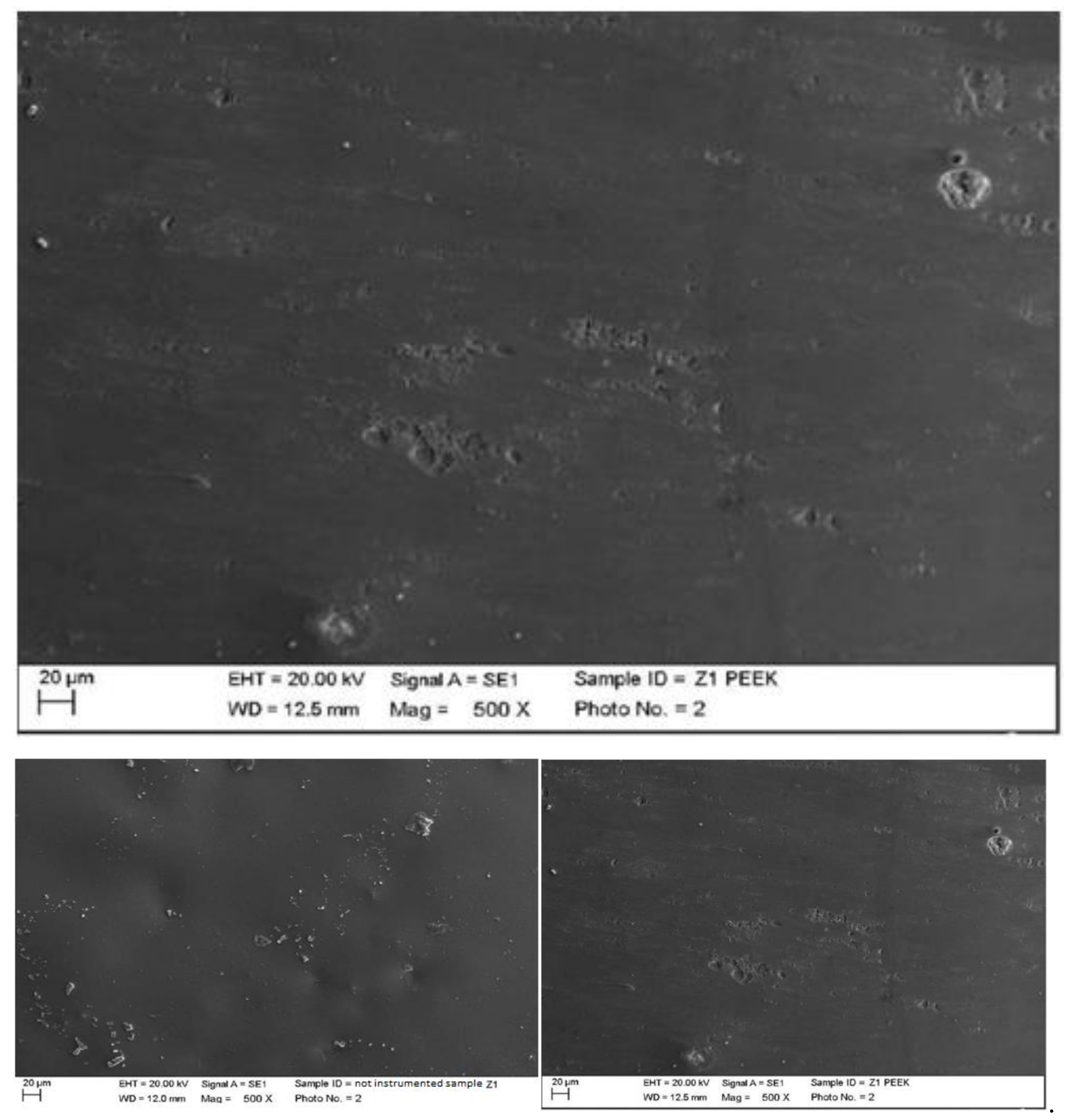


| Instrumentation Performed | Label Table 1 |
|---|---|
| Instrumented composite with ultrasonic steel insert lingual surface first elements | CUA |
| Instrumented composite with ultrasonic peek insert vestibular surface first elements | CUP |
| Instrumented composite with steel curette lingual surfaces of the second elements | CMA |
| Instrumented composite with titanium insert vestibular surfaces of the second elements | CMT |
| Instrumented disilicate with ultrasonic steel insert lingual surface first elements | DUA |
| Instrumented disilicate with ultrasonic peek insert vestibular surface first elements | DUP |
| Instrumented disilicate with steel curette lingual surfaces of the second elements | DMA |
| Instrumented disilicate with titanium insert vestibular surfaces of the second elements | DMT |
| Instrumented ceramic with ultrasonic steel insert lingual surface first elements | MUA |
| Instrumented ceramic with ultrasonic peek insert vestibular surface first elements | MUP |
| Instrumented ceramic with steel curette lingual surfaces of the second elements | MMA |
| Instrumented ceramic with titanium insert vestibular surfaces of the second elements | MMT |
| Instrumented zirconia with ultrasonic steel insert lingual surface first elements | ZUA |
| Instrumented zirconia with ultrasonic peek insert vestibular surface first elements | ZUP |
| Instrumented zirconia with steel curette lingual surfaces of the second elements | ZMA |
| Instrumented zirconia with titanium insert vestibular surfaces of the second elements | ZMT |
| Material | p (Bonferroni) | |
|---|---|---|
| Composite | Lithium disilicate | 1.47 × 10−7 |
| Composite | Metal ceramic | 0.235 |
| Composite | Zirconia ceramic | 0.023 |
| Lithium disilicate | Metal ceramic | 0.003 |
| Lithium disilicate | Zirconia ceramic | 0.054 |
| Metal ceramic | Zirconia ceramic | 1 |
| Instrumentation | p (Bonferroni) | |
|---|---|---|
| Ultrasonic-steel | Ultrasonic-peek | 0.089 |
| Ultrasonic-steel | Manual-steel | 0.032 |
| Ultrasonic-steel | Manual-titanium | 0.002 |
| Ultrasonic-peek | Manual-steel | 1 |
| Ultrasonic-peek | Manual-titanium | 1 |
| Manual-steel | Manual-titanium | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldi, D.; Colombo, J.; Gavoglio, P.; De Giorgis, L.; Motta, F.; Lugas, A.; Lertora, E.; Schierano, G. Roughness and SEM Analysis of Manual and Ultrasonic Instrumentation over Different Crown Materials for Dental Implants Restorations. Materials 2022, 15, 1159. https://doi.org/10.3390/ma15031159
Baldi D, Colombo J, Gavoglio P, De Giorgis L, Motta F, Lugas A, Lertora E, Schierano G. Roughness and SEM Analysis of Manual and Ultrasonic Instrumentation over Different Crown Materials for Dental Implants Restorations. Materials. 2022; 15(3):1159. https://doi.org/10.3390/ma15031159
Chicago/Turabian StyleBaldi, Domenico, Jacopo Colombo, Paola Gavoglio, Luisa De Giorgis, Franco Motta, Andrea Lugas, Enrico Lertora, and Gianmario Schierano. 2022. "Roughness and SEM Analysis of Manual and Ultrasonic Instrumentation over Different Crown Materials for Dental Implants Restorations" Materials 15, no. 3: 1159. https://doi.org/10.3390/ma15031159
APA StyleBaldi, D., Colombo, J., Gavoglio, P., De Giorgis, L., Motta, F., Lugas, A., Lertora, E., & Schierano, G. (2022). Roughness and SEM Analysis of Manual and Ultrasonic Instrumentation over Different Crown Materials for Dental Implants Restorations. Materials, 15(3), 1159. https://doi.org/10.3390/ma15031159







