Achieving Persistent Luminescence Performance Based on the Cation-Tunable Trap Distribution
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Optimization of PersL Decay Rate by Doping Si4+ Ions
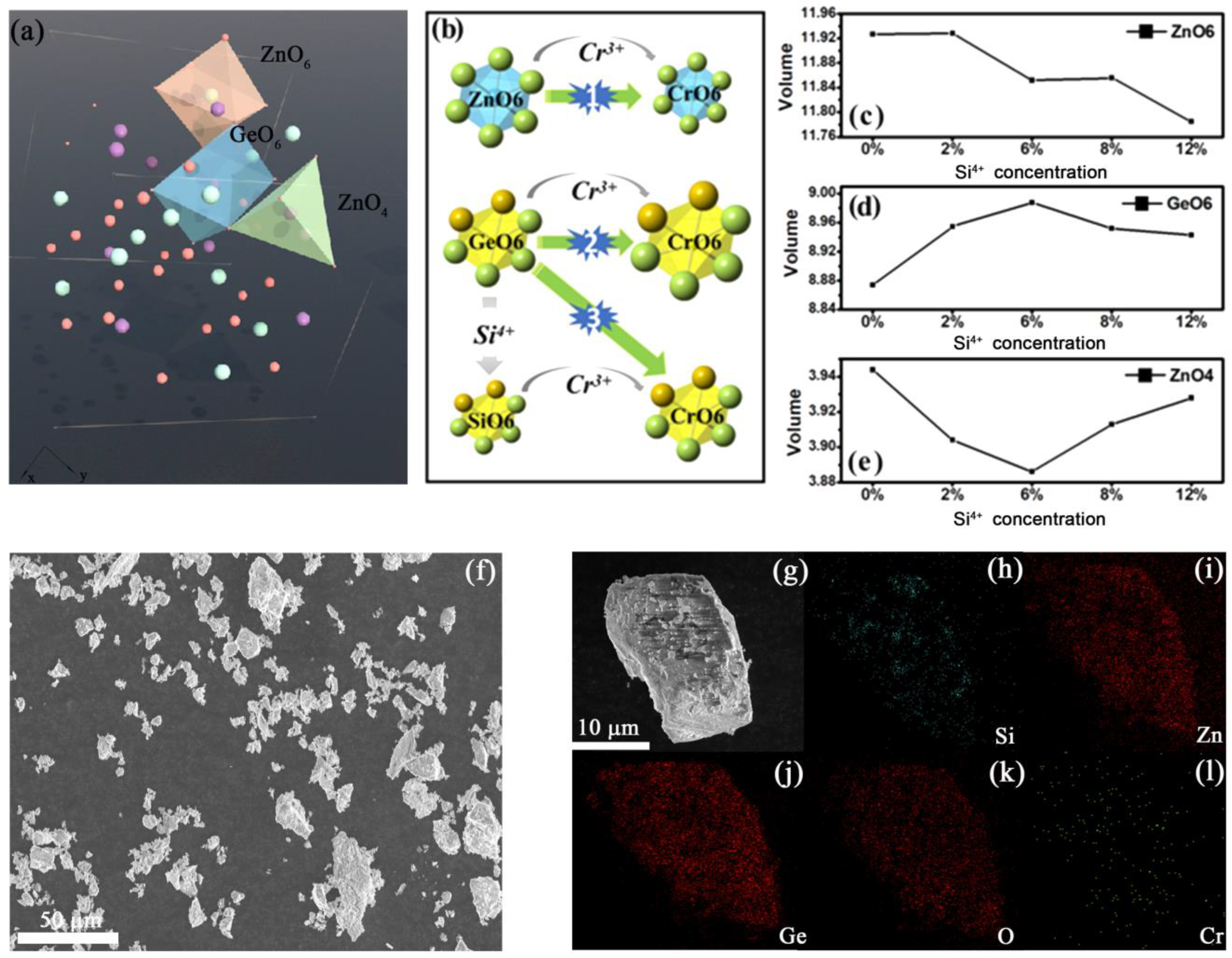
3.1.1. Phase Information of Li2ZnGe3-ySiyO8: 0.8%Cr3+
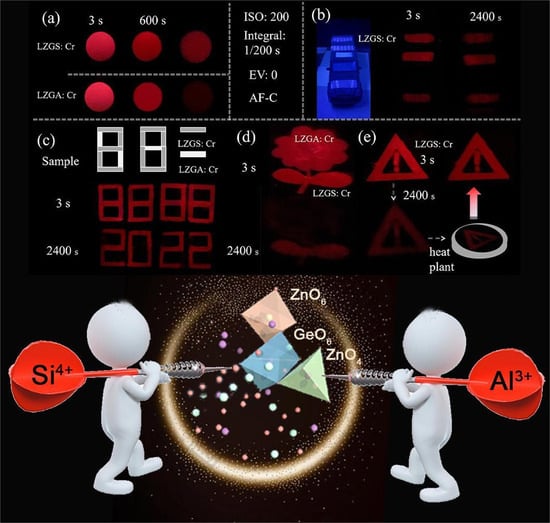
3.1.2. PersL Properties of Li2ZnGe3-ySiyO8: 0.8%Cr3+
3.2. Optimization of PersL Emission Intensity by Doping Al3+ Ions
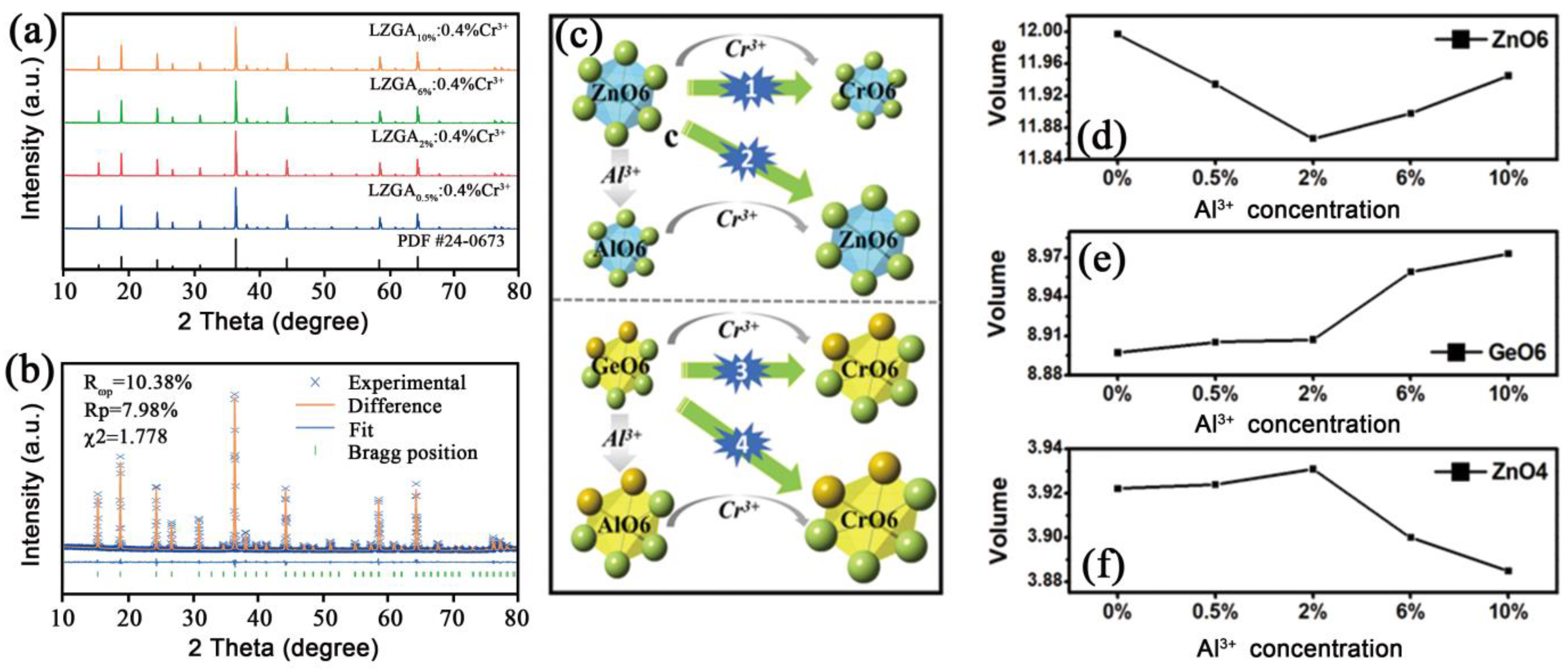
3.2.1. Phase Information of Li2Zn1-zGe3-zAl2zO8: 0.4%Cr3+
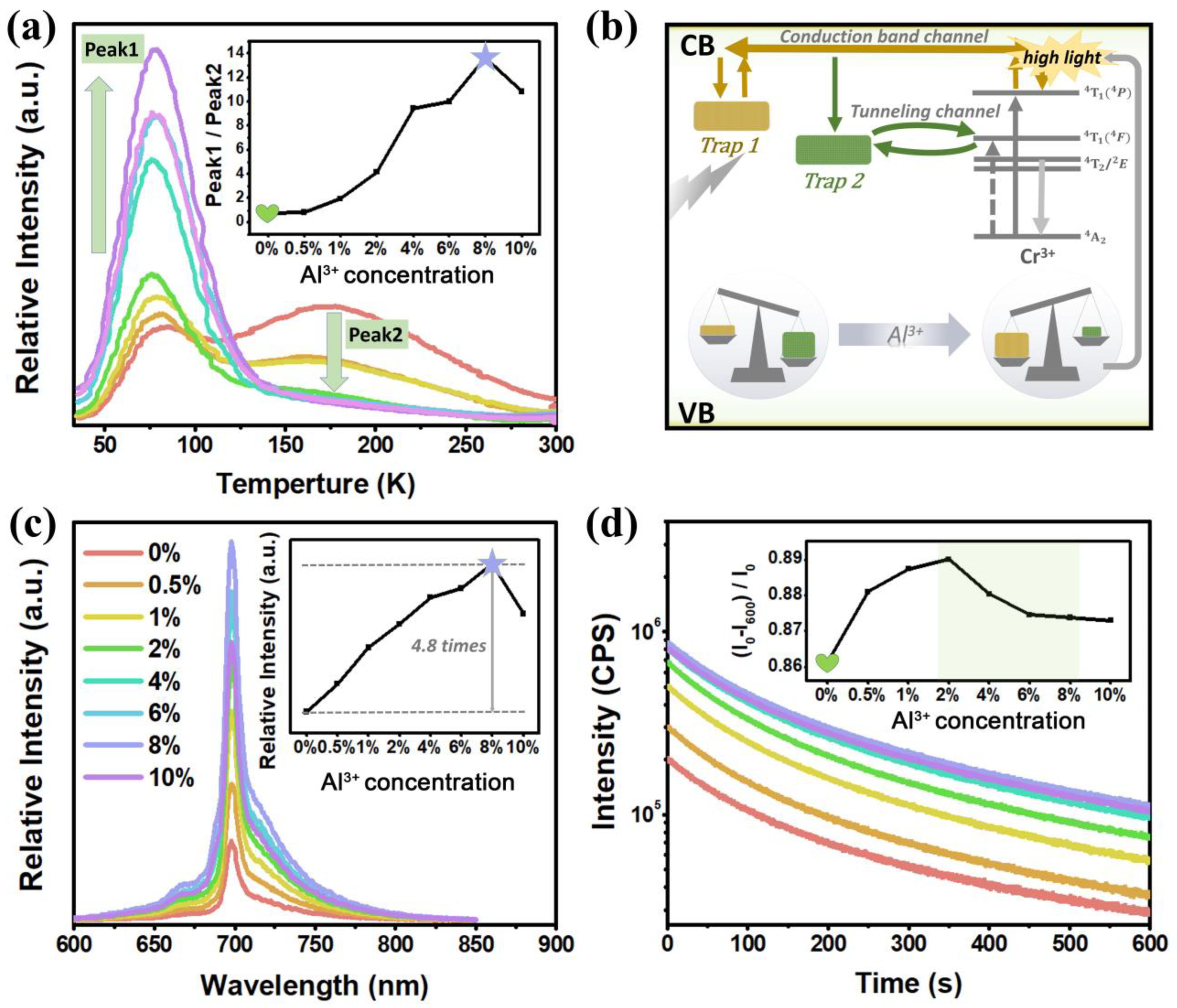
3.2.2. PersL Properties of Li2Zn1-zGe3-zAl2zO8: 0.4%Cr3+
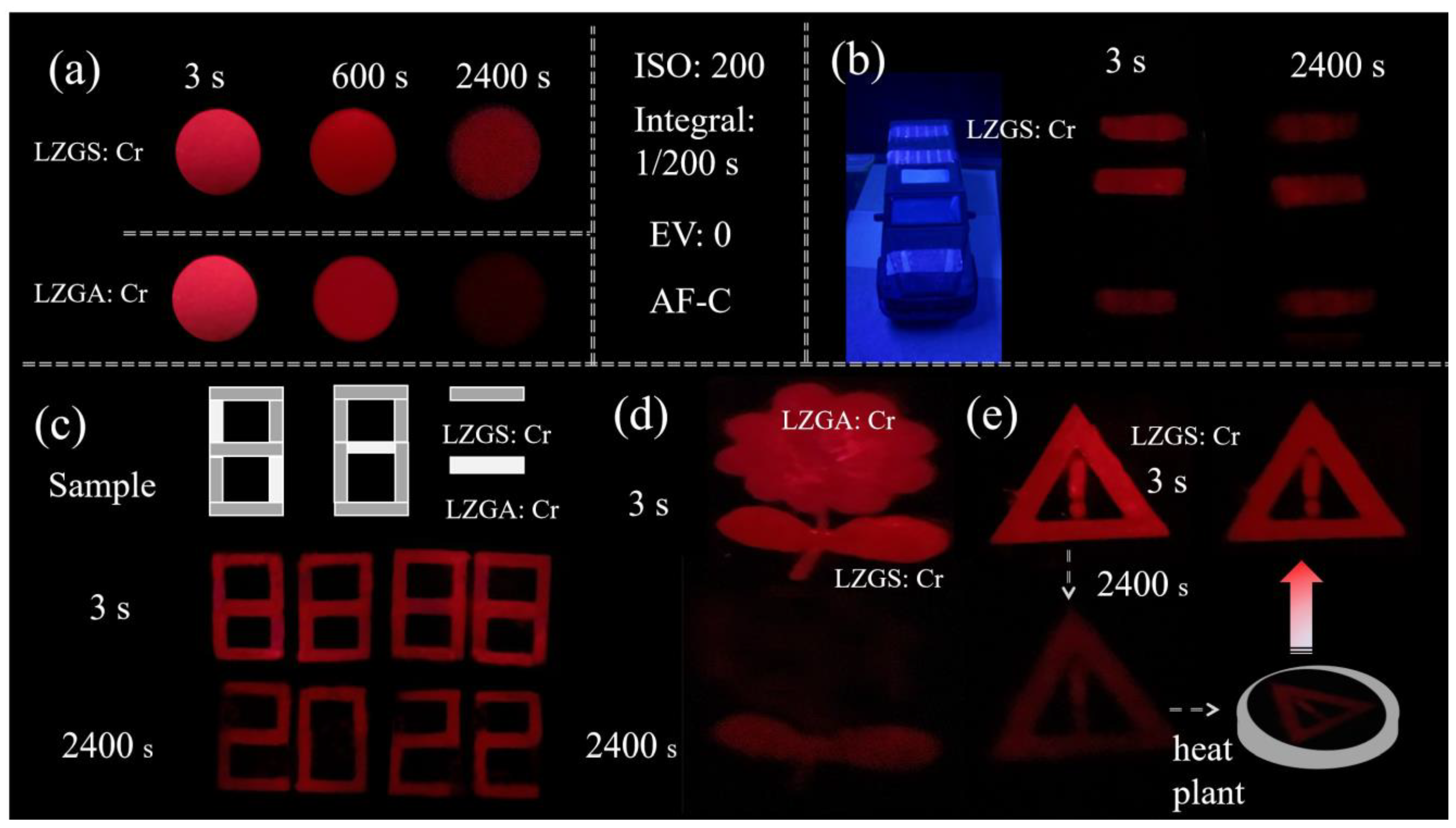
3.3. Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dincer, C.; Bruch, R.; Costa-Rama, E.; Fernández-Abedul, M.T.; Merkoçi, A.; Manz, A.; Urban, G.A.; Güder, F. Disposable sensors in diagnostics, food, and environmental monitoring. Adv. Mater. 2019, 31, 1806739. [Google Scholar] [CrossRef]
- Liang, Y.-J.; Liu, F.; Chen, Y.-F.; Wang, X.-J.; Sun, K.-N.; Pan, Z. New function of the Yb3+ ion as an efficient emitter of persistent luminescence in the short-wave infrared. Light-Sci. Appl. 2016, 5, e16124. [Google Scholar] [CrossRef] [PubMed]
- Tuong Ly, K.; Chen-Cheng, R.-W.; Lin, H.-W.; Shiau, Y.-J.; Liu, S.-H.; Chou, P.-T.; Tsao, C.-S.; Huang, Y.-C.; Chi, Y. Near-infrared organic light-emitting diodes with very high external quantum efficiency and radiance. Nat. Photon. 2017, 11, 63–68. [Google Scholar] [CrossRef]
- Gu, Y.; Guo, Z.; Yuan, W.; Kong, M.; Liu, Y.; Liu, Y.; Gao, Y.; Feng, W.; Wang, F.; Zhou, J. High-sensitivity imaging of time-domain near-infrared light transducer. Nat. Photon. 2019, 13, 525–531. [Google Scholar] [CrossRef]
- Basore, E.T.; Wu, H.; Xiao, W.; Zheng, G.; Liu, X.; Qiu, J. High-Power Broadband NIR LEDs Enabled by Highly Efficient Blue-to-NIR Conversion. Adv. Opt. Mater. 2021, 9, 2001660. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, R.; Li, H.; Lin, Z.; Hou, D.; Guo, Y.; Song, J.; Song, C.; Lin, Z.; Zhang, W. Triple-Mode Emissions with Invisible Near-Infrared After-Glow from Cr3+-Doped Zinc Aluminum Germanium Nanoparticles for Advanced Anti-Counterfeiting Applications. Small 2020, 16, 2003121. [Google Scholar] [CrossRef] [PubMed]
- Basore, E.T.; Xiao, W.; Liu, X.; Wu, J.; Qiu, J. Broadband near-infrared garnet phosphors with near-unity internal quantum efficiency. Adv. Opt. Mater. 2020, 8, 2000296. [Google Scholar] [CrossRef]
- Zhong, J.; Zhuo, Y.; Du, F.; Zhang, H.; Zhao, W.; Brgoch, J. Efficient and Tunable Luminescence in Ga2–xInxO3: Cr3+ for Near-Infrared Imaging. ACS Appl. Mater. Int. 2021, 13, 31835–31842. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, Y.; Yuan, C.; Leniec, G.; Miao, L.; Sun, P.; Liu, Z.; Luo, Z.; Dong, R.; Jiang, J. Thermally stable CaLu2Mg2Si3O12: Cr3+ phosphors for NIR LEDs. Adv. Opt. Mater. 2021, 9, 2100388. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Seto, T.; Wang, Y. A high efficiency trivalent chromium-doped near-infrared-emitting phosphor and its NIR spectroscopy application. ACS Sustain. Chem. Eng. 2021, 9, 3145–3156. [Google Scholar] [CrossRef]
- Kabe, R.; Adachi, C. Organic long persistent luminescence. Nature 2017, 550, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gecevicius, M.; Qiu, J. Long persistent phosphors—From fundamentals to applications. Chem. Soc. Rev. 2016, 45, 2090–2136. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Mao, N.; Song, Z.; Liu, Q. UV-Red Light-Chargeable Near-Infrared-Persistent Phosphors and Their Applications. ACS Appl. Mater. Interfaces 2021, 14, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qiu, Z.; Mo, Y.; Zhang, H.; Lian, H.; Zhang, J.; Lian, S. Self-reduction-induced BaMgP2O7: Eu2+/3+: A multi-stimuli-responsive phosphor for X-ray detection, anti-counterfeiting and optical thermometry. Dalton Trans. 2022, 51, 6622–6630. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Wang, L.; Lv, Y.; Zhou, T.L.; Xie, R.J. Optical data storage and multicolor emission readout on flexible films using deep-trap persistent luminescence materials. Adv. Funct. Mater. 2018, 28, 1705769. [Google Scholar] [CrossRef]
- Le Masne de Chermont, Q.; Chanéac, C.; Seguin, J.; Pellé, F.; Maîtrejean, S.; Jolivet, J.-P.; Gourier, D.; Bessodes, M.; Scherman, D. Nanoprobes with near-infrared persistent luminescence for in vivo imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 9266–9271. [Google Scholar] [CrossRef]
- Wu, B.-Y.; Wang, H.-F.; Chen, J.-T.; Yan, X.-P. Fluorescence resonance energy transfer inhibition assay for α-fetoprotein excreted during cancer cell growth using functionalized persistent luminescence nanoparticles. J. Am. Chem. Soc. 2011, 133, 686–688. [Google Scholar] [CrossRef]
- Yamamoto, H.; Matsuzawa, T. Mechanism of long phosphorescence of SrAl2O4: Eu2+, Dy3+ and CaAl2O4: Eu2+, Nd3+. J. Lumin. 1997, 72, 287–289. [Google Scholar] [CrossRef]
- Matsuzawa, T.; Aoki, Y.; Takeuchi, N.; Murayama, Y. A new long phosphorescent phosphor with high brightness, SrAl2O4: Eu2+, Dy3+. J. Electrochem. Soc. 1996, 143, 2670. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z. Characterization of Y2O2S: Eu3+, Mg2+, Ti4+ long-lasting phosphor synthesized by flux method. J. Rare Earths 2006, 24, 25–28. [Google Scholar] [CrossRef]
- Allix, M.; Chenu, S.; Véron, E.; Poumeyrol, T.; Kouadri-Boudjelthia, E.A.; Alahrache, S.; Porcher, F.; Massiot, D.; Fayon, F. Considerable improvement of long-persistent luminescence in germanium and tin substituted ZnGa2O4. Chem. Mater. 2013, 25, 1600–1606. [Google Scholar] [CrossRef]
- Wei, J.; Chen, L.; Zhang, W.; Yang, Y.; Li, Z. The orange–red persistent luminescence of Ba1−xCaxS: Yb2+. J. Lumin. 2017, 181, 427–432. [Google Scholar] [CrossRef]
- Xu, H.; Chen, G. Enhancing near infrared persistent luminescence from Cr3+-activated zinc gallogermanate powders through Ca2+ doping. Opt. Mater. Express 2017, 7, 2783–2792. [Google Scholar] [CrossRef]
- Yan, W.; Liu, F.; Lu, Y.-Y.; Wang, X.-J.; Yin, M.; Pan, Z. Near infrared long-persistent phosphorescence in La3Ga5 GeO14: Cr3+ phosphor. Opt. Express 2010, 18, 20215–20221. [Google Scholar] [CrossRef]
- Zou, Z.; Zhou, H.; Wang, W.; Zhang, J.; Cao, C.; Zhang, T.; Ci, Z.; Zhao, Z.; Wang, Y. A vivid example of turning waste into treasure: Persistent luminescence of Ca2Ga2 (Si, Ge) O7: Pr3+, Yb3+ phosphor tailored by band gap engineering. J. Mater. Chem. C 2016, 4, 10026–10031. [Google Scholar] [CrossRef]
- Xiahou, J.; Zhu, Q.; Zhu, L.; Li, S.; Li, J.-G. Local structure regulation in near-infrared persistent phosphor of ZnGa2O4: Cr3+ to fabricate natural-light rechargeable optical thermometer. ACS Appl. Electr. Mater. 2021, 3, 3789–3803. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Qin, X.; Chen, R.; Wu, D.; Liu, S.; Qiu, J. Dual mode NIR long persistent phosphorescence and NIR-to-NIR Stokes luminescence in La3Ga5GeO14: Cr3+, Nd3+ phosphor. J. Alloys Compd. 2015, 649, 62–66. [Google Scholar] [CrossRef]
- Lin, Y.; Tang, Z.; Zhang, Z. Preparation of long-afterglow Sr4Al14O25-based luminescent material and its optical properties. Mater. Lett. 2001, 51, 14–18. [Google Scholar] [CrossRef]
- Lin, Y.; Tang, Z.; Zhang, Z.; Nan, C.W. Anomalous luminescence in Sr4Al14O25: Eu, Dy phosphors. Appl. Phys. Lett. 2002, 81, 996–998. [Google Scholar] [CrossRef]
- Zuo, Z.-H.; Peng, Y.-Y.; Li, J.; Wang, X.; Liu, Z.-Q.; Chen, Y. Thermal-responsive dynamic color-tunable persistent luminescence from green to deep red for advanced anti-counterfeiting. Chem. Eng. J. 2022, 2022, 136976. [Google Scholar] [CrossRef]
- Katayama, Y. Red to near-infrared persistent luminescence in transition metal ion activated phosphors. J. Ceram. Soc. Jpn. 2017, 125, 793–798. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Z.; Wang, X.; Suo, H.; Cao, L.; Yao, Y.; Zheng, M.; Cui, J.; Yang, Z.; Li, P. Competitive Cr3+ occupation in persistent phosphors toward tunable traps distribution for dynamic anti-counterfeiting. J. Am. Ceram. Soc. 2021, 104, 5224–5234. [Google Scholar] [CrossRef]
- Gartia, R.; Chandrasekhar, N. Physical basis of persistent luminescence: The case of europium doped Ca1−xSrxS. J. Alloys Compd. 2016, 683, 157–163. [Google Scholar] [CrossRef]
- Jin, Y.; Hu, Y.; Yuan, L.; Chen, L.; Wu, H.; Ju, G.; Duan, H.; Mu, Z. Multifunctional near-infrared emitting Cr3+-doped Mg4Ga8 Ge2O20 particles with long persistent and photostimulated persistent luminescence, and photochromic properties. J. Mater. Chem. C 2016, 4, 6614–6625. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, S.; Li, Y.; Sharafudeen, K.; Ma, Z.; Dong, G.; Peng, M.; Qiu, J. Long persistent and photo-stimulated luminescence in Cr3+-doped Zn–Ga–Sn–O phosphors for deep and reproducible tissue imaging. J. Mater. Chem. C 2014, 2, 2657–2663. [Google Scholar] [CrossRef]
- Pathak, N.; Ghosh, P.S.; Saxena, S.; Dutta, D.; Yadav, A.K.; Bhattacharyya, D.; Jha, S.N.; Kadam, R.M. Exploring defect-induced emission in ZnAl2O4: An exceptional color-tunable phosphor material with diverse lifetimes. Inorg. Chem. 2018, 57, 3963–3982. [Google Scholar] [CrossRef] [PubMed]
- Cui, E.; Meng, Q.; Ge, C.; Yu, G.; Hou, G.; Xu, N.; Zhang, F.; Wu, Y. The roles of surface oxygen vacancy over Mg4Ta2O9-x photocatalyst in enhancing visible-light photocatalytic hydrogen evolution performance. Catal. Commun. 2018, 103, 29–33. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Li, R.; Zhang, M.; Li, P.; Wang, Z. Achieving Persistent Luminescence Performance Based on the Cation-Tunable Trap Distribution. Materials 2022, 15, 9083. https://doi.org/10.3390/ma15249083
Wang T, Li R, Zhang M, Li P, Wang Z. Achieving Persistent Luminescence Performance Based on the Cation-Tunable Trap Distribution. Materials. 2022; 15(24):9083. https://doi.org/10.3390/ma15249083
Chicago/Turabian StyleWang, Tao, Rui Li, Mengya Zhang, Panlai Li, and Zhijun Wang. 2022. "Achieving Persistent Luminescence Performance Based on the Cation-Tunable Trap Distribution" Materials 15, no. 24: 9083. https://doi.org/10.3390/ma15249083
APA StyleWang, T., Li, R., Zhang, M., Li, P., & Wang, Z. (2022). Achieving Persistent Luminescence Performance Based on the Cation-Tunable Trap Distribution. Materials, 15(24), 9083. https://doi.org/10.3390/ma15249083