K2O-Metakaolin-Based Geopolymer Foams: Production, Porosity Characterization and Permeability Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Foam Preparation
2.2. Characterization of the Foams
2.3. Permeability Tests
3. Results and Discussion
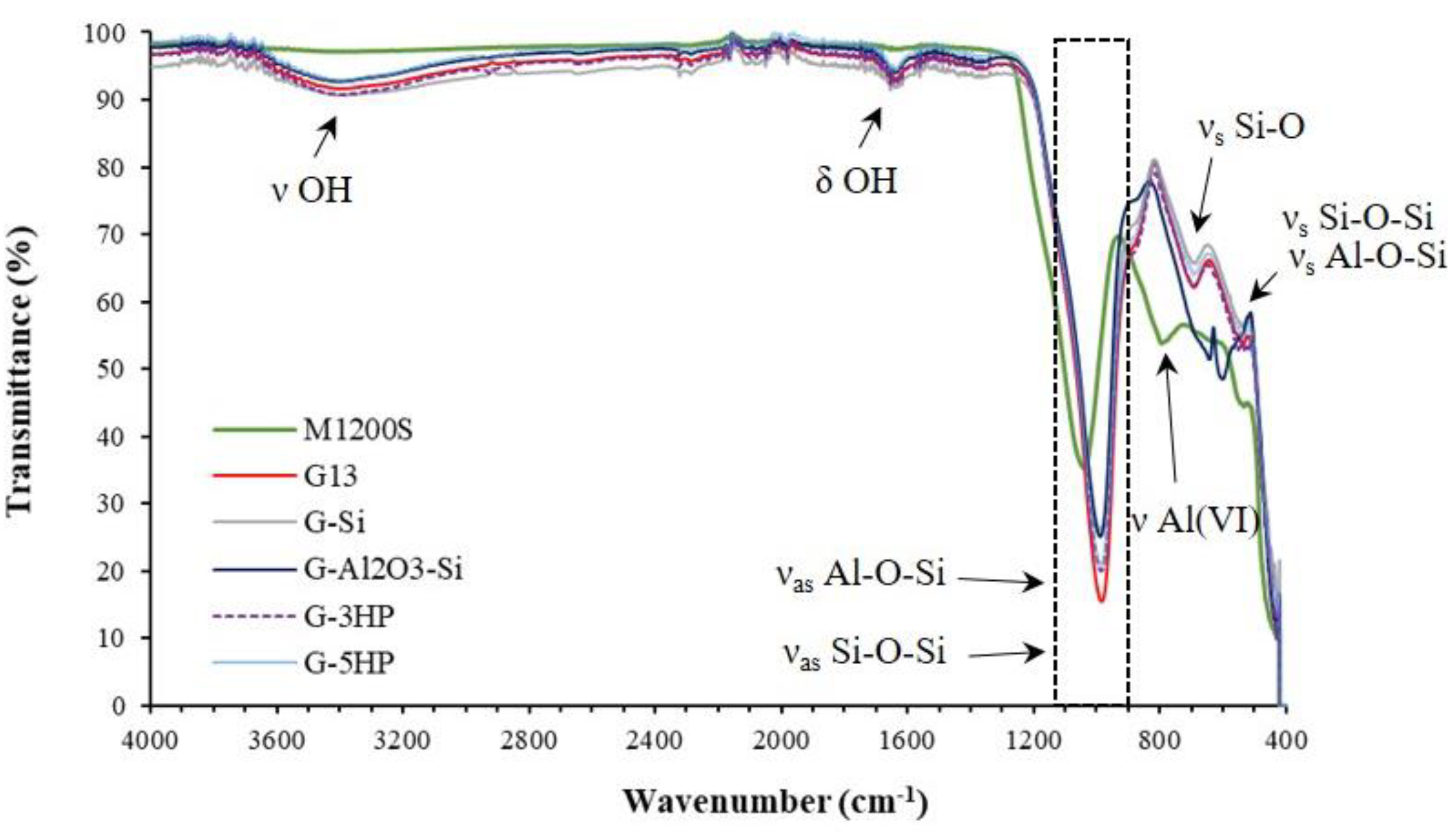
3.1. ATR-FTIR Measurements: Geopolymerization of Foams
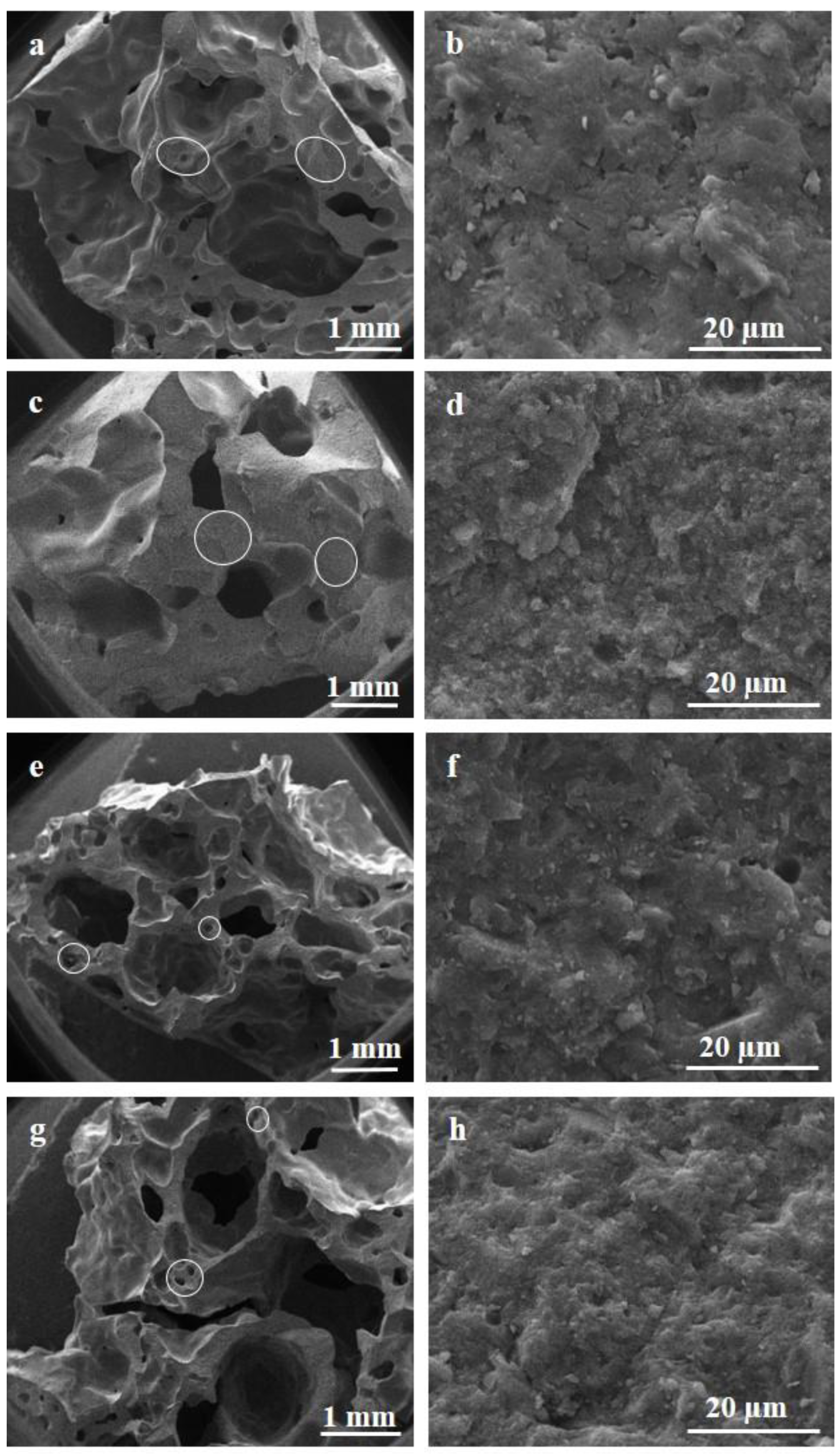
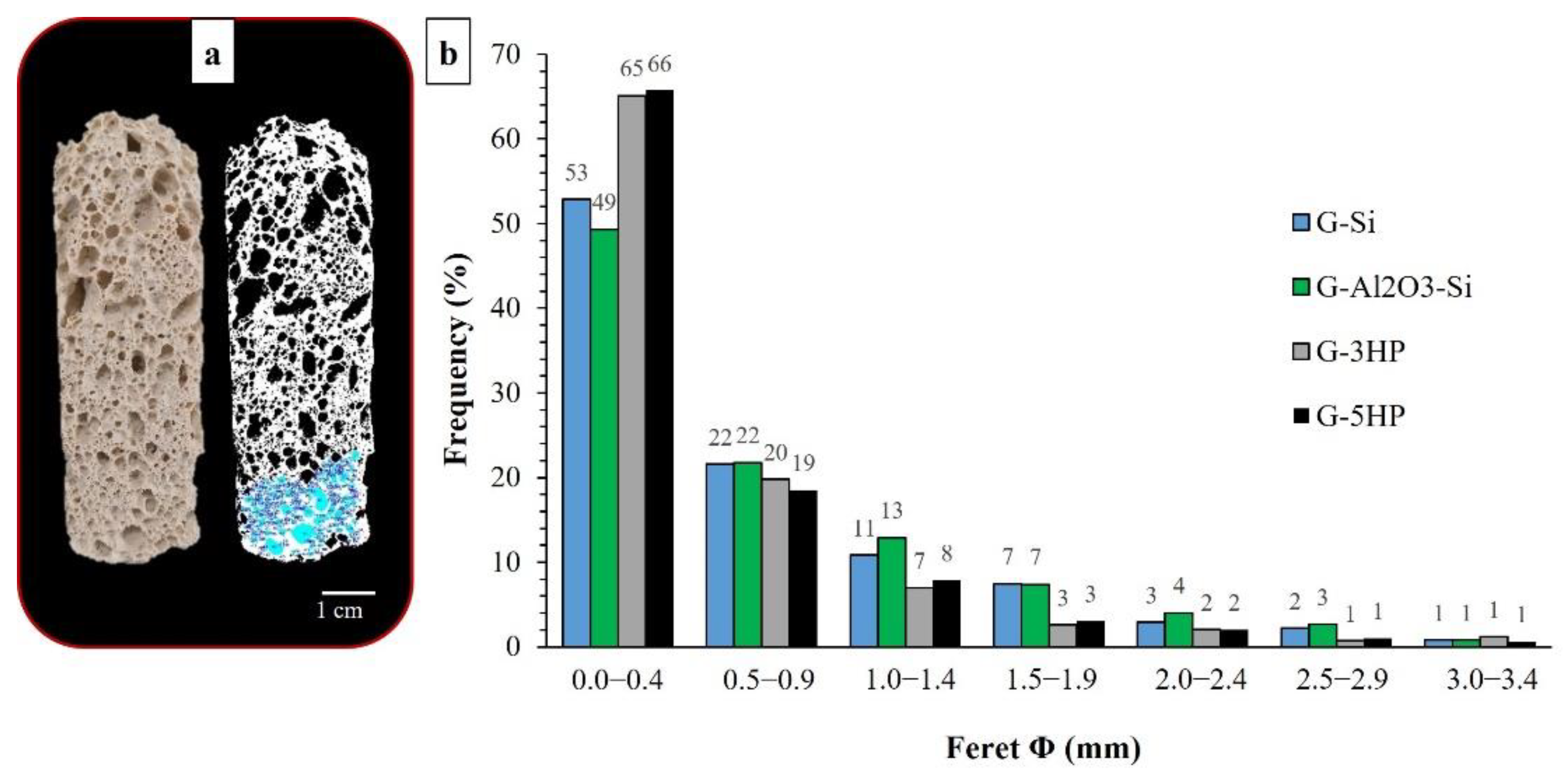
3.2. Macro-Microstructure of the Foams and Porosity
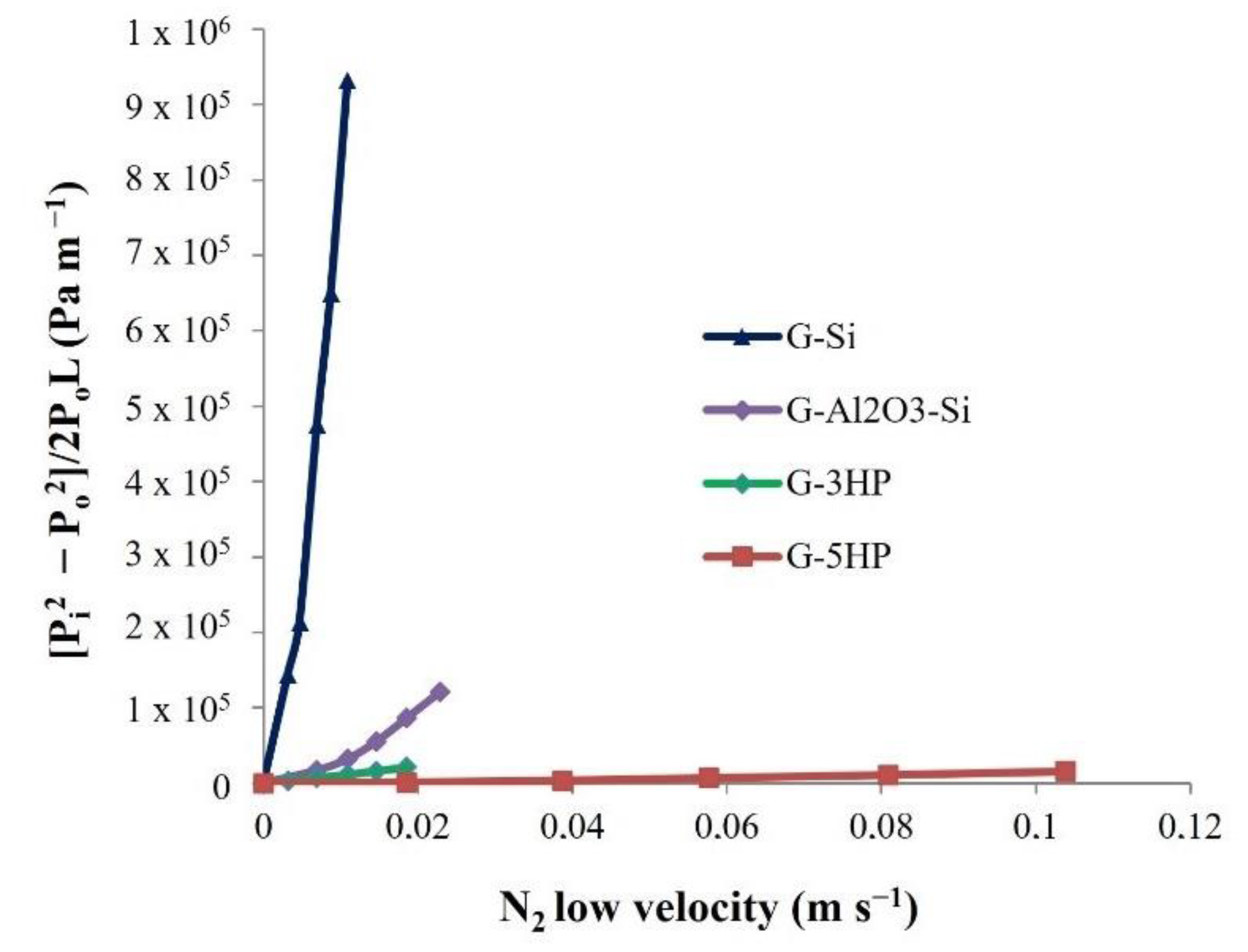
3.3. Permeability Test
4. Conclusions
- High L/S ratios in the geopolymer slurry increase the permeability of the foams by increasing the total porosity. In general, the foams from hydrogen peroxides, which is added as an aqueous solution and which releases water, are more porous than foams from metallic silicon, which is water consuming.
- The porosity of the struts influences the permeability due to changes in the frictional area. The addition of inert fillers, such as micrometric alumina, stabilizes the foam with the production of uniform pores and struts that favor the interconnectivity and permeability, although the foam has a higher density and lower expansion.
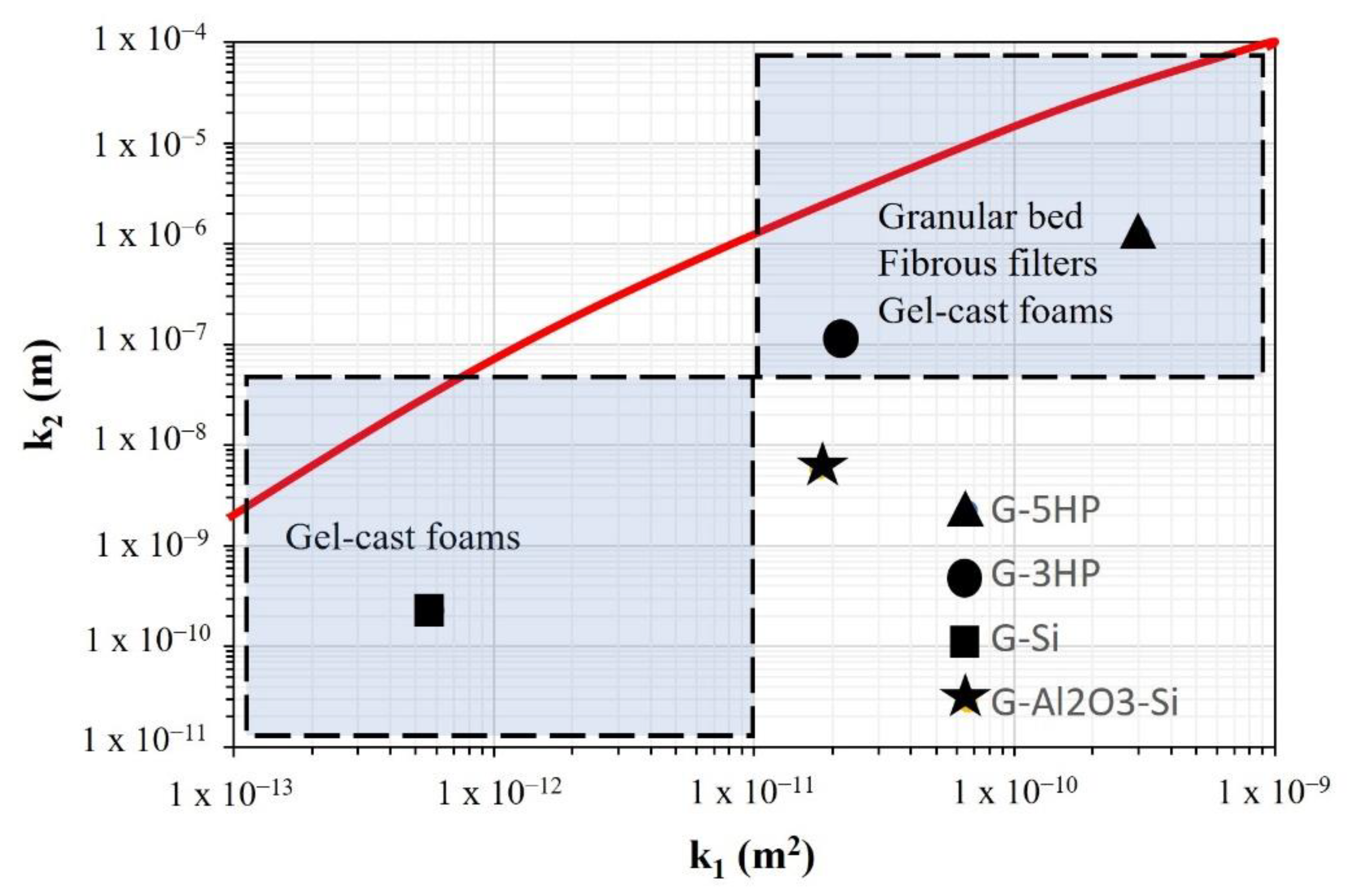
- Concerning the evaluation of permeability, this showed that the produced geopolymer foams are in the range of granular beds, fibrous filters and gel-cast foams (on the permeability map), with these differences caused by changing the blowing agent in the processing method.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Stochero, N.P.; de Moraes, E.G.; Moreira, A.C.; Fernandes, C.P.; Innocentini, M.D.M.; Novaes de Oliveira, A.P. Ceramic shell foams produced by direct foaming and gelcasting of proteins: Permeability and microstructural characterization by X-ray microtomography. J. Eur. Ceram. Soc. 2020, 40, 4224–4231. [Google Scholar] [CrossRef]
- Scheffler, M.; Colombo, P. Cellular Ceramics: Structure, Manufacturing, Properties and Application; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Studart, A.R.; Gonzenbach, U.T.; Tervoort, E.; Gauckler, L.J. Processing routes to macroporous ceramics: A review. J. Am. Ceram. Soc. 2006, 89, 1771–1789. [Google Scholar] [CrossRef]
- Arumugham, T.; Kaleekkal, N.J.; Gopal, S.; Nambikkattu, J.; Rambabu, K.; Aboulella, A.M.; Ranil Wickramasinghe, S.; Banat, F. Recent developments in porous ceramic membranes for wastewater treatment and desalination: A review. J. Environ. Manag. 2021, 293, 112925. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers Chemistry and Applications; Institut Geopolymere: Saint-Quentin, France, 2008. [Google Scholar]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Nodehi, M. A comparative review on foam-based versus lightweight aggregate-based alkali-activated materials and geopolymer. Innov. Infrastruct. Solut. 2021, 6, 231. [Google Scholar] [CrossRef]
- Novais, R.M.; Pullar, R.C.; Labrincha, J.A. Geopolymer foams: An overview of recent advancements. Prog. Mater. Sci. 2020, 109, 100621. [Google Scholar] [CrossRef]
- Kriven, W.M.; Bell, J.L.; Gordon, M. Microstructure and microchemistry of fully-reacted geopolymers and geopolymer matrix composites. Ceram. Trans. 2003, 153, 227–250. [Google Scholar]
- Landi, E.; Medri, V.; Papa, E.; Dedecek, J.; Klein, P.; Benito, P.; Vaccari, A. Alkali-bonded ceramics with hierarchical tailored porosity. Appl. Clay Sci. 2013, 73, 56–64. [Google Scholar] [CrossRef]
- Bai, C.; Colombo, P. Processing, properties and applications of highly porous geopolymers: A review. Ceram. Int. 2018, 44, 16103–16118. [Google Scholar] [CrossRef]
- Papa, E.; Medri, V.; Benito, P.; Vaccari, A.; Bugani, S.; Jaroszewicz, J.; Swieszkowski, W.; Landi, E. Synthesis of porous hierarchical geopolymer monoliths by ice-templating. Micropor. Mesopor. Mat. 2015, 215, 206–214. [Google Scholar] [CrossRef]
- Papa, E.; Medri, V.; Benito, P.; Vaccari, A.; Bugani, S.; Jaroszewicz, J.; Landi, E. Insights into the macroporosity of freeze-cast hierarchical geopolymers. RSC Adv. 2016, 6, 24635–24644. [Google Scholar] [CrossRef]
- Papa, E.; Mor, M.; Natali Murri, A.; Landi, E.; Medri, V. Ice-templated geopolymer beads for dye removal. J. Colloid Interface Sci. 2020, 15, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Franchin, G.; Scanferla, P.; Zeffiro, L.; Elsayed, H.; Baliello, A.; Giacomello, G.; Pasetto, M.; Colombo, P. Direct ink writing of geopolymeric inks. J. Eur. Ceram. Soc. 2017, 37, 2481–2489. [Google Scholar] [CrossRef]
- Medri, V.; Papa, E.; Lizion, J.; Landi, E. Metakaolin-based geopolymer beads: Production methods and characterization. J. Clean. Prod. 2020, 244, 118844. [Google Scholar] [CrossRef]
- Medri, V.; Papa, E.; Mor, M.; Vaccari, A.; Natali Murri, A.; Piotte, L.; Melandri, C.; Landi, E. Mechanical strength and cationic dye adsorption ability of metakaolin-based geopolymer spheres. Appl. Clay Sci. 2020, 193, 105678. [Google Scholar] [CrossRef]
- Tang, Q.; Ge, Y.-Y.; Wang, K.-T.; He, Y.; Cui, X.-M. Preparation of porous metakaolin-based inorganic polymer spheres as an adsorbent. Mater. Design 2015, 88, 1244–1249. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, C.; Qiao, Y.; Wang, X.; Jia, D.; Li, H.; Colombo, P. Porous geopolymer composites: A review. Compos.-A Appl. Sci. Manuf. 2021, 150, 106629. [Google Scholar] [CrossRef]
- Degefu, D.M.; Liao, Z.; Berardi, U.; Doan, H. Salient parameters affecting the performance of foamed geopolymers as sustainable insulating materials. Constr. Build. Mater. 2021, 313, 125400. [Google Scholar] [CrossRef]
- Dhasindrakrishna, K.; Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. Progress, current thinking and challenges in geopolymer foam concrete technology. Cement Concrete Comp. 2021, 116, 103886. [Google Scholar] [CrossRef]
- Bai, C.Y.; Franchin, G.; Elsayed, H.; Conte, A.; Colombo, P. High strength metakaolin-based geopolymer foams with variable macroporous structure. J. Eur. Ceram. Soc. 2016, 36, 4243–4249. [Google Scholar] [CrossRef]
- Muath, A.; Syeda, T. Experimental Measurements of Permeability of Open Foam. In Proceedings of the 5th international Conference of Fluid Flow, Heat and Mass Transfer (FFHMT’18), Niagara Falls, ON, Canada, 7–9 June 2018; p. 186. [Google Scholar] [CrossRef]
- Stauffer, D.; Aharony, A. Introduction to Percolation Theory: Second Edition, 2nd ed.; Taylor & Francis: Abingdon, UK, 1992. [Google Scholar] [CrossRef]
- Kohout, J.; Koutník, P. Effect of Filler Type on the Thermo-Mechanical Properties of Metakaolinite-Based Geopolymer Composites. Materials 2020, 13, 2395. [Google Scholar] [CrossRef] [PubMed]
- Musil, S.S.; Kriven, W.M. In Situ Mechanical Properties of Chamotte Particulate Reinforced, Potassium Geopolymer. J. Am. Ceram. Soc. 2014, 97, 907–915. [Google Scholar] [CrossRef]
- Sarkar, M.; Dana, K.; Das, S. Microstructural and phase evolution in metakaolin geopolymers with different activators and added aluminosilicate fillers. J. Mol. Struct. 2015, 1098, 110–118. [Google Scholar] [CrossRef]
- Available online: https://imagej.nih.gov/ij/docs/menus/analyze.html (accessed on 21 December 2021).
- Boumaza, A.; Djelloul, A.; Guerrab, F. Specific signatures of α-alumina powders prepared by calcination of boehmite or gibbsite. Powder Technol. 2010, 201, 177–180. [Google Scholar] [CrossRef]
- Papa, E.; Medri, V.; Kpogbemabou, D.; Morinière, V.; Laumonier, J.; Vaccari, A.; Rossignol, S. Porosity and insulating properties of silica-fume based foams. Energy Build. 2016, 131, 223–232. [Google Scholar] [CrossRef]
- Prud’homme, E.; Michaud, P.; Joussein, E.; Clacens, J.M.; Rossignol, S. Role of alkaline cations and water content on geomaterial foams: Monitoring during formation. J. Non-Cryst. Solids 2011, 357, 1270–1278. [Google Scholar] [CrossRef]
- Tchakoute Kouamo, H.; Elimbi, A.; Mbey, J.A.; Ngally Sabouang, C.J.; Njopwouo, D. The effect of adding alumina-oxide to metakaolin and volcanic ash on geopolymer products: A comparative study. Constr. Build. Mater. 2012, 35, 960–969. [Google Scholar] [CrossRef]
- Gouny, F.; Fouchal, F.; Maillard, P.; Rossignol, S. A geopolymer mortar for wood and earth structures. Constr. Build. Mater. 2012, 36, 188–195. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, S.; Zongjin, L. Composition design and microstructural characterization of calcined kaolin-based geopolymer cement. Appl. Clay Sci. 2010, 47, 271–275. [Google Scholar]
- Medri, V.; Ruffini, A. The influence of process parameters on in situ inorganic foaming of alkali-bonded SiC based foams. Ceram. Int. 2012, 38, 3351–3359. [Google Scholar] [CrossRef]
- Park, S.; Pour-Ghaz, M. What is the role of water in the geopolymerization of metakaolin? Constr. Build. Mater. 2018, 182, 360–370. [Google Scholar] [CrossRef]
- Marzzacco, C.J. The Enthalpy of Decomposition of Hydrogen Peroxide: A General Chemistry Calorimetry Experiment. J. Chem. Educ. 1999, 76, 1517. [Google Scholar] [CrossRef]
- Mo, B.; Zhu, H.; Cui, X.; He, Y.; Gong, S. Effect of curing temperature on geopolymerization of metakaolin-based geopolymers. Appl. Clay Sci. 2014, 99, 144–148. [Google Scholar] [CrossRef]
- Muñiz-Villarreal, M.S.; Manzano-Ramírez, A.; Sampieri-Bulbarela, S.; Ramón Gasca-Tirado, J.; Reyes-Araiza, J.L.; Rubio-Ávalos, J.C.; Pérez-Bueno, J.J.; Apatiga, L.M.; Zaldivar-Cadena, A.; Amigó-Borrás, V. The effect of temperature on the geopolymerization process of a metakaolin-based geopolymer. Mater. Lett. 2011, 65, 995–998. [Google Scholar] [CrossRef]
- Cilla, M.; Morelli, M.; Colombo, P. Effect of process parameters on the physical properties of porous geopolymers obtained by gelcasting. Ceram. Int. 2014, 40, 13585–13590. [Google Scholar] [CrossRef]
- Innocentini, M.D.M.; Pandolfelli, V.C. Issues concerning the evaluation of permeability in refractory concretes with Darcy and Forchheimer equations. Cerâmica 1999, 45, 61–67. [Google Scholar] [CrossRef]


| Foam Code | Blowing Agent (wt.%) | Liquid/Solid Ratio |
|---|---|---|
| G-Si | Silicon—0.05 | 0.42 |
| G-Al2O3-Si | Silicon—0.03 | 0.51 |
| G-3HP | Hydrogen peroxide—3 | 0.46 |
| G-5HP | Hydrogen peroxide—5 | 0.49 |
| Foam Code | True D (g cm−3) | Bulk D (g cm−3) | Total Porosity (%) | Expansion (%) | Porosity by Hg * (%) | Total Pore Volume * (mm3 g−1) | Modal Pore Ø * (µm) | S.S.A. (m2 g−1) |
|---|---|---|---|---|---|---|---|---|
| G-Si | 2.22 | 0.66 | 70 | 79 | 41 | 338 | 0.01 | 94 |
| G-Al2O3-Si | 2.35 | 0.69 | 71 | 57 | 49 | 418 | 0.02 | 65 |
| G-3HP | 2.13 | 0.35 | 84 | 153 | 52 | 484 | 0.02 | 47 |
| G-5HP | 2.16 | 0.34 | 84 | 164 | 52 | 449 | 0.03 | 81 |
| Foam Code | k1 (m2) | k2 (m) |
|---|---|---|
| G-Si | 5.94 × 10−13 | 2.29 × 10−10 |
| G-Al2O3-Si | 1.78 × 10−11 | 5.73 × 10−9 |
| G-3HP | 2.04 × 10−11 | 1.15 × 10−7 |
| G-5HP | 3.03 × 10−10 | 1.24 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papa, E.; Landi, E.; Miccio, F.; Medri, V. K2O-Metakaolin-Based Geopolymer Foams: Production, Porosity Characterization and Permeability Test. Materials 2022, 15, 1008. https://doi.org/10.3390/ma15031008
Papa E, Landi E, Miccio F, Medri V. K2O-Metakaolin-Based Geopolymer Foams: Production, Porosity Characterization and Permeability Test. Materials. 2022; 15(3):1008. https://doi.org/10.3390/ma15031008
Chicago/Turabian StylePapa, Elettra, Elena Landi, Francesco Miccio, and Valentina Medri. 2022. "K2O-Metakaolin-Based Geopolymer Foams: Production, Porosity Characterization and Permeability Test" Materials 15, no. 3: 1008. https://doi.org/10.3390/ma15031008
APA StylePapa, E., Landi, E., Miccio, F., & Medri, V. (2022). K2O-Metakaolin-Based Geopolymer Foams: Production, Porosity Characterization and Permeability Test. Materials, 15(3), 1008. https://doi.org/10.3390/ma15031008







