Silsesquioxanes in the Cosmetics Industry—Applications and Perspectives †
Abstract
:1. Introduction
- Silanes
- Hydroxyl-containing compounds (silanols)
- Cyclic dimethyl siloxanes (cyclomethicones)
- Linear Polysiloxanes (trimethylsiloxy end-capped, non-trimethylsiloxy end-capped)
- Silicates (inorganic)
- Copolymers and silsesquioxanes (SSQs) [6].
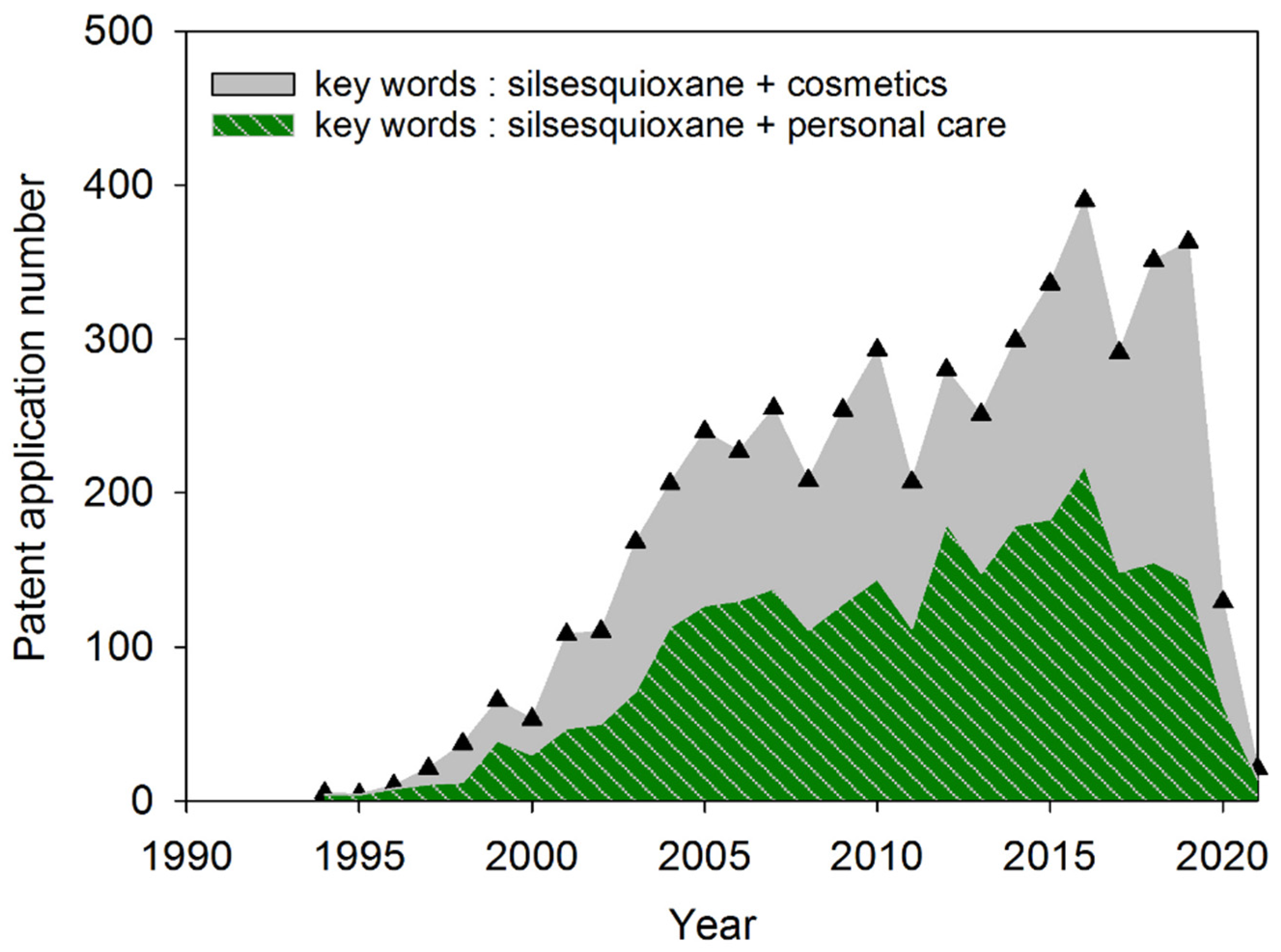
2. Data Sources and Search Model for the Study
3. Structure of Molecular and Macromolecular POSS Derivatives and Their Polymeric Hybrids
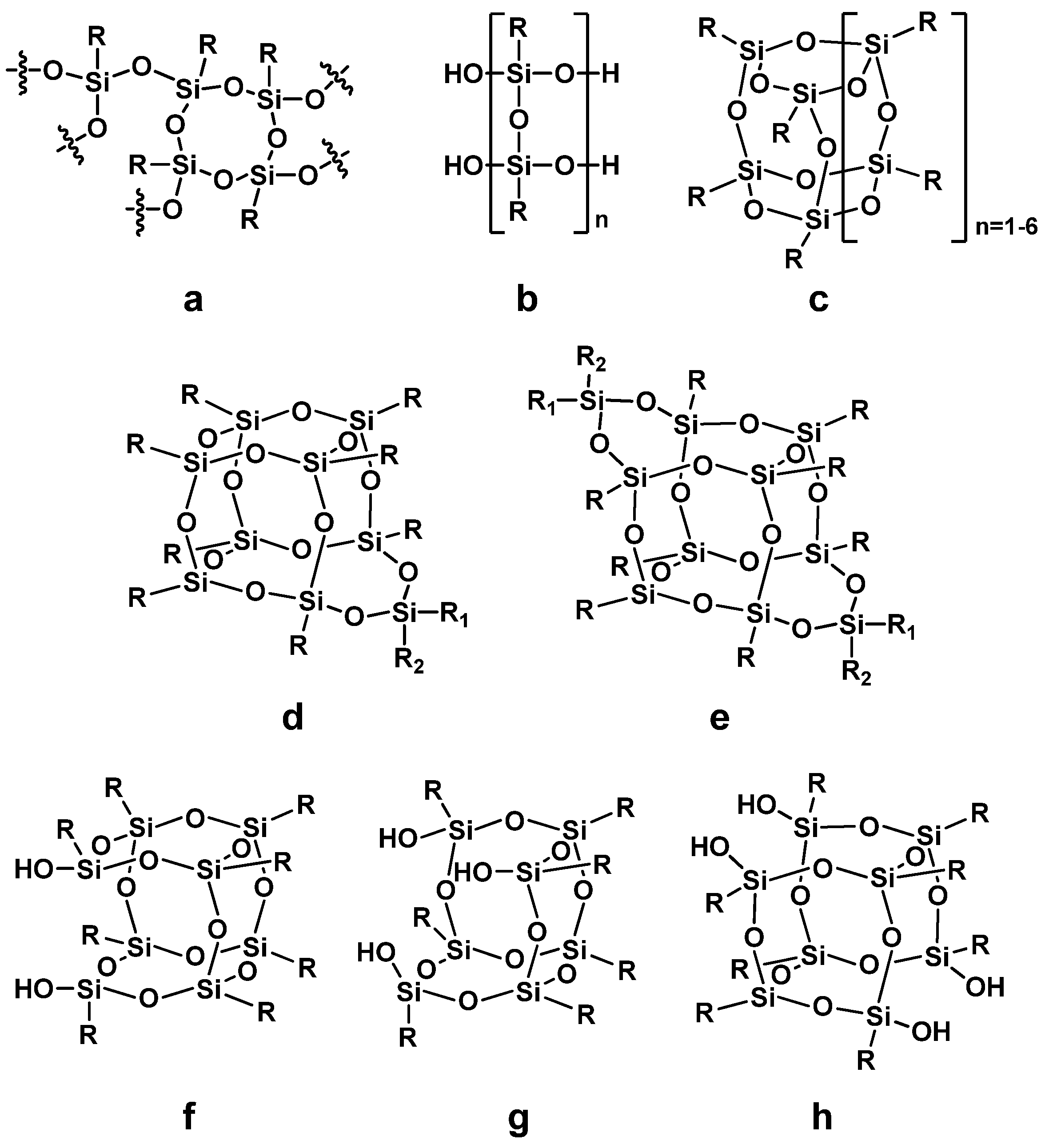
3.1. POSS Structure and Nomenclature
3.2. Methods of Synthesis of Silsesquioxanes
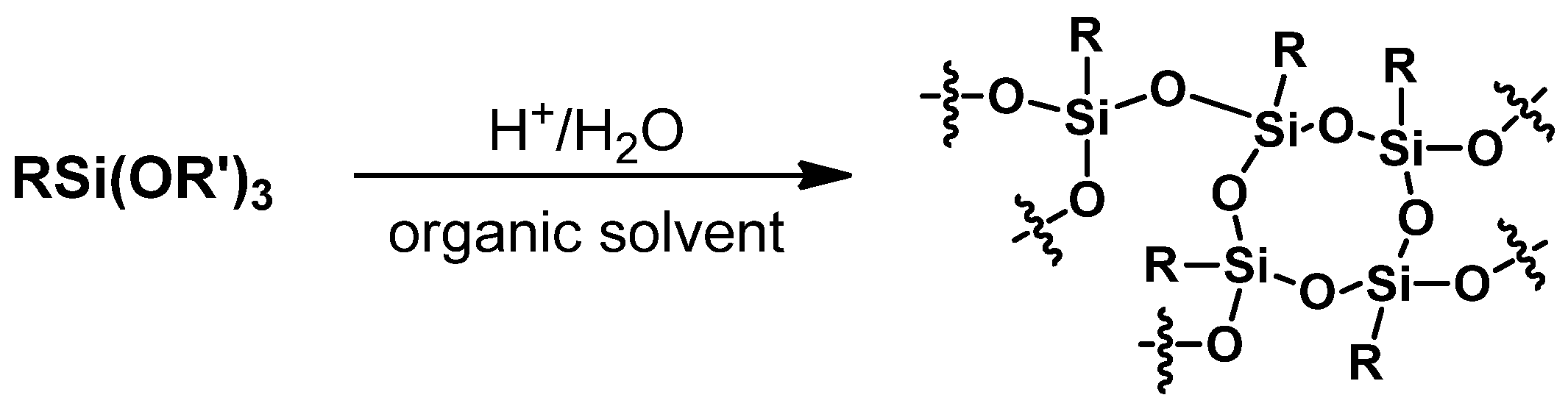
3.2.1. Hydrolitic (olygo)condensation
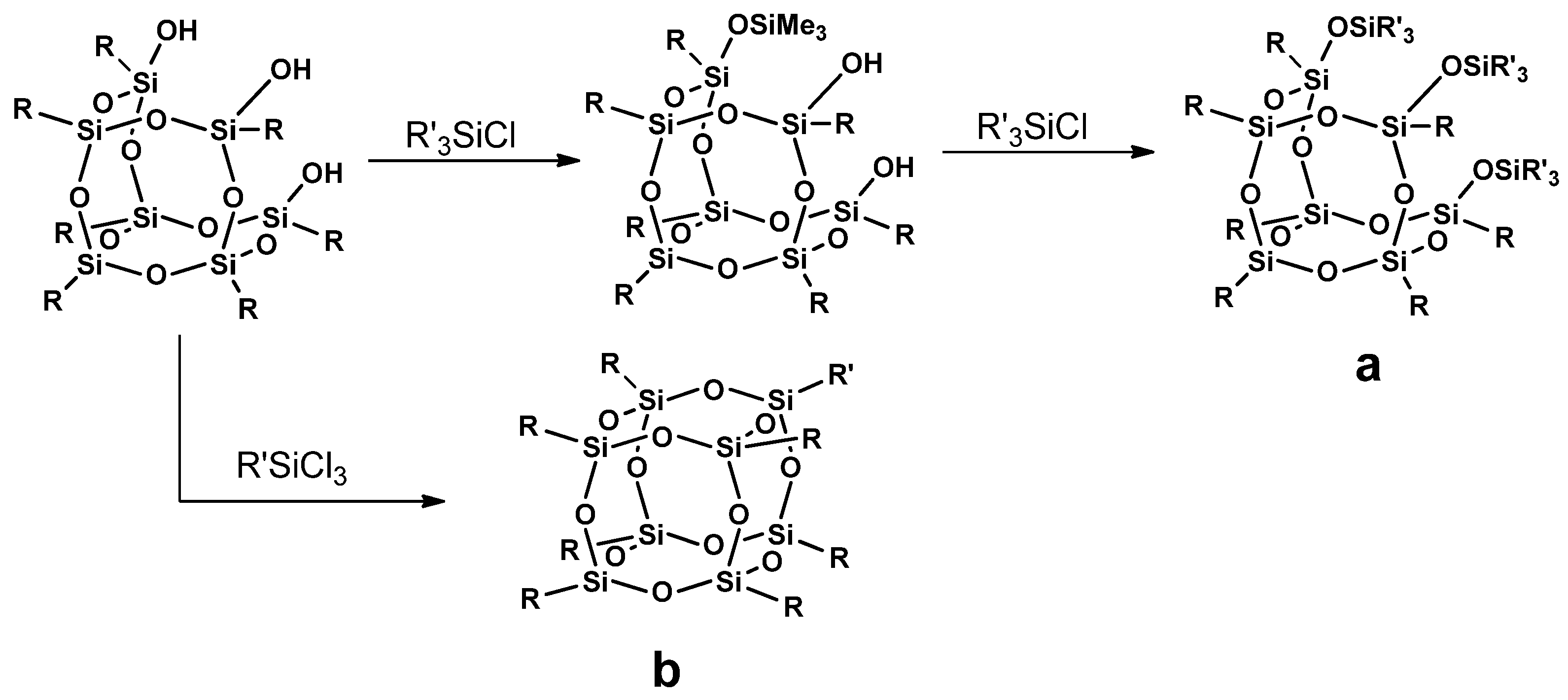
3.2.2. Corner Capping
3.2.3. Synthesis of Silsesquioxane Resins/Polysilsesquioxanes
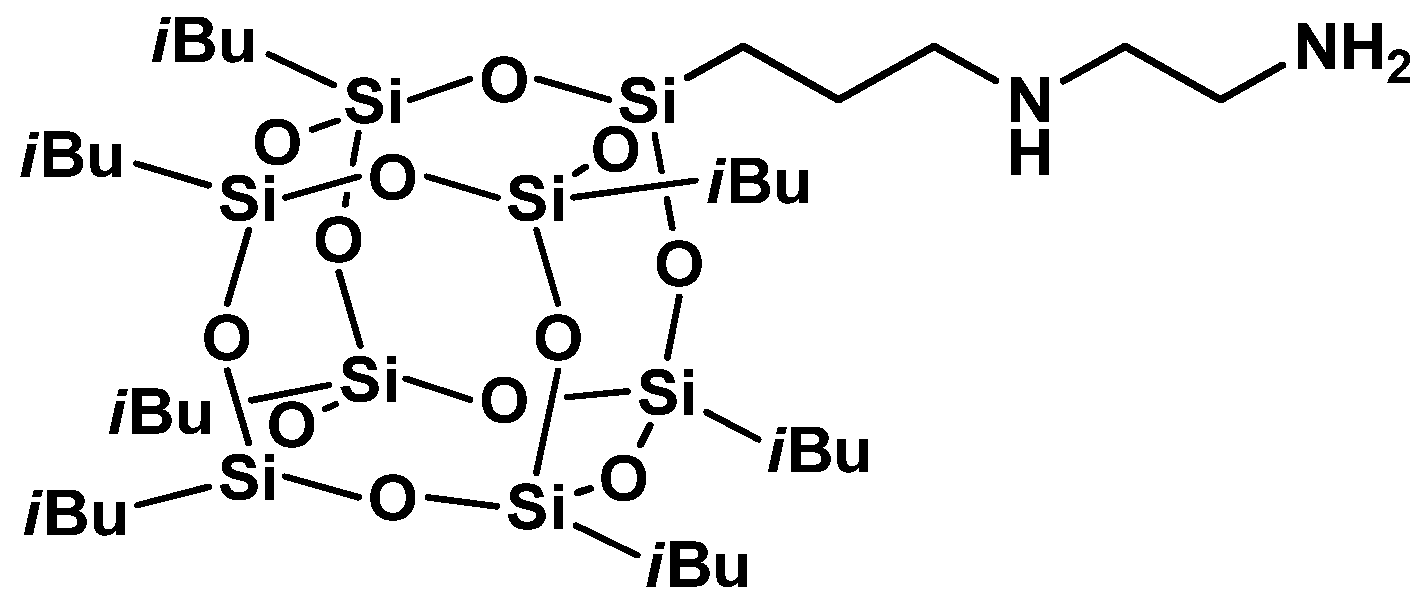
3.2.4. Post-Functionalization of POSS Cage
4. Literature Review on Patent Analysis Regarding Silsesquioxanes in Cosmetics
5. Application of POSS Derivatives in Cosmetic Products
5.1. Hair Care Products
- products that provide temporary effects such as shampoos, conditioners, and sprays
- products with long-lasting effects such as permanent waves and permanent colors [85].
5.2. Color Cosmetics
5.3. Nail Care Products
- MercaptopropylIsooctyl-POSS
- EpoxyCyclohexylCyclopentyl-POSS
- EpoxyCyclohexylCyclohexyl-POSS
- EpoxyCyclohexylIsobutyl-POSS
- GlycidylIsooctyl-POSS
- Octaepoxy-POSS.
5.4. Skincare Products
6. Future Outlooks
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nitesh, C.; Himanshu, V.; Roshan, D. Cosmetics Market by Category (Skin and Sun Care Products, Hair Care Products, Deodorants & Fragrances, and Makeup & Color Cosmetics), Gender (Men, Women, and Unisex), and Distribution Channel (Hypermarkets/Supermarkets, Specialty Stores, Pharmacies, Online Sales Channels, and Others): Global Opportunity Analysis and Industry Forecast, 2021–2027; Allied Market Research: Portland, OR, USA, 2021. [Google Scholar]
- Pawar, A.B.; Falk, B. Use of Advanced Silicone Materials in Long-Lasting Cosmetics. In Surface Science and Adhesion in Cosmetics; Wiley, Scrivener Publishing LLC: Beverly, MA, USA, 2021; pp. 151–182. ISBN 978-1-119-65482-7. [Google Scholar]
- Goddard, E.D.; Gruber, J.V. Principles of Polymer Science and Technology in Cosmetics and Personal Care, Cosmetic Science and Technology; Marcel Dekker, Inc.: New York, NY, USA, 1999; Volume 22, ISBN 0824719239. [Google Scholar]
- O’Lenick, A.J.; O’Lenick, K.A. Silicone Polymers in Skin Care. MRS Bull. 2007, 32, 801–806. [Google Scholar] [CrossRef]
- Mojsiewicz-Pieńkowska, K.; Jamrógiewicz, M.; Szymkowska, K.; Krenczkowska, D. Direct Human Contact with Siloxanes (Silicones)–Safety or Risk Part 1. Characteristics of Siloxanes (Silicones). Front. Pharmacol. 2016, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- O’Lenick, A.J. Silicones for Personal Care; Allured Pub.: Carol Stream, IL, USA, 2008; ISBN 1932633367. [Google Scholar]
- Lardy, F.; Vennat, B.; Pouget, M.P.; Pourrat, A. Functionalization of Hydrocolloids: Principal Component Analysis Applied to the Study of Correlations between Parameters Describing the Consistency of Hydrogels. Drug Dev. Ind. Pharm. 2000, 26, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Budiasih, S.; Masyitah, I.; Jiyauddin, K.; Kaleemullah, M.; Samer, A.D.; Fadli, A.M.; Eddy, Y. Formulation and Characterization of Cosmetic Serum Containing Argan Oil as Moisturizing Agent. In Proceedings of the BROMO Conference, Surabaya, Indonesia, 11–12 July 2018; SciTePress: Setubal, Portugal, 2018; pp. 297–304. [Google Scholar]
- Fulton, J.E. Comedogenicity and Irritancy of Commonly Used Ingredients in Skin Care Products. J. Soc. Cosmet. Chem. 1989, 40, 321–333. [Google Scholar]
- Adams, F.; Romero, I.L.; da Silva, C.B.; de Almeida Manzano, R.P. Evaluation of Silicon Oil on Bacterial Growth. Arq. Bras. Oftalmol. 2012, 75, 89–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kis, N.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Csányi, E.; Csóka, I.; Berkó, S. Investigation of Silicone-Containing Semisolid in Situ Film-Forming Systems Using QbD Tools. Pharmaceutics 2019, 11, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojsiewicz-Pieńkowska, K. Review of Current Pharmaceutical Applications of Polysiloxanes (Silicones). In Handbook of Polymers for Pharmaceutical Technologies; Scrivener Publishing LLC: Beverly, MA, USA, 2015; pp. 363–382. ISBN 9781119041429. [Google Scholar]
- Barel, A.O.; Paye, M.; Maibach, H.I. Handbook of Cosmetic Science and Technology; CRC Press: Boca Raton, FL, USA, 2014; ISBN 1842145649. [Google Scholar]
- Eeman, M.; van Reeth, I. Silicone Film-Formers: New Approaches for Measuring Film Barrier Properties; Poster at IFSCC Congress: Paris, France, 2014. [Google Scholar]
- Bleasdale, B.; Finnegan, S.; Murray, K.; Kelly, S.; Percival, S.L. The Use of Silicone Adhesives for Scar Reduction. Adv. Wound Care 2015, 4, 422–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clugston, P.A.; Vistnes, M.D.; Perry, L.C.; Maxwell, G.P.; Fisher, J. Evaluation of Silicone-Gel Sheeting on Early Wound Healing of Linear Incisions. Ann. Plast. Surg. 1995, 34, 12–15. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, L.A.; Addor, F.; Campos, P.M.B.G.M. Use of Silicon for Skin and Hair Care: An Approach of Chemical Forms Available and Efficacy. An. Bras. Dermatol. 2016, 91, 331–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, D.; Tian, F.; Ozkan, C.S.; Wang, M.; Gao, H. Effect of Single Wall Carbon Nanotubes on Human HEK293 Cells. Toxicol. Lett. 2005, 155, 73–85. [Google Scholar] [CrossRef]
- Arkles, B. Commercial Applications of Sol-Gel-Derived Hybrid Materials. MRS Bull. 2001, 26, 402–408. [Google Scholar] [CrossRef]
- Lungu, A.; Cernencu, A.I.; Vlasceanu, G.M.; Florea, N.M.; Ionita, M.; Iovu, H. 3D POSS Cages Decorated 2D Graphenic Sheets: A Versatile Platform for Silicon-Carbonaceous Nano-Additives Design. Compos. B Eng. 2021, 207, 108578. [Google Scholar] [CrossRef]
- DeArmitt, C. Polyhedral Oligomericsilsequioxanes: Additives for Unique Cosmetic Properties. Cosmet. Toilet. 2008, 123, 51–56. [Google Scholar]
- Blanco, I. The Rediscovery of POSS: A Molecule Rather than a Filler. Polymers 2018, 10, 904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quadir, M. Poss and Eposs Containing Cosmetics and Personal Care Products. EP1603518A4, 22 November 2006. [Google Scholar]
- Loman-Cortes, P.; Binte Huq, T.; Vivero-Escoto, J.L. Use of Polyhedral Oligomeric Silsesquioxane (POSS) in Drug Delivery, Photodynamic Therapy and Bioimaging. Molecules 2021, 26, 6453. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ye, Q.; Xu, J. Polyhedral Oligomeric Silsesquioxane-Based Hybrid Materials and Their Applications. Mater. Chem. Front. 2017, 1, 212–230. [Google Scholar] [CrossRef]
- DeArmitt, C. Polyhedral Oligomeric Silsesquioxanes in Plastics. In Applications of Polyhedral Oligomeric Silsesquioxanes; Springer: Berlin/Heidelberg, Germany, 2011; pp. 209–228. ISBN 9789048137862. [Google Scholar]
- Punshon, G.; Vara, D.S.; Sales, K.M.; Kidane, A.G.; Salacinski, H.J.; Seifalian, A.M. Interactions between Endothelial Cells and a Poly (Carbonate-Silsesquioxane-Bridge-Urea) Urethane. Biomaterials 2005, 26, 6271–6279. [Google Scholar] [CrossRef]
- Brząkalski, D.; Przekop, R.E.; Dobrosielska, M.; Sztorch, B.; Marciniak, P.; Marciniec, B. Highly Bulky Spherosilicates as Functional Additives for Polyethylene Processing—Influence on Mechanical and Thermal Properties. Polym. Compos. 2020, 41, 3389–3402. [Google Scholar] [CrossRef]
- Hartmann-Thompson, C. Applications of Polyhedral Oligomeric Silsesquioxanes; Springer Science & Business Media: Berlin, Germany, 2011; Volume 3, ISBN 904813787X. [Google Scholar]
- Pescarmona, P.P.; Maschmeyer, T. Oligomeric Silsesquioxanes: Synthesis, Characterization and Selected Applications. Aust. J. Chem. 2001, 54, 583–596. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef]
- Marciniec, B.; Dutkiewicz, M.; Maciejewski, H.; Kubicki, M. New, Effective Method of Synthesis and Structural Characterization of Octakis (3-Chloropropyl) Octasilsesquioxane. Organometallics 2008, 27, 793–794. [Google Scholar] [CrossRef]
- Brefort, J.L.; Corriu, R.J.P.; Guerin, C.; Henner, B.J.L.; Wong Chi Man, W.W.C. Pentacoordinated Silicon Anions: Synthesis and Reactivity. Organometallics 1990, 9, 2080–2085. [Google Scholar] [CrossRef]
- Voronkov, M.G.; Lavrent’yev, V.I. Polyhedral Oligosilsesquioxanes and Their Homo Derivatives. In Inorganic Ring Systems; Springer: Berlin/Heidelberg, Germany, 1982; pp. 199–236. ISBN 978-3-540-11345-4. [Google Scholar]
- Hoque, M.A.; Kakihana, Y.; Shinke, S.; Kawakami, Y. Polysiloxanes with Periodically Distributed Isomeric Double-Decker Silsesquioxane in the Main Chain. Macromolecules 2009, 42, 3309–3315. [Google Scholar] [CrossRef]
- Yoshida, K.; Hattori, T.; Ootake, N.; Tanaka, R.; Matsumoto, H. Silsesquioxane-Based Polymers: Synthesis of Phenylsilsesquioxanes with Double-Decker Structure and Their Polymers. In Silicon Based Polymers; Springer: Berlin/Heidelberg, Germany, 2008; pp. 205–211. ISBN 978-1-4020-8527-7. [Google Scholar]
- Shimizu, T.; Kanamori, K.; Nakanishi, K. Transparent Polyvinylsilsesquioxane Aerogels: Investigations on Synthetic Parameters and Surface Modification. J. Solgel Sci. Technol. 2017, 82, 2–14. [Google Scholar] [CrossRef]
- Anoop, V.; Sankaraiah, S.; Jaisankar, S.N.; Chakraborty, S.; Mary, N.L. Enhanced Mechanical, Thermal and Adhesion Properties of Polysilsesquioxane Spheres Reinforced Epoxy Nanocomposite Adhesives. J. Adhes. 2021, 97, 1–18. [Google Scholar]
- Shimizu, T.; Kanamori, K.; Maeno, A.; Kaji, H.; Doherty, C.M.; Falcaro, P.; Nakanishi, K. Transparent, Highly Insulating Polyethyl-and Polyvinylsilsesquioxane Aerogels: Mechanical Improvements by Vulcanization for Ambient Pressure Drying. Chem. Mater. 2016, 28, 6860–6868. [Google Scholar] [CrossRef]
- Tsukada, S.; Wada, K.; Miura, H.; Hosokawa, S.; Abe, R.; Inoue, M. Phosphine-Stabilized, Oxide-Supported Rhodium Catalysts for Highly Efficient Silylative Coupling Reactions. Res. Chem. Intermed. 2015, 41, 9575–9586. [Google Scholar] [CrossRef]
- Frydrych, M.; Sztorch, B.; Brząkalski, D.; Pakuła, D.; Frąckowiak, D.; Majchrzak, M.; Marciniec, B. New Functionalized Borasilsesquioxanes Obtained via Metathetical Transformation of 4-Vinylphenylborasilsesquioxanes. Eur. J. Inorg. Chem. 2020, 3597, 3600. [Google Scholar] [CrossRef]
- Chan, K.L.; Sonar, P.; Sellinger, A. Cubic Silsesquioxanes for Use in Solution Processable Organic Light Emitting Diodes (OLED). J. Mater. Chem. 2009, 19, 9103–9120. [Google Scholar] [CrossRef]
- Shen, R.; Du, Y.; Yang, X.; Liu, H. Silsesquioxanes-Based Porous Functional Polymers for Water Purification. J. Mater. Sci. 2020, 55, 7518–7529. [Google Scholar] [CrossRef]
- Hou, K.; Zeng, Y.; Zhou, C.; Chen, J.; Wen, X.; Xu, S.; Cheng, J.; Pi, P. Facile Generation of Robust POSS-Based Superhydrophobic Fabrics via Thiol-Ene Click Chemistry. Chem. Eng. J. 2018, 332, 150–159. [Google Scholar] [CrossRef]
- Brząkalski, D.; Przekop, R.E.; Sztorch, B.; Jakubowska, P.; Jałbrzykowski, M.; Marciniec, B. Silsesquioxane Derivatives as Functional Additives for Preparation of Polyethylene-Based Composites: A Case of Trisilanol Melt-Condensation. Polymers 2020, 12, 2269. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, M.; Maciejewski, H.; Marciniec, B. Functionalization of Polyhedral Oligomeric Silsesquioxane (POSS) via Nucleophilic Substitution. Synthesis 2009, 2009, 2019–2024. [Google Scholar]
- Chen, G.; Zhou, Y.; Wang, X.; Li, J.; Xue, S.; Liu, Y.; Wang, Q.; Wang, J. Construction of Porous Cationic Frameworks by Crosslinking Polyhedral Oligomeric Silsesquioxane Units with N-Heterocyclic Linkers. Sci. Rep. 2015, 5, 11236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaroentomeechai, T.; Yingsukkamol, P.; Phurat, C.; Somsook, E.; Osotchan, T.; Ervithayasuporn, V. Synthesis and Reactivity of Nitrogen Nucleophiles-Induced Cage-Rearrangement Silsesquioxanes. Inorg. Chem. 2012, 51, 12266–12272. [Google Scholar] [CrossRef]
- Misasi, J.M.; Jin, Q.; Knauer, K.M.; Morgan, S.E.; Wiggins, J.S. Hybrid POSS-Hyperbranched Polymer Additives for Simultaneous Reinforcement and Toughness Improvements in Epoxy Networks. Polymer 2017, 117, 54–63. [Google Scholar] [CrossRef]
- Hu, M.-B.; Hou, Z.-Y.; Hao, W.-Q.; Xiao, Y.; Yu, W.; Ma, C.; Ren, L.-J.; Zheng, P.; Wang, W. POM–Organic–POSS Cocluster: Creating a Dumbbell-Shaped Hybrid Molecule for Programming Hierarchical Supramolecular Nanostructures. Langmuir 2013, 29, 5714–5722. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.-H.; Zou, Y.; Wang, Z.; Yue, K.; Huang, M.; Liu, H.; Feng, X.; Lin, Z.; Zhang, W. Polyhedral Oligomeric Silsesquioxane Meets “Click” Chemistry: Rational Design and Facile Preparation of Functional Hybrid Materials. Polymer 2017, 125, 303–329. [Google Scholar] [CrossRef]
- Marciniec, B. Hydrosilylation: A Comprehensive Review on Recent Advances; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-9048177912. [Google Scholar]
- Cekada, J.; Weyenberg, D.R. Verfahren zur Herstellung Kolloidaler Suspenionen von Silsesquioxannen. DE1595471A1, 5 February 1970. [Google Scholar]
- Krantz, K.W. Organopolysiloxanes and Processes for Their Preparation. DE1520029A1, 14 May 1969. [Google Scholar]
- Yawat, Y.; Kumagaya, S.; Yokoyama, H.; Nakane, T.; Okunuki, Y. Non-Liquid Makeup Cosmetics. JPH0723287B2, 22 February 1985. [Google Scholar]
- Defossez, B.; Gagnebien, D. Cosmetic Composition in the Form of a Compacted Powder Containing Hollow Microspheres of a Thermoplastic Synthetic Material. AU636202B2, 22 April 1993. [Google Scholar]
- Takezawa, Y. Emulsion Composition. JP2831877B2, 2 December 1998. [Google Scholar]
- Shimizu, C. Complex Globular Powder and Cosmetic Containing the Same. JPH0725727A, 27 January 1995. [Google Scholar]
- Halloran, J.D.; Vincent, M.J. Organosilicon Hair Care Products. US5225190A, 7 June 1993. [Google Scholar]
- Bekemeier, T.; Deklippel, L.; Dimitrova, T.; Elms, R.; Galeone, F.; Lenoble, B.; Marteaux, L.; Roidl, J.; Severance, M.; Ugazio, S.; et al. Process for Preparing Silicate Shell Microcapsules. US9192549B2, 24 November 2015. [Google Scholar]
- Iwami, S.; Watanabe, Y.; Watanabe, T. Sunscreen Cosmetic. WO2021125211A1, 24 June 2021. [Google Scholar]
- Garrison, M.S.; Robinson, F.E.; Sandstrom, G.A.; Buckridge, K.A.; Yang, S.; Luo, X. Methods and Compositions for Preventing or Reducing Frizzy Appearance of Hair. US9622945B2, 18 April 2017. [Google Scholar]
- Wagner, R.; Simon, W.; Kropfgans, M.; Nienstedt, S.; Schnering, A.; Streicher, K.; Sockel, K.-H.; Maass, S. Hydrophilic/Lipophilic Modified Polysiloxanes as Emsulsifiers. CN102712757, 7 January 2015. [Google Scholar]
- Baldo, F.; Didier, C. Make-Up Methods and Compositions for Controlling Browning of Skin Induced by Uv Radiation. 2018. JP2018048210A, 29 March 2018. [Google Scholar]
- Tamura, S.; Souda, T.; Iimura, T.; Sawayama, S.; Hori, S.; Furukawa, H. Novel Liquid Organo Polysiloxane and Use Therefor. EP 2716685 A4, 29 October 2014. [Google Scholar]
- Tachon, R.; Shimizu, M. Pore Hiding Cosmetic Composition Comprising a Plate Type Filler, a Silicon Elastomer and an Oil Absorbing Filler. WO2013190709 A1, 27 December 2013. [Google Scholar]
- Mendoza, R. Cosmetic Composition. CN105250154B, 21 August 2020. [Google Scholar]
- Jansen, J.H.; Zukowski, J.M.; Tanner, P.R. Cosmetic Composition. CN105916489B, 11 December 2020. [Google Scholar]
- Cohen, I.D.; Cummins, P.; Sojka, M.F.; Culhane, D.W.; Fthenakis, C.G.; Sobel, J.L.; Dreher, J.D.; Mikhaylova, Y. Method and Compositions for Improving Appearance of Skin. US-9283154-B2, 15 March 2016. [Google Scholar]
- Nicholson, J.R.; Fieschi-Corso, L.P. Transfer Resistant Cosmetic Composition with Improved Feel. WO2009002498, 31 December 2008. [Google Scholar]
- Kanji, M.; Orr, C.; Robert, V. Cosmetic Compositions Comprising at Least One Polymethylsilsesquioxane Film Former. US6991782B2, 31 January 2006. [Google Scholar]
- Glenn Corp. Available online: Https://Glenncorp.Com/Wp-Content/Uploads/2016/11/BRB-PMS-2.Pdf (accessed on 30 September 2021).
- Glenn Corp. Available online: Https://Glenncorp.Com/Wp-Content/Uploads/2016/11/BRB-PMS-5.Pdf (accessed on 30 September 2021).
- Schlosser, A. Cosmetic Formulation Comprising Alkyl Phenyl Silsesquioxane Resins. US20040180011A1, 16 September 2004. [Google Scholar]
- Dow Chemical. 670 F. (Online) Dow Chemical. Available online: https://Dow.Com/En-Us/Document-Viewer.Html?RamdomVar=5246173742923931473&docPath=/Content/Dam/Dcc/Documents/En-Us/Productdatasheet/27/27-11/27-1158-0-1-Dowsil-670-Fluid.Pdf (accessed on 4 October 2021).
- Kimble, E.; Arkles, B.; Zazyczny, J.; Dick, D. Quaternary Functional Silsesquioxanes for Use in Personal Care: Polysilsesquioxane Steardimonium Chloride. SCC Technical Showcase, 6–7 December 2012. Available online: https://www.gelest.com/wp-content/uploads/pdf-04.pdf (accessed on 4 October 2021).
- Cho, H.J.; Kim, K.N.; Choi, K.H.; Choi, Y.J. Make-up Cosmetic Composition Containing MQ Silicone Resin and Propyl Silsesquioxane Resin. US9463152B2, 11 October 2016. [Google Scholar]
- Patel, S.; Amin, A.; Voorhees-Nordhaus, L. Cosmetic Compositions Containing Polypropylsilsesquioxane, a Volatile Solvent, Boron Nitride, and Silica. 2014. US20110243867A1, 18 November 2014. [Google Scholar]
- Becker, L.C. Safety Assessment of Polysilsesquioxanes as Used in Cosmetics; Cosmetic Ingredient Review: Washington, DC, USA, 2017. [Google Scholar]
- Hibrid Plastics. Available online: Https://Hybridplastics.Com/Wp-Content/Uploads/2018/10/MS0805_0702161.Pdf (accessed on 5 October 2021).
- Hibrid Plastics. Available online: Https://Hybridplastics.Com/Product/Ep0408-2/ (accessed on 6 October 2021).
- Hibrid Plastics. Available online: Https://Hybridplastics.Com/Wp-Content/Uploads/2019/03/EP0409-TDS-1020161.Pdf (accessed on 6 October 2021).
- European Commission. Available online: Https://Ec.Europa.Eu/Growth/Tools-Databases/Cosing/Pdf/COSING_Ingredients-Fragrance%20Inventory_v2.Pdf (accessed on 6 October 2021).
- Hibrid Plastics. Available online: Https://Hybridplastics.Com/Wp-Content/Uploads/2016/07/PG1190_070216.Pdf (accessed on 6 October 2021).
- Alessandrini, A.; Piraccini, B.M. Essential of Hair Care Cosmetics. Cosmetics 2016, 3, 34. [Google Scholar] [CrossRef] [Green Version]
- Thomson, B.; Halloran, D.; Vincent, J. Use of Aqueous Silsesquioxanes for Providing Body and Volume Effects from Hair Conditioners. In Proceedings of the 17th IFSCC Congres, Yokohama, Japan, 13–16 October 1992; pp. 352–360. [Google Scholar]
- Halloran, D.J.; Vincent, J.M. Hair Fixatives Comprising Nonpolar Silsesquioxanes. US5075103A, 24 December 1991. [Google Scholar]
- Castrogiovanni, A.; Barone, S.J.; Krog, A.; McCulley, M.L.; Callelo, J.F. Cosmetic Compositions with Improved Transfer Resistance. US5505937A, 9 April 1996. [Google Scholar]
- Dimotakis, E.; Bui, H.S.; Kanji, M. Long Wear Cosmetic Compositions Containing Poss Thermoplastic Elastomers. WO2012078751A3, 20 September 2012. [Google Scholar]
- Maitra, P.; Zheng, T. Cosmetic Nanocomposites Based on In-Situ Cross-Linked POSS Materials. US8545823 B2, 1 October 2013. [Google Scholar]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing Superoleophobic Surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef] [Green Version]
- Flagg, D.H.; McCarthy, T.J. Rediscovering Silicones: MQ Copolymers. Macromolecules 2016, 49, 8581–8592. [Google Scholar] [CrossRef]
- Yu, W.H.; Quadir, M. Poss Containing Cosmetic Compositions Having Improved Wear And/Or Pliability And Methods Of Making Improved Cosmetic Compositions. EP1794233A1, 13 June 2007. [Google Scholar]
- Arora, H.; Tosti, A. Safety and Efficacy of Nail Products. Cosmetics 2017, 4, 24. [Google Scholar] [CrossRef] [Green Version]
- Welton, J.; Dixon, K.; Lichtenhan, J.; Hait, S.; Schwab, J. Silsesquioxanes and Use in Nail Coatings Thereof. WO2019126781, 27 June 2019. [Google Scholar]
- Kim, K.-M.; Oh, H.M.; Lee, J.H. Controlling the Emulsion Stability of Cosmetics through Shear Mixing Process. Korea-Aust. Rheol. J. 2020, 32, 243–249. [Google Scholar] [CrossRef]
- Muenchow, L.; Lemmer, K.; Kruse, U. Cosmetic W/S Emulsion. EP2319482A2, 11 May 2011. [Google Scholar]
- Yeon, J.Y.; Shin, B.R.; Kim, T.G.; Seo, J.M.; Lee, C.H.; Lee, S.G.; Pyo, H.B. A Study on Emulsion Stability of O/W and W/S Emulsion According to HLB of Emulsifier. J. Soc. Cosmet. Sci. Korea 2014, 40, 227–236. [Google Scholar] [CrossRef]
- Montenegro, L.; Paolino, D.; Puglisi, G. Effects of Silicone Emulsifiers on in Vitro Skin Permeation of Sunscreens from Cosmetic Emulsions. J. Cosmet. Sci. 2004, 55, 509–518. [Google Scholar] [PubMed]
- Tcholakova, S.; Denkov, N.D.; Ivanov, I.B.; Campbell, B. Coalescence Stability of Emulsions Containing Globular Milk Proteins. Adv. Colloid Interface Sci. 2006, 123, 259–293. [Google Scholar] [CrossRef]
- Olejnik, A.; Schroeder, G.; Nowak, I. The Tetrapeptide N-Acetyl-Pro-Pro-Tyr-Leu in Skin Care Formulations-Physicochemical and Release Studies. Int. J. Pharm. 2015, 492, 161–168. [Google Scholar] [CrossRef]
- Dyab, A.K.F. Destabilisation of Pickering Emulsions Using PH. Colloids Surf. A: Physicochem. Eng. Asp. 2012, 402, 2–12. [Google Scholar] [CrossRef]
- Prakash, N.K.S.; Mahendra, C.; Prashanth, S.J.; Manral, K.; Babu, U.V.; Gowda, D.V.S. Emulsions and Emulsifiers. Asian J. Exp. Chem. 2013, 8, 30–45. [Google Scholar]
- Feng, X.; Zhu, S.; Yue, K.; Su, H.; Guo, K.; Wesdemiotis, C.; Zhang, W.-B.; Cheng, S.Z.D.; Li, Y. T10 Polyhedral Oligomeric Silsesquioxane-Based Shape Amphiphiles with Diverse Head Functionalities via “Click” Chemistry. ACS Macro Lett. 2014, 3, 900–905. [Google Scholar] [CrossRef]
- Yue, K.; Liu, C.; Guo, K.; Yu, X.; Huang, M.; Li, Y.; Wesdemiotis, C.; Cheng, S.Z.D.; Zhang, W.-B. Sequential “Click” Approach to Polyhedral Oligomeric Silsesquioxane-Based Shape Amphiphiles. Macromolecules 2012, 45, 8126–8134. [Google Scholar] [CrossRef]
- Imoto, H.; Nakao, Y.; Nishizawa, N.; Fujii, S.; Nakamura, Y.; Naka, K. Tripodal Polyhedral Oligomeric Silsesquioxanes as a Novel Class of Three-Dimensional Emulsifiers. Polym. J. 2015, 47, 609–615. [Google Scholar] [CrossRef]
- Tadros, T.F. Applied Surfactants: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 3527604537. [Google Scholar]
- Masalova, I.; Tshilumbu, N.N.; Mamedov, E.; Kharatyan, E.; Katende, J.K. Stabilisation of Highly Concentrated Water-in-Oil Emulsions by Polyhedral Oligomeric Silsesquioxane Nanomolecules. J. Mol. Liq. 2019, 279, 351–360. [Google Scholar] [CrossRef]
- Sakamoto, K.; Lochhead, H.; Maibach, H.; Yamashita, Y. Cosmetic Science and Technology: Theoretical Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128020050. [Google Scholar]
- Kockler, J.; Oelgemöller, M.; Robertson, S.; Glass, B.D. Photostability of Sunscreens. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 91–110. [Google Scholar] [CrossRef]
- Sambandan, D.R.; Ratner, D. Sunscreens: An Overview and Update. J. Am. Acad. Dermatol. 2011, 64, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Latha, M.S.; Martis, J.; Shobha, V.; Shinde, R.S.; Bangera, S.; Krishnankutty, B.; Bellary, S.; Varughese, S.; Rao, P.; Kumar, B.R.N. Sunscreening Agents: A Review. J. Clin. Aesthet. Dermatol. 2013, 6, 16–26. [Google Scholar] [PubMed]
- Sabzevari, N.; Qiblawi, S.; Norton, S.A.; Fivenson, D. Sunscreens: UV Filters to Protect Us: Part 1: Changing Regulations and Choices for Optimal Sun Protection. Int. J. Women’s Dermatol. 2021, 7, 28–44. [Google Scholar] [CrossRef]
- Ngoc, L.T.N.; van Tran, V.; Moon, J.-Y.; Chae, M.; Park, D.; Lee, Y.-C. Recent Trends of Sunscreen Cosmetic: An Update Review. Cosmetics 2019, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Tampucci, S.; Burgalassi, S.; Chetoni, P.; Monti, D. Cutaneous Permeation and Penetration of Sunscreens: Formulation Strategies and in Vitro Methods. Cosmetics 2018, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Stiefel, C.; Schwack, W. Photoprotection in Changing Times–UV Filter Efficacy and Safety, Sensitization Processes and Regulatory Aspects. Int. J. Cosmet. Sci. 2015, 37, 2–30. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.B.; Yoon, K.-S.; Cosmetics, S.; Yeongdong, C.S.K. Organic-Inorganic Hybrid UV Blocking Powders Based on Polysilsesquioxane. Proc. NSTI Nanotech 2007 2007, 4, 254–256. [Google Scholar]
- Yoon, K.-S.; Jeong, T.-G.; Kim, Y.-B.; Lim, M.S. Cosmetic Composition Containing UV-Screening Polysilsesquioxane Sphere. KR20060102225A, 27 September 2007. [Google Scholar]
- Loy, D.A.; Tolbert, S.H. Photochemically Stable, Non-Leaching, Bridged Polysilsesquioxane Based Sunscreens. WO2017132655, 3 August 2018. [Google Scholar]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a Historical Perspective: From Simple Networks to Smart Materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A. Biomedical Applications of Hydrogels Handbook; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010; ISBN 144195919X. [Google Scholar]
- Zhang, D.; Chen, L.; Wang, Z.; Wei, T. Recent Progress on the Research of High Strength Hydrogels. In Proceedings of the 5th International Conference on Advanced Design and Manufacturing Engineering, Shenzhen, China, 19 September 2015; pp. 19–20. [Google Scholar]
- Ghanbari, H.; Cousins, B.G.; Seifalian, A.M. A Nanocage for Nanomedicine: Polyhedral Oligomeric Silsesquioxane (POSS). Macromol. Rapid Commun. 2011, 32, 1032–1046. [Google Scholar] [CrossRef]
- Tanaka, K.; Chujo, Y. Advanced Functional Materials Based on Polyhedral Oligomeric Silsesquioxane (POSS). J. Mater. Chem. 2012, 22, 1733–1746. [Google Scholar] [CrossRef]
- Shi, H.; Yang, J.; You, M.; Li, Z.; He, C. Polyhedral Oligomeric Silsesquioxanes (POSS)-Based Hybrid Soft Gels: Molecular Design, Material Advantages, and Emerging Applications. ACS Mater. Lett. 2020, 2, 296–316. [Google Scholar] [CrossRef]
- Wu, J.; Hou, S.; Ren, D.; Mather, P.T. Antimicrobial Properties of Nanostructured Hydrogel Webs Containing Silver. Biomacromolecules 2009, 10, 2686–2693. [Google Scholar] [CrossRef] [PubMed]


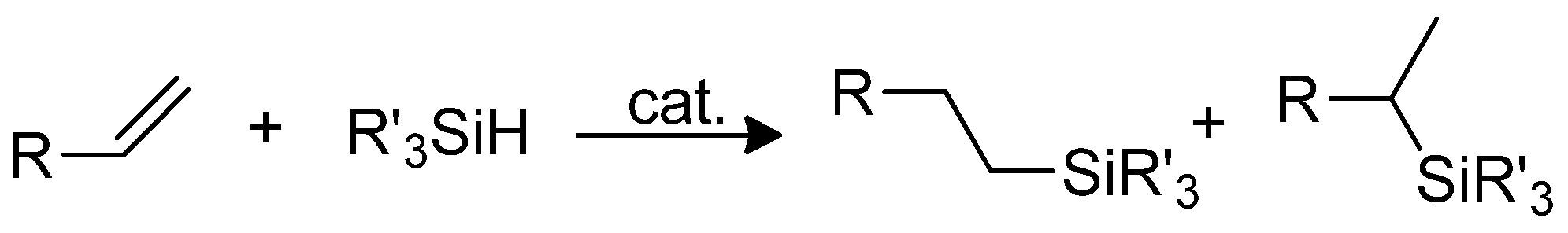
| International Nomenclature of Cosmetic Ingredients (INCI) | Commercial Name | Producer | Cosmetic Product Category | Functions | Refs. |
|---|---|---|---|---|---|
| Polymethylsilsesquioxane | SilForm*Flexible Resin | Momentive Performance Materials | color cosmetics, creams, lotions, sun care | opacifying agent, film former, provide long-lasitng wearability | [2,70] |
| BELSIL® B 110 | Wacker Chemie AG | skin care, sunscreen | providing softness, enhanced spreading | [71] | |
| BRB PMS-2 | BRB International BV | color cosmetics, skin care | pigment stabilizer | [72] | |
| BRB PMS-5 | BRB International BV | color cosmetics, skin care | pigment stabilizer | [73] | |
| BELSIL® SPR 45 VP | Wacker Chemie AG | color cosmetics, skin care, sunscreen | providing softness, water repellence, film former | [74] | |
| Blend of cyclopentasiloxane and polypropylsilsesquioxane | DOWSIL 670 Fluid | Dow | color cosmetics, suncare, skin care | film former, provide long-lasting color, shine enhancer | [2,75] |
| Polysilsesquioxane Steardimonium Chloride | SiQubeTM Q1850 | Paradigm Science Inc. | skincare, haircare | cleansing ability, skin barrier function improver | [76] |
| Blend of MQ (trimethylsiloxysilicate and T propyl (polypropylsilsesquioxane) silicone resins | MQ-1640 Flake Resin | Dow Corning | color cosmetics, skin care, hair care | provide hair long lasting colour | [77] |
| Isobutyl Polysilsesquioxane | PSS-Octaisobutyl substituted 97 | Boc Sciences | nail care | nail conditioning agent | [78] |
| Acryloyloxypropyl Polysilsesquioxane | SLT-3R01 | Gelest | nail care | nail conditioning agent | [79] |
| Trimethylpentyl Polysilsesquioxane | Isooctyl POSS | Hybrid Plastics | nail care | nail conditioning agent | [79,80] |
| Epoxycyclohexylethyl Polysilsesquioxane | EP0408 | Hybrid Plastics | nail care | nail conditioning agent | [81] |
| Glycidoxypropyl Polysilsesquioxane | EP0408 | Hybrid Plastics | nail care | film forming ability | [82,83] |
| Methoxy PEG-10 Polysilsesquioxane | PG1190 | Hybrid Plastics | skin care | skin conditioning agent, cleansing agent, solubilizing agent | [79,83,84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olejnik, A.; Sztorch, B.; Brząkalski, D.; Przekop, R.E. Silsesquioxanes in the Cosmetics Industry—Applications and Perspectives. Materials 2022, 15, 1126. https://doi.org/10.3390/ma15031126
Olejnik A, Sztorch B, Brząkalski D, Przekop RE. Silsesquioxanes in the Cosmetics Industry—Applications and Perspectives. Materials. 2022; 15(3):1126. https://doi.org/10.3390/ma15031126
Chicago/Turabian StyleOlejnik, Anna, Bogna Sztorch, Dariusz Brząkalski, and Robert E. Przekop. 2022. "Silsesquioxanes in the Cosmetics Industry—Applications and Perspectives" Materials 15, no. 3: 1126. https://doi.org/10.3390/ma15031126
APA StyleOlejnik, A., Sztorch, B., Brząkalski, D., & Przekop, R. E. (2022). Silsesquioxanes in the Cosmetics Industry—Applications and Perspectives. Materials, 15(3), 1126. https://doi.org/10.3390/ma15031126







