Improving Polysaccharide-Based Chitin/Chitosan-Aerogel Materials by Learning from Genetics and Molecular Biology
Abstract
:1. Introduction
2. Chitin/Chitosan Matrices Provide the Potential for Multiple Biomedical Applications
3. Natural and Synthetic Chitin/Chitosan Matrices Support Wound Closure
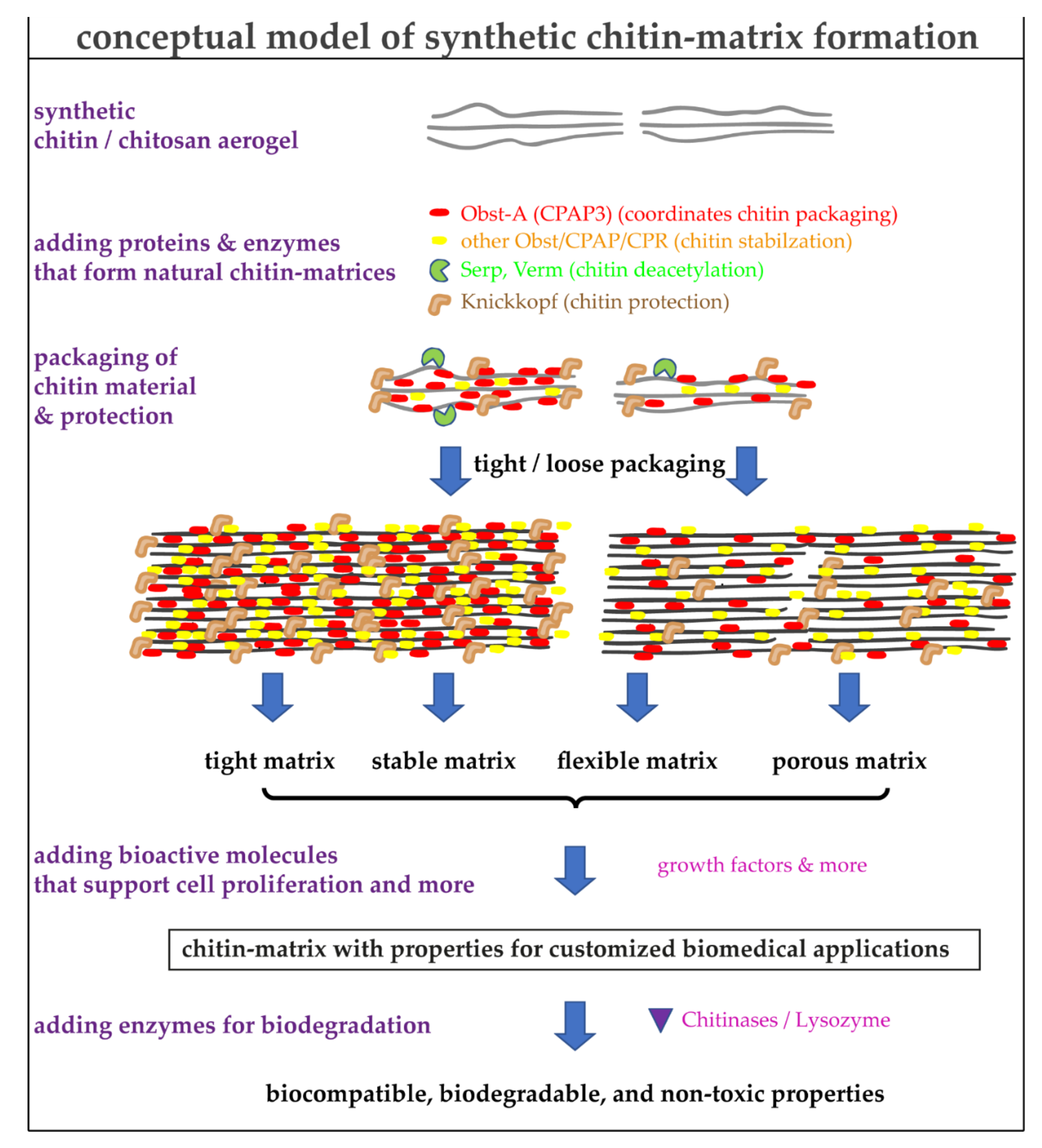
4. Using the Natural Toolbox to Improve the Quality of Synthetic Chitin/Chitosan Matrices
5. Production of Polysaccharide-Based Chitin/Chitosan Aerogel Matrices
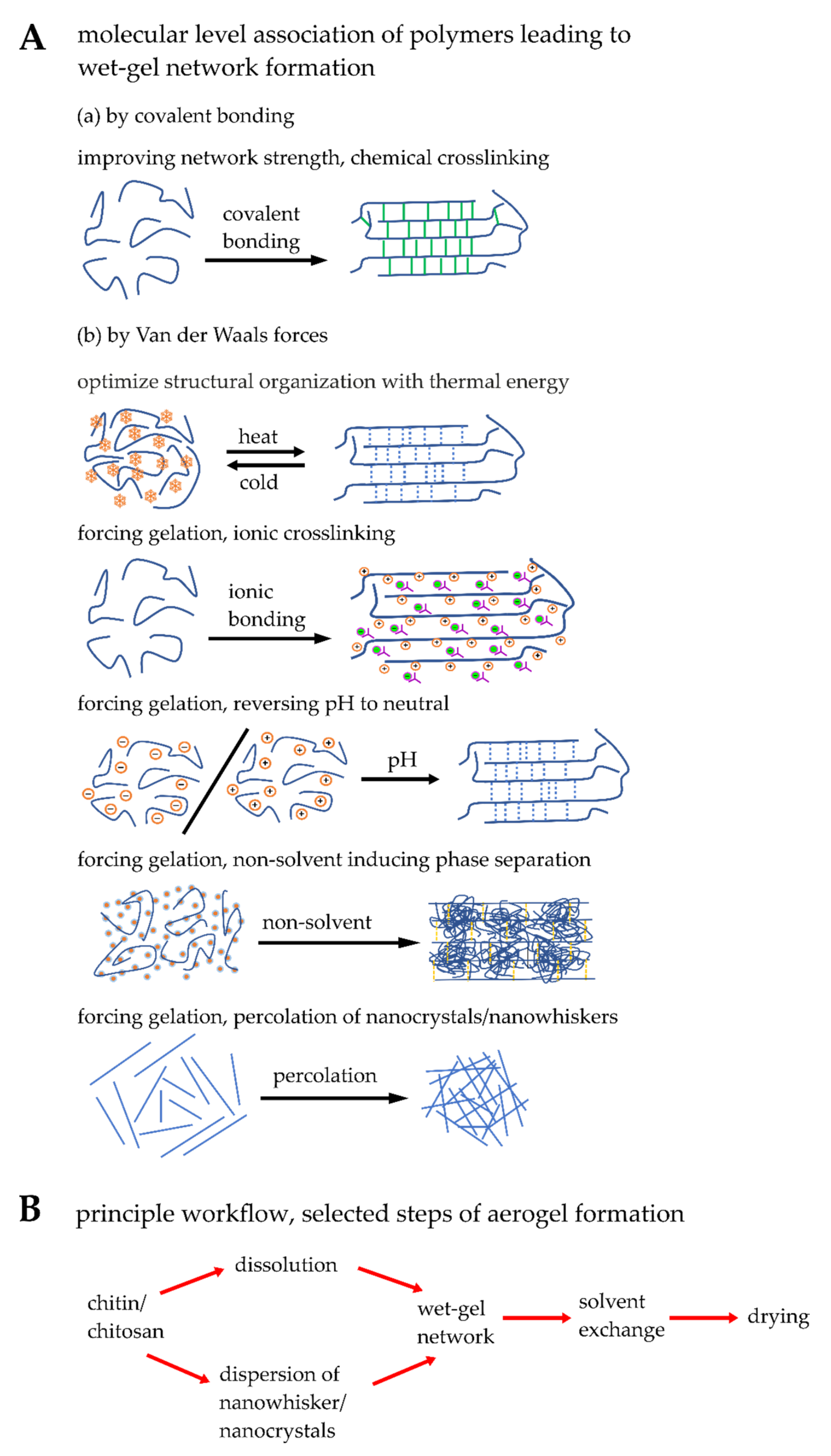
5.1. Engineering Synthetic Chitin/Chitosan Matrices
5.2. Blending, Physical and Chemical Crosslinking of Chitin/Chitosan Matrices
5.3. Modifying Microstructural and Physical Properties of Synthetic Matrices
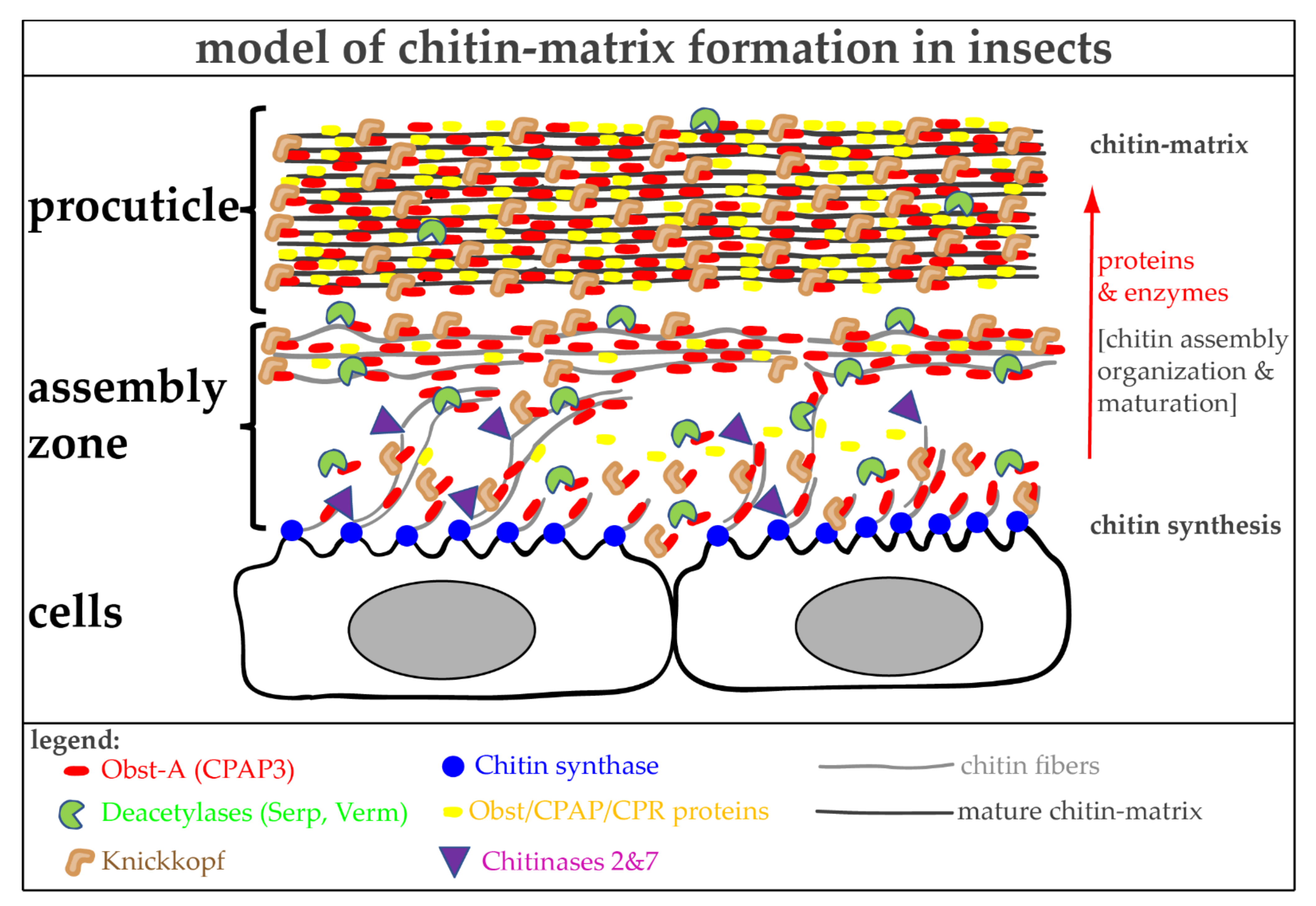
6. The Molecular Toolbox for Natural Chitin Matrix Production
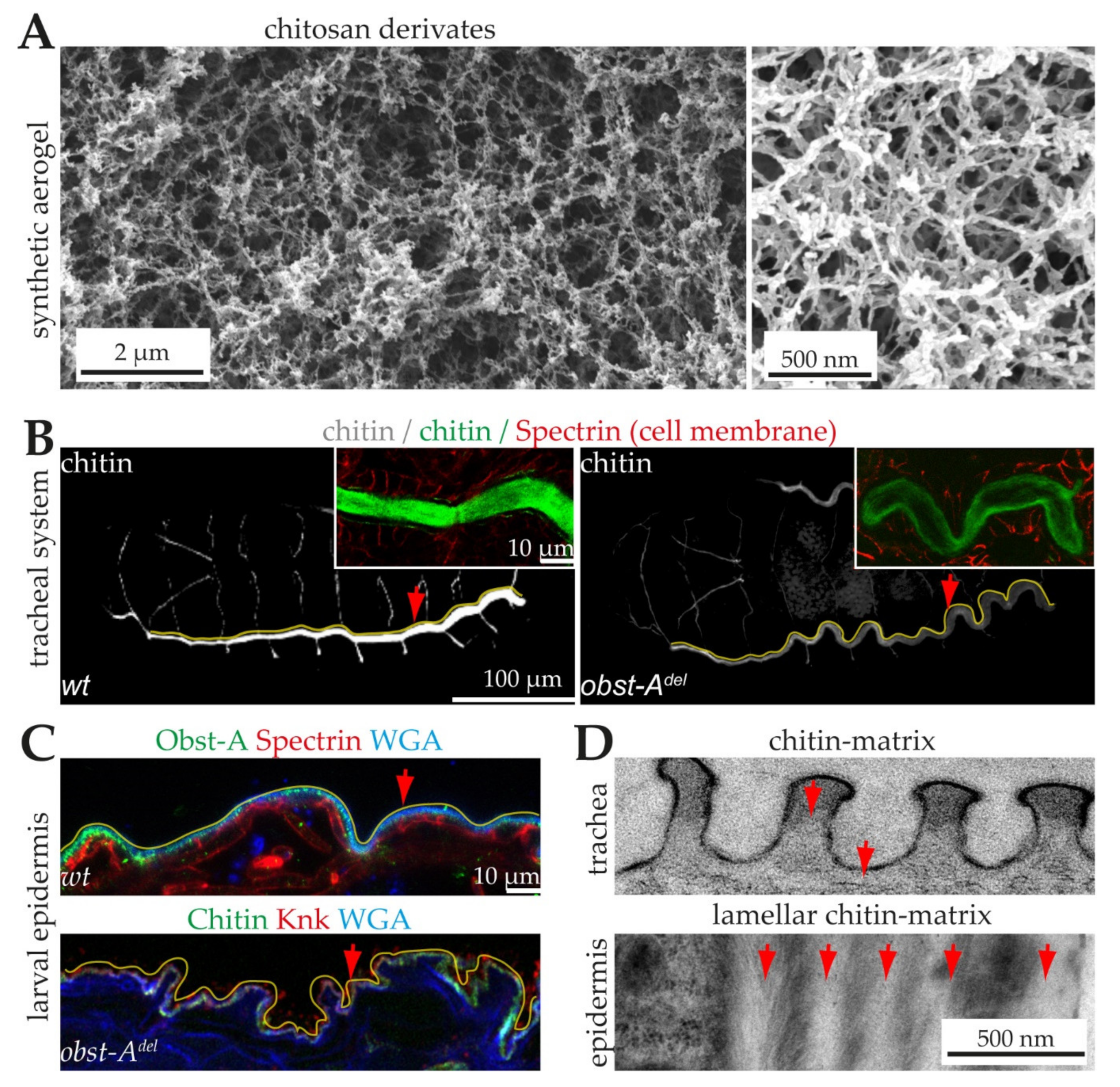
6.1. The Architecture of Cuticular Chitin Matrices
6.2. Natural Chitin Production
6.3. Proteins Control Proper Chitin Matrix Formation
7. Advantages and Limitations of Chitin/Chitosan-Protein Materials
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merzendorfer, H. The cellular basis of chitin synthesis in fungi and insects: Common principles and differences. Eur. J. Cell Biol. 2011, 90, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, G.; Kim, Y.-S.; Hwang, J.-W.; Kim, S.-K.; Jeon, Y.-J.; Je, J.-Y.; Ahn, C.-B.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Chitooli-gosaccharide and its derivatives: Preparation and biological applications. BioMed Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wegst, U.G.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, B.G.; Del Gaudio, P.; Alves, P.; Costa, R.; García-Gonzaléz, C.A.; Oliveira, A.L. Bioaerogels: Promising nanostructured materials in fluid management, healing and regeneration of wounds. Molecules 2021, 26, 3834. [Google Scholar] [CrossRef]
- Hong, M.-S.; Choi, G.-M.; Kim, J.; Jang, J.; Choi, B.; Kim, J.-K.; Jeong, S.; Leem, S.; Kwon, H.-Y.; Hwang, H.-B.; et al. Biomimetic chitin–silk hybrids: An optically transparent structural platform for wearable devices and advanced electronics. Adv. Funct. Mater. 2018, 28, 1705480. [Google Scholar] [CrossRef]
- Hou, J.; Aydemir, B.E.; Dumanli, A.G. Understanding the structural diversity of chitins as a versatile biomaterial. Phil. Trans. R. Soc. A 2021, 379, 20200331. [Google Scholar] [CrossRef]
- Merzendorfer, H.; Cohen, E. Chitin/Chitosan: Versatile Ecological, Industrial, and Biomedical Applications. In Extracellular Sugar-Based Biopolymers Matrices; Cohen, E., Merzendorfer, H., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 541–624. ISBN 978-3-030-12918-7. [Google Scholar]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a wound dressing starting material: Antimicrobial properties and mode of action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [Green Version]
- Muzzarelli, R.A.A. Chitins and chitosans as immunoadjuvants and non-allergenic drug carriers. Mar. Drugs 2010, 8, 292–312. [Google Scholar] [CrossRef] [Green Version]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Rudall, K.M. The Chitin/Protein complexes of insect cuticles. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 1963; pp. 257–313. [Google Scholar]
- Focher, B.; Naggi, A.; Torri, G.; Cosani, A.; Terbojevich, M. Structural differences between chitin polymorphs and their pre-cipitates from solutions—Evidence from CP-MAS 13C-NMR, FT-IR and FT-Raman spectroscopy. Carbohydr. Polym. 1992, 17, 97–102. [Google Scholar] [CrossRef]
- Kameda, T.; Miyazawa, M.; Ono, H.; Yoshida, M. Hydrogen bonding structure and stability of alpha-chitin studied by 13C solid-state NMR. Macromol. Biosci. 2005, 5, 103–106. [Google Scholar] [CrossRef]
- Zhang, M.; Haga, A.; Sekiguchi, H.; Hirano, S. Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Int. J. Biol. Macromol. 2000, 27, 99–105. [Google Scholar] [CrossRef]
- Minke, R.; Blackwell, J. The structure of α-chitin. J. Mol. Biol. 1978, 120, 167–181. [Google Scholar] [CrossRef]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On chemistry of γ-chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef]
- Carlström, D. The crystal structure of alpha-chitin (poly-N-acetyl-D-glucosamine). J. Biophys. Biochem. Cytol. 1957, 3, 669–683. [Google Scholar] [CrossRef] [Green Version]
- Hackman, R.H.; Goldberg, M. Studies on chitin. VI. The nature of alpha- and beta-chitins. Aust. J. Biol. Sci. 1965, 18, 935–946. [Google Scholar] [CrossRef] [Green Version]
- Blackwell, J. Structure of beta-chitin or parallel chain systems of poly-beta-(1-4)-N-acetyl-D-glucosamine. Biopolymers 1969, 7, 281–298. [Google Scholar] [CrossRef]
- Sugiyama, J.; Boisset, C.; Hashimoto, M.; Watanabe, T. Molecular directionality of beta-chitin biosynthesis. J. Mol. Biol. 1999, 286, 247–255. [Google Scholar] [CrossRef]
- Jang, M.-K.; Kong, B.-G.; Jeong, Y.-I.; Lee, C.H.; Nah, J.-W. Physicochemical characterization of α-chitin, β-chitin, and γ-chitinseparated from natural resources. J. Polym. Sci. A Polym. Chem. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Rudall, K.M.; Kenchington, W. The chitin system. Biol. Rev. 1973, 48, 597–633. [Google Scholar] [CrossRef]
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. mushroom: A review of crustacean and fungal chitin in wound treatment. Mar. Drugs 2020, 18, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostag, M.; El Seoud, O.A. Sustainable biomaterials based on cellulose, chitin and chitosan composites—A review. Carbohydr. Polym. Tech. Applic. 2021, 2, 100079. [Google Scholar] [CrossRef]
- Peter, M.G. Chitin and Chitosan from Animal Sources. In Biopolymers: Biology, Chemistry, Biotechnology, Applications; Steinbüchel, A., Ed.; Wiley-VCH: Weinheim, Germany, 2001; ISBN 9783527302901. [Google Scholar]
- Gooday, G.W. The Ecology of Chitin Degradation. In Advances in Microbial Ecology; Marshall, K.C., Ed.; Springer: Boston, MA, USA, 1990; pp. 387–430. ISBN 978-1-4684-7614-9. [Google Scholar]
- Peter, M.G. Chitin and Chitosan in Fungi. In Biopolymers: Biology, Chemistry, Biotechnology, Applications; Steinbüchel, A., Ed.; Wiley-VCH: Weinheim, Germany, 2001; ISBN 9783527302901. [Google Scholar]
- Latgé, J.-P. The cell wall: A carbohydrate armour for the fungal cell. Mol. Microbiol. 2007, 66, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Halfar, J. First evidence of chitin in calcified coralline algae: New insights into the calcification process of Clathromorphum compactum. Sci. Rep. 2014, 4, 6162. [Google Scholar] [CrossRef]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Jiang, C.; Yang, Q. Extraction and characterization of chitin from the beetle Holotrichia parallela Motschulsky. Molecules 2012, 17, 4604–4611. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K.; Verma, M. Green synthesis approach: Extraction of chitosan from fungus mycelia. Crit. Rev. Biotechnol. 2013, 33, 379–403. [Google Scholar] [CrossRef]
- Joseph, S.M.; Krishnamoorthy, S.; Paranthaman, R.; Moses, J.A.; Anandharamakrishnan, C. A review on source-specific chemistry, functionality, and applications of chitin and chitosan. Carbohydr. Polym. Technol. Appl. 2021, 2, 100036. [Google Scholar] [CrossRef]
- Castro, R.; Guerrero-Legarreta, I.; Bórquez, R. Chitin extraction from Allopetrolisthes punctatus crab using lactic fermentation. Biotechnol. Rep. 2018, 20, e00287. [Google Scholar] [CrossRef]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Mehmood Khan, T.; Akabar, M.D.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef]
- Cottaz, S.; Samain, E. Genetic engineering of Escherichia coli for the production of NI, NII-diacetylchitobiose (chitinbiose) and its utilization as a primer for the synthesis of complex carbohydrates. Metab. Eng. 2005, 7, 311–317. [Google Scholar] [CrossRef]
- Yang, T.-L. Chitin-based materials in tissue engineering: Applications in soft tissue and epithelial organ. Int. J. Mol. Sci. 2011, 12, 1936–1963. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.Z.; Chen, X.G.; Liu, N.; Wang, S.X.; Liu, C.S.; Meng, X.H.; Liu, C.G. Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohydr. Polym. 2006, 65, 194–201. [Google Scholar] [CrossRef]
- Madihally, S.V.; Flake, A.W.; Matthew, H.W.T. Maintenance of CD34 expression during proliferation of CD34 & +; cord blood cells on glycosaminoglycan surfaces. Stem Cells 1999, 17, 295–305. [Google Scholar] [CrossRef]
- Masuoka, K.; Ishihara, M.; Asazuma, T.; Hattori, H.; Matsui, T.; Takase, B.; Kanatani, Y.; Fujita, M.; Saito, Y.; Yura, H.; et al. The interaction of chitosan with fibroblast growth factor-2 and its protection from inactivation. Biomaterials 2005, 26, 3277–3284. [Google Scholar] [CrossRef]
- Rondon, E.P.; Benabdoun, H.A.; Vallières, F.; Segalla Petrônio, M.; Tiera, M.J.; Benderdour, M.; Fernandes, J.C. Evidence supporting the safety of pegylated Diethylaminoethyl-Chitosan polymer as a nanovector for gene therapy applications. Int. J. Nanomed. 2020, 15, 6183–6200. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Seo, S.-J.; Moon, H.-S.; Yoo, M.-K.; Park, I.-Y.; Kim, B.-C.; Cho, C.-S. Chitosan and its derivatives for tissue engi-neering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef]
- Kim, S.; Cui, Z.-K.; Koo, B.; Zheng, J.; Aghaloo, T.; Lee, M. Chitosan-Lysozyme conjugates for Enzyme-Triggered Hydrogel degradation in tissue engineering applications. ACS Appl. Mater. Interfaces 2018, 10, 41138–41145. [Google Scholar] [CrossRef]
- Nordtveit, R.J.; Vårum, K.M.; Smidsrød, O. Degradation of fully water-soluble, partially N-acetylated chitosans with lysozyme. Carbohydr. Polym. 1994, 23, 253–260. [Google Scholar] [CrossRef]
- Roman, D.L.; Ostafe, V.; Isvoran, A. Deeper inside the specificity of lysozyme when degrading chitosan. A structural bioinformatics study. J. Mol. Graph. Model. 2020, 100, 107676. [Google Scholar] [CrossRef]
- Ren, D.; Yi, H.; Wang, W.; Ma, X. The enzymatic degradation and swelling properties of chitosan matrices with different degrees of N-acetylation. Carbohydr. Res. 2005, 340, 2403–2410. [Google Scholar] [CrossRef]
- Mason, D.Y.; Taylor, C.R. The distribution of muramidase (lysozyme) in human tissues. J. Clin. Pathol. 1975, 28, 124–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geyer, G. Lysozyme in Paneth cell secretions. Acta Histochem. 1973, 45, 126–132. [Google Scholar] [PubMed]
- Saraiva, S.M.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Correia, I.J. Synthesis and characterization of a photocrosslinkable chitosan–gelatin hydrogel aimed for tissue regeneration. RSC Adv. 2015, 5, 63478–63488. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications-A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- Li, S.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. Chitosans for tissue repair and organ three-dimensional (3D) bioprinting. Micromachines 2019, 10, 765. [Google Scholar] [CrossRef] [Green Version]
- Rejinold, N.S.; Chennazhi, K.P.; Tamura, H.; Nair, S.V.; Rangasamy, J. Multifunctional chitin nanogels for simultaneous drug delivery, bioimaging, and biosensing. Acs Appl. Mater. Interfaces 2011, 3, 3654–3665. [Google Scholar] [CrossRef]
- Hanagata, N.; Zhang, H.; Chen, S.; Zhi, C.; Yamazaki, T. Chitosan-coated boron nitride nanospheres enhance delivery of CpG oligodeoxynucleotides and induction of cytokines. Int. J. Nanomed. 2013, 8, 1783–1793. [Google Scholar] [CrossRef] [Green Version]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Takeshita, S.; Zhao, S.; Malfait, W.J.; Koebel, M.M. Chemistry of Chitosan Aerogels: Three-dimensional pore control for tailored applications. Angew. Chem. Int. Ed. 2021, 60, 9828–9851. [Google Scholar] [CrossRef]
- Singh, R.; Shitiz, K.; Singh, A. Chitin and chitosan: Biopolymers for wound management. Int. Wound J. 2017, 14, 1276–1289. [Google Scholar] [CrossRef]
- Song, X.; Huang, X.; Li, Z.; Li, Z.; Wu, K.; Jiao, Y.; Zhou, C. Construction of blood compatible chitin/graphene oxide composite aerogel beads for the adsorption of bilirubin. Carbohydr. Polym. 2019, 207, 704–712. [Google Scholar] [CrossRef]
- Wu, S.; Duan, B.; Lu, A.; Wang, Y.; Ye, Q.; Zhang, L. Biocompatible chitin/carbon nanotubes composite hydrogels as neuronal growth substrates. Carbohydr. Polym. 2017, 174, 830–840. [Google Scholar] [CrossRef]
- Ratanajiajaroen, P.; Ohshima, M. Synthesis, release ability and bioactivity evaluation of chitin beads incorporated with curcumin for drug delivery applications. J. Microencapsul. 2012, 29, 549–558. [Google Scholar] [CrossRef]
- McCarthy, P.C.; Zhang, Y.; Abebe, F. Recent applications of dual-stimuli responsive chitosan hydrogel nanocomposites as drug delivery tools. Molecules 2021, 26, 4735. [Google Scholar] [CrossRef]
- Narmani, A.; Jafari, S.M. Chitosan-based nanodelivery systems for cancer therapy: Recent advances. Carbohydr. Polym. 2021, 272, 118464. [Google Scholar] [CrossRef]
- Pathak, K.; Misra, S.K.; Sehgal, A.; Singh, S.; Bungau, S.; Najda, A.; Gruszecki, R.; Behl, T. Biomedical Applications of Quaternized Chitosan. Polymers 2021, 13, 2514. [Google Scholar] [CrossRef]
- Araujo, V.H.S.; de Souza, M.P.C.; Carvalho, G.C.; Duarte, J.L.; Chorilli, M. Chitosan-based systems aimed at local application for vaginal infections. Carbohydr. Polym. 2021, 261, 117919. [Google Scholar] [CrossRef]
- Boroumand, H.; Badie, F.; Mazaheri, S.; Seyedi, Z.S.; Nahand, J.S.; Nejati, M.; Baghi, H.B.; Abbasi-Kolli, M.; Badehnoosh, B.; Ghandali, M.; et al. Chitosan-based nanoparticles against viral infections. Front. Cell. Infect. Microbiol. 2021, 11, 643953. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yamada, S.; Yanagi Guchi, K.; Koyama, Z.; Ikeda, T. Chitosan and Fish Collagen as Biomaterials for Regenerative Medicine. In Marine Medicinal Foods: Implications and Applications—Animals and Microbes; Kim, S.-K., Ed.; Elsevier Academy Press: Amsterdam, The Netherlands, 2012; pp. 107–120. ISBN 9780124160033. [Google Scholar]
- Liu, X.; Howard, K.A.; Dong, M.; Andersen, M.Ø.; Rahbek, U.L.; Johnsen, M.G.; Hansen, O.C.; Besenbacher, F.; Kjems, J. The influence of polymeric properties on chitosan/siRNA nanoparticle formulation and gene silencing. Biomaterials 2007, 28, 1280–1288. [Google Scholar] [CrossRef]
- Koide, M.; Osaki, K.; Konishi, J.; Oyamada, K.; Katakura, T.; Takahashi, A.; Yoshizato, K. A new type of biomaterial for artificial skin: Dehydrothermally cross-linked composites of fibrillar and denatured collagens. J. Biomed. Mater. Res. 1993, 27, 79–87. [Google Scholar] [CrossRef]
- Aibibu, D.; Hild, M.; Wöltje, M.; Cherif, C. Textile cell-free scaffolds for in situ tissue engineering applications. J. Mater. Sci. Mater. Med. 2016, 27, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadeghi, A.M.M.; Dorkoosh, F.A.; Avadi, M.R.; Saadat, P.; Rafiee-Tehrani, M.; Junginger, H.E. Preparation, characterization and antibacterial activities of chitosan, N-trimethyl chitosan (TMC) and N-diethylmethyl chitosan (DEMC) nanoparticles loaded with insulin using both the ionotropic gelation and polyelectrolyte complexation methods. Int. J. Pharm. 2008, 355, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, P.; Gupta, M.S.; Jayakumar, R.; Gowda, D.V. Prospection of chitosan and its derivatives in wound healing: Proof of patent analysis (2010–2020). Int. J. Biol. Macromol. 2021, 184, 701–712. [Google Scholar] [CrossRef]
- Xiao, B.; Ma, P.; Viennois, E.; Merlin, D. Urocanic acid-modified chitosan nanoparticles can confer anti-inflammatory effect by delivering CD98 siRNA to macrophages. Colloids Surf. B 2016, 143, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic evaluation of regenerative endodontic procedures with the use of chitosan scaffolds in immature dog teeth with apical periodontitis. J. Endod. 2017, 43, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, M.G.; Karimian, R.; Rakhshaei, R.; Pakdel, F.; Eslami, H.; Fakhrzadeh, V.; Rahimi, M.; Salehi, R.; Kafil, H.S. Chitin/silk fibroin/TiO2 bio-nanocomposite as a biocompatible wound dressing bandage with strong antimicrobial activity. Int. J. Biol. Macromol. 2018, 116, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Tallawi, M.; Rosellini, E.; Barbani, N.; Cascone, M.G.; Rai, R.; Saint-Pierre, G.; Boccaccini, A.R. Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: A review. J. R. Soc. Interface 2015, 12, 20150254. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.P.; Espiga, A.; Silva, D.; Baptista, P.; Henriques, J.; Ferreira, C.; Silva, J.C.; Borges, J.P.; Pires, E.; Chaves, P.; et al. Development of a new chitosan hydrogel for wound dressing. Wound Repair Regen. 2009, 17, 817–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinemann, C.; Heinemann, S.; Bernhardt, A.; Lode, A.; Worch, H.; Hanke, T. In vitro osteoclastogenesis on textile chitosan scaffold. Eur. Cells Mater. 2010, 19, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Ostadhossein, F.; Mahmoudi, N.; Morales-Cid, G.; Tamjid, E.; Navas-Martos, F.J.; Soriano-Cuadrado, B.; Paniza, J.M.L.; Simchi, A. Development of Chitosan/Bacterial cellulose composite films containing nanodiamonds as a potential flexible platform for wound dressing. Materials 2015, 8, 6401–6418. [Google Scholar] [CrossRef]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for wound healing applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef]
- Peluso, G.; Petillo, O.; Ranieri, M.; Santin, M.; Ambrosic, L.; Calabró, D.; Avallone, B.; Balsamo, G. Chitosan-mediated stimulation of macrophage function. Biomaterials 1994, 15, 1215–1220. [Google Scholar] [CrossRef]
- Shibata, Y.; Metzger, W.J.; Myrvik, Q.N. Chitin particle-induced cell-mediated immunity is inhibited by soluble mannan: Mannose receptor-mediated phagocytosis initiates IL-12 production. J. Immunol. 1997, 159, 2462. [Google Scholar]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Kortekaas, K.I.; Seys, S.F.; Lund, G.; Jonckheere, A.-C.; Dierckx de Casterlé, I.; Ceuppens, J.L.; Steelant, B.; Hellings, P.W. Nasal epithelial barrier dysfunction increases sensitization and mast cell degranulation in the absence of allergic inflammation. Allergy 2020, 75, 1155–1164. [Google Scholar] [CrossRef]
- Lopata, A.L.; Lehrer, S.B. New insights into seafood allergy. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 270–277. [Google Scholar] [CrossRef]
- Yusof, N.L.B.M.; Wee, A.; Lim, L.Y.; Khor, E. Flexible chitin films as potential wound-dressing materials: Wound model studies. J. Biomed. Mater. Res. 2003, 66, 224–232. [Google Scholar] [CrossRef]
- Moussian, B.; Uv, A.E. An ancient control of epithelial barrier formation and wound healing. Bioessays 2005, 27, 987–990. [Google Scholar] [CrossRef]
- Pesch, Y.-Y.; Riedel, D.; Patil, K.R.; Loch, G.; Behr, M. Chitinases and imaginal disc growth factors organize the extracellular matrix formation at barrier tissues in insects. Sci. Rep. 2016, 6, 18340. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Behr, M.; Kiritsi, D.; Scheffschick, A.; Grahnert, A.; Homberg, M.; Schwieger-Briel, A.; Jakob, T.; Bruck-ner-Tuderman, L.; Magin, T.M. Keratin-dependent thymic stromal lymphopoietin expression suggests a link between skin blistering and atopic disease. J. Allergy Clin. Immunol. 2016, 133, 1461–1464. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, T.; Rothoeft, T.; Teig, N.; Bauer, J.W.; Pellegrini, G.; De, R.L.; Scaglione, D.; Reichelt, J.; Klausegger, A.; Kneisz, D.; et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature 2017, 551, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Ngoc-Hai, V.C.; Hong-My, D.D.; Bach, P.T.; Ngoc, T.Q.; van Vo, T.; Nguyen, T.-H. Simple fabrication of a chitin wound healing membrane from Soft-Shell crab carapace. Mater. Lett. 2021, 297, 129995. [Google Scholar] [CrossRef]
- Baxter, R.M.; Dai, T.; Kimball, J.; Wang, E.; Hamblin, M.R.; Wiesmann, W.P.; McCarthy, S.J.; Baker, S.M. Chitosan dressing promotes healing in third degree burns in mice: Gene expression analysis shows biphasic effects for rapid tissue regeneration and decreased fibrotic signaling. J. Biomed. Mater. Res. A 2013, 101, 340–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muzzarelli, R.A.; Morganti, P.; Morganti, G.; Palombo, P.; Palombo, M.; Biagini, G.; Mattioli Belmonte, M.; Giantomassi, F.; Orlandi, F.; Muzzarelli, C. Chitin nanofibrils/chitosan glycolate composites as wound medicaments. Carbohydr. Polym. 2007, 70, 274–284. [Google Scholar] [CrossRef]
- Miguel, S.P.; Figueira, D.R.; Simões, D.; Ribeiro, M.P.; Coutinho, P.; Ferreira, P.; Correia, I.J. Electrospun polymeric nanofibres as wound dressings: A review. Colloids Surf. B 2018, 169, 60–71. [Google Scholar] [CrossRef]
- Mezzana, P. Clinical efficacy of a new chitin nanofibrils-based gel in wound healing. Acta Chir. Plast. 2008, 50, 81–84. [Google Scholar]
- Paddle-Ledinek, J.E.; Nasa, Z.; Cleland, H.J. Effect of different wound dressings on cell viability and proliferation. Plast. Reconstr. Surg. 2006, 117, 110S–118S. [Google Scholar] [CrossRef]
- Enoch, S.; Leaper, D.J. Basic science of wound healing. Surgery 2008, 26, 31–37. [Google Scholar] [CrossRef]
- Barnes, L.A.; Marshall, C.D.; Leavitt, T.; Hu, M.S.; Moore, A.L.; Gonzalez, J.G.; Longaker, M.T.; Gurtner, G.C. Mechanical forces in cutaneous wound healing: Emerging therapies to minimize scar formation. Adv. Wound Care 2018, 7, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New trends in bio-based aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef]
- Batista, M.P.; Gonçalves, V.S.S.; Gaspar, F.B.; Nogueira, I.D.; Matias, A.A.; Gurikov, P. Novel alginate-chitosan aerogel fibres for potential wound healing applications. Int. J. Biol. Macromol. 2020, 156, 773–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keil, C.; Hübner, C.; Richter, C.; Lier, S.; Barthel, L.; Meyer, V.; Subrahmanyam, R.; Gurikov, P.; Smirnova, I.; Haase, H. Ca-Zn-Ag alginate aerogels for wound healing applications: Swelling behavior in simulated human body fluids and effect on macrophages. Polymers 2020, 12, 2741. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.A.; Ilyas, K.; Dlouhý, I.; Siska, F.; Boccaccini, A.R. Electrophoretic deposition of copper (II)-chitosan complexes for antibacterial coatings. Int. J. Mol. Sci. 2020, 21, 2637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gritsch, L.; Maqbool, M.; Mouriño, V.; Ciraldo, F.E.; Cresswell, M.; Jackson, P.R.; Lovell, C.; Boccaccini, A.R. Chitosan/hydroxyapatite composite bone tissue engineering scaffolds with dual and decoupled therapeutic ion delivery: Copper and strontium. J. Mater. Chem. B 2019, 7, 6109–6124. [Google Scholar] [CrossRef] [Green Version]
- Kruppke, B.; Heinemann, C.; Farack, J.; Weil, S.; Aflalo, E.D.; Sagi, A.; Hanke, T. Hemocyanin modification of chitosan scaffolds with calcium phosphate phases increase the osteoblast/osteoclast activity ratio—A co-culture study. Molecules 2020, 25, 4580. [Google Scholar] [CrossRef]
- Radwan-Pragłowska, J.; Piątkowski, M.; Janus, Ł.; Bogdał, D.; Matysek, D.; Cablik, V. Microwave-assisted synthesis and characterization of antioxidant chitosan-based aerogels for biomedical applications. Int. J. Polym. Anal. Charact. 2018, 23, 721–729. [Google Scholar] [CrossRef]
- Ryu, S.; Song, P.I.; Seo, C.H.; Cheong, H.; Park, Y. Colonization and infection of the skin by S. aureus: Immune system evasion and the response to cationic antimicrobial peptides. Int. J. Mol. Sci. 2014, 15, 8753–8772. [Google Scholar] [CrossRef] [Green Version]
- Öztürk-Çolak, A.; Moussian, B.; Araújo, S.J.; Casanova, J. A feedback mechanism converts individual cell features into a supracellular ECM structure in Drosophila trachea. eLife 2016, 5, 179. [Google Scholar] [CrossRef]
- Moussian, B.; Seifarth, C.; Müller, U.; Berger, J.; Schwarz, H. Cuticle differentiation during Drosophila embryogenesis. Arthropod Struct. Dev. 2006, 35, 137–152. [Google Scholar] [CrossRef]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef] [Green Version]
- Romano, P.; Fabritius, H.; Raabe, D. The exoskeleton of the lobster Homarus americanus as an example of a smart anisotropic biological material. Acta Biomater. 2007, 3, 301–309. [Google Scholar] [CrossRef]
- Kaya, M.; Sargin, I.; Al-Jaf, I.; Erdogan, S.; Arslan, G. Characteristics of corneal lens chitin in dragonfly compound eyes. Int. J. Biol. Macromol. 2016, 89, 54–61. [Google Scholar] [CrossRef]
- Nikolov, S.; Petrov, M.; Lymperakis, L.; Friák, M.; Sachs, C.; Fabritius, H.-O.; Raabe, D.; Neugebauer, J. Revealing the design principles of high-performance biological composites using ab initio and multiscale simulations: The example of lobster cuticle. Adv. Mater. 2010, 22, 519–526. [Google Scholar] [CrossRef]
- Fabritius, H.-O.; Ziegler, A.; Friák, M.; Nikolov, S.; Huber, J.; Seidl, B.H.M.; Ruangchai, S.; Alagboso, F.I.; Karsten, S.; Lu, J.; et al. Functional adaptation of crustacean exoskeletal elements through structural and compositional diversity: A combined experimental and theoretical study. Bioinspir. Biomim. 2016, 11, 55006. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Merzendorfer, H.; Arakane, Y.; Yang, Q. Chitin organizing and modifying enzymes and proteins involved in remodeling of the insect cuticle. Adv. Exp. Med. Biol. 2019, 1142, 83–114. [Google Scholar] [CrossRef]
- Sviben, S.; Spaeker, O.; Bennet, M.; Albéric, M.; Dirks, J.-H.; Moussian, B.; Fratzl, P.; Bertinetti, L.; Politi, Y. Epidermal cell surface structure and chitin-protein coassembly determine fiber architecture in the locust cuticle. ACS Appl. Mater. Interfaces 2020, 12, 25581–25590. [Google Scholar] [CrossRef]
- Shen, X.; Shamshina, J.L.; Berton, P.; Gurau, G.; Rogers, R.D. Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green Chem. 2016, 18, 53–75. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the production of polysaccharide aerogel particles. Materials 2018, 11, 2144. [Google Scholar] [CrossRef] [Green Version]
- Rani, M.; Agarwal, A.; Negi, Y.S. Review: Chitosan based hydrogel polymeric beads—As drug delivery system. BioResource 2010, 5, 276–2807. [Google Scholar]
- Hozumi, K.; Nomizu, M. Mixed peptide-conjugated chitosan matrices as multi-receptor targeted cell-adhesive scaffolds. Int. J. Mol. Sci. 2018, 19, 2713. [Google Scholar] [CrossRef] [Green Version]
- Higashi, B.; Mariano, T.B.; de Abreu Filho, B.A.; Gonçalves, R.A.C.; de Oliveira, A.J.B. Effects of fructans and probiotics on the inhibition of Klebsiella oxytoca and the production of short-chain fatty acids assessed by NMR spectroscopy. Carbohydr. Polym. 2020, 248, 116832. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Silk fibroin protein and chitosan polyelectrolyte complex porous scaffolds for tissue engineering applications. Carbohydr. Polym. 2011, 85, 325–333. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Yang, M.; Zhang, H.; Zhu, L. Fabrication of a novel blended membrane with chitosan and silk microfibers for wound healing: Characterization, in vitro and in vivo studies. J. Mater. Chem. B 2015, 3, 3634–3642. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Li, M.G.; Guan, Y.J.; Schreyer, D.J.; Chen, X.B. Effects of laminin blended with chitosan on axon guidance on patterned substrates. Biofabrication 2010, 2, 45002. [Google Scholar] [CrossRef]
- Orellana, S.L.; Giacaman, A.; Vidal, A.; Morales, C.; Oyarzun-Ampuero, F.; Lisoni, J.G.; Henríquez-Báez, C.; Morán-Trujillo, L.; Concha, M.; Moreno-Villoslada, I. Chitosan/chondroitin sulfate aerogels with high polymeric electroneutralization degree: Formation and mechanical properties. Pure Appl. Chem. 2018, 90, 901–911. [Google Scholar] [CrossRef]
- Mehrabani, M.G.; Karimian, R.; Mehramouz, B.; Rahimi, M.; Kafil, H.S. Preparation of biocompatible and biodegradable silk fibroin/chitin/silver nanoparticles 3D scaffolds as a bandage for antimicrobial wound dressing. Int. J. Biol. Macromol. 2018, 114, 961–971. [Google Scholar] [CrossRef]
- Cui, L.; Xiong, Z.; Guo, Y.; Liu, Y.; Zhao, J.; Zhang, C.; Zhu, P. Fabrication of interpenetrating polymer network chi-tosan/gelatin porous materials and study on dye adsorption properties. Carbohydr. Polym. 2015, 132, 330–337. [Google Scholar] [CrossRef]
- Gilarska, A.; Lewandowska-Łańcucka, J.; Horak, W.; Nowakowska, M. Collagen/chitosan/hyaluronic acid-based injectable hydrogels for tissue engineering applications-design, physicochemical and biological characterization. Colloids Surf. B 2018, 170, 152–162. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.; Tang, B.; Yang, W.-J.; Jin, D.-C. Identification and functional analysis of chitinase 7 gene in white-backed planthopper, Sogatella furcifera. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2017, 208–209, 19–28. [Google Scholar] [CrossRef]
- Ho, M.-H.; Wang, D.-M.; Hsieh, H.-J.; Liu, H.-C.; Hsien, T.-Y.; Lai, J.-Y.; Hou, L.-T. Preparation and characterization of RGD-immobilized chitosan scaffolds. Biomaterials 2005, 26, 3197–3206. [Google Scholar] [CrossRef]
- Tiğli, R.S.; Gümüşderelioğlu, M. Evaluation of RGD- or EGF-immobilized chitosan scaffolds for chondrogenic activity. Int. J. Biol. Macromol. 2008, 43, 121–128. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Thiolation of chitosan. Attachment of proteins via thioether formation. Biomacromolecules 2005, 6, 880–884. [Google Scholar] [CrossRef]
- Hozumi, K.; Nomizu, M. Cell adhesion activity of peptides conjugated to polysaccharides. Curr. Protoc. Cell Biol. 2018, 80, e53. [Google Scholar] [CrossRef]
- Silva, S.S.; Motta, A.; Rodrigues, M.T.; Pinheiro, A.F.M.; Gomes, M.E.; Mano, J.F.; Reis, R.L.; Migliaresi, C. Novel genipin-cross-linked chitosan/silk fibroin sponges for cartilage engineering strategies. Biomacromolecules 2008, 9, 2764–2774. [Google Scholar] [CrossRef] [Green Version]
- Butler, M.F.; Clark, A.H.; Adams, S. Swelling and mechanical properties of biopolymer hydrogels containing chitosan and bovine serum albumin. Biomacromolecules 2006, 7, 2961–2970. [Google Scholar] [CrossRef]
- Cheng, Y.; Hu, Z.; Zhao, Y.; Zou, Z.; Lu, S.; Zhang, B.; Li, S. Sponges of carboxymethyl chitosan grafted with collagen peptides for wound healing. Int. J. Mol. Sci. 2019, 20, 3890. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, K.; Heyer, M.; Ratke, L.; Milow, B. Facile preparation of nanofibrillar networks of ‘’Ureido-Chitin’’ containing ureido and amine as chelating functional groups. Chemistry 2018, 24, 19332–19340. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Leventis, N. Aerogels Handbook; Springer Science Business Media LLC: New York, NY, USA, 2011; ISBN 9781441975898. [Google Scholar]
- Yamasaki, S.; Sakuma, W.; Yasui, H.; Daicho, K.; Saito, T.; Fujisawa, S.; Isogai, A.; Kanamori, K. Nanocellulose xerogels with high porosities and large specific surface areas. Front. Chem. 2019, 7, 316. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, K.; Dennstedt, A.; Barowski, A.; Ratke, L. Design of aerogels, cryogels and xerogels of cellulose with hierarchical porous structures. Mater. Des. 2016, 92, 345–355. [Google Scholar] [CrossRef]
- Li, J.; Sun, X.; Zhang, K.; Yang, G.; Mu, Y.; Su, C.; Pang, J.; Chen, T.; Chen, X.; Feng, C. Chitosan/Diatom-Biosilica aerogel with controlled porous structure for rapid hemostasis. Adv. Healthc. Mater. 2020, 9, e2000951. [Google Scholar] [CrossRef]
- Guo, X.; Xu, D.; Zhao, Y.; Gao, H.; Shi, X.; Cai, J.; Deng, H.; Chen, Y.; Du, Y. Electroassembly of Chitin nanoparticles to construct freestanding hydrogels and high porous aerogels for wound healing. ACS Appl. Mater. Interfaces 2019, 11, 34766–34776. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-based functional materials for skin wound repair: Mechanisms and applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Jing, X.; Sun, Y.; Ma, X.; Hu, H. Marine polysaccharides: Green and recyclable resources as wound dressings. Mater. Chem. Front. 2021, 5, 5595–5616. [Google Scholar] [CrossRef]
- Jin, T.; Liu, T.; Lam, E.; Moores, A. Chitin and chitosan on the nanoscale. Nanoscale Horiz. 2021, 6, 505–542. [Google Scholar] [CrossRef]
- Ueno, H.; Mori, T.; Fujinaga, T. Topical formulations and wound healing applications of chitosan. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef]
- Lovskaya, D.; Menshutina, N.; Mochalova, M.; Nosov, A.; Grebenyuk, A. Chitosan-based aerogel particles as highly effective local hemostatic agents. Production process and in vivo evaluations. Polymers 2020, 12, 2055. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Gurikov, P.; Monteiro, F.J.; Smirnova, I.; Alvarez-Lorenzo, C.; García-González, C.A. Jet cutting technique for the production of chitosan aerogel microparticles loaded with vancomycin. Polymers 2020, 12, 273. [Google Scholar] [CrossRef] [Green Version]
- Ko, E.; Kim, H. Preparation of chitosan aerogel crosslinked in chemical and ionical ways by non-acid condition for wound dressing. Int. J. Biol. Macromol. 2020, 164, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Li, Y.; Li, X.; Wu, Y.; Tang, K.; Liu, J.; Zheng, X.; Wan, G. Injectable antibacterial cellulose nanofiber/chitosan aerogel with rapid shape recovery for noncompressible hemorrhage. Int. J. Biol. Macromol. 2020, 154, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Neville, A.C.; Luke, B.M. A two-system model for chitin-protein complexes in insect cuticles. Tissue Cell 1969, 1, 689–707. [Google Scholar] [CrossRef]
- Mrak, P.; Bogataj, U.; Štrus, J.; Žnidaršič, N. Cuticle morphogenesis in crustacean embryonic and postembryonic stages. Arthropod Struct. Dev. 2017, 46, 77–95. [Google Scholar] [CrossRef]
- Uv, A.; Moussian, B. The apical plasma membrane of Drosophila embryonic epithelia. Eur. J. Cell Biol. 2010, 89, 208–211. [Google Scholar] [CrossRef]
- Wang, Y.; Farine, J.-P.; Yang, Y.; Yang, J.; Tang, W.; Gehring, N.; Ferveur, J.-F.; Moussian, B. Transcriptional control of quality differences in the lipid-based cuticle barrier in Drosophila suzukii and Drosophila melanogaster. Front. Genet. 2020, 11, 887. [Google Scholar] [CrossRef]
- Dong, W.; Dobler, R.; Dowling, D.K.; Moussian, B. The cuticle inward barrier in Drosophila melanogaster is shaped by mitochondrial and nuclear genotypes and a sex-specific effect of diet. PeerJ 2019, 7, e7802. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, P.E.; Walkiewicz, M.; Stern, M. Ras activity in the Drosophila prothoracic gland regulates body size and developmetal rate via ecdysone release. Curr. Biol. 2005, 15, 1785–1795. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Bender, M. A conditional rescue system reveals essential functions for the ecdysone receptor (EcR) gene during molting and metamorphosis in Drosophila. Development 2000, 127, 2897–2905. [Google Scholar] [CrossRef]
- Liu, S.; Li, K.; Gao, Y.; Liu, X.; Chen, W.; Ge, W.; Feng, Q.; Palli, S.R.; Li, S. Antagonistic actions of juvenile hormone and 20-hydroxyecdysone within the ring gland determine developmental transitions in Drosophila. Proc. Natl. Acad. Sci. USA 2018, 115, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 2013, 58, 181–204. [Google Scholar] [CrossRef]
- Pesch, Y.-Y.; Hesse, R.; Ali, T.; Behr, M. A cell surface protein controls endocrine ring gland morphogenesis and steroid production. Dev. Biol. 2019, 445, 16–28. [Google Scholar] [CrossRef]
- Raabe, D.; Al-Sawalmih, A.; Yi, S.B.; Fabritius, H. Preferred crystallographic texture of alpha-chitin as a microscopic and macroscopic design principle of the exoskeleton of the lobster Homarus americanus. Acta Biomater. 2007, 3, 882–895. [Google Scholar] [CrossRef]
- Vincent, J.F. Arthropod cuticle: A natural composite shell system. Compos. Part A Appl. Sci. Manuf. 2002, 33, 1311–1315. [Google Scholar] [CrossRef]
- Bouligand, Y. Twisted fibrous arrangements in biological materials and cholesteric mesophases. Tissue Cell 1972, 4, 189–217. [Google Scholar] [CrossRef]
- Dirks, J.-H.; Taylor, D. Fracture toughness of locust cuticle. J. Exp. Biol. 2012, 215, 1502–1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajabi, H.; Jafarpour, M.; Darvizeh, A.; Dirks, J.-H.; Gorb, S.N. Stiffness distribution in insect cuticle: A continuous or a dis-continuous profile? J. R. Soc. Interface 2017, 14, 20170310. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.F.V.; Wegst, U.G.K. Design and mechanical properties of insect cuticle. Arthropod Struct. Dev. 2004, 33, 187–199. [Google Scholar] [CrossRef]
- Rajabi, H.; Dirks, J.-H.; Gorb, S.N. Insect wing damage: Causes, consequences and compensatory mechanisms. J. Exp. Biol. 2020, 223, 223. [Google Scholar] [CrossRef]
- Behr, M.; Riedel, D. Glycosylhydrolase genes control respiratory tubes sizes and airway stability. Sci. Rep. 2020, 10, 13377. [Google Scholar] [CrossRef]
- Arakane, Y.; Muthukrishnan, S.; Kramer, K.J.; Specht, C.A.; Tomoyasu, Y.; Lorenzen, M.D.; Kanost, M.; Beeman, R.W. The tribolium chitin synthase genes TcCHS1 and TcCHS2 are specialized for synthesis of epidermal cuticle and midgut peritrophic matrix. Insect Mol. Biol. 2005, 14, 453–463. [Google Scholar] [CrossRef]
- Nikolov, S.; Fabritius, H.; Petrov, M.; Friák, M.; Lymperakis, L.; Sachs, C.; Raabe, D.; Neugebauer, J. Robustness and optimal use of design principles of arthropod exoskeletons studied by ab initio-based multiscale simulations. J. Mech. Behav. Biomed. Mater. 2011, 4, 129–145. [Google Scholar] [CrossRef]
- Wang, Y.; Carballo, R.G.; Moussian, B. Double cuticle barrier in two global pests, the whitefly Trialeurodes vaporariorum and the bedbug Cimex lectularius. J. Exp. Biol. 2017, 220, 1396–1399. [Google Scholar] [CrossRef] [Green Version]
- Locke, M. The wigglesworth lecture: Insects for studying fundamental problems in biology. J. Insect Physiol. 2001, 47, 495–507. [Google Scholar] [CrossRef]
- Hegedus, D.; Erlandson, M.; Gillott, C.; Toprak, U. New insights into peritrophic matrix synthesis, architecture, and function. Annu. Rev. Entomol. 2009, 54, 285–302. [Google Scholar] [CrossRef]
- Tonning, A.; Hemphälä, J.; Tång, E.; Nannmark, U.; Samakovlis, C.; Uv, A. A transient luminal chitinous matrix is required to model epithelial tube diameter in the Drosophila trachea. Dev. Cell 2005, 9, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Tsarouhas, V.; Senti, K.-A.; Jayaram, S.A.; Tiklova, K.; Hemphala, J.; Adler, J.; Samakovlis, C. Sequential pulses of apical epithelial secretion and endocytosis drive airway maturation in Drosophila. Dev. Cell 2007, 13, 214–225. [Google Scholar] [CrossRef] [Green Version]
- Dong, B.; Hannezo, E.; Hayashi, S. Balance between apical membrane growth and luminal matrix resistance determines epithlial tubule shape. Cell Rep. 2014, 7, 941–950. [Google Scholar] [CrossRef]
- Behr, M.; Wingen, C.; Wolf, C.; Schuh, R.; Hoch, M. Wurst is essential for airway clearance and respiratory-tube size control. Nat. Cell Biol. 2007, 9, 847–853. [Google Scholar] [CrossRef]
- Luschnig, S.; Bätz, T.; Armbruster, K.; Krasnow, M.A. Serpentine and vermiform encode matrix proteins with chitin binding and deacetylation domains that limit tracheal tube length in Drosophila. Curr. Biol. 2006, 16, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Jayaram, S.A.; Hemphälä, J.; Senti, K.-A.; Tsarouhas, V.; Jin, H.; Samakovlis, C. Septate-junction-dependent luminal deposition of chitin deacetylases restricts tube elongation in the Drosophila trachea. Curr. Biol. 2006, 16, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Kelkenberg, M.; Odman-Naresh, J.; Muthukrishnan, S.; Merzendorfer, H. Chitin is a necessary component to maintain the barier function of the peritrophic matrix in the insect midgut. Insect Biochem. Mol. Biol. 2015, 56, 21–28. [Google Scholar] [CrossRef]
- Jaspers, M.H.J.; Pflanz, R.; Riedel, D.; Kawelke, S.; Feussner, I.; Schuh, R. The fatty acyl-CoA reductase waterproof mediates airway clearance in Drosophila. Dev. Biol. 2014, 385, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Shaik, K.S.; Meyer, F.; Vázquez, Á.V.; Flötenmeyer, M.; Cerdán, M.E.; Moussian, B. δ-Aminolevulinate synthase is required for apical transcellular barrier formation in the skin of the Drosophila larva. Eur. J. Cell Biol. 2012, 91, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Itakura, Y.; Inagaki, S.; Wada, H.; Hayashi, S. Trynity controls epidermal barrier function and respiratory tube maturation in Drosophila by modulating apical extracellular matrix nano-patterning. PLoS ONE 2018, 13, e0209058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, S.; Kelkenberg, M.; Begum, K.; Steinfeld, L.; Williams, C.E.; Kramer, K.J.; Beeman, R.W.; Park, Y.; Muthukrishnan, S.; Merzendorfer, H. Two essential peritrophic matrix proteins mediate matrix barrier functions in the insect midgut. Insect Biochem. Mol. Biol. 2014, 49, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, S.; Mun, S.; Noh, M.Y.; Geisbrecht, E.R.; Arakane, Y. Insect cuticular chitin contributes to form and function. Curr. Pharm. Des. 2020, 26, 3530–3545. [Google Scholar] [CrossRef] [PubMed]
- Moussian, B.; Tang, E.; Tonning, A.; Helms, S.; Schwarz, H.; Nusslein-Volhard, C.; Uv, A.E. Drosophila knickkopf and retroative are needed for epithelial tube growth and cuticle differentiation through their specific requirement for chitin filament organization. Development 2006, 133, 163–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesch, Y.-Y.; Riedel, D.; Behr, M. Obstructor a organizes matrix assembly at the apical cell surface to promote enzymatic cuticle maturation in Drosophila. J. Biol. Chem. 2015, 290, 10071–10082. [Google Scholar] [CrossRef] [Green Version]
- Noh, M.Y.; Muthukrishnan, S.; Kramer, K.J.; Arakane, Y.; Riddiford, L.M. Tribolium castaneum RR-1 cuticular protein TcCPR4 is required for formation of pore canals in rigid cuticle. PLoS Genet. 2015, 11, e1004963. [Google Scholar] [CrossRef] [Green Version]
- Muthukrishnan, S.; Arakane, Y.; Yang, Q.; Zhang, C.-X.; Zhang, J.; Zhang, W.; Moussian, B. Future questions in insect chitin biology: A microreview. Arch. Insect Biochem. Physiol. 2018, 98, e21454. [Google Scholar] [CrossRef]
- Chaudhari, S.S.; Arakane, Y.; Specht, C.A.; Moussian, B.; Kramer, K.J.; Muthukrishnan, S.; Beeman, R.W. Retroactive maintains cuticle integrity by promoting the trafficking of Knickkopf into the procuticle of Tribolium castaneum. PLoS Genet. 2013, 9, e1003268. [Google Scholar] [CrossRef] [Green Version]
- Arakane, Y.; Lomakin, J.; Gehrke, S.H.; Hiromasa, Y.; Tomich, J.M.; Muthukrishnan, S.; Beeman, R.W.; Kramer, K.J.; Kanost, M.R.; Stern, D.L. Formation of rigid, non-flight forewings (Elytra) of a beetle requires two major cuticular proteins. PLoS Genet. 2012, 8, e1002682. [Google Scholar] [CrossRef] [Green Version]
- Noh, M.Y.; Muthukrishnan, S.; Kramer, K.J.; Arakane, Y. Development and ultrastructure of the rigid dorsal and flexible ventral cuticles of the elytron of the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2017, 91, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, N.T.; Hiromasa, Y.; Tomich, J.M.; Lu, N.; Beeman, R.W.; Kramer, K.J.; Kanost, M.R. Proteomic and transcriptomic analyses of rigid and membranous cuticles and epidermis from the Elytra and Hindwings of the red flour beetle, Tribolium castaneum. J. Proteome Res. 2012, 11, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Tetreau, G.; Dittmer, N.T.; Cao, X.; Agrawal, S.; Chen, Y.R.; Muthukrishnan, S.; Haobo, J.; Blissard, G.W.; Kanost, M.R.; Wang, P. Analysis of chitin-binding proteins from Manduca sexta provides new insights into evolution of peritrophin A-type chitin-binding domains in insects. Insect Biochem. Mol. Biol. 2015, 62, 127–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Li, S.; Li, W.; Peng, L.; Chen, Z.; Xiao, Y.; Guo, H.; Zhang, J.; Cheng, T.; Goldsmith, M.R.; et al. Genome-wide annotation and comparative analysis of cuticular protein genes in the noctuid pest Spodoptera litura. Insect Biochem. Mol. Biol. 2019, 110, 90–97. [Google Scholar] [CrossRef]
- Zhou, D.; Duan, B.; Sun, Y.; Ma, L.; Zhu, C.; Shen, B. Preliminary characterization of putative structural cuticular proteins in the malaria vector Anopheles sinensis. Pest Manag. Sci. 2017, 73, 2519–2528. [Google Scholar] [CrossRef]
- Willis, J.H. Structural cuticular proteins from arthropods: Annotation, nomenclature, and sequence characteristics in the genomics era. Insect Biochem. Mol. Biol. 2010, 40, 189–204. [Google Scholar] [CrossRef] [Green Version]
- Jasrapuria, S.; Specht, C.A.; Kramer, K.J.; Beeman, R.W.; Muthukrishnan, S.; Palli, S.R. Gene families of cuticular proteins analogous to Peritrophins (CPAPs) in Tribolium castaneum have diverse functions. PLoS ONE 2012, 7, e49844. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.-L.; Tian, S.; Yang, H.; Zhou, X.; Xu, S.; Zhang, Z.; Gong, J.; Hou, Y.; Xia, Q. Genome-wide identification of chitin-binding proteins and characterization of BmCBP1 in the silkworm, Bombyx mori. Insect Sci. 2019, 26, 400–412. [Google Scholar] [CrossRef] [Green Version]
- Dittmer, N.T.; Tetreau, G.; Cao, X.; Jiang, H.; Wang, P.; Kanost, M.R. Annotation and expression analysis of cuticular proteins from the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 2015, 62, 100–113. [Google Scholar] [CrossRef] [Green Version]
- Jasrapuria, S.; Arakane, Y.; Osman, G.; Kramer, K.J.; Beeman, R.W.; Muthukrishnan, S. Genes encoding proteins with peritrophin A-type chitin-binding domains in Tribolium castaneum are grouped into three distinct families based on phylogeny, expression and function. Insect Biochem. Mol. Biol. 2010, 40, 214–227. [Google Scholar] [CrossRef]
- Tajiri, R.; Ogawa, N.; Fujiwara, H.; Kojima, T. mechanical control of whole body shape by a single cuticular protein obstructor-e in Drosophila melanogaster. PLoS Genet. 2017, 13, e1006548. [Google Scholar] [CrossRef]
- Noh, M.Y.; Kramer, K.J.; Muthukrishnan, S.; Kanost, M.R.; Beeman, R.W.; Arakane, Y. Two major cuticular proteins are required for assembly of horizontal laminae and vertical pore canals in rigid cuticle of Tribolium castaneum. Insect Biochem. Mol. Biol. 2014, 53, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Behr, M.; Hoch, M. Identification of the novel evolutionary conserved obstructor multigene family in invertebrates. FEBS Lett. 2005, 579, 6827–6833. [Google Scholar] [CrossRef] [Green Version]
- Tiklova, K.; Tsarouhas, V.; Samakovlis, C. Control of airway tube diameter and integrity by secreted chitin-binding proteins in Drosophila. PLoS ONE 2013, 8, e67415. [Google Scholar] [CrossRef]
- Petkau, G.; Wingen, C.; Jussen, L.C.A.; Radtke, T.; Behr, M. Obstructor-A is required for epithelial extracellular matrix dynamics, exoskeleton function, and tubulogenesis. J. Biol. Chem. 2012, 287, 21396–21405. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Ji, Y.; Zhang, X.; Ma, P.; Wang, Y.; Moussian, B.; Zhang, J. The putative chitin deacetylases Serpentine and Vermiform have non-redundant functions during Drosophila wing development. Insect Biochem. Mol. Biol. 2019, 110, 128–135. [Google Scholar] [CrossRef]
- Arakane, Y.; Dixit, R.; Begum, K.; Park, Y.; Specht, C.A.; Merzendorfer, H.; Kramer, K.J.; Muthukrishnan, S.; Beeman, R.W. Analysis of functions of the chitin deacetylase gene family in Tribolium castaneum. Insect Biochem. Mol. Biol. 2009, 39, 355–365. [Google Scholar] [CrossRef]
- Yu, R.-R.; Liu, W.-M.; Zhao, X.-M.; Zhang, M.; Li, D.-Q.; Zuber, R.; Ma, E.-B.; Zhu, K.Y.; Moussian, B.; Zhang, J.-Z. LmCDA1 organizes the cuticle by chitin deacetylation in Locusta migratoria. Insect Mol. Biol. 2019, 28, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Yu, R.; Liu, W.; Li, D.; Zhao, X.; Ding, G.; Zhang, M.; Ma, E.; Zhu, K.Y.; Li, S.; Moussian, B.; et al. Helicoidal organization of chitin in the cuticle of the migratory locust requires the function of the chitin deacetylase 2 enzyme (LmCDA2). J. Biol. Chem. 2016, 291, 24352–24363. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Ma, P.; Zhou, J.; He, Y.; Liu, W.; Liu, X.; Zhang, X.; Yu, R.; Zhang, M.; Moussian, B.; et al. Group I CDAs are responsible for a selective CHC-independent cuticular barrier in Locusta migratoria. Pestic. Biochem. Physiol. 2021, 175, 104854. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, P.-J.; Zhang, T.-T.; Gao, Z.-M.; Zhao, P.; Liu, X.-J.; Zhang, X.-Y.; Liu, W.-M.; Yu, R.-R.; Moussian, B.; et al. Roles of LmCDA1 and LmCDA2 in cuticle formation in the foregut and hindgut of Locusta migratoria. Insect Sci. 2020, 28, 1314–1325. [Google Scholar] [CrossRef]
- Chaudhari, S.S.; Arakane, Y.; Specht, C.A.; Moussian, B.; Boyle, D.L.; Park, Y.; Kramer, K.J.; Beeman, R.W.; Muthukrishnan, S. Knickkopf protein protects and organizes chitin in the newly synthesized insect exoskeleton. Proc. Natl. Acad. Sci. USA 2011, 108, 17028–17033. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Zhang, X.; Zuo, Y.; Liu, W.; Zhang, J.; Moussian, B. Timed Knickkopf function is essential for wing cuticle formation in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2017, 89, 1–10. [Google Scholar] [CrossRef]
- Arakane, Y.; Muthukrishnan, S. Insect chitinase and chitinase-like proteins. Cell. Mol. Life Sci. 2010, 67, 201–216. [Google Scholar] [CrossRef]
- Dixit, R.; Arakane, Y.; Specht, C.A.; Richard, C.; Kramer, K.J.; Beeman, R.W.; Muthukrishnan, S. Domain organization and phylogenetic analysis of proteins from the chitin deacetylase gene family of Tribolium castaneum and three other species of insects. Insect Biochem. Mol. Biol. 2008, 38, 440–451. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Arakane, Y.; Beeman, R.W.; Kramer, K.J.; Muthukrishnan, S. Functional specialization among insect chitinase family genes revealed by RNA interference. Proc. Natl. Acad. Sci. USA 2008, 105, 6650–6655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, M.Y.; Muthukrishnan, S.; Kramer, K.J.; Arakane, Y. A chitinase with two catalytic domains is required for organization of the cuticular extracellular matrix of a beetle. PLoS Genet. 2018, 14, e1007307. [Google Scholar] [CrossRef] [Green Version]
- Pesch, Y.-Y.; Riedel, D.; Behr, M. Drosophila Chitinase 2 is expressed in chitin producing organs for cuticle formation. Arthropod Struct. Dev. 2017, 46, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irons, S.L.; Chambers, A.C.; Lissina, O.; King, L.A.; Possee, R.D. Protein production using the baculovirus expression system. Curr. Protoc. Protein Sci. 2018, 91, 5.5.1.–5.5.22. [Google Scholar] [CrossRef]
- Erko, M.; Hartmann, M.A.; Zlotnikov, I.; Valverde Serrano, C.; Fratzl, P.; Politi, Y. Structural and mechanical properties of the arthropod cuticle: Comparison between the fang of the spider Cupiennius salei and the carapace of American lobster Homarus americanus. J. Struct. Biol. 2013, 183, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, S.S.; Moussian, B.; Specht, C.A.; Arakane, Y.; Kramer, K.J.; Beeman, R.W.; Muthukrishnan, S. Functional specialization among members of Knickkopf family of proteins in insect cuticle organization. PLoS Genet. 2014, 10, e1004537. [Google Scholar] [CrossRef] [Green Version]
| Matrix Formation | Bonding | Advantage | Disadvantage |
|---|---|---|---|
| Chemical crosslinking | Covalent bonding | Improving network strength results in rigid matrix formation providing high mechanical strength | May become brittle |
| Thermal energy | Van der Waals force | Reversible in wet-gel matrix formation | Gel transition temperature may be very high (>40 °C) or it is controlled by additives |
| Ionic crosslinking | Van der Waals force | Strong interaction with biological medium; advantage of using the ionic matrix as carrier; buffering at biological medium | High volume shrinkage while supercritically dried aerogel formation |
| Reversing pH of medium | Van der Waals force | Most employed economic path and easy to handle; possible to functionalize with sensitive biological molecules in neutralized post wet-gel matrix | Inappropriate for functionalizing the chitin/chitosan molecules with sensitive biological molecules |
| Non-solvent induced phase separation | Van der Waals force | Macropore channels are formed | Non-aqueous medium was used as non-solvent |
| Percolation | Van der Waals force | Highly crystalline wet-gel matrix is generated | Precursors should be produced in situ; should be stored or transported with high care and caution; not forming wet-gel matrix |
| Aerogels | Crosslinked/Blended with Additives | Observed in Biomedicine Application |
|---|---|---|
| Chitosan | Crosslinked with diatom-biosilica by in situ polymerization of dopamine (Michael-type cycloaddition) [137] | Improved hemostatic performance. |
| No additives [143] | Improved hemostatic performance | |
| Blended with alginate producing polyelectrolyte complex [144] | Efficient antibacterial activity (Staphylococcus aureus and Klebsiella pneumoniae) and effective wound closure | |
| Crosslinked with itaconic acid using epichlorohydrin [145] | Efficient antibacterial activity (Corynebacterium glutamicum and Escherichia coli) | |
| Vancomycin, drug-loaded chitosan aerogel [97] | Efficient antibacterial activity (Staphylococcus aureus) and drug release kinetics | |
| Cellulose nanofibers dry-crosslinked in the chitosan matrix [146] | Shape recoverable foam material under wet conditions showing improved hemostatic performance | |
| Chitin | No additives, electrophoretic deposition of chitin nanoparticles [138] | Accelerate wound healing and reduce scar area in comparison with cryogels |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behr, M.; Ganesan, K. Improving Polysaccharide-Based Chitin/Chitosan-Aerogel Materials by Learning from Genetics and Molecular Biology. Materials 2022, 15, 1041. https://doi.org/10.3390/ma15031041
Behr M, Ganesan K. Improving Polysaccharide-Based Chitin/Chitosan-Aerogel Materials by Learning from Genetics and Molecular Biology. Materials. 2022; 15(3):1041. https://doi.org/10.3390/ma15031041
Chicago/Turabian StyleBehr, Matthias, and Kathirvel Ganesan. 2022. "Improving Polysaccharide-Based Chitin/Chitosan-Aerogel Materials by Learning from Genetics and Molecular Biology" Materials 15, no. 3: 1041. https://doi.org/10.3390/ma15031041
APA StyleBehr, M., & Ganesan, K. (2022). Improving Polysaccharide-Based Chitin/Chitosan-Aerogel Materials by Learning from Genetics and Molecular Biology. Materials, 15(3), 1041. https://doi.org/10.3390/ma15031041







