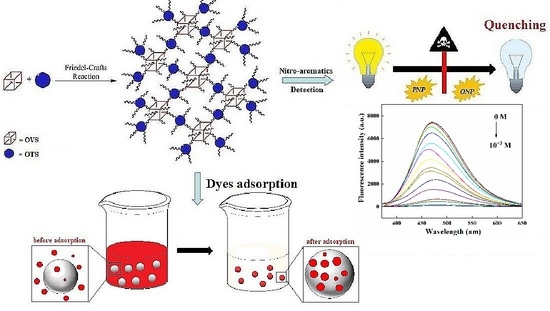
Silsesquioxane-Based Triphenylamine-Linked Fluorescent Porous Polymer for Dyes Adsorption and Nitro-Aromatics Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Characterization and Adsorption Experiments
2.3. Synthesis of Hybrid Luminescent Porous Polymer (PCS-OTS)
3. Results and Discussion
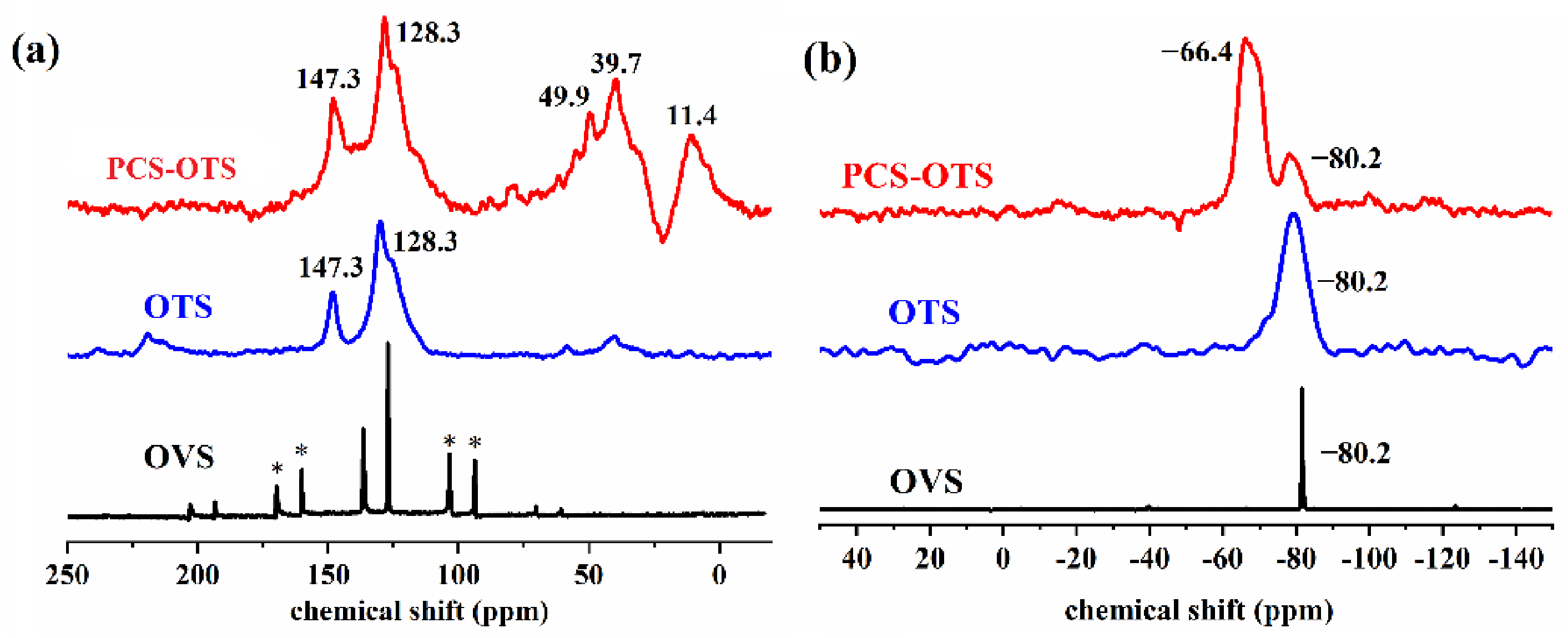
3.1. Preparation and Characterization
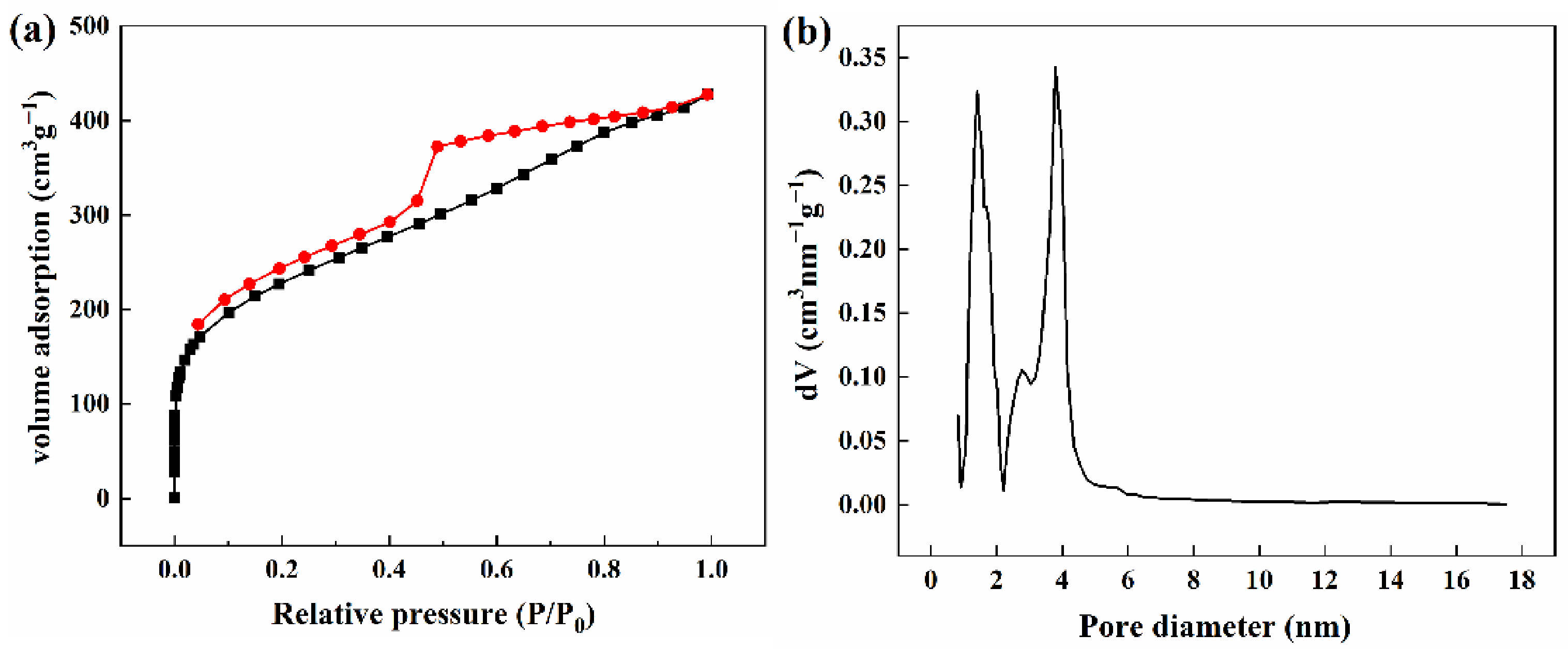
3.2. Porosity
3.3. Thermal Property and Morphology
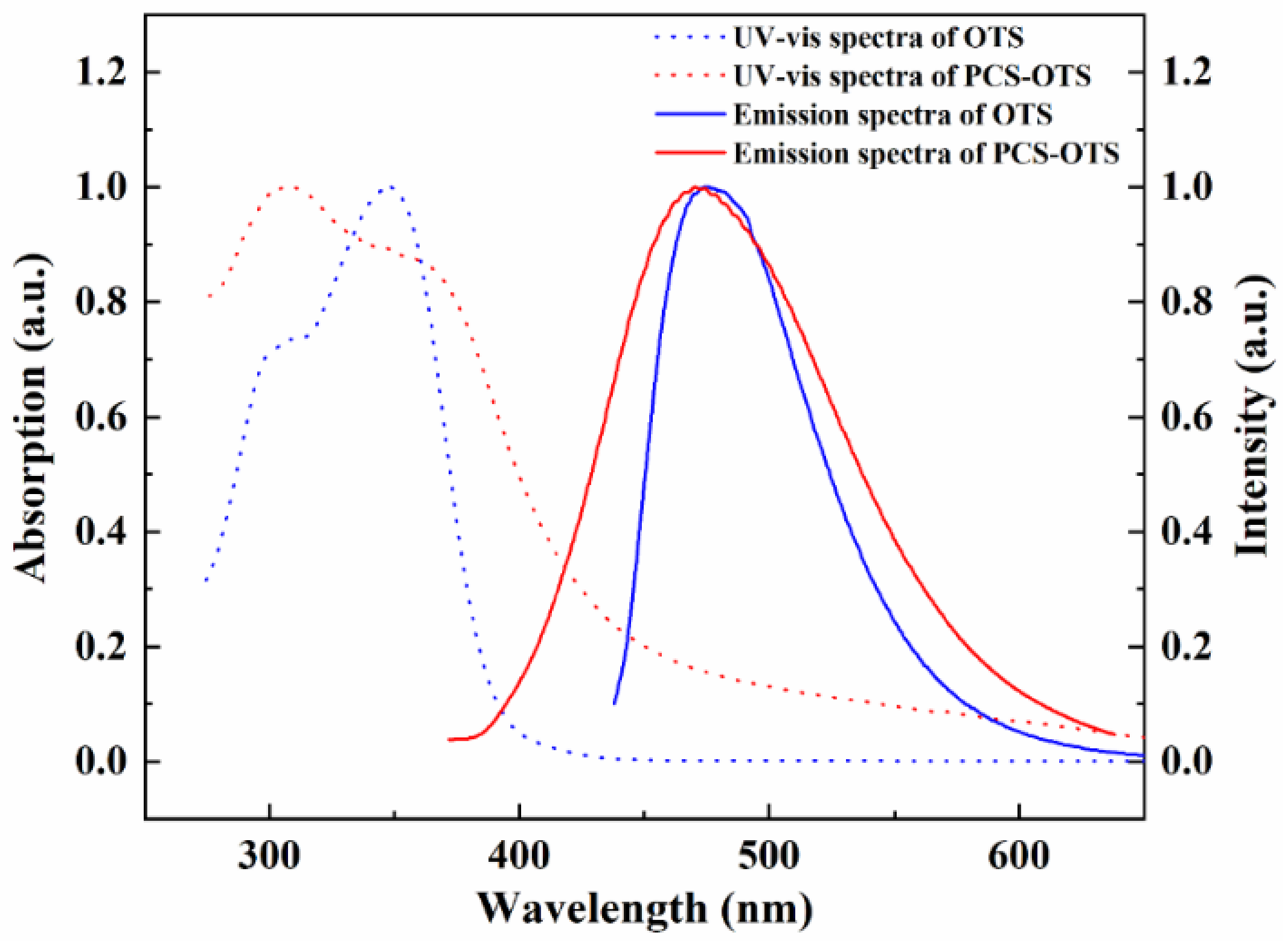
3.4. Photophysical Properties
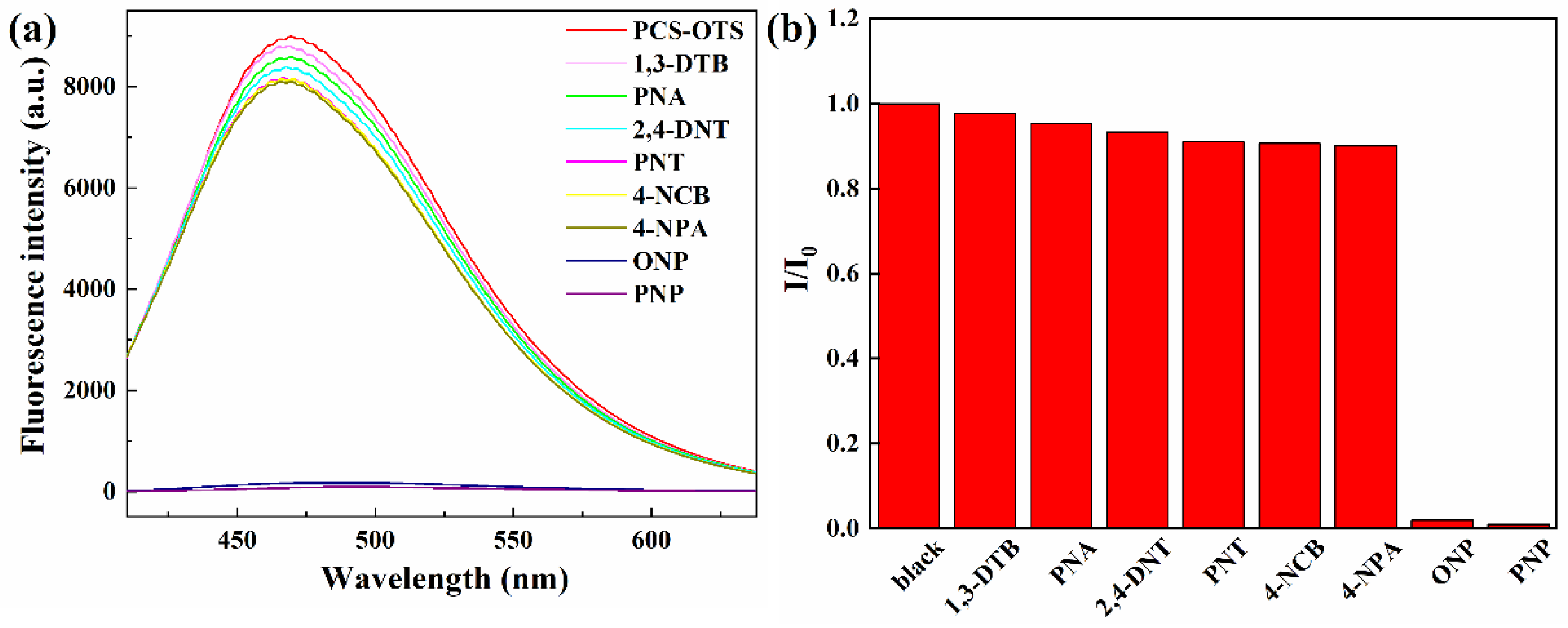
3.5. Detection of Nitro-Aromatics by PCS-OTS Suspension
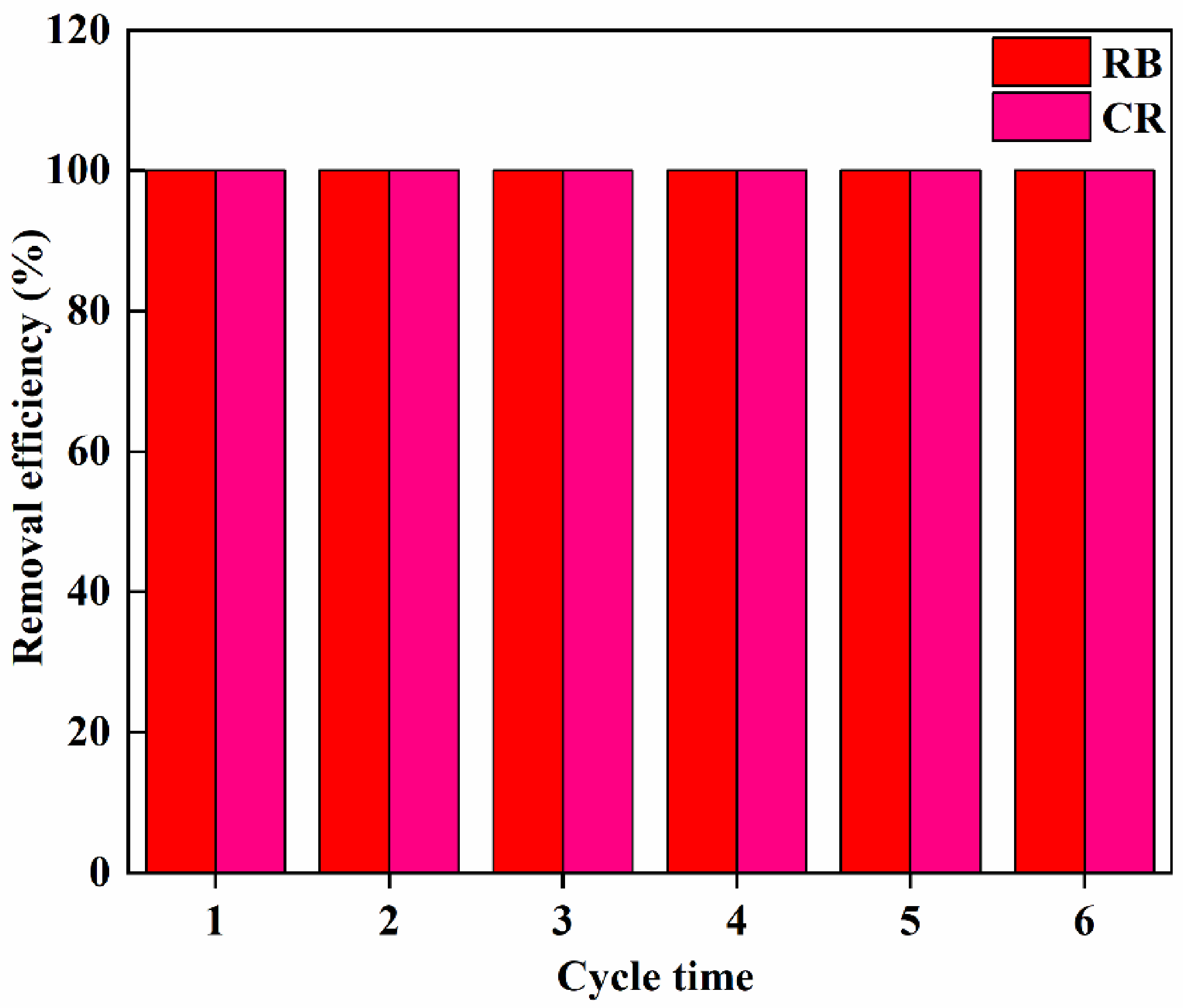
3.6. Adsorption for Dyes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, Y.; Liu, H. Cage-like silsesquioxanes-based hybrid materials. Dalton Trans. 2020, 49, 5396–5405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, J.; Xu, T.; Sima, H.; Hou, J. Effects of Polyhedral Oligomeric Silsesquioxane (POSS) on thermal and mechanical properties of polysiloxane foam. Materials 2020, 13, 5470. [Google Scholar] [CrossRef] [PubMed]
- Romo-Uribe, A.; Albanil, L. POSS-induced dynamic cross-links produced self-healing and shape memory physical hydrogels when copolymerized with N-isopropyl acrylamide. ACS Appl. Mater. Interfaces 2019, 11, 24447–24458. [Google Scholar] [CrossRef] [PubMed]
- Gon, M.; Saotome, S.; Tanaka, K.; Chujo, Y. Paintable hybrids with thermally stable dual emission composed of tetraphenylethene-integrated POSS and MEH-PPV for heat-resistant white-light luminophores. ACS Appl. Mater. Interfaces 2021, 13, 12483–12490. [Google Scholar] [CrossRef]
- Soldatov, M.; Liu, H. Hybrid porous polymers based on cage-like organosiloxanes: Synthesis, properties and applications. Prog. Polym. Sci. 2021, 119, 101419. [Google Scholar] [CrossRef]
- Xiang, K.; Li, Y.; Xu, C.; Li, S. POSS-based organic–inorganic hybrid nanomaterials: Aggregation-enhanced emission, and highly sensitive and selective detection of nitroaromatic explosives in aqueous media. J. Mater. Chem. A 2016, 4, 5578–5583. [Google Scholar] [CrossRef]
- Bendrea, A.-D.; Vacareanu, L.; Grigoras, M. Synthesis, characterization and (electro)chemical polymerization of triphenylamine-end-functionalized poly(ε-caprolactone). Polym. Int. 2010, 59, 624–629. [Google Scholar] [CrossRef]
- Jiang, Z.; Ye, T.; Yang, C.; Yang, D.; Zhu, M.; Zhong, C.; Qin, J.; Ma, D. Star-shaped oligotriarylamines with planarized triphenylamine core: Solution-processable, high-Tg hole-injecting and hole-transporting materials for organic light-emitting devices. Chem. Mater. 2011, 23, 771–777. [Google Scholar] [CrossRef]
- Metri, N.; Sallenave, X.; Plesse, C.; Beouch, L.; Aubert, P.-H.; Goubard, F.; Chevrot, C.; Sini, G. Processable star-shaped molecules with triphenylamine core as hole-transporting materials: Experimental and theoretical approach. J. Phys. Chem. C 2012, 116, 3765–3772. [Google Scholar] [CrossRef]
- Wu, J.H.; Yen, H.J.; Hu, Y.C.; Liou, G.S. Side-chain and linkage-mediated effects of anthraquinone moieties on ambipolar poly(triphenylamine)-based volatile polymeric memory devices. Chem. Commun. 2014, 50, 4915–4917. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, H.; Jiang, C.; Liu, H. Silsesquioxane-based triphenylamine functionalized porous polymer for CO2, I2 capture and nitro-aromatics detection. Polymer 2020, 186, 122004. [Google Scholar] [CrossRef]
- Meng, X.; Liu, Y.; Wang, S.; Du, J.; Ye, Y.; Song, X.; Liang, Z. Silsesquioxane-carbazole-corbelled hybrid porous polymers with flexible nanopores for efficient CO2 conversion and luminescence sensing. ACS Appl. Polym. Mater. 2019, 2, 189–197. [Google Scholar] [CrossRef]
- Peng, Y.; Ben, T.; Xu, J.; Xue, M.; Jing, X.; Deng, F.; Qiu, S.; Zhu, G. A covalently-linked microporous organic-inorganic hybrid framework containing polyhedral oligomeric silsesquioxane moieties. Dalton Trans. 2011, 40, 2720–2724. [Google Scholar] [CrossRef]
- Suenaga, K.; Tanaka, K.; Chujo, Y. Heat-resistant mechanoluminescent chromism of the hybrid molecule based on boron ketoiminate modified octasubstituted polyhedral oligomeric silsesquioxane. Chem. Eur. J. 2017, 23, 1409–1414. [Google Scholar] [CrossRef]
- Roll, M.F.; Kampf, J.W.; Kim, Y.; Yi, E.; Laine, R.M. Nano building blocks via iodination of [PhSiO1.5]n, forming [p-I-C6H4SiO1.5]n (n = 8, 10, 12), and a new route to high-surface-area, thermally stable, microporous materials via thermal elimination of I2. J. Am. Chem. Soc. 2010, 132, 10171–10183. [Google Scholar] [CrossRef]
- Markovic, E.; Ginic-Markovic, M.; Clarke, S.; Matisons, J.; Hussain, M.; Simon, G.P. Poly(ethylene glycol)-octafunctionalized polyhedral oligomeric silsesquioxane: Synthesis and thermal analysis. Macromolecules 2007, 40, 2694–2701. [Google Scholar] [CrossRef]
- Chen, L.-H.; Wang, X.-Y.; Liao, Z.-C.; Wang, T.-Q.; Lin, H.-X.; Wang, Z.-X.; Cui, Y.-M. π-Conjugated twin molecules based on 9,9-diethyl-1-phenyl-1,9-dihydrofluoreno[2,3-d]imidazole module: Synthesis, characterization, and electroluminescence properties. Mon. Chem. Chem. Mon. 2020, 151, 917–924. [Google Scholar] [CrossRef]
- Maragani, R.; Sharma, R.; Misra, R. Donor-acceptor triphenylvinyl and tetraphenyl conjugates: Synthesis, aggregation and computational studies. ChemistrySelect 2017, 2, 10033–10037. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zheng, K.; Zhu, S. FRET-based small-molecule fluorescent probes: Rational design and bioimaging applications. Acc. Chem. Res. 2013, 46, 1462–1473. [Google Scholar] [CrossRef]
- Wang, J.; Mei, J.; Yuan, W.; Lu, P.; Qin, A.; Sun, J.; Ma, Y.; Tang, B.Z. Hyperbranched polytriazoles with high molecular compressibility: Aggregation-induced emission and superamplified explosive detection. J. Mater. Chem. 2011, 21, 4056. [Google Scholar] [CrossRef]
- Li, D.; Liu, J.; Kwok, R.T.; Liang, Z.; Tang, B.Z.; Yu, J. Supersensitive detection of explosives by recyclable AIE luminogen-functionalized mesoporous materials. Chem. Commun. 2012, 48, 7167–7169. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Wang, L.; Zhang, X.; Yang, W.; Song, G. Enhanced adsorption of Congo red dye by functionalized carbon nanotube/mixed metal oxides nanocomposites derived from layered double hydroxide precursor. Chem. Eng. J. 2015, 275, 315–321. [Google Scholar] [CrossRef]
- Xue, F.; Tang, B.; Bin, L.; Ye, J.; Huang, S.; Fu, F.; Li, P.; Cui, J. Residual micro organic pollutants and their biotoxicity of the effluent from the typical textile wastewater treatment plants at Pearl River Delta. Sci. Total Environ. 2019, 657, 696–703. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, H.; Chen, L.; Chen, Y. N,N′-bicarbazole: A versatile building block toward the construction of conjugated porous polymers for CO2 capture and dyes adsorption. Macromolecules 2017, 50, 4993–5003. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Wang, J.; Liu, F.; Hu, N.; Pei, H.; Yang, W.; Li, Z.; Suo, Y.; Wang, J. High effective adsorption/removal of illegal food dyes from contaminated aqueous solution by Zr-MOFs (UiO-67). Food Chem. 2018, 254, 241–248. [Google Scholar] [CrossRef]
- Zhuang, X.; Wan, Y.; Feng, C.; Shen, Y.; Zhao, D. Highly efficient adsorption of bulky dye molecules in wastewater on ordered mesoporous carbons. Chem. Mater. 2009, 21, 706–716. [Google Scholar] [CrossRef]
- Pelekani, C.; Snoeyink, V. A kinetic and equilibrium study of competitive adsorption between atrazine and Congo red dye on activated carbon: The importance of pore size distribution. Carbon 2001, 39, 25–37. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, P.-C.; Tan, L.; Liu, J.-M.; Tan, B.; Yang, X.-L.; Xu, H.-B. Triptycene-based hyper-cross-linked polymer sponge for gas storage and water treatment. Macromolecules 2015, 48, 8509–8514. [Google Scholar] [CrossRef]
- Lei, C.; Pi, M.; Jiang, C.; Cheng, B.; Yu, J. Synthesis of hierarchical porous zinc oxide (ZnO) microspheres with highly efficient adsorption of Congo red. J. Colloid Interface Sci. 2017, 490, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, S.; Tavakoli, O.; Khoobi, M.; Ansari, A.; Faramarzi, M.A. Application of novel magnetic β-cyclodextrin-anhydride polymer nano-adsorbent in cationic dye removal from aqueous solution. J. Taiwan Inst. Chem. Eng. 2017, 80, 452–463. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, G.; Gao, W.-Y.; Niu, Z.; Wojtas, L.; Ma, S. Anionic metal-organic framework for selective dye removal and CO2 fixation. Eur. J. Inorg. Chem. 2016, 27, 4373–4377. [Google Scholar] [CrossRef]
- Byun, J.; Patel, H.A.; Thirion, D.; Yavuz, C.T. Charge-specific size-dependent separation of water-soluble organic molecules by fluorinated nanoporous networks. Nat. Commun. 2016, 7, 13377. [Google Scholar] [CrossRef]
- Shi, W.; Tao, S.; Yu, Y.; Wang, Y.; Ma, W. High performance adsorbents based on hierarchically porous silica for purifying multicomponent wastewater. J. Mater. Chem. 2011, 21, 15567. [Google Scholar] [CrossRef]
- Han, Y.; Li, W.; Zhang, J.; Meng, H.; Xu, Y.; Zhang, X. Adsorption behavior of Rhodamine B on nanoporous polymers. RSC Adv. 2015, 5, 104915–104922. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Unno, M.; Liu, H. Silsesquioxane-Based Triphenylamine-Linked Fluorescent Porous Polymer for Dyes Adsorption and Nitro-Aromatics Detection. Materials 2021, 14, 3851. https://doi.org/10.3390/ma14143851
Wang Q, Unno M, Liu H. Silsesquioxane-Based Triphenylamine-Linked Fluorescent Porous Polymer for Dyes Adsorption and Nitro-Aromatics Detection. Materials. 2021; 14(14):3851. https://doi.org/10.3390/ma14143851
Chicago/Turabian StyleWang, Qingzheng, Masafumi Unno, and Hongzhi Liu. 2021. "Silsesquioxane-Based Triphenylamine-Linked Fluorescent Porous Polymer for Dyes Adsorption and Nitro-Aromatics Detection" Materials 14, no. 14: 3851. https://doi.org/10.3390/ma14143851
APA StyleWang, Q., Unno, M., & Liu, H. (2021). Silsesquioxane-Based Triphenylamine-Linked Fluorescent Porous Polymer for Dyes Adsorption and Nitro-Aromatics Detection. Materials, 14(14), 3851. https://doi.org/10.3390/ma14143851