Biphasic Properties of PVAH (Polyvinyl Alcohol Hydrogel) Reflecting Biomechanical Behavior of the Nucleus Pulposus of the Human Intervertebral Disc
Abstract
1. Introduction
2. Materials and Methods
2.1. PVAH Specimen Preparation
2.2. Holmes–Mow Model
2.3. Unconfined Compression Experiments
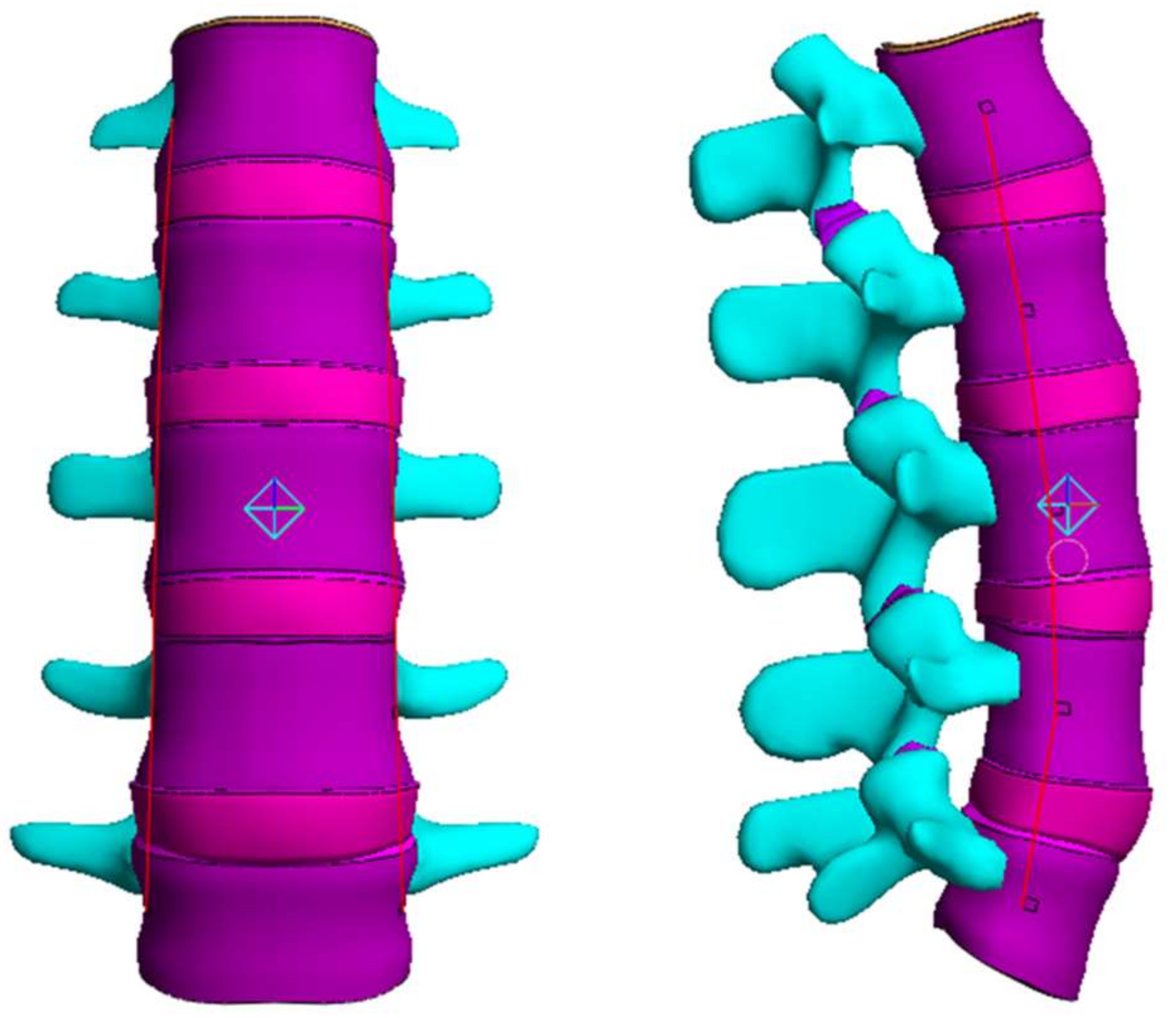
2.4. Range of Motion (ROM), Facet Joint Force (FJF), and Nucleus Pulposus (NP) Pressures
3. Results
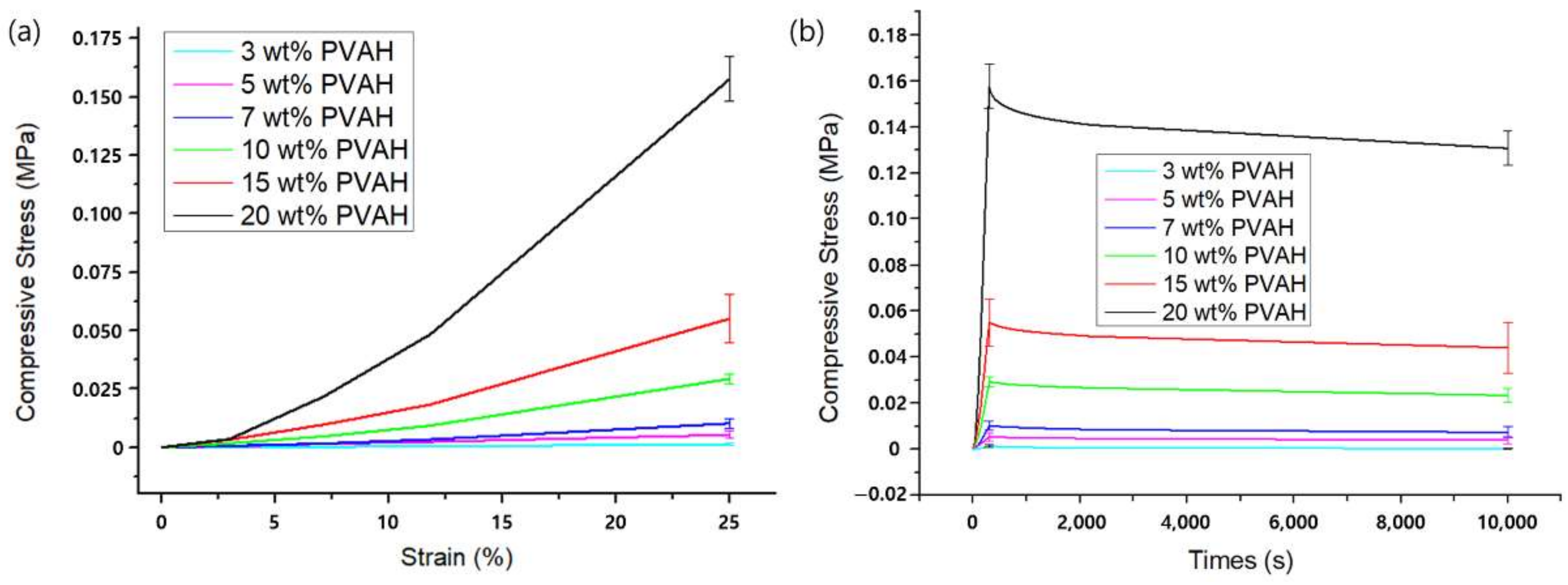
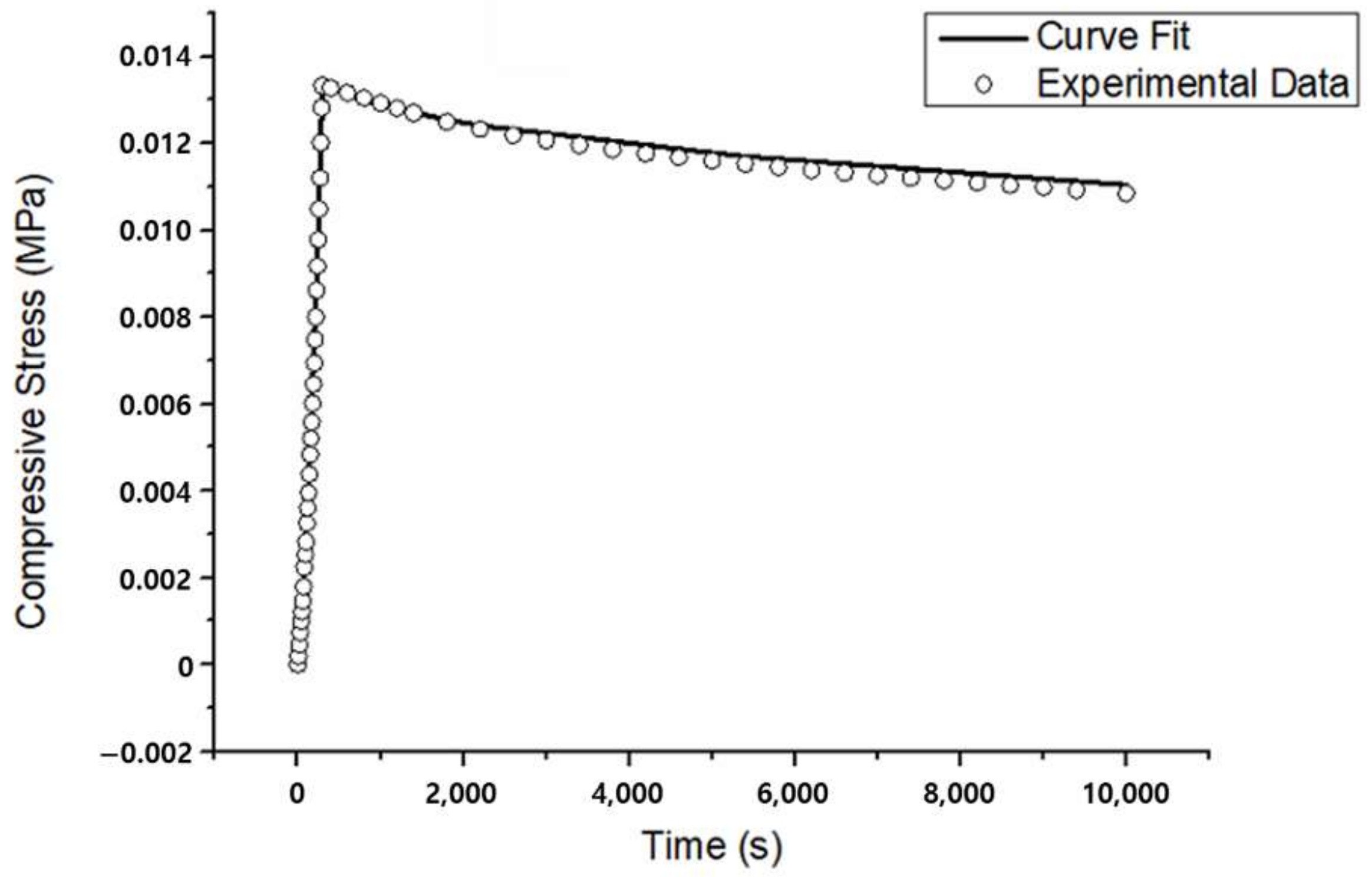
3.1. Unconfined Compression Experiments
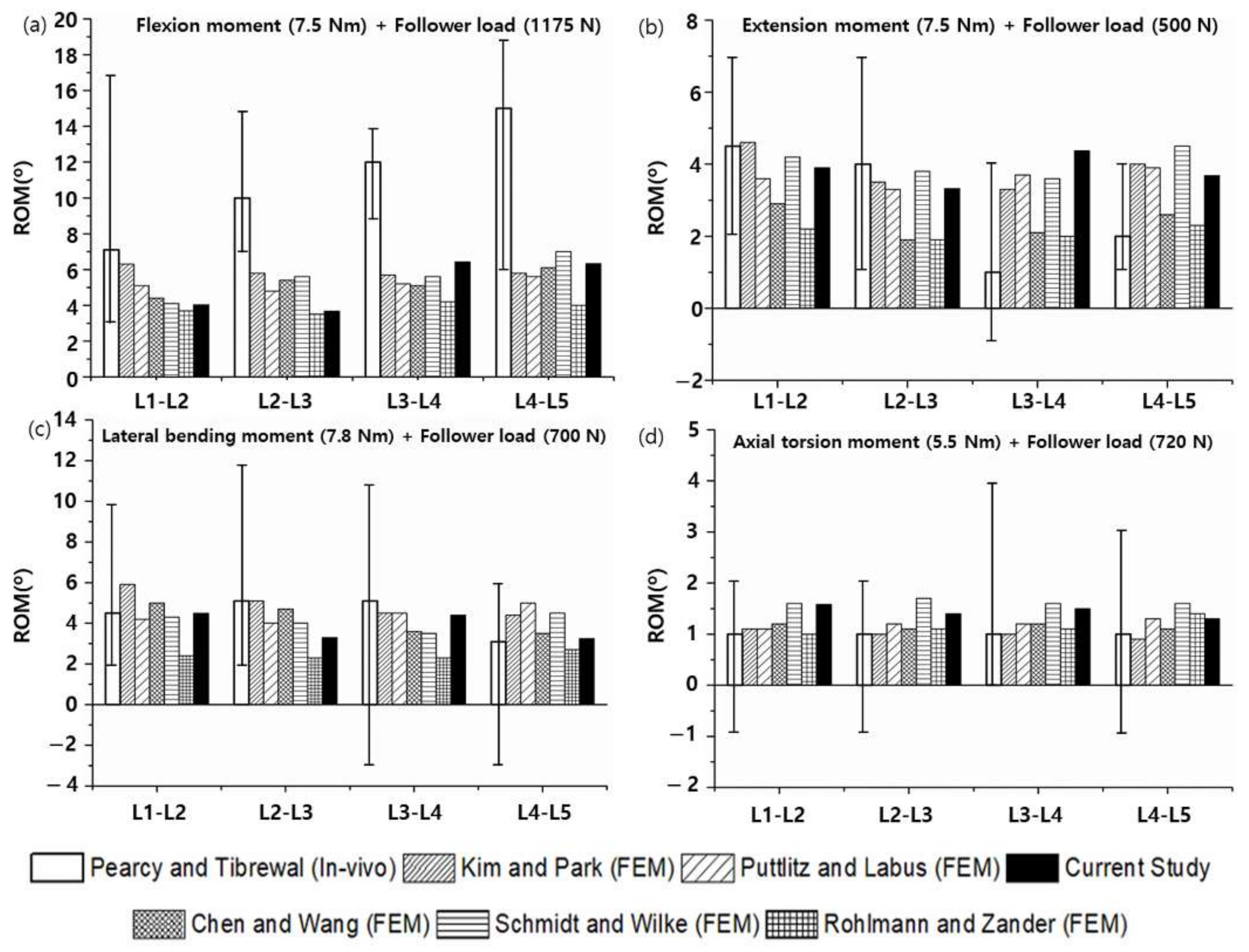
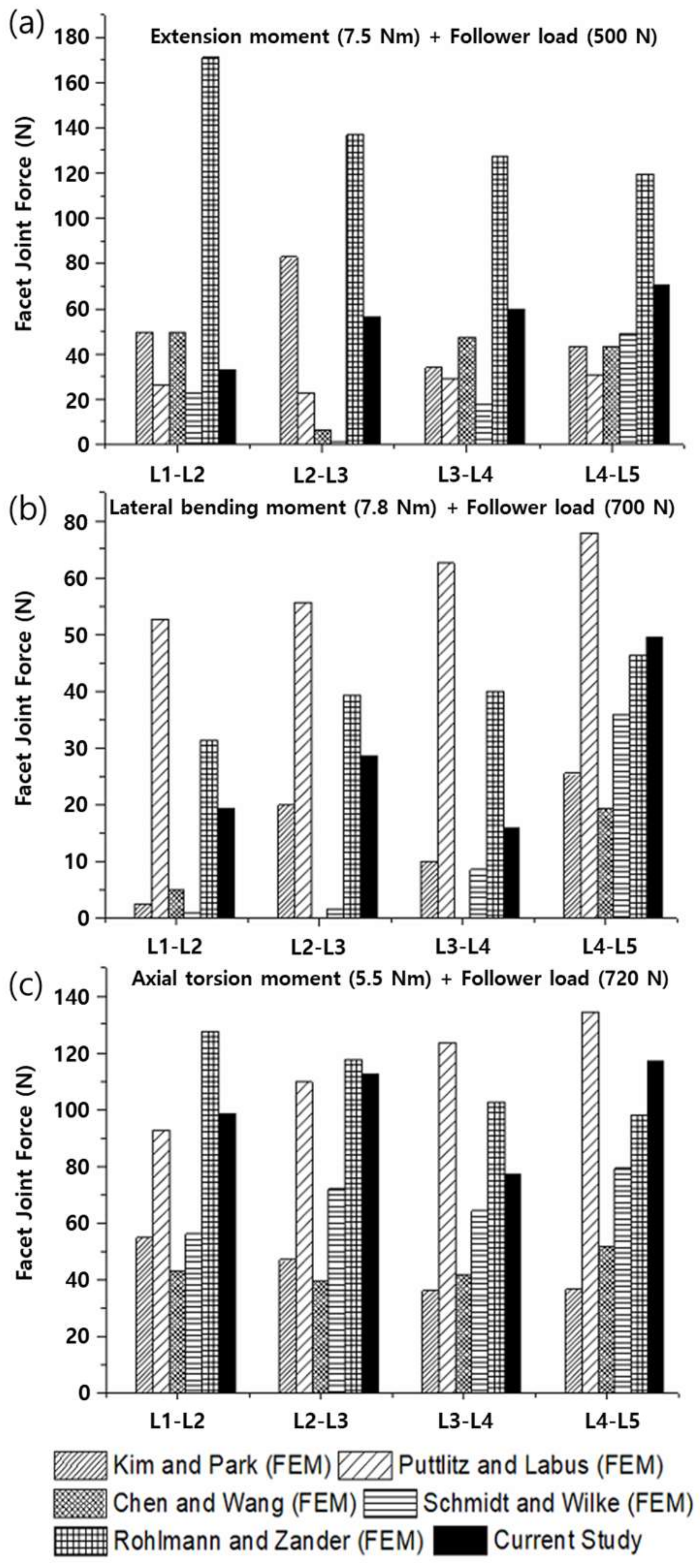
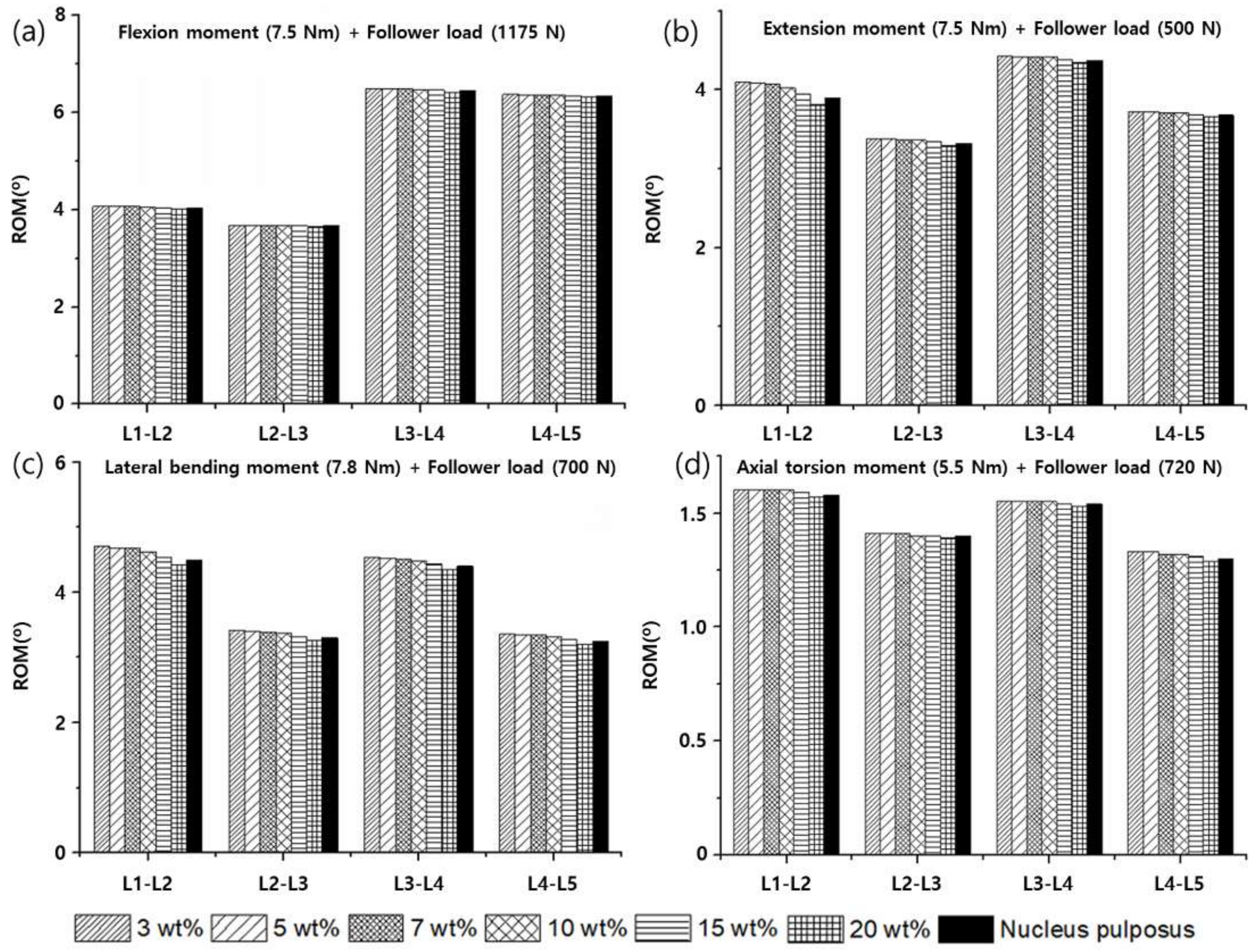
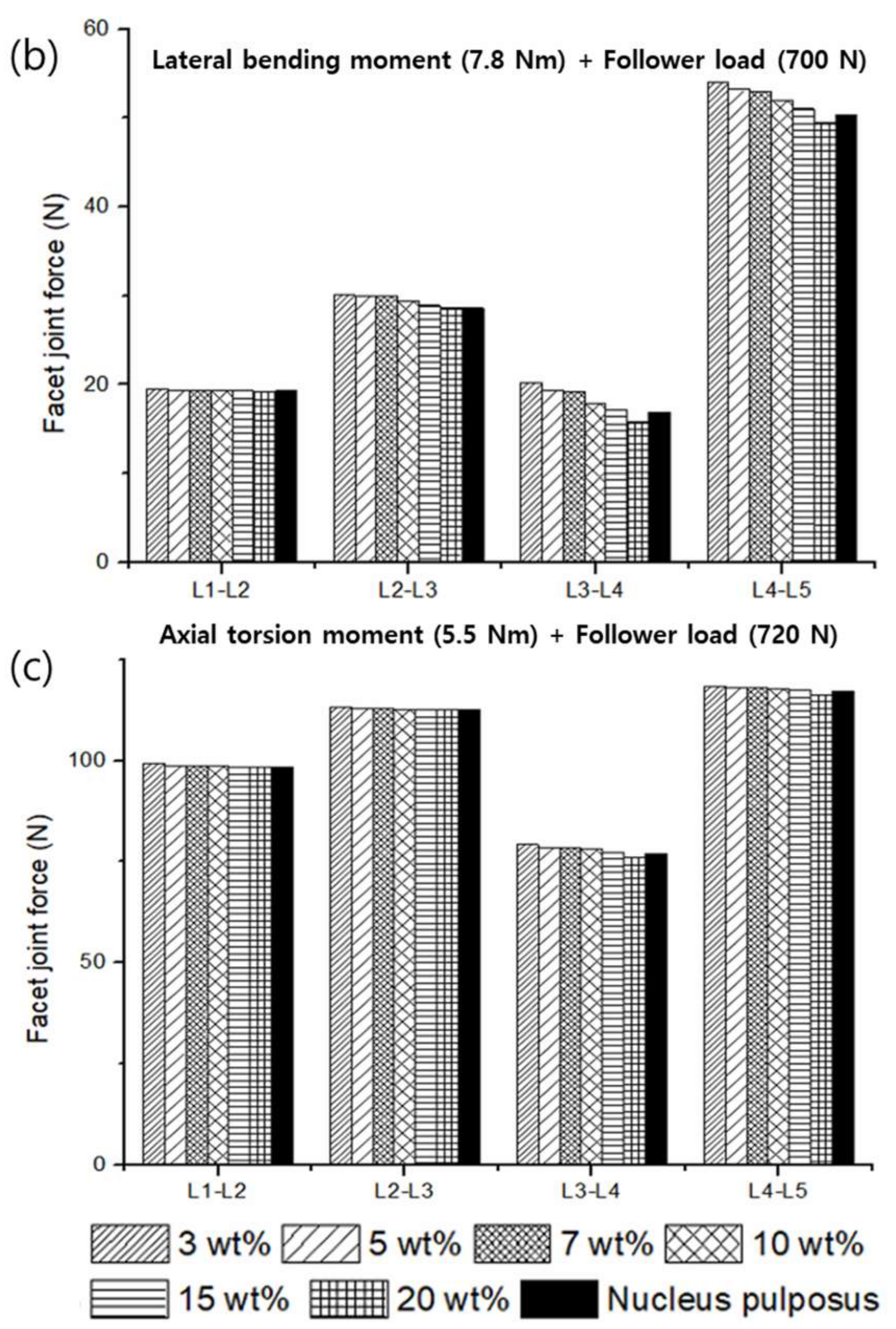
3.2. Range of Motion (ROM), Facet Joint Force (FJF), and Nucleus Pulposus (NP) Pressures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joshi, A.; Fussell, G.; Thomas, J.; Hsuan, A.; Lowman, A.; Karduna, A.; Vresilovic, E.; Marcolongo, M. Functional compressive mechanics of a PVA/PVP nucleus pulposus replacement. Biomaterials 2006, 27, 176–184. [Google Scholar] [CrossRef] [PubMed]
- White, A.A., III; Panjabi, M.M. Clinical Biomechanics of the Spine; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1990. [Google Scholar]
- Bao, Q.B.; McCullen, G.M.; Higham, P.A.; Dumbleton, J.H.; Yuan, H.A. The artificial disc: Theory, design and materials. Biomaterials 1996, 17, 1157–1167. [Google Scholar] [CrossRef]
- Iatridis, J.C.; Weidenbaum, M.; Setton, L.A.; Mow, V.C. Is the nucleus pulposus a solid or a fluid? Mechanical behaviors of the nucleus pulposus of the human intervertebral disc. Spine 1996, 21, 1174–1184. [Google Scholar] [CrossRef]
- Nachemson, A. Some mechanical properties of the lumbar intervertebral disc. Bull. Hosp. Joint Dis. 1962, 23, 130–132. [Google Scholar] [PubMed]
- Boyd, L.M.; Carter, A.J. Injectable biomaterials and vertebral endplate treatment for repair and regeneration of the intervertebral disc. Eur. Spine J. 2006, 15 (Suppl. 3), S414–S421. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Vaccaro, A.R.; Lee, J.Y.; Denaro, V.; Lim, M.R. Nucleus pulposus replacement: Basic science and indications for clinical use. Spine 2005, 30, S16–S22. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.C.; Wright, T.M.; Panjabi, M.M.; Lipman, J.D. Biomechanics of nonfusion implants. Orthop. Clin. N. Am. 2005, 36, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Mehta, S.; Vresilovic, E.; Karduna, A.; Marcolongo, M. Nucleus implant parameters significantly change the compressive stiffness of the human lumbar intervertebral disc. J. Biomech. Eng. 2005, 127, 536–540. [Google Scholar] [CrossRef][Green Version]
- Klara, P.M.; Ray, C.D. Artificial nucleus replacement: Clinical experience. Spine 2002, 27, 1374–1377. [Google Scholar] [CrossRef]
- Larson, J.; Chadderon, R.; Georgescu, H.; Lee, D.; Hubert, M.; Werkmeister-Lewis, L.; Irrang, J.; Gilbertson, L.; Kang, J. Prevention of Intervertebral Disc Degeneration after Surgical Discectomy Using an Injectable Nucleus Pulposus Prosthesis. In Proceedings of the 52nd Annual Meeting of the Orthopaedic Research Society, Chicago, IL, USA, 19–22 March 2006. [Google Scholar]
- Argoubi, M.; Shirazi-Adl, A. Poroelastic creep response analysis of a lumbar motion segment in compression. J. Biomech. 1996, 29, 1331–1339. [Google Scholar] [CrossRef]
- Bao, Q.B.; Yuan, H.A. Prosthetic disc replacement: The future? Clin. Orthop. Relat. Res. 2002, 394, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, W.; Elliott, D.M. Effects of degeneration on the biphasic material properties of human nucleus pulposus in confined compression. Spine 2005, 30, E724–E729. [Google Scholar] [CrossRef]
- Lee, C.K.; Kim, Y.E.; Lee, C.S.; Hong, Y.M.; Jung, J.M.; Goel, V.K. Impact response of the intervertebral disc in a finite-element model. Spine 2000, 25, 2431–2439. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.B.; Oloyede, V.O.; Broom, N.D. Biomechanics of load-bearing of the intervertebral disc: An experimental and finite element model. Med. Eng. Phys. 1997, 19, 145–156. [Google Scholar] [CrossRef]
- Iatridis, J.C.; Setton, L.A.; Weidenbaum, M.; Mow, V.C. Alterations in the mechanical behavior of the human lumbar nucleus pulposus with degeneration and aging. J. Orthop. Res. 1997, 15, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Iatridis, J.C.; Setton, L.A.; Weidenbaum, M.; Mow, V.C. The viscoelastic behavior of the non-degenerate human lumbar nucleus pulposus in shear. J. Biomech. 1997, 30, 1005–1013. [Google Scholar] [CrossRef]
- Cortes, D.H.; Jacobs, N.T.; DeLucca, J.F.; Elliott, D.M. Elastic, permeability and swelling properties of human intervertebral disc tissues: A benchmark for tissue engineering. J. Biomech. 2014, 47, 2088–2094. [Google Scholar] [CrossRef]
- Jacobs, N.T.; Cortes, D.H.; Peloquin, J.M.; Vresilovic, E.J.; Elliott, D.M. Validation and application of an intervertebral disc finite element model utilizing independently constructed tissue-level constitutive formulations that are nonlinear, anisotropic, and time-dependent. J. Biomech. 2014, 47, 2540–2546. [Google Scholar] [CrossRef]
- Nerurkar, N.L.; Elliott, D.M.; Mauck, R.L. Mechanical design criteria for intervertebral disc tissue engineering. J. Biomech. 2010, 43, 1017–1030. [Google Scholar] [CrossRef]
- del Palomar, A.P.; Calvo, B.; Doblare, M. An accurate finite element model of the cervical spine under quasi-static loading. J. Biomech. 2008, 41, 523–531. [Google Scholar] [CrossRef]
- Malandrino, A.; Planell, J.A.; Lacroix, D. Statistical factorial analysis on the poroelastic material properties sensitivity of the lumbar intervertebral disc under compression, flexion and axial rotation. J. Biomech. 2009, 42, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, Y.; Huyghe, J.M.; van Donkelaar, C.C.; Ito, K. A biochemical/biophysical 3D FE intervertebral disc model. Biomech. Model. Mechanobiol. 2010, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Stokes, I.A.; Laible, J.P.; Gardner-Morse, M.G.; Costi, J.J.; Iatridis, J.C. Refinement of elastic, poroelastic, and osmotic tissue properties of intervertebral disks to analyze behavior in compression. Ann. Biomed. Eng. 2011, 39, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.C.; Rutt, B.K. Polyvinyl alcohol cryogel: An ideal phantom material for MR studies of arterial flow and elasticity. Magn. Reson. Med. 1997, 37, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Campbell, G.; Boughner, D.; Wan, W.K.; Quantz, M. Design and manufacture of a polyvinyl alcohol (PVA) cryogel tri-leaflet heart valve prosthesis. Med. Eng. Phys. 2004, 26, 269–277. [Google Scholar] [CrossRef]
- Vijayasekaran, S.; Fitton, J.H.; Hicks, C.R.; Chirila, T.V.; Crawford, G.J.; Constable, I.J. Cell viability and inflammatory response in hydrogel sponges implanted in the rabbit cornea. Biomaterials 1998, 19, 2255–2267. [Google Scholar] [CrossRef]
- Stammen, J.A.; Williams, S.; Ku, D.N.; Guldberg, R.E. Mechanical properties of a novel PVA hydrogel in shear and unconfined compression. Biomaterials 2001, 22, 799–806. [Google Scholar] [CrossRef]
- Allen, M.J.; Schoonmaker, J.E.; Bauer, T.W.; Williams, P.F.; Higham, P.A.; Yuan, H.A. Preclinical evaluation of a poly (vinyl alcohol) hydrogel implant as a replacement for the nucleus pulposus. Spine 2004, 29, 515–523. [Google Scholar] [CrossRef]
- Thomas, J.; Lowman, A.; Marcolongo, M. Novel associated hydrogels for nucleus pulposus replacement. J. Biomed. Mater. Res. A 2003, 67, 1329–1337. [Google Scholar] [CrossRef]
- Sasson, A.; Patchornik, S.; Eliasy, R.; Robinson, D.; Haj-Ali, R. Hyperelastic mechanical behavior of chitosan hydrogels for nucleus pulposus replacement-experimental testing and constitutive modeling. J. Mech. Behav. Biomed. Mater. 2012, 8, 143–153. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Malhotra, N.R.; Weng, L.; Chen, W.; Mauck, R.L.; Elliott, D.M. Material properties in unconfined compression of human nucleus pulposus, injectable hyaluronic acid-based hydrogels and tissue engineering scaffolds. Eur. Spine J. 2007, 16, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Toguchida, J.; Oka, M. Development of an artificial meniscus using polyvinyl alcohol-hydrogel for early return to, and continuance of, athletic life in sportspersons with severe meniscus injury. I: Mechanical evaluation. Knee 2003, 10, 47–51. [Google Scholar] [CrossRef]
- Nakashima, K.; Sawae, Y.; Murakami, T. Study on wear reduction mechanisms of artificial cartilage by synergistic protein boundary film formation. JSME Int. J. Ser. C Mech. Syst. Mach. Elem. Manuf. 2005, 48, 555–561. [Google Scholar] [CrossRef]
- Cortes, D.H.; Elliott, D.M. Extra-fibrillar matrix mechanics of annulus fibrosus in tension and compression. Biomech. Model. Mechanobiol. 2012, 11, 781–790. [Google Scholar] [CrossRef]
- Garcia, J.J.; Cortes, D.H. A nonlinear biphasic viscohyperelastic model for articular cartilage. J. Biomech. 2006, 39, 2991–2998. [Google Scholar] [CrossRef]
- Holmes, M.H.; Mow, V.C. The nonlinear characteristics of soft gels and hydrated connective tissues in ultrafiltration. J. Biomech. 1990, 23, 1145–1156. [Google Scholar] [CrossRef]
- Iatridis, J.C.; Setton, L.A.; Foster, R.J.; Rawlins, B.A.; Weidenbaum, M.; Mow, V.C. Degeneration affects the anisotropic and nonlinear behaviors of human anulus fibrosus in compression. J. Biomech. 1998, 31, 535–544. [Google Scholar] [CrossRef]
- Masharawi, Y.; Rothschild, B.; Dar, G.; Peleg, S.; Robinson, D.; Been, E.; Hershkovitz, I. Facet orientation in the thoracolumbar spine-Three-dimensional anatomic and biomechanical analysis. Spine 2004, 29, 1755–1763. [Google Scholar] [CrossRef]
- Sharma, M.; Langrana, N.A.; Rodriguez, J. Role of ligaments and facets in lumbar spinal stability. Spine 1995, 20, 887–900. [Google Scholar] [CrossRef]
- Senegas, J. Mechanical supplementation by non-rigid fixation in degenerative intervertebral lumbar segments: The Wallis system. Eur. Spine J. 2002, 11, S164–S169. [Google Scholar] [CrossRef]
- Sengupta, D.K. Dynamic stabilization devices in the treatment of low back pain. Neurol. India 2005, 53, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; O’Connell, G.D. Effect of collagen fibre orientation on intervertebral disc torsion mechanics. Biomech. Model. Mechanobiol. 2017, 16, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, M.C.; Feng, F.; Pang, M.; Xie, P.G.; Chen, R.Q.; Rong, L.M. Stress distribution over lumbosacral vertebrae and axial transsacral rod after axial lumbar interbody fusion (AxiaLIF): Finite element analysis. Int. J. Clin. Exp. Med. 2016, 9, 13372–13383. [Google Scholar]
- Kim, Y.; Kim, T.W. Finite element analysis of the effects of pedicle screw fixation nut loosening on lumbar interbody fusion based on the elasto-plateau plasticity of bone characteristics. Spine 2010, 35, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yang, Y.H.; Lou, S.L.; Zhang, D.S.; Liao, S.H. Construction and validation of a three-dimensional finite element model of degenerative scoliosis. J. Orthop. Surg. Res. 2015, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.N.; Chen, B.H.; An, H.S.; Andersson, G.B. Anterior cervical fusion: A finite element model study on motion segment stability including the effect of osteoporosis. Spine 2000, 25, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Goel, V.K.; Monroe, B.T.; Gilbertson, L.G.; Brinckmann, P.; Nat, R. Interlaminar shear stresses and laminae separation in a disc-Finite-element analysis of the L3-L4 motion segment subjected to axial compressive loads. Spine 1995, 20, 689–698. [Google Scholar] [CrossRef]
- Shiraziadl, A.; Ahmed, A.M.; Shrivastava, S.C. Mechanical response of a lumbar motion segment in axial torque alone and combined with compression. Spine 1986, 11, 914–927. [Google Scholar] [CrossRef]
- Zander, T.; Rohlmann, A.; Calisse, J.; Bergmann, G. Estimation of muscle forces in the lumbar spine during upper-body inclination. Clin. Biomech. 2001, 16, S73–S80. [Google Scholar] [CrossRef]
- Rohlmann, A.; Zander, T.; Rao, M.; Bergmann, G. Realistic loading conditions for upper body bending. J. Biomech. 2009, 42, 884–890. [Google Scholar] [CrossRef]
- Dreischarf, M.; Rohlmann, A.; Bergmann, G.; Zander, T. Optimised in vitro applicable loads for the simulation of lateral bending in the lumbar spine. Med. Eng. Phys. 2012, 34, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Dreischarf, M.; Rohlmann, A.; Bergmann, G.; Zander, T. Optimised loads for the simulation of axial rotation in the lumbar spine. J. Biomech. 2011, 44, 2323–2327. [Google Scholar] [CrossRef] [PubMed]
- Dreischarf, M.; Zander, T.; Shirazi-Adl, A.; Puttlitz, C.M.; Adam, C.J.; Chen, C.S.; Goel, V.K.; Kiapour, A.; Kim, Y.H.; Labus, K.M.; et al. Comparison of eight published static finite element models of the intact lumbar spine: Predictive power of models improves when combined together. J. Biomech. 2014, 47, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Pearcy, M.; Portek, I.; Shepherd, J. Three-dimensional x-ray analysis of normal movement in the lumbar spine. Spine 1984, 9, 294–297. [Google Scholar] [CrossRef]
- Pearcy, M.J. Stereo radiography of lumbar spine motion. Acta Orthop. Scand. Suppl. 1985, 212, 1–45. [Google Scholar] [CrossRef]
- Pearcy, M.J.; Tibrewal, S.B. Axial rotation and lateral bending in the normal lumbar spine measured by three-dimensional radiography. Spine 1984, 9, 582–587. [Google Scholar] [CrossRef]
- Anseth, K.S.; Bowman, C.N.; Brannon-Peppas, L. Mechanical properties of hydrogels and their experimental determination. Biomaterials 1996, 17, 1647–1657. [Google Scholar] [CrossRef]
- Lai, W.M.; Mow, V.C.; Roth, V. Effects of nonlinear strain-dependent permeability and rate of compression on the stress behavior of articular cartilage. J. Biomech. Eng. 1981, 103, 61–66. [Google Scholar] [CrossRef]
- Gao, X.; Kusmierczyk, P.; Shi, Z.; Liu, C.; Yang, G.; Sevostianov, I.; Silberschmidt, V.V. Through-thickness stress relaxation in bacterial cellulose hydrogel. J. Mech. Behav. Biomed. Mater. 2016, 59, 90–98. [Google Scholar] [CrossRef]
- Adams, P.; Eyre, D.R.; Muir, H. Biochemical aspects of development and ageing of human lumbar intervertebral discs. Rheumatol. Rehabil. 1977, 16, 22–29. [Google Scholar] [CrossRef]
- Cassidy, J.J.; Hiltner, A.; Baer, E. Hierarchical structure of the intervertebral disc. Connect. Tissue Res. 1989, 23, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Hsu, E.W.; Setton, L.A. Diffusion tensor microscopy of the intervertebral disc anulus fibrosus. Magn. Reson. Med. 1999, 41, 992–999. [Google Scholar] [CrossRef]
- Yu, J.; Fairbank, J.C.; Roberts, S.; Urban, J.P. The elastic fiber network of the anulus fibrosus of the normal and scoliotic human intervertebral disc. Spine 2005, 30, 1815–1820. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tirlapur, U.; Fairbank, J.; Handford, P.; Roberts, S.; Winlove, C.P.; Cui, Z.; Urban, J. Microfibrils, elastin fibres and collagen fibres in the human intervertebral disc and bovine tail disc. J. Anat. 2007, 210, 460–471. [Google Scholar] [CrossRef]





| Component | Young’s Modulus (MPa) | Poisson Ratio | Reference |
|---|---|---|---|
| Cortical bone | 12,000 | 0.3 | [45,46,47] |
| Cancellous bone | 100 | 0.2 | [45,46,47] |
| Posterior elements | 3500 | 0.25 | [45,46,47] |
| Cartilage endplate | Holmes–Mow = 0.5218, = 0.38, = 0.0028, = 5.5 × 10−16 (m4/Ns), = 0.22 | [19] | |
| Bony endplate | 1000 | 0.3 | [19] |
| Cartilage | 11 | 0.4 | [48] |
| Nucleus pulposus | Holmes–Mow = 0.202, = 0.36, = 1.46, = 18.7 × 10−16 (m4/Ns), = 4.8 | [19] | |
| Inner anterior | Mooney–Rivlin = 0.2, = 0.01, = 6 Fiber-pow-linear = 12.5, = 5.5, = 1.135 | [44] | |
| Outer anterior | Mooney–Rivlin = 0.2, = 0.01, = 6 Fiber-pow-linear = 46, = 4, = 1.065 | [44] | |
| Ligament | Nonlinear stress–strain curve | [49,50,51] | |
| Follower Load (N) | Moment (N∙m) | References | |
|---|---|---|---|
| Flexion | 1175 | 7.5 | [52] |
| Extension | 500 | 7.5 | [52] |
| Lateral bending | 700 | 7.8 | [53] |
| Axial rotation | 720 | 5.5 | [54] |
| 3 wt% (n = 5) | 5 wt% (n = 5) | 7 wt% (n = 5) | 10 wt% (n = 5) | 15 wt% (n = 5) | 20 wt% (n = 5) | |
|---|---|---|---|---|---|---|
| (kPa) | ||||||
| × 10−16 (m4/N∙s) | ||||||
(Curve-fitting) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, M.; Park, S. Biphasic Properties of PVAH (Polyvinyl Alcohol Hydrogel) Reflecting Biomechanical Behavior of the Nucleus Pulposus of the Human Intervertebral Disc. Materials 2022, 15, 1125. https://doi.org/10.3390/ma15031125
Heo M, Park S. Biphasic Properties of PVAH (Polyvinyl Alcohol Hydrogel) Reflecting Biomechanical Behavior of the Nucleus Pulposus of the Human Intervertebral Disc. Materials. 2022; 15(3):1125. https://doi.org/10.3390/ma15031125
Chicago/Turabian StyleHeo, Minhyeok, and Seonghun Park. 2022. "Biphasic Properties of PVAH (Polyvinyl Alcohol Hydrogel) Reflecting Biomechanical Behavior of the Nucleus Pulposus of the Human Intervertebral Disc" Materials 15, no. 3: 1125. https://doi.org/10.3390/ma15031125
APA StyleHeo, M., & Park, S. (2022). Biphasic Properties of PVAH (Polyvinyl Alcohol Hydrogel) Reflecting Biomechanical Behavior of the Nucleus Pulposus of the Human Intervertebral Disc. Materials, 15(3), 1125. https://doi.org/10.3390/ma15031125






