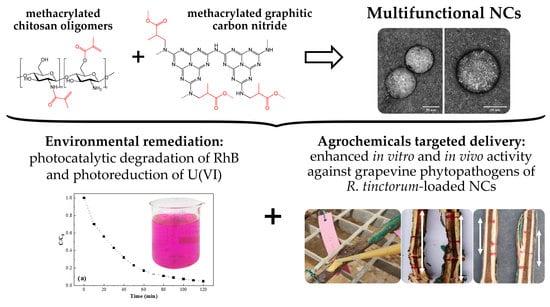
Multifunctional Nanocarriers Based on Chitosan Oligomers and Graphitic Carbon Nitride Assembly
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Fungal and Bacterial Isolates
2.3. Chitosan Oligomers and g-C3N4 Preparation
2.4. Plant Material and Preparation of Extract
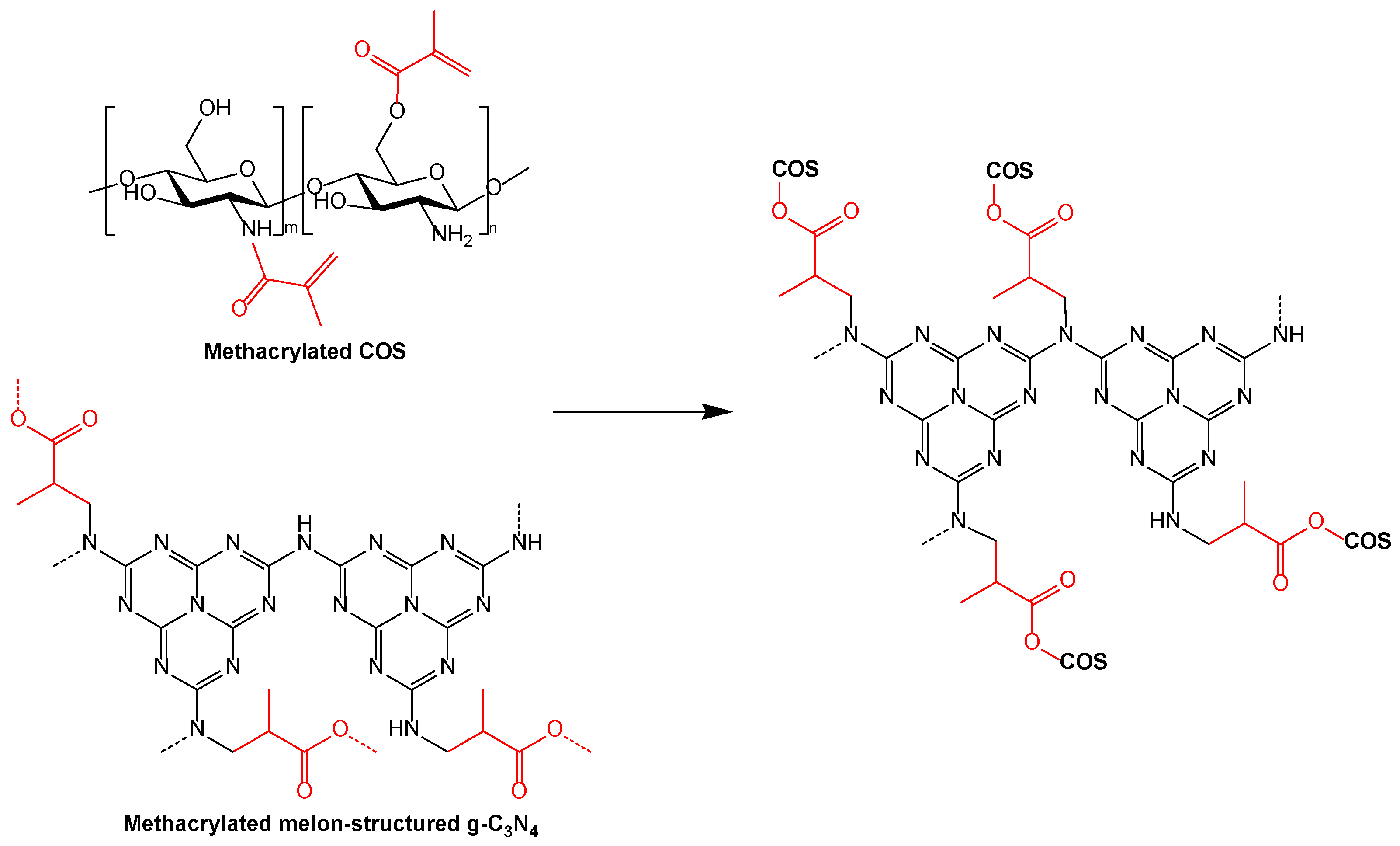
2.5. Synthesis of the g-C3N4-MA-COS Nanocarriers
2.6. Encapsulation and Release of R. tinctorum Extract
2.7. Characterization
2.8. Photocatalytic Activity
2.9. In Vitro Antimicrobial Activity
2.10. In Planta Bioassays
2.11. Statistical Analyses
3. Results
3.1. Nanocarriers Characterization
3.1.1. Elemental Analysis
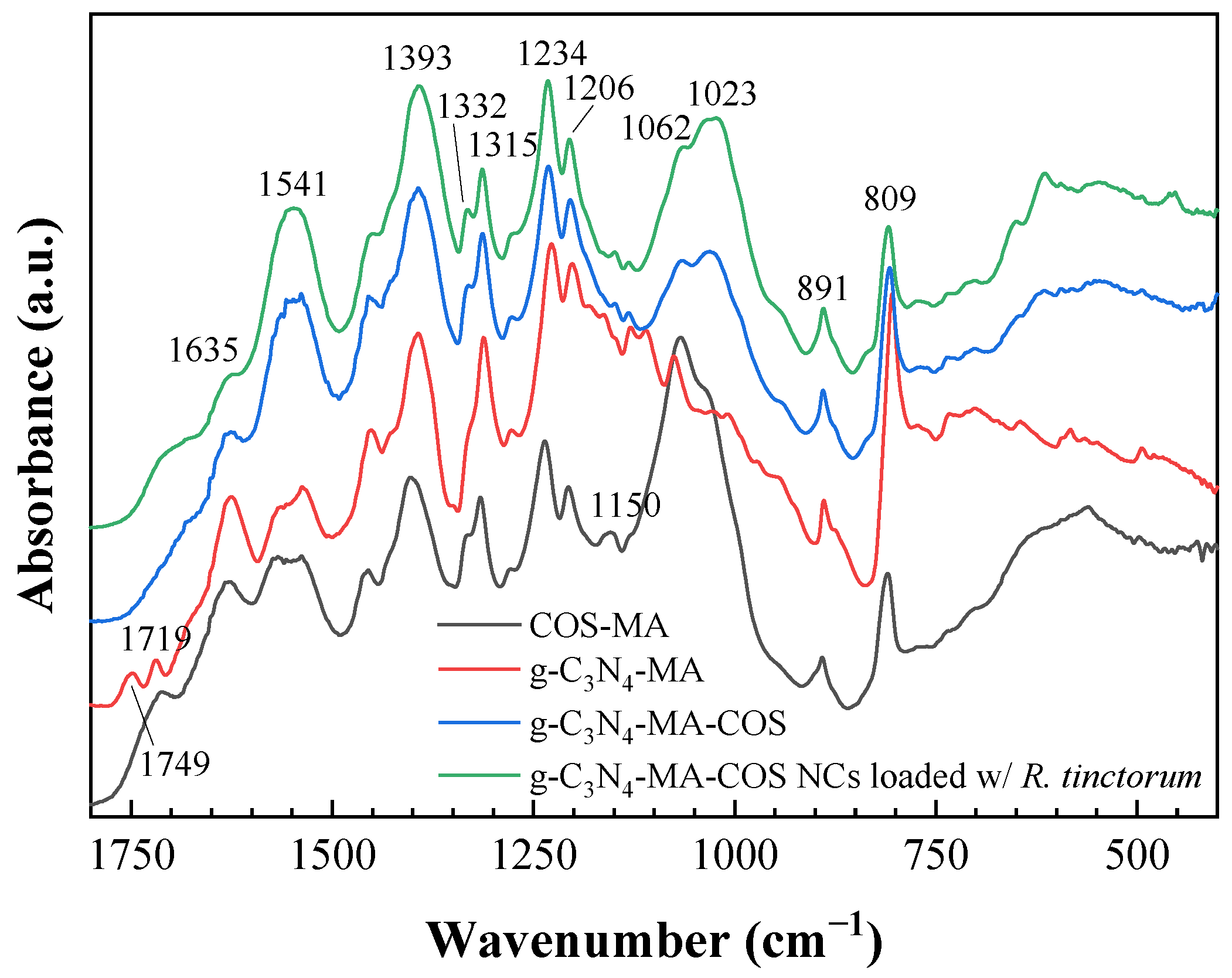
3.1.2. Vibrational Characterization
3.1.3. Morphology
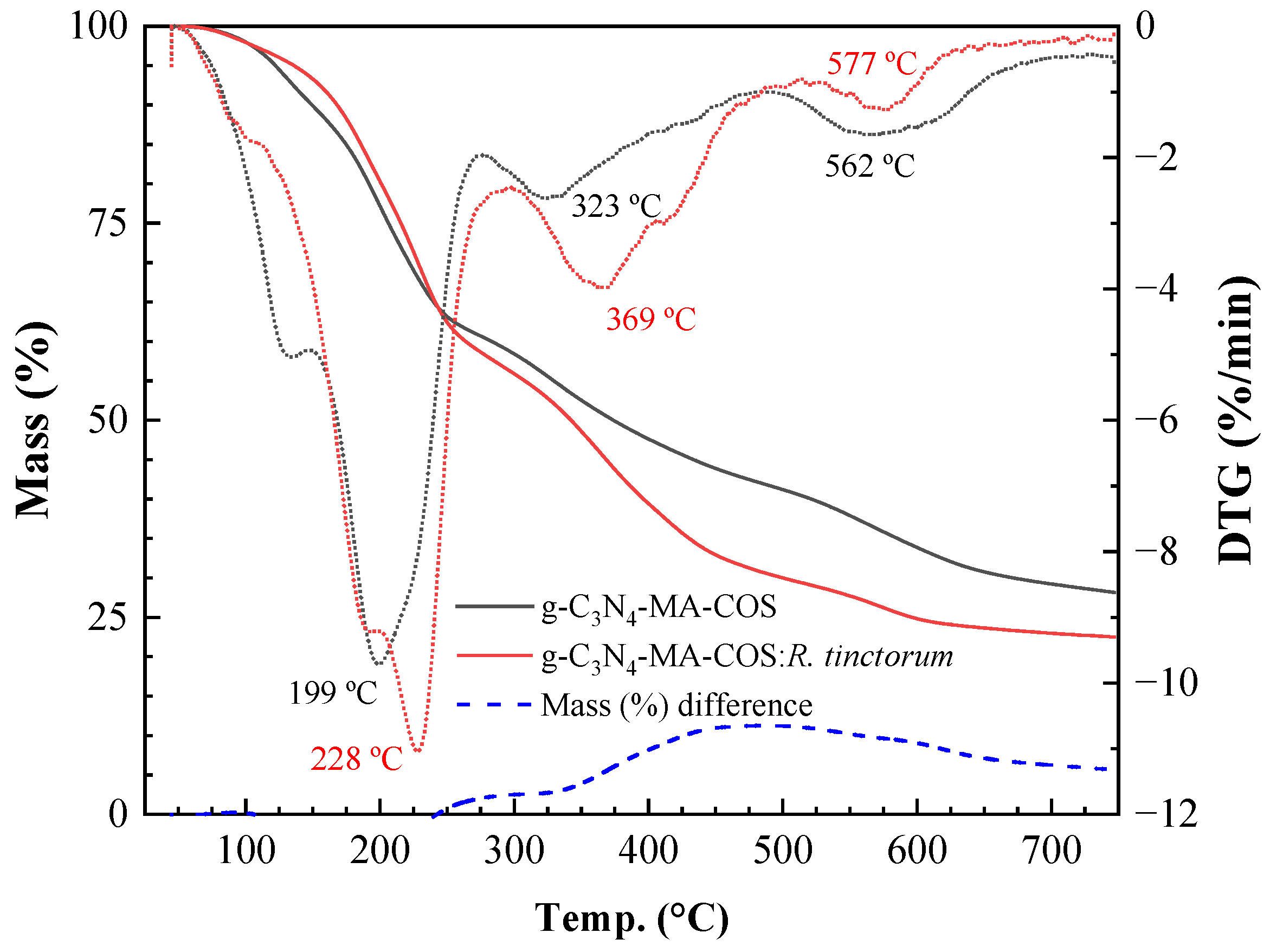
3.1.4. Thermal Analysis
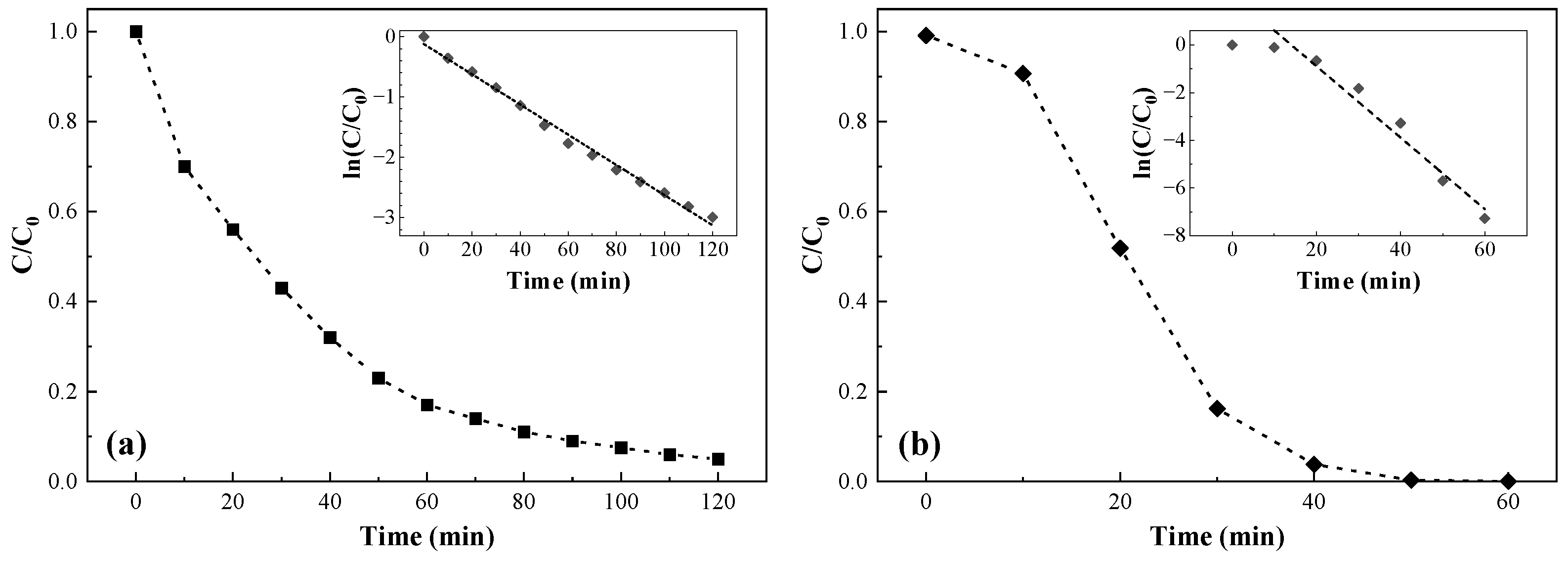
3.2. Photocatalytic Activity
3.2.1. Rhodamine B Degradation
3.2.2. Uranium(VI) Reduction
3.3. Encapsulation and Release Efficiencies
3.4. Antimicrobial Activity
3.4.1. Antibacterial Activity
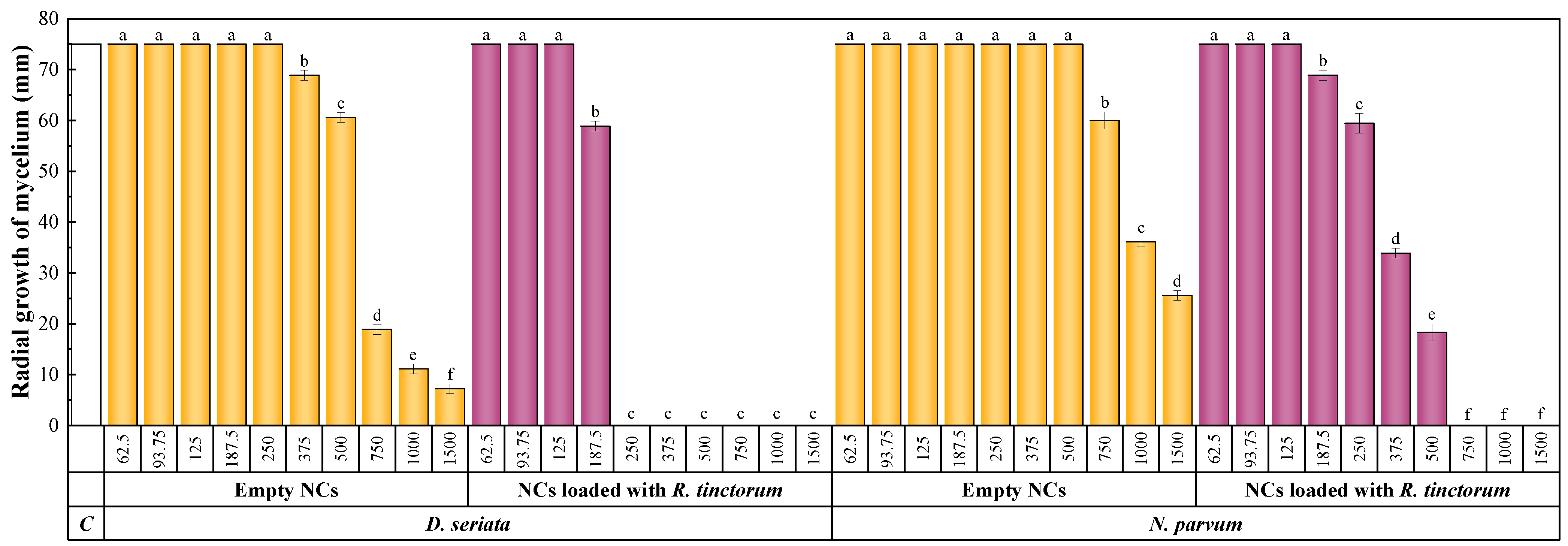
3.4.2. Antifungal Activity
3.4.3. In Planta Bioassays
4. Discussion
4.1. On the Nanocarriers Structure and the Encapsulation Mechanism
4.2. Photocatalytic Activity
4.3. On the Antimicrobial Activity
4.4. Mechanism of Action
4.5. Limitations of the Study and Prospects of Nanocarriers for Agrochemical Delivery
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, A.; Aftab, S.; Nisar, J.; Ashiq, M.N.; Iftikhar, F.J. Nanocarriers for targeted drug delivery. J. Drug Deliv. Sci. Technol. 2021, 62, 102426. [Google Scholar] [CrossRef]
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale drug delivery systems: From Medicine to Agriculture. Front. Bioeng. Biotechnol. 2020, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Pinto, T.V.; Silva, C.A.; Siquenique, S.; Learmonth, D.A. Micro- and nanocarriers for encapsulation of biological plant protection agents: A systematic literature review. ACS Agric. Sci. Technol. 2022, 2, 838–857. [Google Scholar] [CrossRef]
- Machado, T.O.; Grabow, J.; Sayer, C.; de Araújo, P.H.H.; Ehrenhard, M.L.; Wurm, F.R. Biopolymer-based nanocarriers for sustained release of agrochemicals: A review on materials and social science perspectives for a sustainable future of agri- and horticulture. Adv. Colloid Interface Sci. 2022, 303, 102645. [Google Scholar] [CrossRef] [PubMed]
- Maluin, F.N.; Hussein, M.Z. Chitosan-based agronanochemicals as a sustainable alternative in crop protection. Molecules 2020, 25, 1611. [Google Scholar] [CrossRef]
- Jakhar, A.M.; Aziz, I.; Kaleri, A.R.; Hasnain, M.; Haider, G.; Ma, J.; Abideen, Z. Nano-fertilizers: A sustainable technology for improving crop nutrition and food security. NanoImpact 2022, 27, 100411. [Google Scholar] [CrossRef]
- Pal, P.; Pal, A.; Nakashima, K.; Yadav, B.K. Applications of chitosan in environmental remediation: A review. Chemosphere 2021, 266, 128934. [Google Scholar] [CrossRef]
- Benettayeb, A.; Ghosh, S.; Usman, M.; Seihoub, F.Z.; Sohoo, I.; Chia, C.H.; Sillanpää, M. Some well-known alginate and chitosan modifications used in adsorption: A review. Water 2022, 14, 1353. [Google Scholar] [CrossRef]
- Li, P.-W.; Wang, G.; Yang, Z.-M.; Duan, W.; Peng, Z.; Kong, L.-X.; Wang, Q.-H. Development of drug-loaded chitosan–vanillin nanoparticles and its cytotoxicity against HT-29 cells. Drug Deliv. 2014, 23, 30–35. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, Y.; Lapitsky, Y. Factors affecting the stability of chitosan/tripolyphosphate micro- and nanogels: Resolving the opposing findings. J. Mater. Chem. B 2015, 3, 5957–5970. [Google Scholar] [CrossRef]
- Samani, S.; Bonakdar, S.; Farzin, A.; Hadjati, J.; Azami, M. A facile way to synthesize a photocrosslinkable methacrylated chitosan hydrogel for biomedical applications. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 730–741. [Google Scholar] [CrossRef]
- Gruppuso, M.; Iorio, F.; Turco, G.; Marsich, E.; Porrelli, D. Hyaluronic acid/lactose-modified chitosan electrospun wound dressings–Crosslinking and stability criticalities. Carbohydr. Polym. 2022, 288, 119375. [Google Scholar] [CrossRef]
- D’Amora, U.; Ronca, A.; Raucci, M.G.; Lin, H.; Soriente, A.; Fan, Y.; Zhang, X.; Ambrosio, L. Bioactive composites based on double network approach with tailored mechanical, physico-chemical, and biological features. J. Biomed. Mater. Res. Part A 2018, 106, 3079–3089. [Google Scholar] [CrossRef]
- Yu, L.M.Y.; Kazazian, K.; Shoichet, M.S. Peptide surface modification of methacrylamide chitosan for neural tissue engineering applications. J. Biomed. Mater. Res. Part A 2007, 82A, 243–255. [Google Scholar] [CrossRef]
- Machado, T.O.; Beckers, S.J.; Fischer, J.; Müller, B.; Sayer, C.; de Araújo, P.H.H.; Landfester, K.; Wurm, F.R. Bio-based lignin nanocarriers loaded with fungicides as a versatile platform for drug delivery in plants. Biomacromolecules 2020, 21, 2755–2763. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Langa-Lomba, N.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Santiago-Aliste, A.; Torres-Sánchez, S.; Martín-Ramos, P. Lignin–chitosan nanocarriers for the delivery of bioactive natural products against wood-decay phytopathogens. Agronomy 2022, 12, 461. [Google Scholar] [CrossRef]
- Rajabzadeh-Khosroshahi, M.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Navaei-Nigjeh, M.; Rasekh, B. Chitosan/agarose/graphitic carbon nitride nanocomposite as an efficient pH-sensitive drug delivery system for anticancer curcumin releasing. J. Drug Deliv. Sci. Technol. 2022, 74, 103443. [Google Scholar] [CrossRef]
- Yang, X.; Ye, Y.; Sun, J.; Li, Z.; Ping, J.; Sun, X. Recent advances in g-C3N4-based photocatalysts for pollutant degradation and bacterial disinfection: Design strategies, mechanisms, and applications. Small 2021, 18, 2105089. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Zhuang, L.; Hu, B.; Chen, J.; Liu, X.; Wang, X. Recent developments of doped g-C3N4 photocatalysts for the degradation of organic pollutants. Crit. Rev. Environ. Sci. Technol. 2020, 51, 751–790. [Google Scholar] [CrossRef]
- Gupta, B.; Gupta, A.K. Photocatalytic performance of 3D engineered chitosan hydrogels embedded with sulfur-doped C3N4/ZnO nanoparticles for Ciprofloxacin removal: Degradation and mechanistic pathways. Int. J. Biol. Macromol. 2022, 198, 87–100. [Google Scholar] [CrossRef]
- Ni, Y.; Nie, H.; Wang, J.; Lin, J.; Wang, Q.; Sun, J.; Zhang, W.; Wang, J. Enhanced functional properties of chitosan films incorporated with curcumin-loaded hollow graphitic carbon nitride nanoparticles for bananas preservation. Food Chem. 2022, 366, 130539. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz Baran, N.; Baran, T.; Çalışkan, M. Production of Pd nanoparticles embedded on micro-sized chitosan/graphitic carbon nitride hybrid spheres for treatment of environmental pollutants in aqueous medium. Ceram. Int. 2021, 47, 27736–27747. [Google Scholar] [CrossRef]
- Santos-Moriano, P.; Fernandez-Arrojo, L.; Mengibar, M.; Belmonte-Reche, E.; Peñalver, P.; Acosta, F.N.; Ballesteros, A.O.; Morales, J.C.; Kidibule, P.; Fernandez-Lobato, M.; et al. Enzymatic production of fully deacetylated chitooligosaccharides and their neuroprotective and anti-inflammatory properties. Biocatal. Biotransform. 2017, 36, 57–67. [Google Scholar] [CrossRef]
- Tian, M.; Tan, H.; Li, H.; You, C. Molecular weight dependence of structure and properties of chitosan oligomers. RSC Adv. 2015, 5, 69445–69452. [Google Scholar] [CrossRef]
- Dante, R.C.; Martín-Ramos, P.; Sánchez-Arévalo, F.M.; Huerta, L.; Bizarro, M.; Navas-Gracia, L.M.; Martín-Gil, J. Synthesis of crumpled nanosheets of polymeric carbon nitride from melamine cyanurate. J. Solid State Chem. 2013, 201, 153–163. [Google Scholar] [CrossRef]
- Langa-Lomba, N.; Sánchez-Hernández, E.; Buzón-Durán, L.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Martín-Ramos, P. Activity of anthracenediones and flavoring phenols in hydromethanolic extracts of Rubia tinctorum against grapevine phytopathogenic fungi. Plants 2021, 10, 1527. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Santiago-Aliste, A.; Martín-Gil, J.; Martín-Ramos, P. Nanomaterial Basado en el Autoensamblaje de g-C3N4 y Oligómeros de Quitosano, Proceso de Obtención y Usos. Patent P202230668, 20 July 2022. [Google Scholar]
- Fischer, J.; Beckers, S.J.; Yiamsawas, D.; Thines, E.; Landfester, K.; Wurm, F.R. Targeted drug delivery in plants: Enzyme-responsive lignin nanocarriers for the curative treatment of the worldwide grapevine trunk disease Esca. Adv. Sci. 2019, 6, 1802315. [Google Scholar] [CrossRef]
- Krizsán, K.; Szókán, G.; Toth, Z.A.; Hollósy, F.; László, M.; Khlafulla, A. HPLC analysis of anthraquinone derivatives in madder root (Rubia tinctorum) and its cell cultures. J. Liq. Chromatogr. Relat. Technol. 2006, 19, 2295–2314. [Google Scholar] [CrossRef]
- Tiunov, I.A.; Gorbachevskyy, M.V.; Kopitsyn, D.S.; Kotelev, M.S.; Ivanov, E.V.; Vinokurov, V.A.; Novikov, A.A. Synthesis of large uniform gold and core–shell gold–silver nanoparticles: Effect of temperature control. Russ. J. Phys. Chem. A 2016, 90, 152–157. [Google Scholar] [CrossRef]
- Dong, G.; Zhang, L. Porous structure dependent photoreactivity of graphitic carbon nitride under visible light. J. Mater. Chem. 2012, 22, 1160–1166. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Wang, W.; Ding, Z.; Geng, R.; Li, P.; Pan, D.; Liang, J.; Qin, H.; Fan, Q. Tunable mesoporous g-C3N4 nanosheets as a metal-free catalyst for enhanced visible-light-driven photocatalytic reduction of U(VI). Chem. Eng. J. 2020, 383, 123193. [Google Scholar] [CrossRef]
- CLSI Standard M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. 11th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; 112p.
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Cankaya, N. Grafting of chitosan: Structural, thermal and antimicrobial properties. J. Chem. Soc. Pak. 2019, 41, 240. [Google Scholar] [CrossRef]
- Alwin, E.; Kočí, K.; Wojcieszak, R.; Zieliński, M.; Edelmannová, M.; Pietrowski, M. Influence of high temperature synthesis on the structure of graphitic carbon nitride and its hydrogen generation ability. Materials 2020, 13, 2756. [Google Scholar] [CrossRef]
- Kolawole, O.M.; Lau, W.M.; Khutoryanskiy, V.V. Methacrylated chitosan as a polymer with enhanced mucoadhesive properties for transmucosal drug delivery. Int. J. Pharm. 2018, 550, 123–129. [Google Scholar] [CrossRef]
- Shcherban, N.D.; Mäki-Arvela, P.; Aho, A.; Sergiienko, S.A.; Yaremov, P.S.; Eränen, K.; Murzin, D.Y. Melamine-derived graphitic carbon nitride as a new effective metal-free catalyst for Knoevenagel condensation of benzaldehyde with ethylcyanoacetate. Catal. Sci. Technol. 2018, 8, 2928–2937. [Google Scholar] [CrossRef]
- Xu, R.; Peng, Y. Preparation of magnetic g-C3N4/Fe3O4 composite and its application in the separation of catechol from water. Materials 2019, 12, 2844. [Google Scholar] [CrossRef]
- Guo, Q.; Xie, Y.; Wang, X.; Lv, S.; Hou, T.; Liu, X. Characterization of well-crystallized graphitic carbon nitride nanocrystallites via a benzene-thermal route at low temperatures. Chem. Phys. Lett. 2003, 380, 84–87. [Google Scholar] [CrossRef]
- Sadeghi, R.; Etemad, S.G.; Keshavarzi, E.; Haghshenasfard, M. Investigation of alumina nanofluid stability by UV–vis spectrum. Microfluid. Nanofluidics 2015, 18, 1023–1030. [Google Scholar] [CrossRef]
- Nisticò, R.; Lavagna, L.; Versaci, D.; Ivanchenko, P.; Benzi, P. Chitosan and its char as fillers in cement-base composites: A case study. Bol. Soc. Española Cerám. Vidr. 2020, 59, 186–192. [Google Scholar] [CrossRef]
- Esmaeili, A.; Asgari, A. In vitro release and biological activities of Carum copticum essential oil (CEO) loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2015, 81, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: Encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2019, 126, 731–742. [Google Scholar] [CrossRef]
- Soltanzadeh, M.; Peighambardoust, S.H.; Ghanbarzadeh, B.; Mohammadi, M.; Lorenzo, J.M. Chitosan nanoparticles as a promising nanomaterial for encapsulation of pomegranate (Punica granatum L.) peel extract as a natural source of antioxidants. Nanomaterials 2021, 11, 1439. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Gu, J.; Hou, K.; Li, J.; Wang, Y.; Yue, Y. High-efficiency, compressible, and recyclable reduced graphene oxide/chitosan composite aerogels supported g-C3N4/BiOBr photocatalyst for adsorption and degradation of rhodamine B. J. Environ. Chem. Eng. 2022, 10, 107157. [Google Scholar] [CrossRef]
- Xu, S.; Xiao, G.; Wang, Z.; Wang, Y.; Liu, Z.; Su, H. A reusable chitosan/TiO2@g-C3N4 nanocomposite membrane for photocatalytic removal of multiple toxic water pollutants under visible light. Water Sci. Technol. 2021, 83, 3063–3074. [Google Scholar] [CrossRef]
- Shi, W.; Fang, W.-X.; Wang, J.-C.; Qiao, X.; Wang, B.; Guo, X. pH-controlled mechanism of photocatalytic RhB degradation over g-C3N4 under sunlight irradiation. Photochem. Photobiol. Sci. 2021, 20, 303–313. [Google Scholar] [CrossRef]
- Gong, J.; Xie, Z.; Wang, B.; Li, Z.; Zhu, Y.; Xue, J.; Le, Z. Fabrication of g-C3N4-based conjugated copolymers for efficient photocatalytic reduction of U(VI). J. Environ. Chem. Eng. 2021, 9, 104638. [Google Scholar] [CrossRef]
- Zhang, W.; Li, L.; Gao, Y.; Zhang, D. Graphitic carbon nitride-based materials for photocatalytic reduction of U(VI). New J. Chem. 2020, 44, 19961–19976. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Buzón-Durán, L.; Andrés-Juan, C.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Physicochemical characterization of Crithmum maritimum L. and Daucus carota subsp. gummifer (Syme) Hook.fil. and their antimicrobial activity against apple tree and grapevine phytopathogens. Agronomy 2021, 11, 886. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Buzón-Durán, L.; Langa-Lomba, N.; Casanova-Gascón, J.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Characterization and antimicrobial activity of a halophyte from the Asturian coast (Spain): Limonium binervosum (G.E.Sm.) C.E.Salmon. Plants 2021, 10, 1852. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Buzón-Durán, L.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Physicochemical characterization and antimicrobial activity against Erwinia amylovora, Erwinia vitivora, and Diplodia seriata of a light purple Hibiscus syriacus L. cultivar. Plants 2021, 10, 1876. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Buzón-Durán, L.; Cuchí-Oterino, J.A.; Martín-Gil, J.; Lorenzo-Vidal, B.; Martín-Ramos, P. Dwarf pomegranate (Punica granatum L. var. nana): Source of 5-HMF and bioactive compounds with applications in the protection of woody crops. Plants 2022, 11, 550. [Google Scholar] [CrossRef]
- Langa-Lomba, N.; Buzón-Durán, L.; Martín-Ramos, P.; Casanova-Gascón, J.; Martín-Gil, J.; Sánchez-Hernández, E.; González-García, V. Assessment of conjugate complexes of chitosan and Urtica dioica or Equisetum arvense extracts for the control of grapevine trunk pathogens. Agronomy 2021, 11, 976. [Google Scholar] [CrossRef]
- Tran, D.A.; Nguyen Pham, C.T.; Nguyen Ngoc, T.; Nguyen Phi, H.; Hoai Ta, Q.T.; Truong, D.H.; Nguyen, V.T.; Luc, H.H.; Nguyen, L.T.; Dao, N.N.; et al. One-step synthesis of oxygen doped g-C3N4 for enhanced visible-light photodegradation of Rhodamine B. J. Phys. Chem. Solids 2021, 151, 109900. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation of rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation. Langmuir 2010, 26, 3894–3901. [Google Scholar] [CrossRef]
- Kong, X.; Liu, X.; Zheng, Y.; Chu, P.K.; Zhang, Y.; Wu, S. Graphitic carbon nitride-based materials for photocatalytic antibacterial application. Mater. Sci. Eng. R Rep. 2021, 145, 100610. [Google Scholar] [CrossRef]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef]
- Nazar Pour, F.; Pedrosa, B.; Oliveira, M.; Fidalgo, C.; Devreese, B.; Driessche, G.V.; Félix, C.; Rosa, N.; Alves, A.; Duarte, A.S.; et al. Unveiling the secretome of the fungal plant pathogen Neofusicoccum parvum induced by in vitro host mimicry. J. Fungi 2022, 8, 971. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Liu, J. Advance in chitosan hydrolysis by non-specific cellulases. Bioresour. Technol. 2008, 99, 6751–6762. [Google Scholar] [CrossRef]
- Labois, C.; Stempien, E.; Schneider, J.; Schaeffer-Reiss, C.; Bertsch, C.; Goddard, M.-L.; Chong, J. Comparative study of secreted proteins, enzymatic activities of wood degradation and stilbene metabolization in grapevine botryosphaeria dieback fungi. J. Fungi 2021, 7, 568. [Google Scholar] [CrossRef]
- An, C.; Sun, C.; Li, N.; Huang, B.; Jiang, J.; Shen, Y.; Wang, C.; Zhao, X.; Cui, B.; Wang, C.; et al. Nanomaterials and nanotechnology for the delivery of agrochemicals: Strategies towards sustainable agriculture. J. Nanobiotechnology 2022, 20, 11. [Google Scholar] [CrossRef] [PubMed]


| Samples | Elemental Composition (%) | Ref. | |||
|---|---|---|---|---|---|
| C | H | N | O | ||
| Methacrylic acid | 55.7 | 7.0 | - | 37.3 | - |
| COS | 42.9 | 6.3 | 6.8 | 44.0 | [36] |
| g-C3N4 (g-C3N4 – g-C3N4.2 range) | 39.4 (32.6–46.1) | 10.1 (0–20.3) | 50.4 (47.1–53.8) | - | [37] |
| Methacrylated COS | 48.8 (45.5–52.1) | 6.5 (5.2–7.8) | 3.8 (1.1–6.5) | 40.9 (39.0–42.8) | [36] |
| Methacrylated g-C3N4 | 43.7 | - | 40.8 | 15.5 | This work |
| g-C3N4-MA-COS assembly | 47.1 | - | 16.1 | 36.8 | This work |
| g-C3N4-MA-COS:R. tinctorum | 52.1 | - | 16.1 | 31.8 | This work |
| Treatment | Concentration (µg·mL−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 62.5 | 93.75 | 125 | 187.5 | 250 | 375 | 500 | 750 | 1000 | 1500 | |
| Empty NCs | + | + | + | + | + | + | + | + | + | − |
| NCs loaded with R. tinctorum | + | + | − | − | − | − | − | − | − | − |
| Treatment | D. seriata | N. parvum | ||||
|---|---|---|---|---|---|---|
| Mean of Ranks | Groups | Mean of Ranks | Groups | |||
| NCs-R. tinctorum | 30.750 | A | 30.607 | A | ||
| Control | 44.750 | B | 45.750 | B | ||
| Pathogen | Effective Concentration | g-C3N4-MA-COS- R. tinctorum | Encapsulated R. tinctorum (ca. 14 wt%) | R. tinctorum Extract [26] |
|---|---|---|---|---|
| D. seriata | EC50 | 193.1 | 27.0 | 78.0 |
| EC90 | 241.0 | 33.7 | 87.8 | |
| N. parvum | EC50 | 362.7 | 50.8 | 92.3 |
| EC90 | 631.5 | 88.4 | 184.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago-Aliste, A.; Sánchez-Hernández, E.; Langa-Lomba, N.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Martín-Ramos, P. Multifunctional Nanocarriers Based on Chitosan Oligomers and Graphitic Carbon Nitride Assembly. Materials 2022, 15, 8981. https://doi.org/10.3390/ma15248981
Santiago-Aliste A, Sánchez-Hernández E, Langa-Lomba N, González-García V, Casanova-Gascón J, Martín-Gil J, Martín-Ramos P. Multifunctional Nanocarriers Based on Chitosan Oligomers and Graphitic Carbon Nitride Assembly. Materials. 2022; 15(24):8981. https://doi.org/10.3390/ma15248981
Chicago/Turabian StyleSantiago-Aliste, Alberto, Eva Sánchez-Hernández, Natalia Langa-Lomba, Vicente González-García, José Casanova-Gascón, Jesús Martín-Gil, and Pablo Martín-Ramos. 2022. "Multifunctional Nanocarriers Based on Chitosan Oligomers and Graphitic Carbon Nitride Assembly" Materials 15, no. 24: 8981. https://doi.org/10.3390/ma15248981
APA StyleSantiago-Aliste, A., Sánchez-Hernández, E., Langa-Lomba, N., González-García, V., Casanova-Gascón, J., Martín-Gil, J., & Martín-Ramos, P. (2022). Multifunctional Nanocarriers Based on Chitosan Oligomers and Graphitic Carbon Nitride Assembly. Materials, 15(24), 8981. https://doi.org/10.3390/ma15248981