Study on the Roasting Process of Guisha Limonite Pellets
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Experimental Materials
3.2. Single-Factor Test
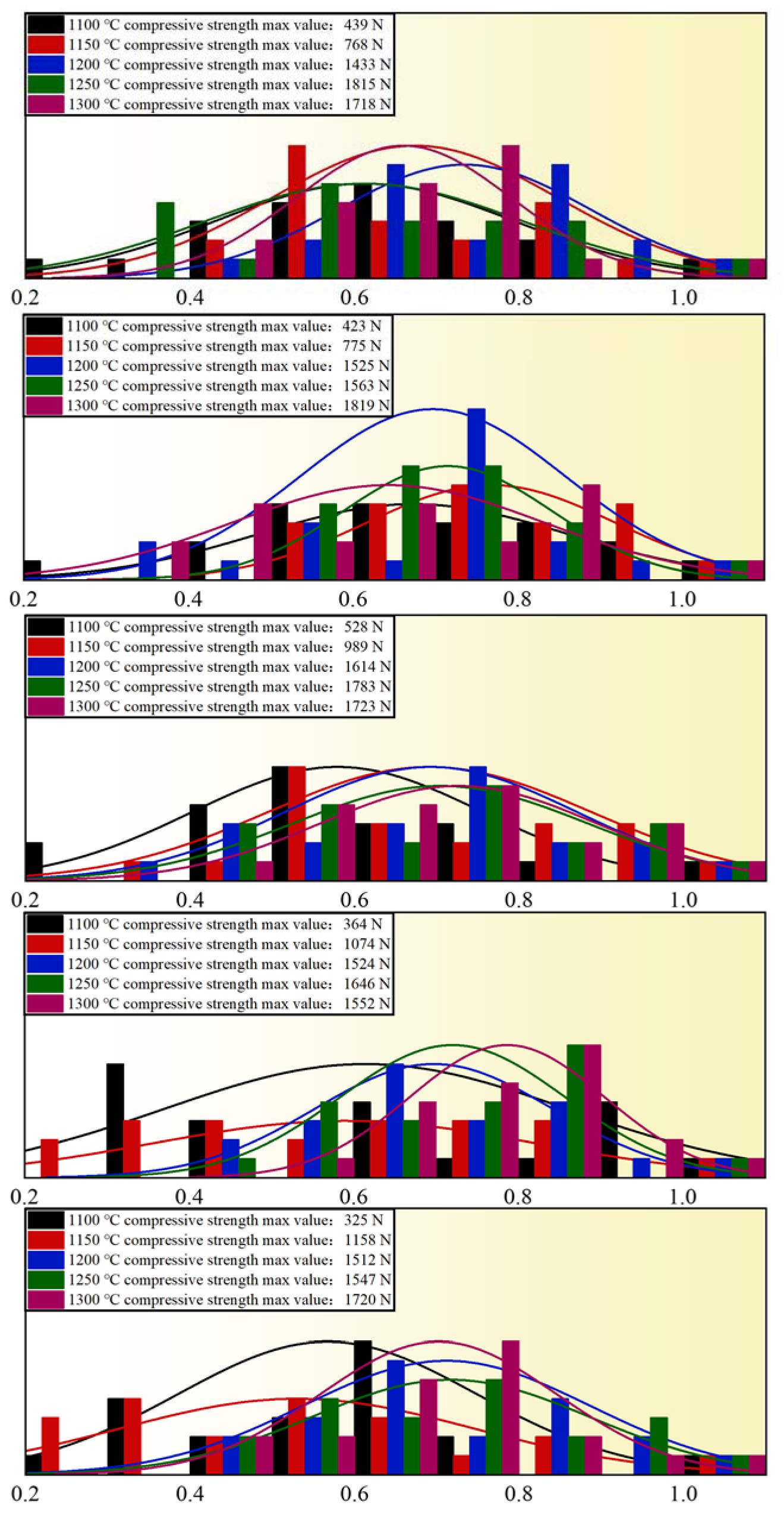
3.2.1. Influence of Roasting Temperature on Compressive Strength of Pellets
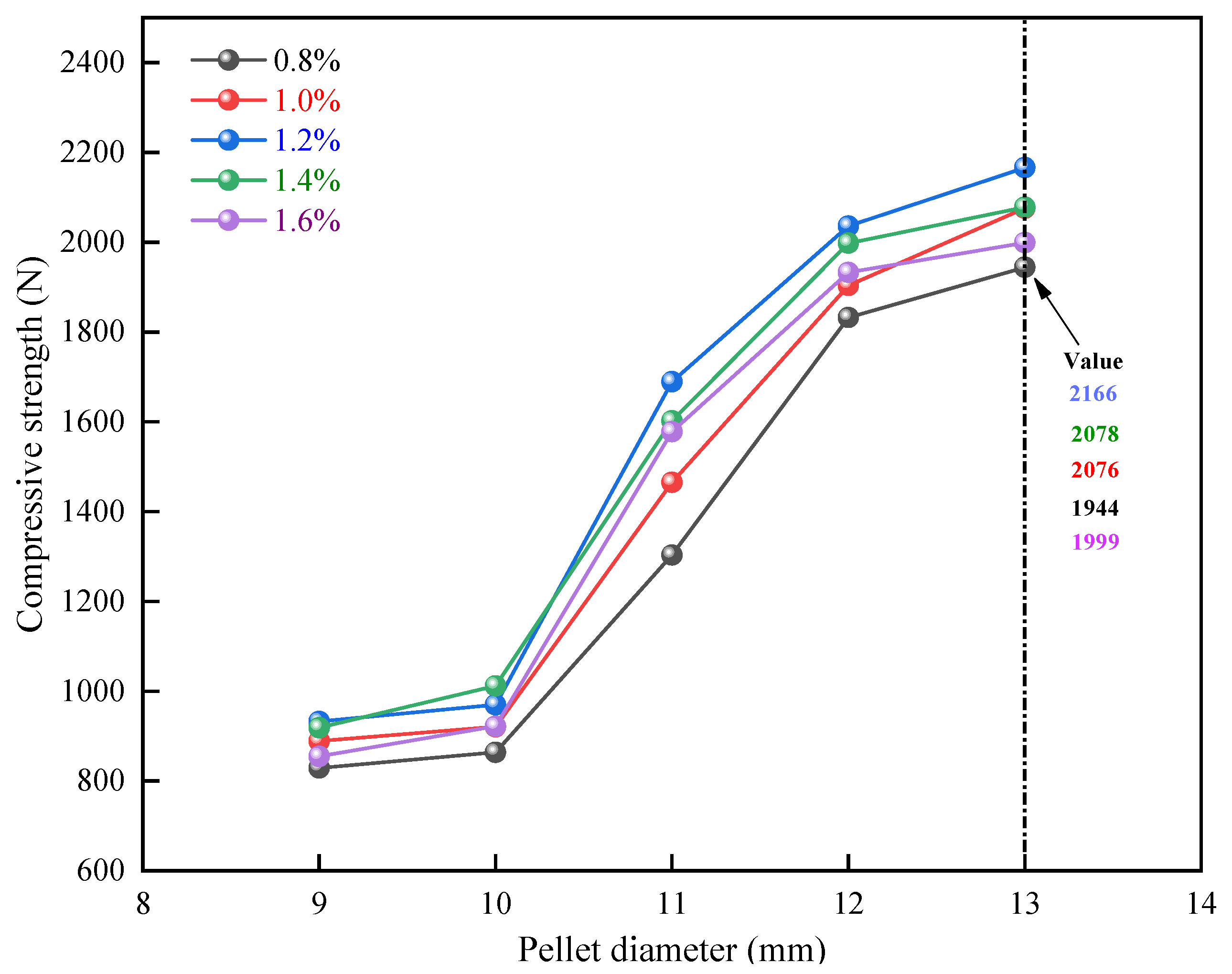
3.2.2. Influence of Roasting Temperature on Compressive Strength of Pellets
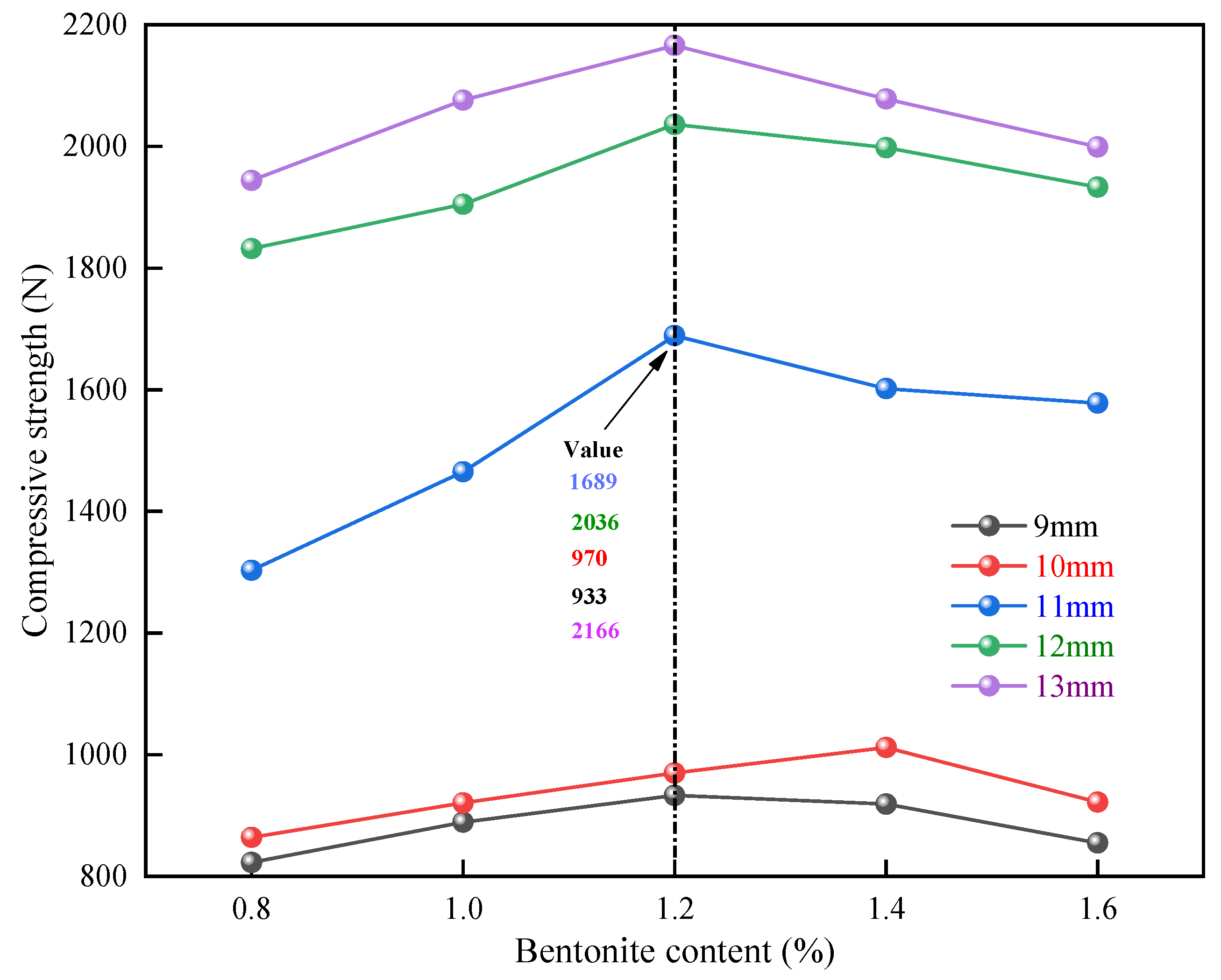
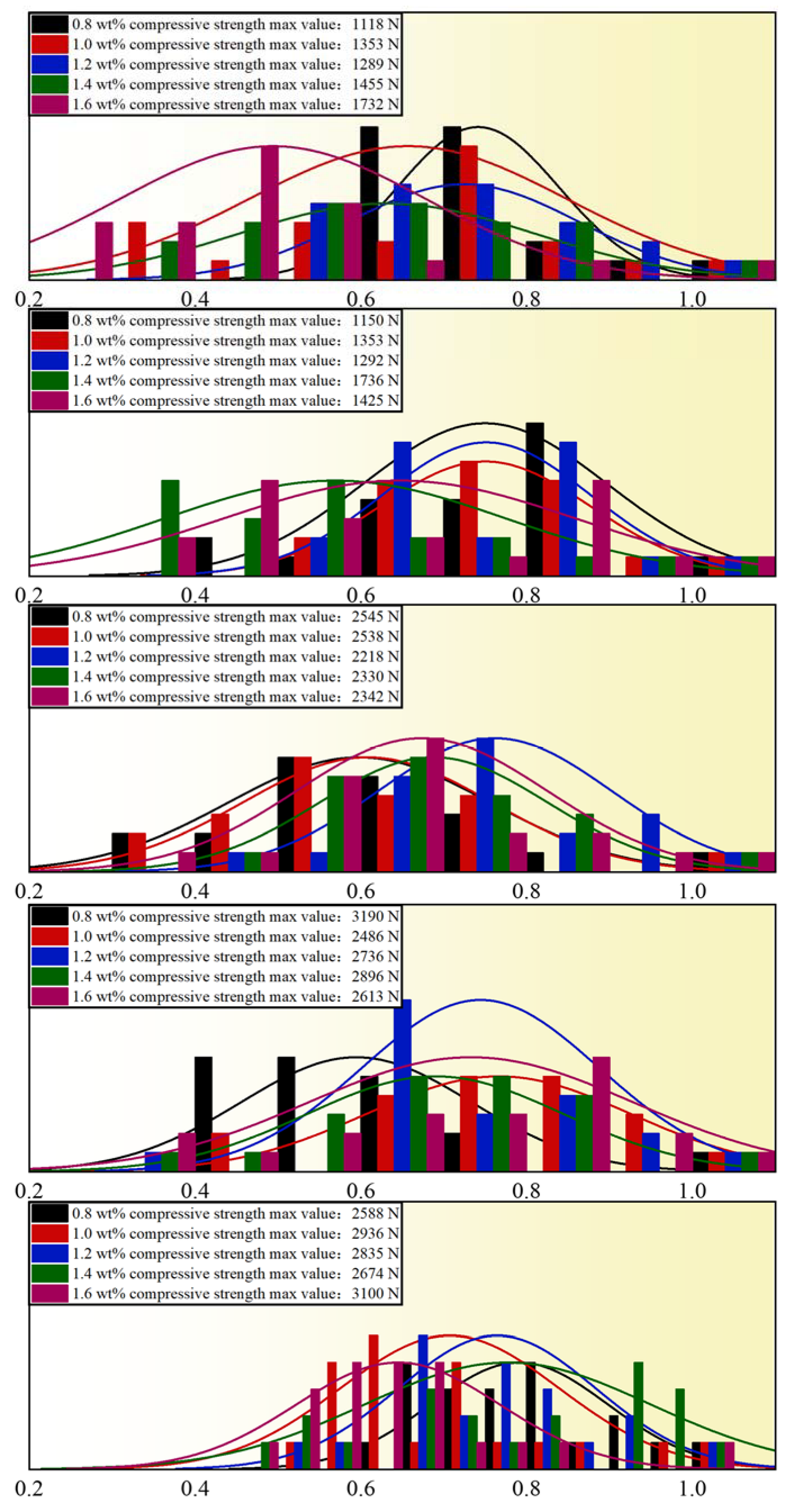
3.2.3. Influence of Bentonite Addition on Compressive Strength of Pellets
3.3. Multifactorial Test
3.3.1. Response Surface Design
3.3.2. Response Surface Method Design Results
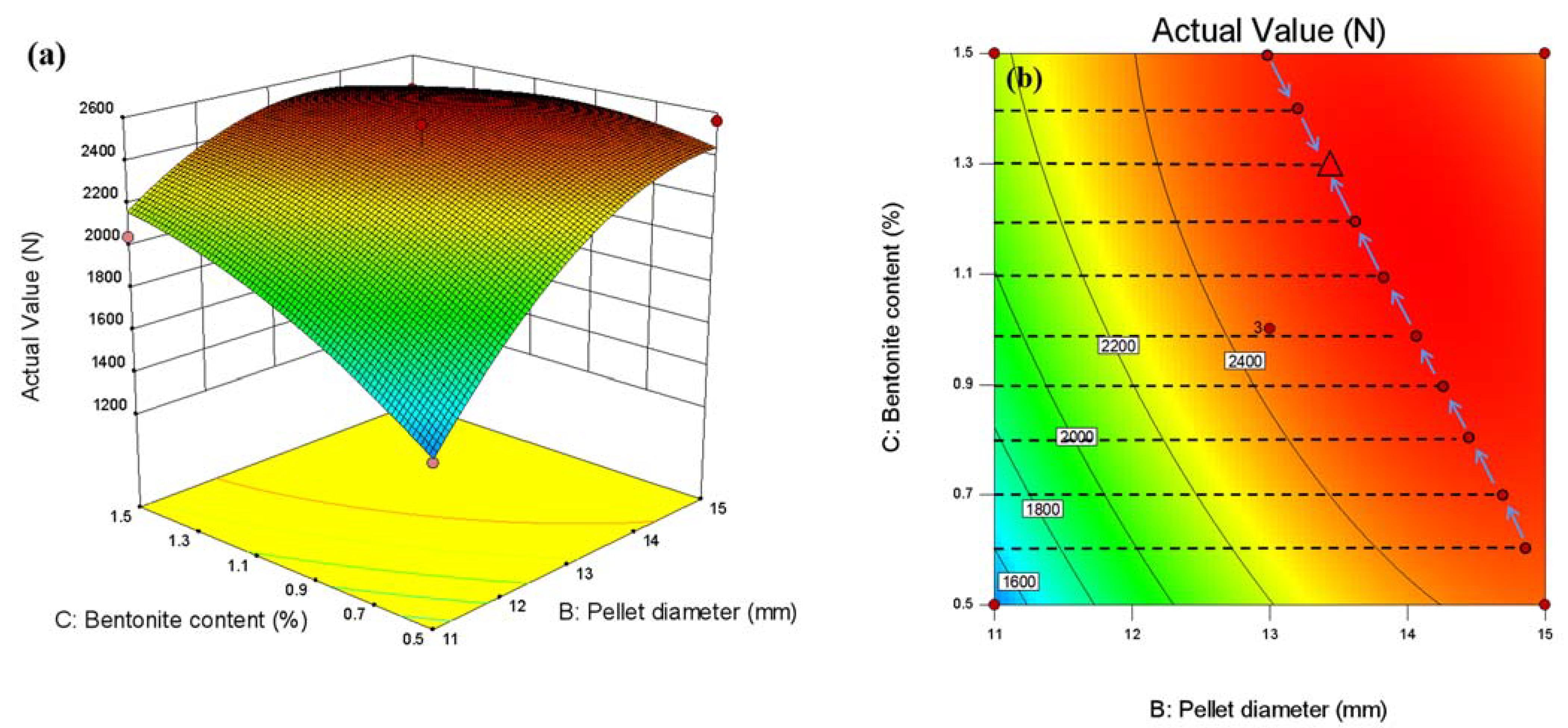
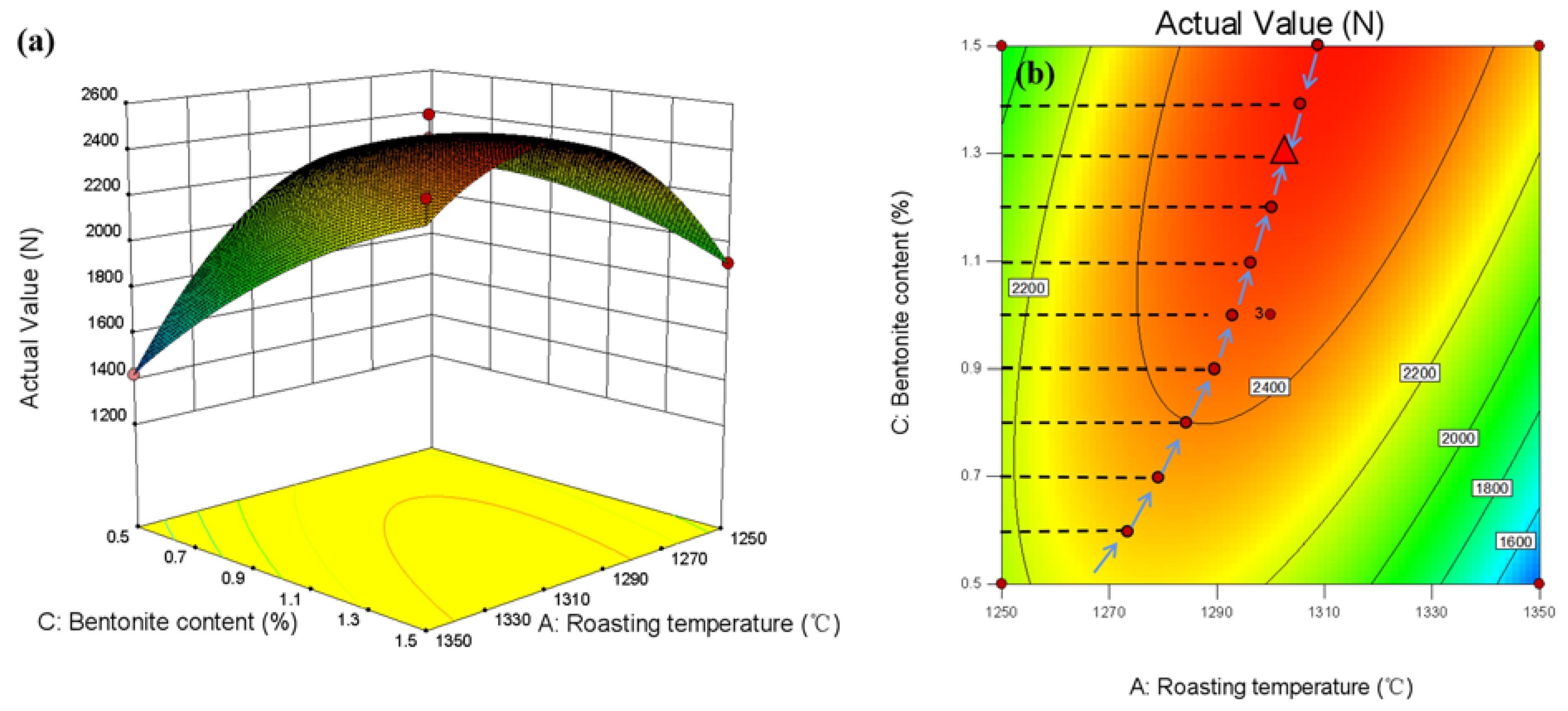
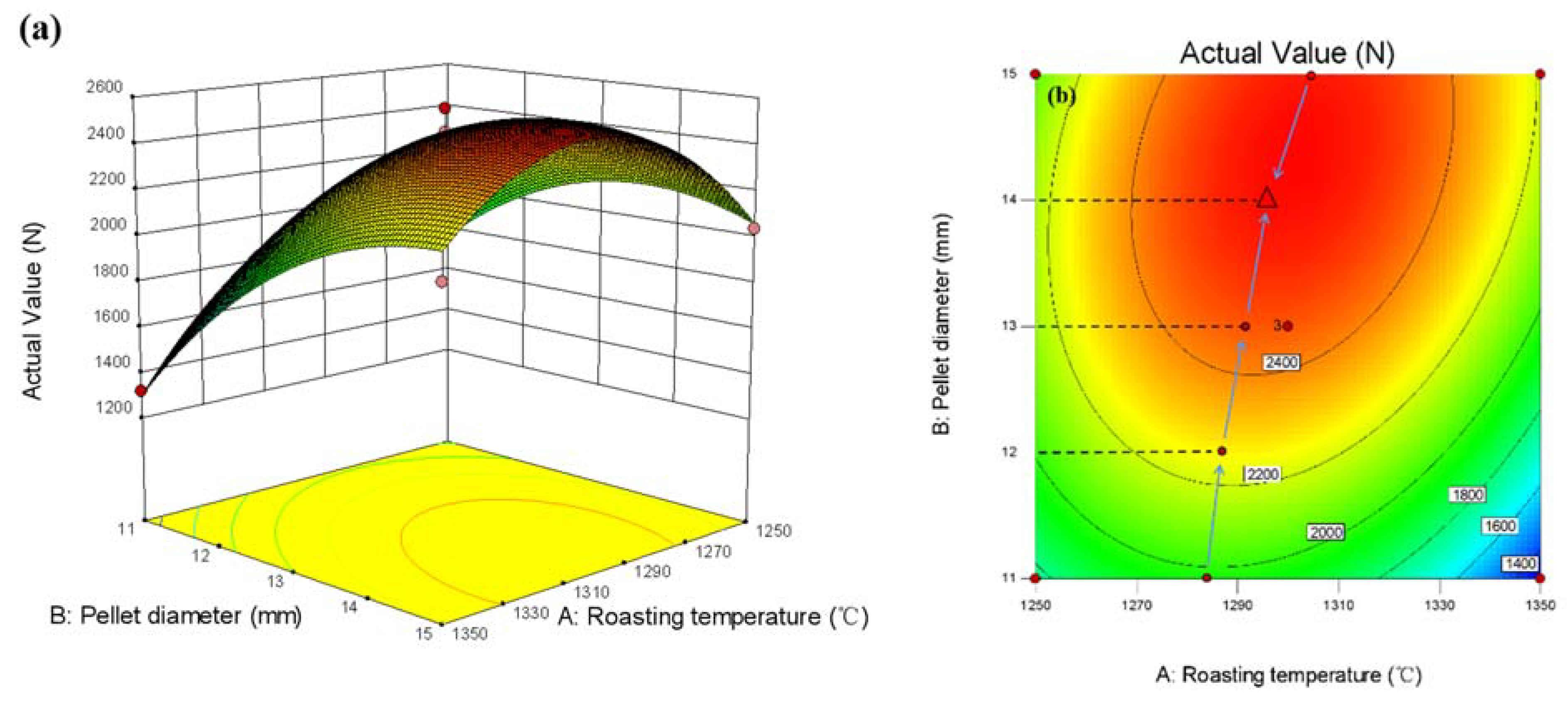
3.3.3. Response Surface Method Optimization
3.3.4. Response Surface Method Optimization
4. Conclusions
- The compressive strength of pure limonite pellets can reach 2700 N, which can be used in small blast furnaces and rotary hearth furnace processes.
- Within the set factor range (calcination temperature 1250–1350 °C, bentonite content 0.5–1.5%, pellet diameter 11–15 mm), pellet diameter has the most significant influence on the compressive strength of pure limonite pellets, followed by the bentonite content, and the calcination temperature has no significant impact on the compressive strength of the pellets.
- The roasting process of pure limonite pellets was optimized by statistical methods. Within the set factor range (calcination temperature 1250–1350 °C, bentonite content 0.5–1.5%, pellet diameter 11–15 mm), the optimized experimental conditions obtained are: bentonite content 1.32828%, calcination temperature 1310.61 °C, pellet diameter 13.9495 mm. The predicted value of pellet compressive strength is 2570.3 N/piece, and the experimentally verified value of pellet compressive strength is 2705 N/piece.
- The content of bentonite does not affect the desorption process of limonite crystal water.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sahu, S.N.; Sharma, K.; Barma, S.D.; Pradhan, P.; Nayak, B.K.; Biswal, S.K. Utilization of low-grade BHQ iron ore by reduction roasting followed by magnetic separation for the production of magnetite-based pellet feed. Metall. Res. Technol. 2019, 116, 611. [Google Scholar] [CrossRef]
- Nabeel, M.; Karasev, A.; Glaser, B.; Jönsson, P.G. Characterization of Dust Generated during Mechanical Wear of Partially Reduced Iron Ore Pellets. Steel Res. Int. 2017, 88, 1600442. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, J.; Chang, X.; Guo, S.; Srinivasakannan, C.; Chen, G.; Peng, J. Effect of microwave irradiation on selective heating behavior and magnetic separation characteristics of Panzhihua ilmenite. Appl. Surf. Sci. 2014, 300, 171–177. [Google Scholar] [CrossRef]
- Prusti, P.; Barik, K.; Dash, N.; Biswal, S.K.; Meikap, B.C. Effect of limestone and dolomite flux on the quality of pellets using high LOI iron ore. Powder Technol. 2021, 379, 154–164. [Google Scholar] [CrossRef]
- Gao, Q.J.; Shen, F.M.; Wei, G.; Jiang, X.; Zheng, H.Y. Effects of MgO containing additive on low-temperature metallurgical properties of oxidized pellet. J. Iron Steel Res. Int. 2013, 20, 25–28. [Google Scholar] [CrossRef]
- de Morais Oliveira, V.; de Resende, V.G.; Domingues, A.L.A.; Bagatini, M.C.; de Castro, L.F.A. Alternative to deal with high level of fine materials in iron ore sintering process. J. Mater. Res. Technol. 2019, 8, 4985–4994. [Google Scholar] [CrossRef]
- Agrawal, S.; Rayapudi, V.; Dhawan, N. Comparative study of low-grade banded iron ores for iron recovery. Metall. Res. Technol. 2020, 117, 403. [Google Scholar] [CrossRef]
- Iljana, M.; Kemppainen, A.; Paananen, T.; Mattila, O.; Pisilä, E.; Kondrakov, M.; Fabritius, T. Effect of adding limestone on the metallurgical properties of iron ore pellets. Int. J. Miner. Process. 2015, 141, 34–43. [Google Scholar] [CrossRef]
- Zhang, K.; Ge, Y.; Guo, W.; Li, N.; Wang, Z.; Luo, H.; Shang, S. Phase transition and magnetic properties of low-grade limonite during reductive roasting. Vacuum 2019, 167, 163–174. [Google Scholar] [CrossRef]
- Sagar, R.K.; Sah, R.; Maribasappanavar, B.; Desai, S.; Balachandran, G. Optimization of Drying and Preheating Temperatures During Pellet Induration for Utilizing Goethitic Iron Ores. Min. Metall. Explor. 2022, 39, 1667–1678. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, G.S.; Jin, M.F.; Shen, F. Effect of MgO content on softening-melting property of sinter. J.-Northeast. Univ. Nat. Sci. 2006, 27, 1358. [Google Scholar]
- Ghadi, A.Z.; Vahedi, S.M.; Sohn, H.Y. Analysis of the Gaseous Reduction of Porous Wustite Pellets by Response Surface Methodology. Steel Res. Int. 2021, 92, 2100048. [Google Scholar] [CrossRef]
- Aslani, H.; Nabizadeh, R.; Nasseri, S.; Mesdaghinia, A.; Alimohammadi, M.; Mahvi, A.H.; Nazmara, S. Application of response surface methodology for modeling and optimization of trichloroacetic acid and turbidity removal using potassium ferrate (VI). Desalination Water Treat. 2016, 57, 25317–25328. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, J.; Srinivasakannan, C.; Zhang, Z.; Zhang, L.; Fernández, Y.; Menéndez, J.A. Leaching zinc from spent catalyst: Process optimization using response surface methodology. J. Hazard. Mater. 2010, 176, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Suman, S.K. Compressive strength of fired pellets using organic binder: Response surface approach for analyzing the performance. Trans. Indian Inst. Met. 2018, 71, 1629–1634. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, C.; Xing, Y.; Kou, J.; Su, M. Thermal decomposition behavior of pyrite in a microwave field and feasibility of gold leaching with generated elemental sulfur from the decomposition of gold-bearing sulfides. Hydrometallurgy. 2018, 180, 210–220. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, G.; Tong, L.; Lin, Y.; Yang, H. Optimization of Knelson gravity separation of a quartz vein type gold ore using response surface methodology. J. Cent. South Univ. Sci. Technol. 2019, 50, 2925–2931. [Google Scholar]
- Wu, S.; Yu, X.; Hu, Z.; Zhang, L.; Chen, J. Optimizing aerobic biodegradation of dichloromethane using response surface methodology. J. Environ. Sci. 2009, 21, 1276–1283. [Google Scholar] [CrossRef]
- Lorente, E.; Herguido, J.; Peña, J.A. Steam-iron process: Influence of steam on the kinetics of iron oxide reduction. Int. J. Hydrog. Energy 2011, 36, 13425–13434. [Google Scholar] [CrossRef]
- Fan, J.J.; Qiu, G.Z.; Jiang, T.; Guo, Y.F.; Cai, M.X. Roasting properties of pellets with iron concentrate of complex mineral composition. J. Iron Steel Res. Int. 2011, 18, 1–7. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Lu, M.M.; Fu, J.T. Oxidation and roasting characteristics of artificial magnetite pellets. J. Cent. South Univ. 2016, 23, 2999–3005. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Hou, X.; Shao, J.; Geng, W. Study on CO2 gasification properties and kinetics of biomass chars and anthracite char. Bioresour. Technol. 2015, 177, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, J.; Shao, J.; Ren, S. Characterisation and model fitting kinetic analysis of coal/biomass co-combustion. Thermochim. Acta 2014, 591, 68–74. [Google Scholar] [CrossRef]
- Wang, L.; Li, T.; Várhegyi, G.; Skreiberg, Ø.; Løvås, T. CO2 gasification of chars prepared by fast and slow pyrolysis from wood and forest residue: A kinetic study. Energy Fuels 2018, 32, 588–597. [Google Scholar] [CrossRef]
- Rousset, P.; Figueiredo, C.; De Souza, M.; Quirino, W. Pressure effect on the quality of eucalyptus wood charcoal for the steel industry: A statistical analysis approach. Fuel Process. Technol. 2011, 92, 1890–1897. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Wang, E. Novel hydrogen-bonded three-dimensional network complexes containing copper-pyridine-2, 6-dicarboxylic acid. J. Coord. Chem. 2014, 57, 1353–1359. [Google Scholar] [CrossRef]

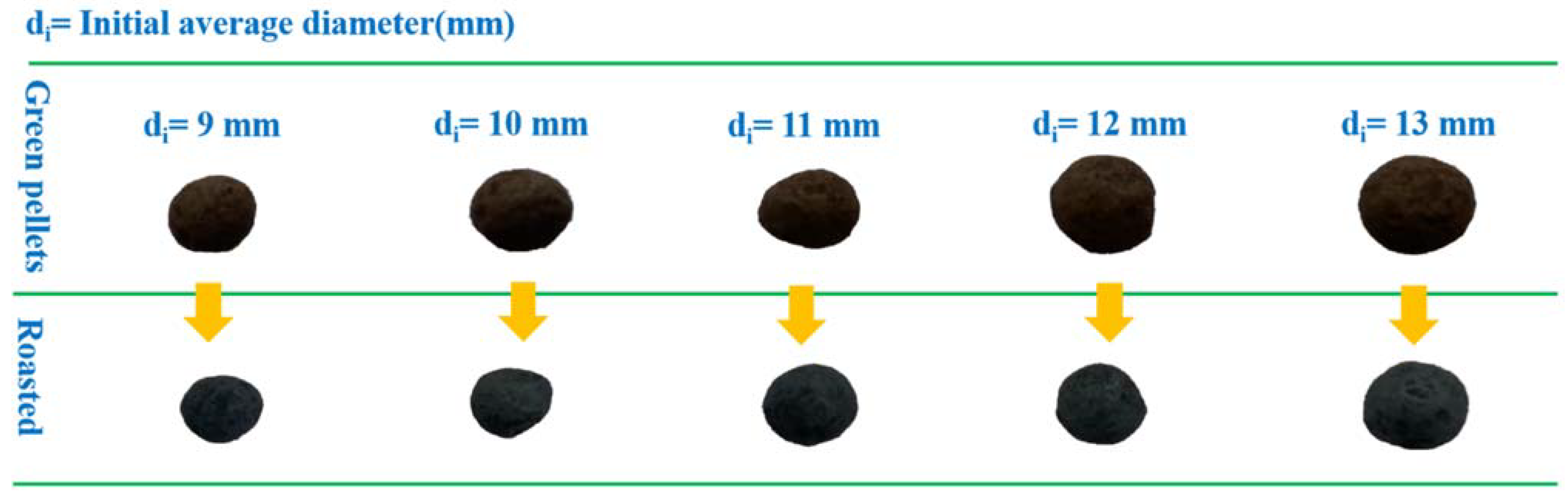
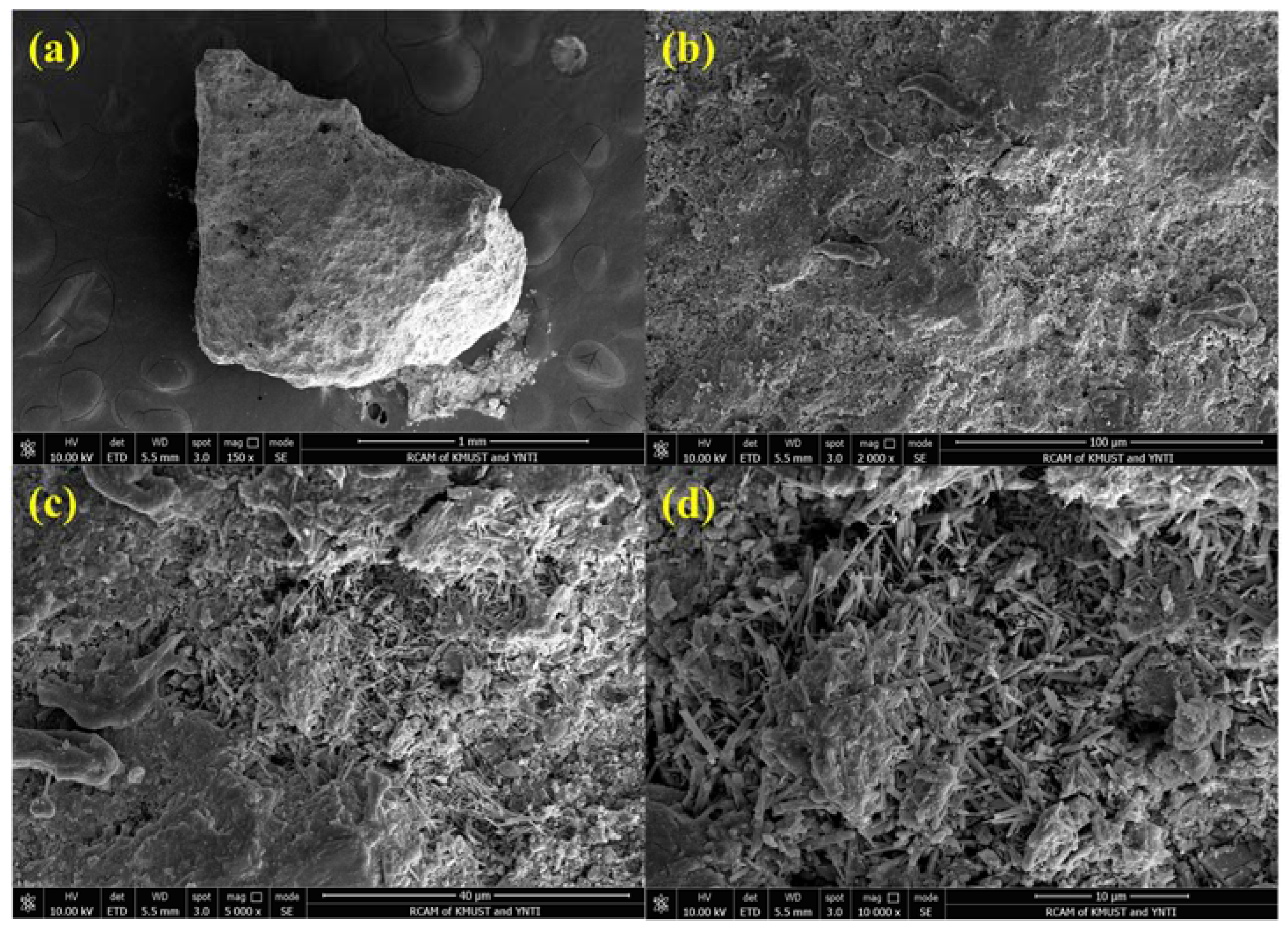

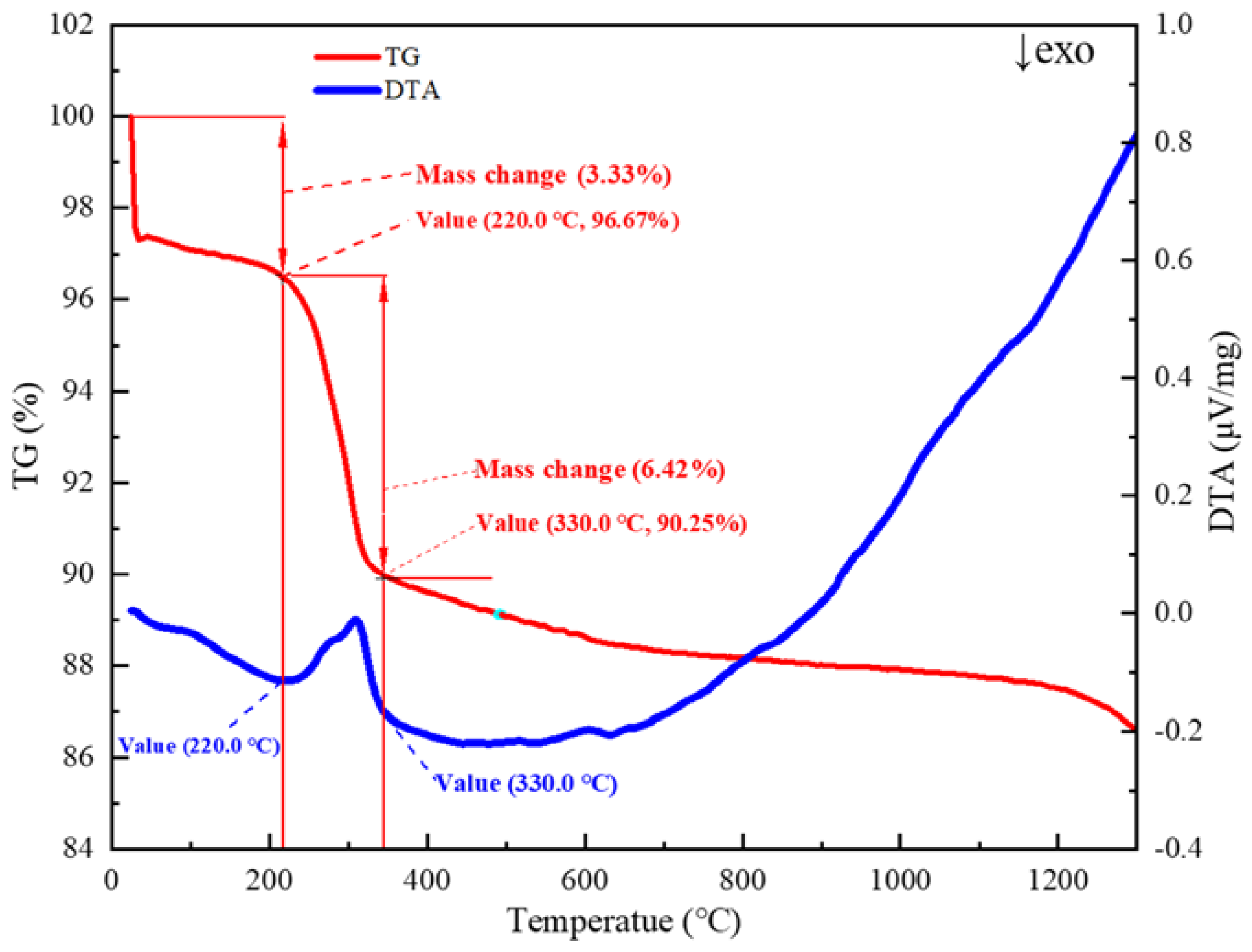
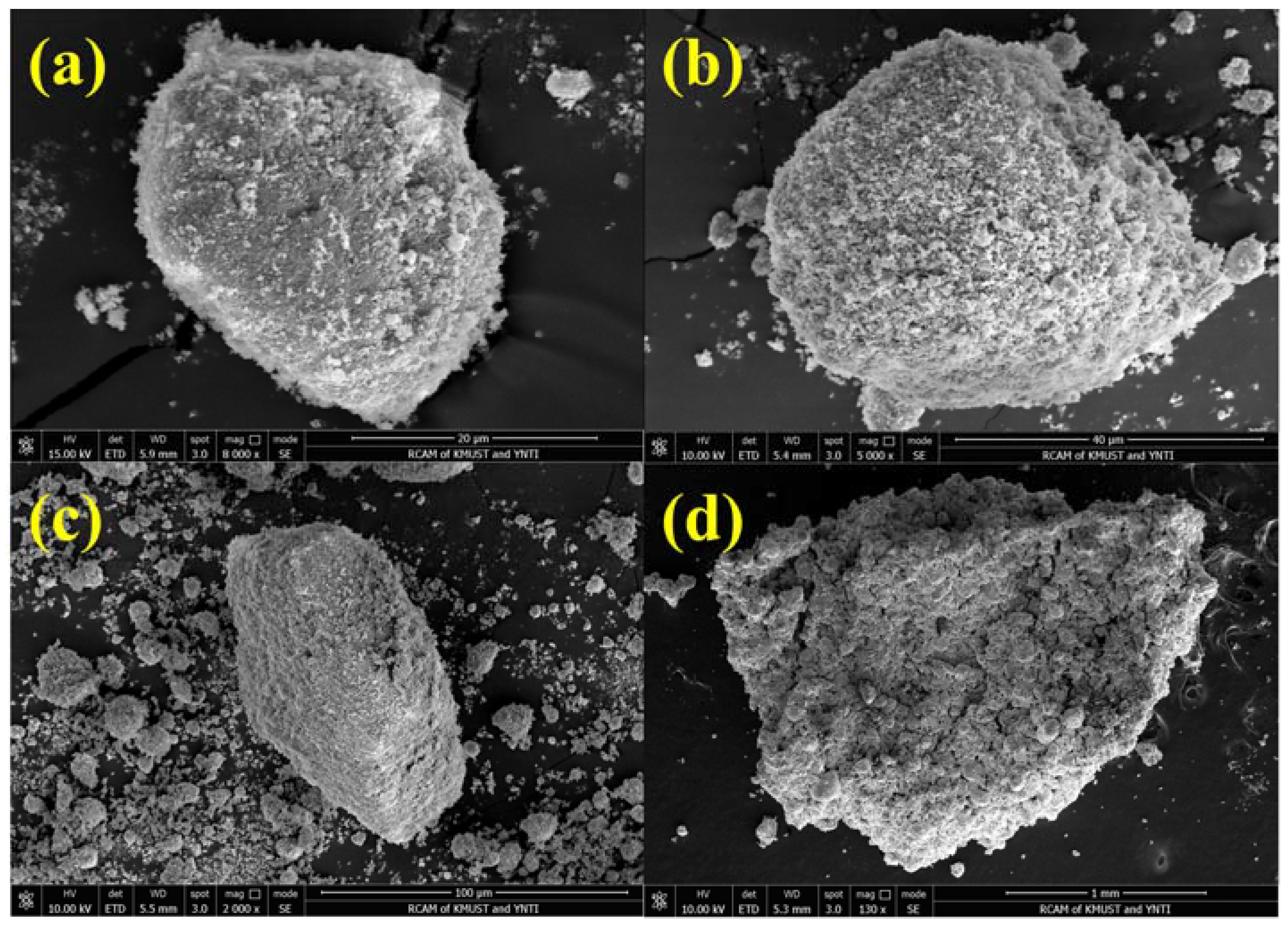


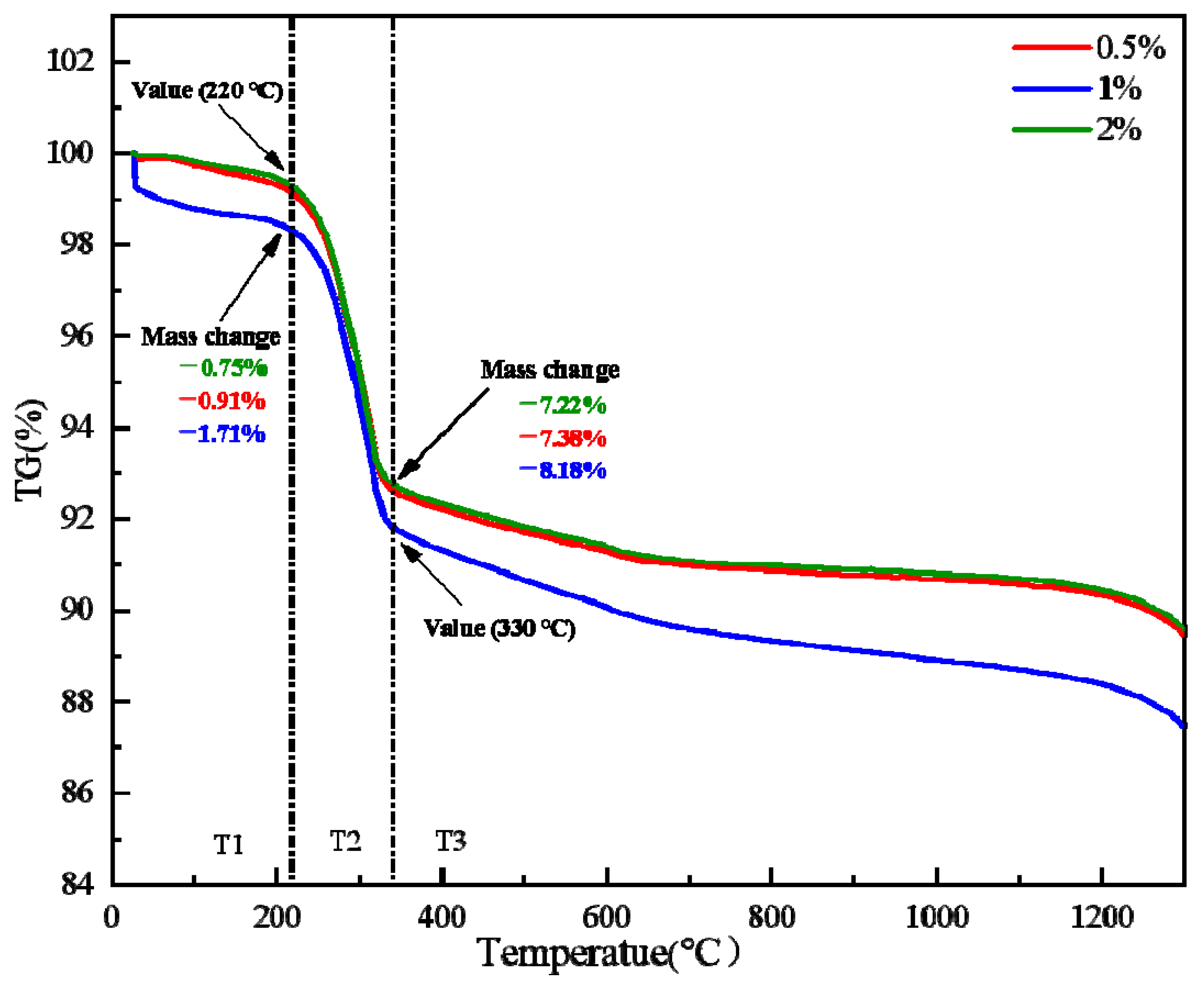
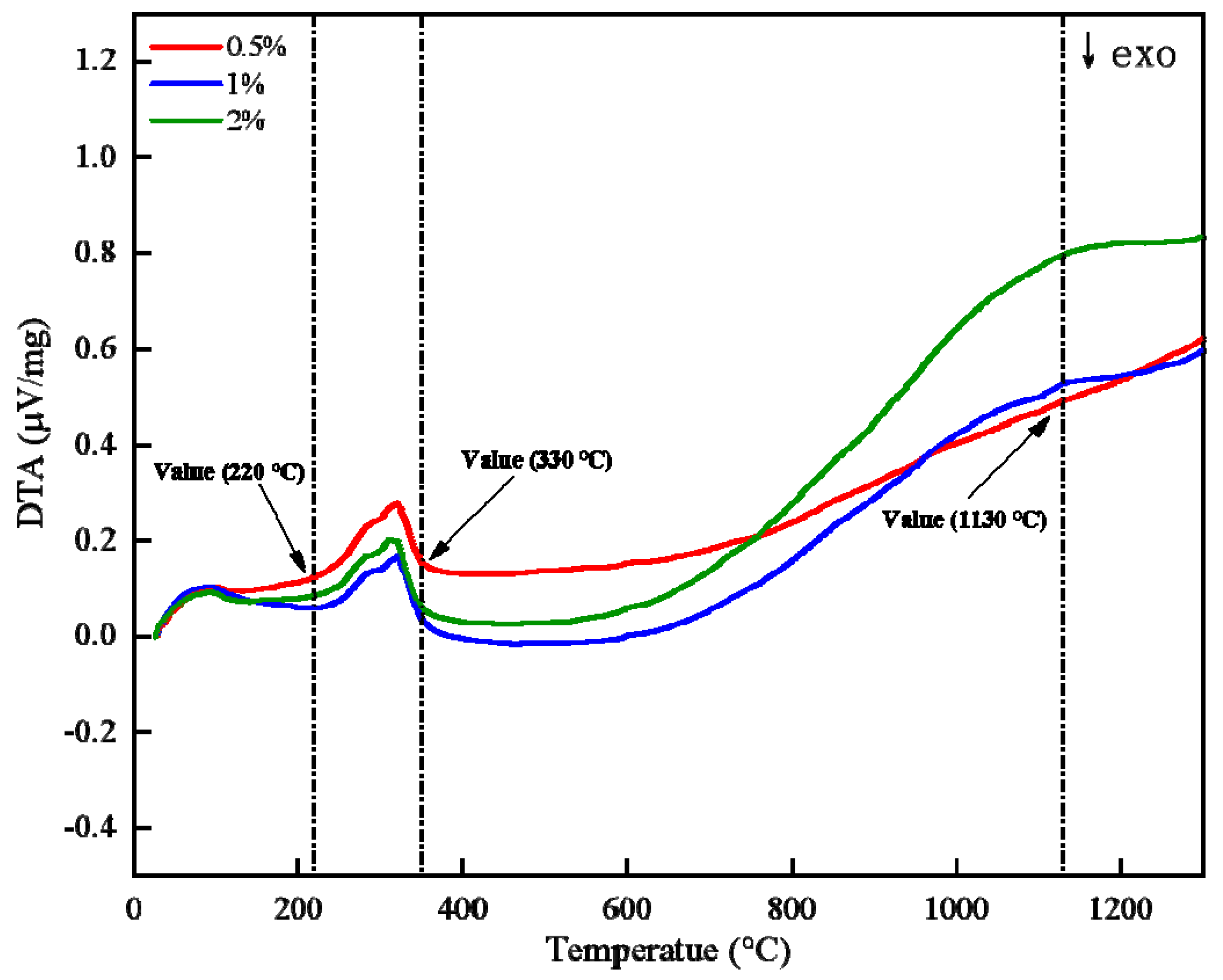
| Factor | Variable | Level | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Roasting temperature/℃ | A | 1100 | 1150 | 1200 | 1250 | 1300 |
| Pellet diameter/mm | B | 9 | 10 | 11 | 12 | 13 |
| Bentonite content/% | C | 0.8 | 1.0 | 1.2 | 1.4 | 1.6 |
| w(TFe) | w(FeO) | w(SiO2) | w(S) | w(MnO) | w(TiO2) | w(Pb) | w(Zn) | w(K2O) | w(Na2O) | w(Cu) | w(V2O5) | Burning Loss |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 54.67 | 0.29 | 4.04 | 0.048 | 3.47 | 0.27 | 0.009 | 0.018 | 0.095 | 0.001 | 0.009 | 0.040 | 14.82 |
| Moisture/% | Colloid Index/(%· (3g)−1) | Swelling Capacity/(mL·g−1) | Water Absorption/% | Methylene Blue Index/(g·(100g)−1) | Montmorillonite Mass Fraction/% |
|---|---|---|---|---|---|
| 9.38 | 20.0 | 34.5 | 408 | 38.0 | 85.97 |
| Factor | Variable | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Roasting temperature/°C | A | 1250 | 1300 | 1350 |
| Pellet diameter/mm | B | 11 | 13 | 15 |
| Bentonite content/% | C | 0.5 | 1.0 | 1.5 |
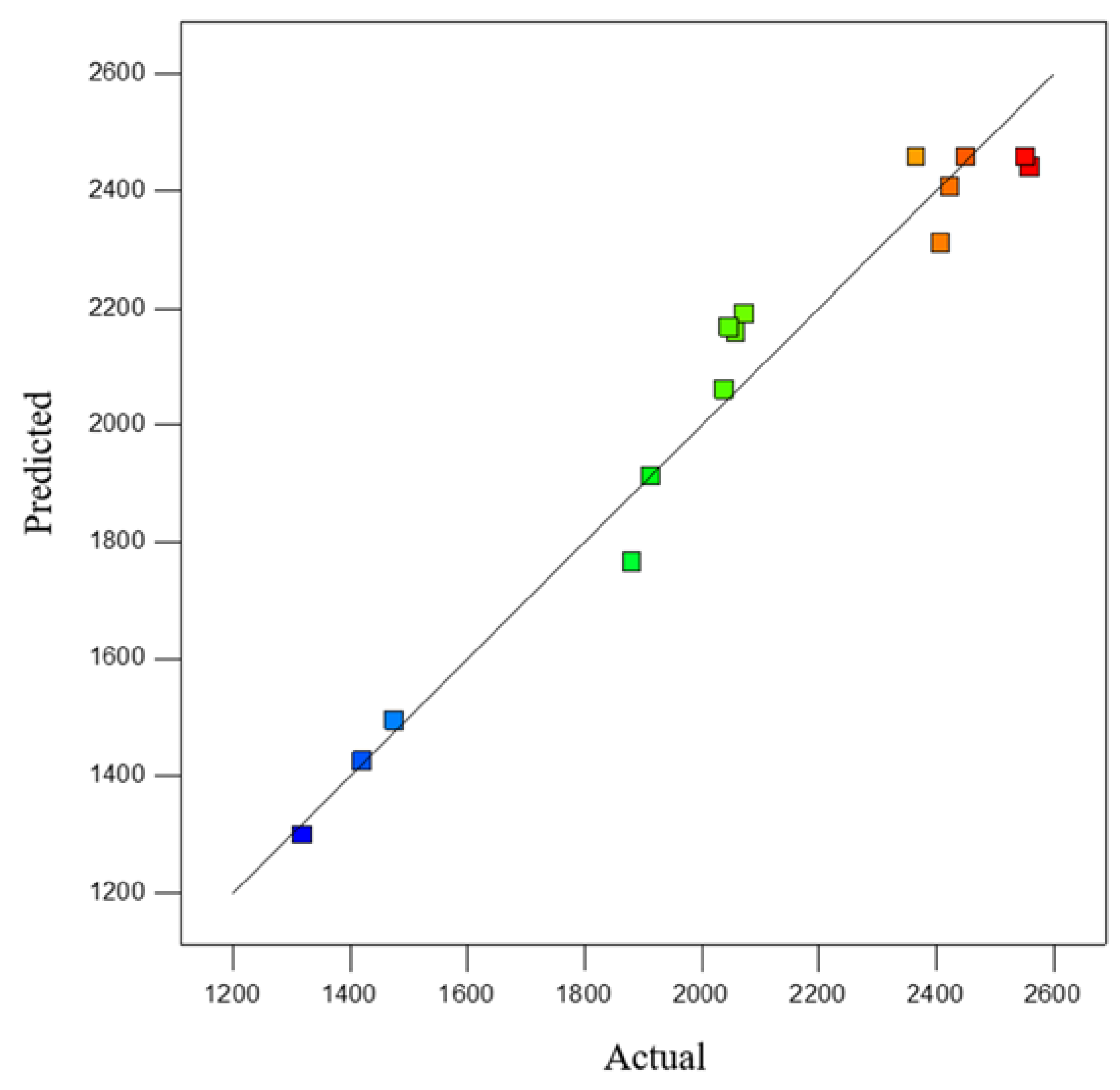
| Run | Factor | Predicted Value/N | Actual Value/N | ||
|---|---|---|---|---|---|
| Roasting Temperature/°C A | Pellet Diameter/mm B | Bentonite Content/% C | |||
| 1 | −1 | 0 | −1 | 2111.05 | 2059.00 |
| 2 | 0 | 1 | −1 | 2392.50 | 2560.00 |
| 3 | −1 | −1 | 0 | 1717.20 | 1881.00 |
| 4 | 0 | −1 | −1 | 1444.30 | 1476.00 |
| 5 | 0 | 1 | 1 | 2359.00 | 2424.00 |
| 6 | 0 | 0 | 0 | 2408.50 | 2452.00 |
| 7 | 0 | −1 | 1 | 2117.60 | 2047.00 |
| 8 | 0 | 0 | 0 | 2408.50 | 2366.00 |
| 9 | 1 | −1 | 0 | 1247.20 | 1319.00 |
| 10 | 1 | 0 | −1 | 1373.55 | 1421.00 |
| 11 | −1 | 1 | 0 | 2014.00 | 2039.00 |
| 12 | 0 | 0 | 0 | 2408.50 | 2553.00 |
| 13 | 1 | 1 | 0 | 2140.00 | 2073.00 |
| 14 | −1 | 0 | 1 | 1865.45 | 1915.00 |
| 15 | 1 | 0 | 1 | 2258.95 | 2408.00 |
| Variable | Statistical Analysis | ||||
|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | F-Value | p-Value | |
| Model | 2,251,335.15 | 9 | 250,148.35 | 13.26 | 0.0055 |
| A | 56,616.13 | 1 | 56,616.13 | 3.00 | 0.1438 |
| B | 703,891.12 | 1 | 703,891.12 | 37.31 | 0.0017 |
| C | 204,160.51 | 1 | 204,160.51 | 10.82 | 0.0217 |
| AB | 88,804.00 | 1 | 88,804.00 | 4.71 | 0.0822 |
| AC | 319,790.25 | 1 | 319,790.25 | 16.95 | 0.0092 |
| BC | 124,962.25 | 1 | 124,962.25 | 6.62 | 0.0498 |
| A2 | 598,176.92 | 1 | 598,176.92 | 31.70 | 0.0024 |
| B2 | 189,423.69 | 1 | 189,423.69 | 10.04 | 0.0249 |
| C2 | 39,744.23 | 1 | 39,744.23 | 2.11 | 0.2064 |
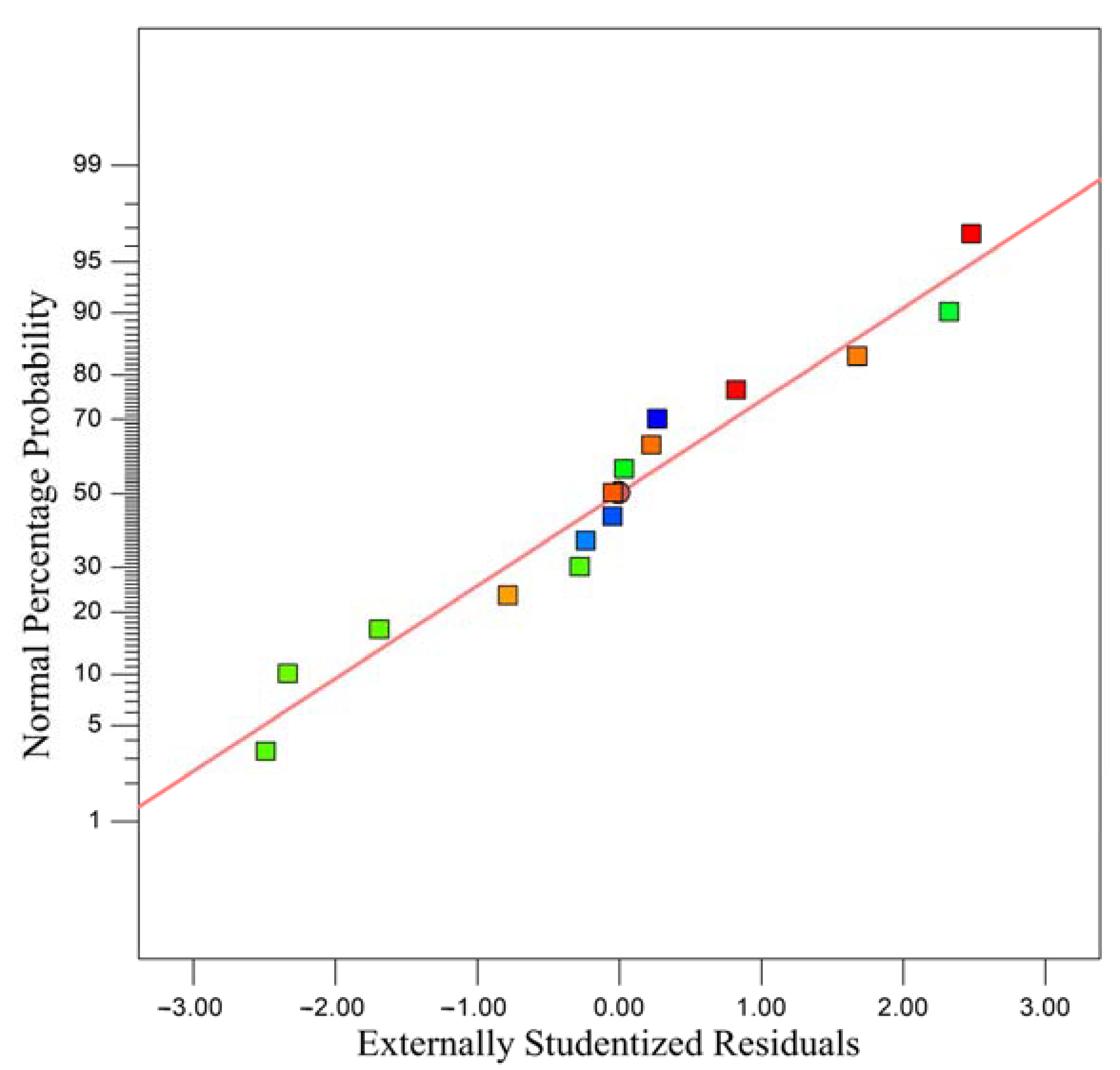
| Residual | 94,341.25 | 5 | 18,868.25 | ||
| Lack of Fit | 76,819.25 | 3 | 25,606.42 | 2.92 | 0.2652 |
| Pure Error | 17,522.00 | 2 | 8761.00 | ||
| Cor total | 12,345,676.40 | 14 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhou, X.; Gao, L.; Fang, H. Study on the Roasting Process of Guisha Limonite Pellets. Materials 2022, 15, 8845. https://doi.org/10.3390/ma15248845
Zhang C, Zhou X, Gao L, Fang H. Study on the Roasting Process of Guisha Limonite Pellets. Materials. 2022; 15(24):8845. https://doi.org/10.3390/ma15248845
Chicago/Turabian StyleZhang, Chuang, Xiaolei Zhou, Lei Gao, and Haoyu Fang. 2022. "Study on the Roasting Process of Guisha Limonite Pellets" Materials 15, no. 24: 8845. https://doi.org/10.3390/ma15248845
APA StyleZhang, C., Zhou, X., Gao, L., & Fang, H. (2022). Study on the Roasting Process of Guisha Limonite Pellets. Materials, 15(24), 8845. https://doi.org/10.3390/ma15248845






