The Role of Mg Content and Aging Treatment on the Tensile and Fatigue Properties of Die-Cast 380 Alloy
Abstract
1. Introduction
2. Experimental Procedure
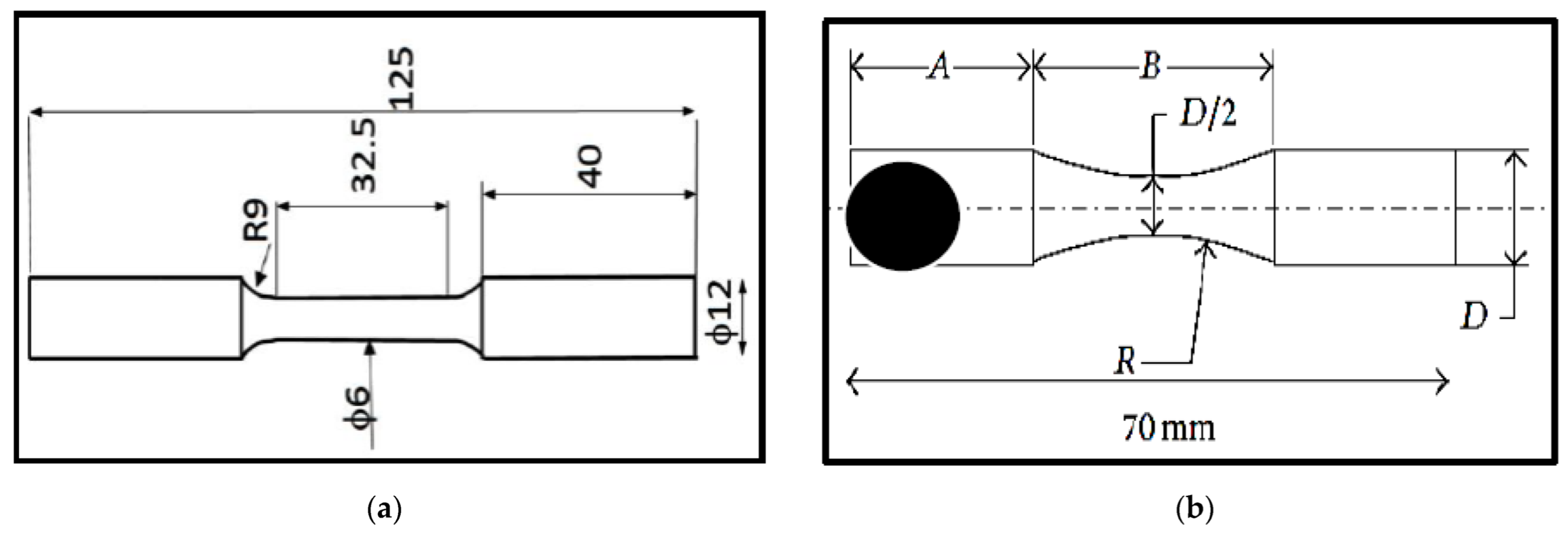
2.1. Preparation of Tensile and Fatigue Samples
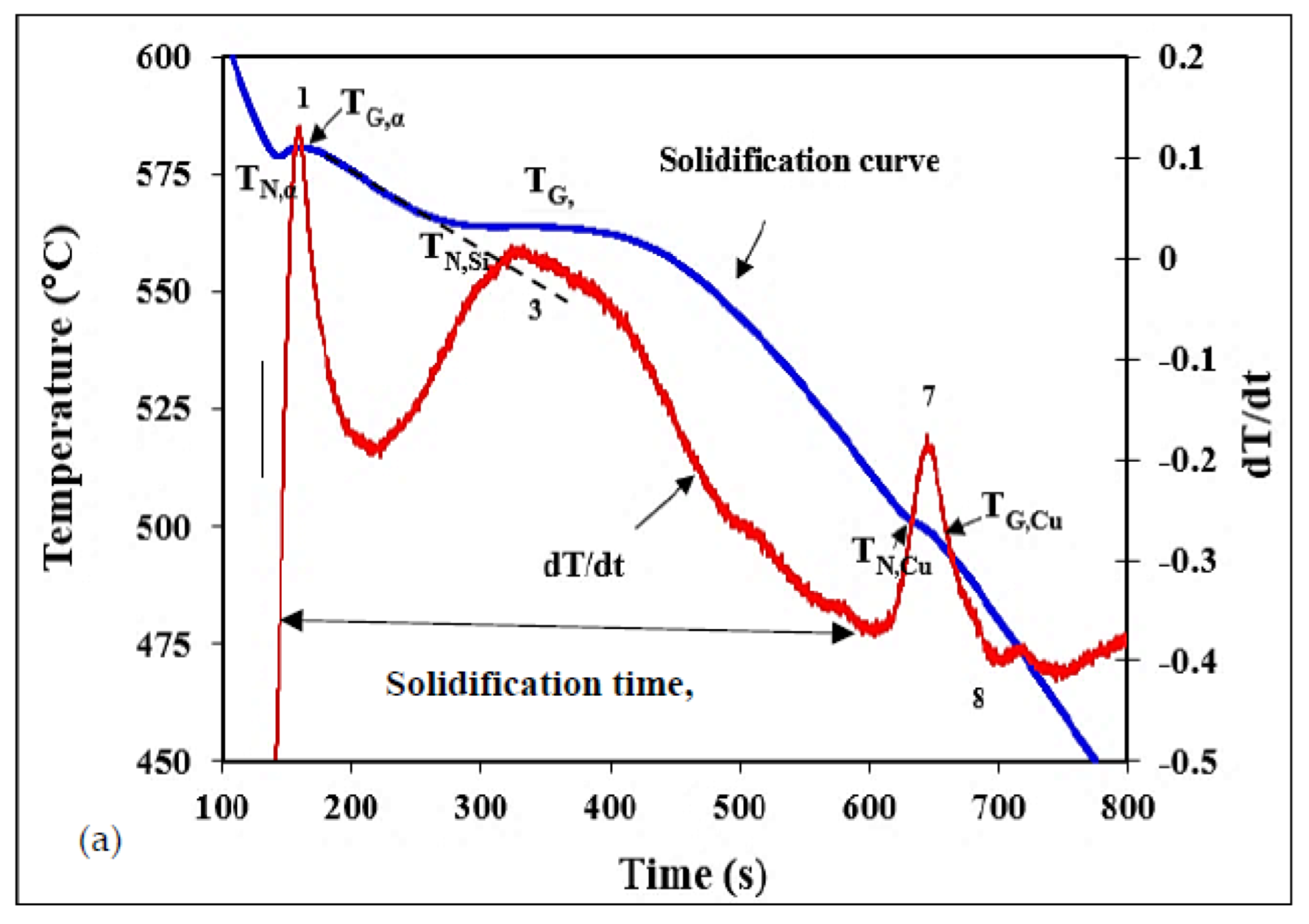
2.2. Thermal Analysis
2.3. Metallography and Fractography
3. Results and Discussion
3.1. Thermal Analysis
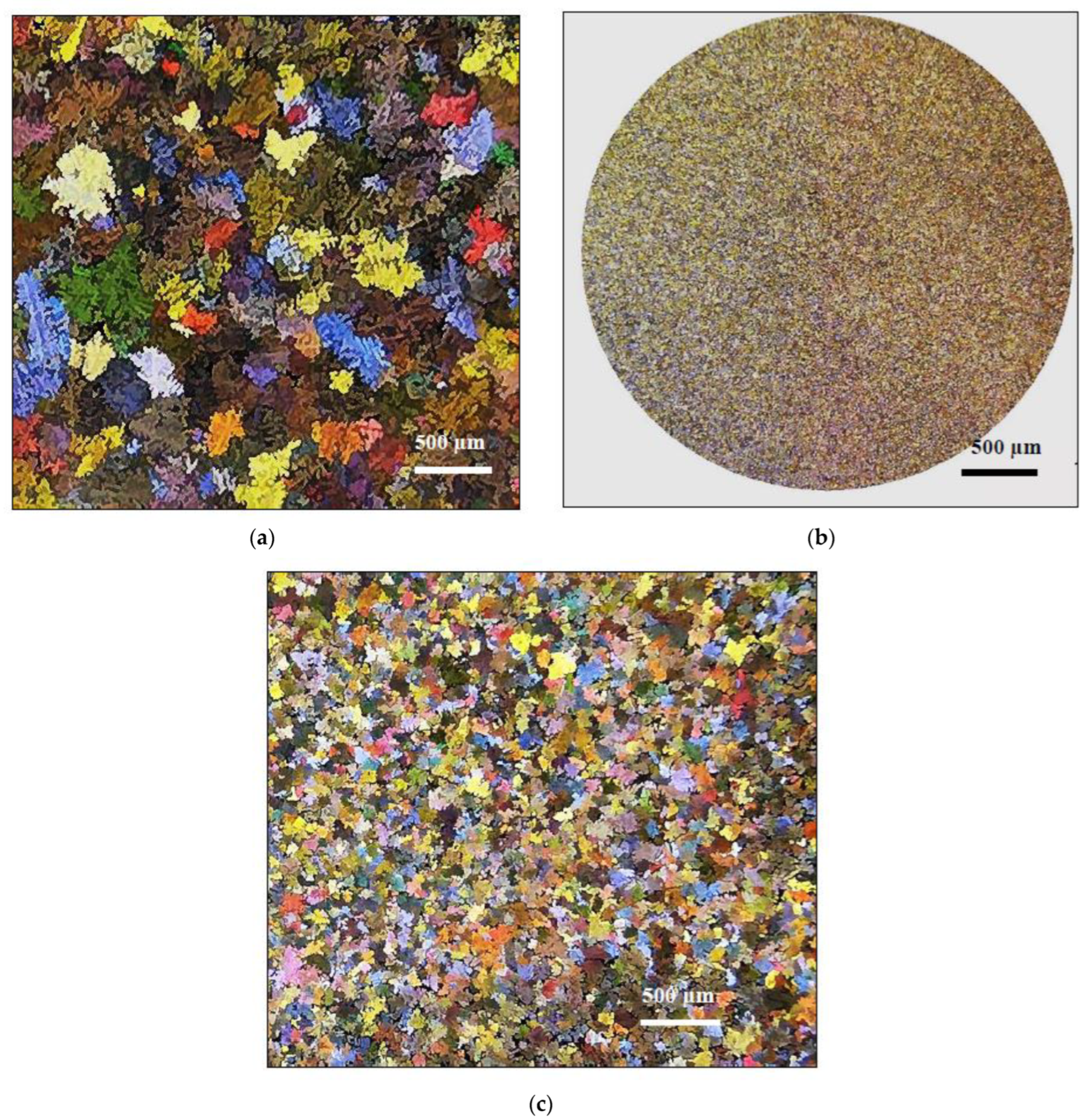
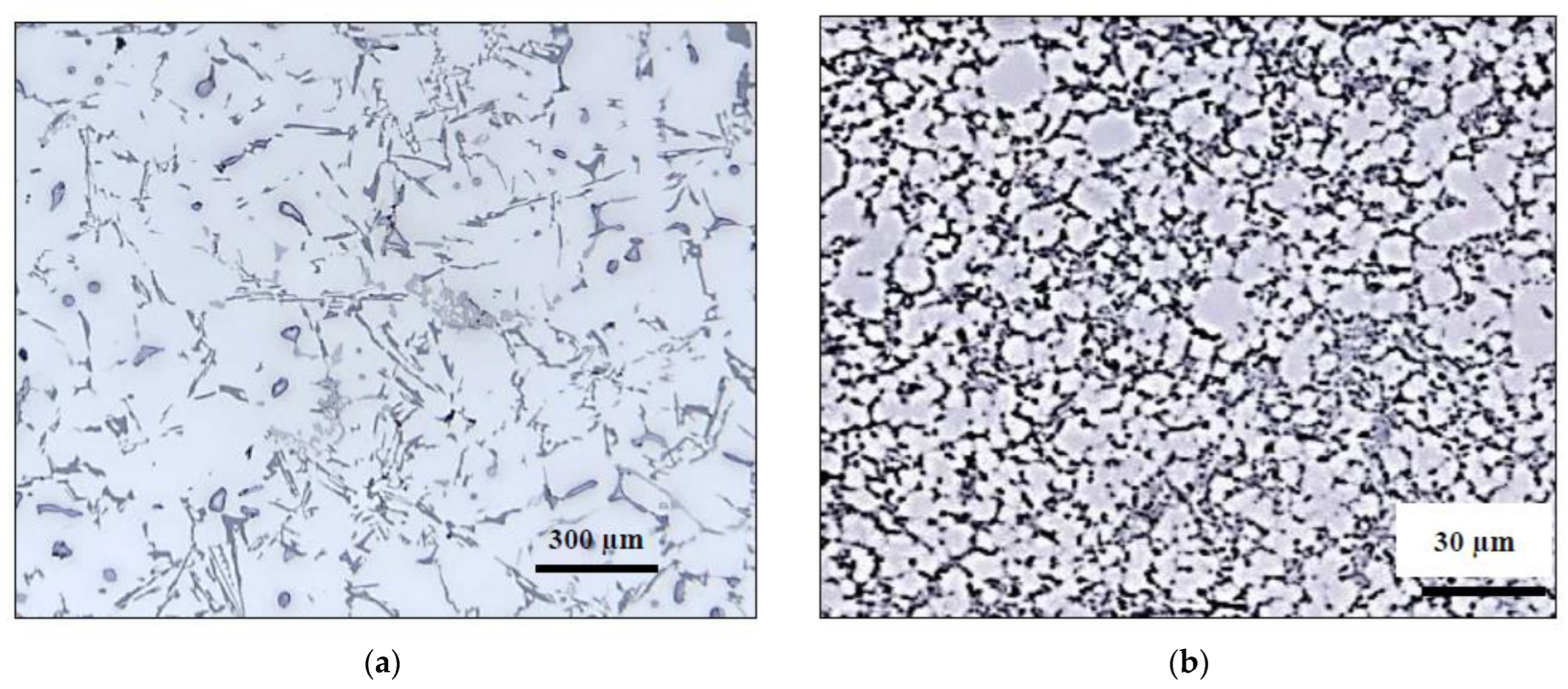
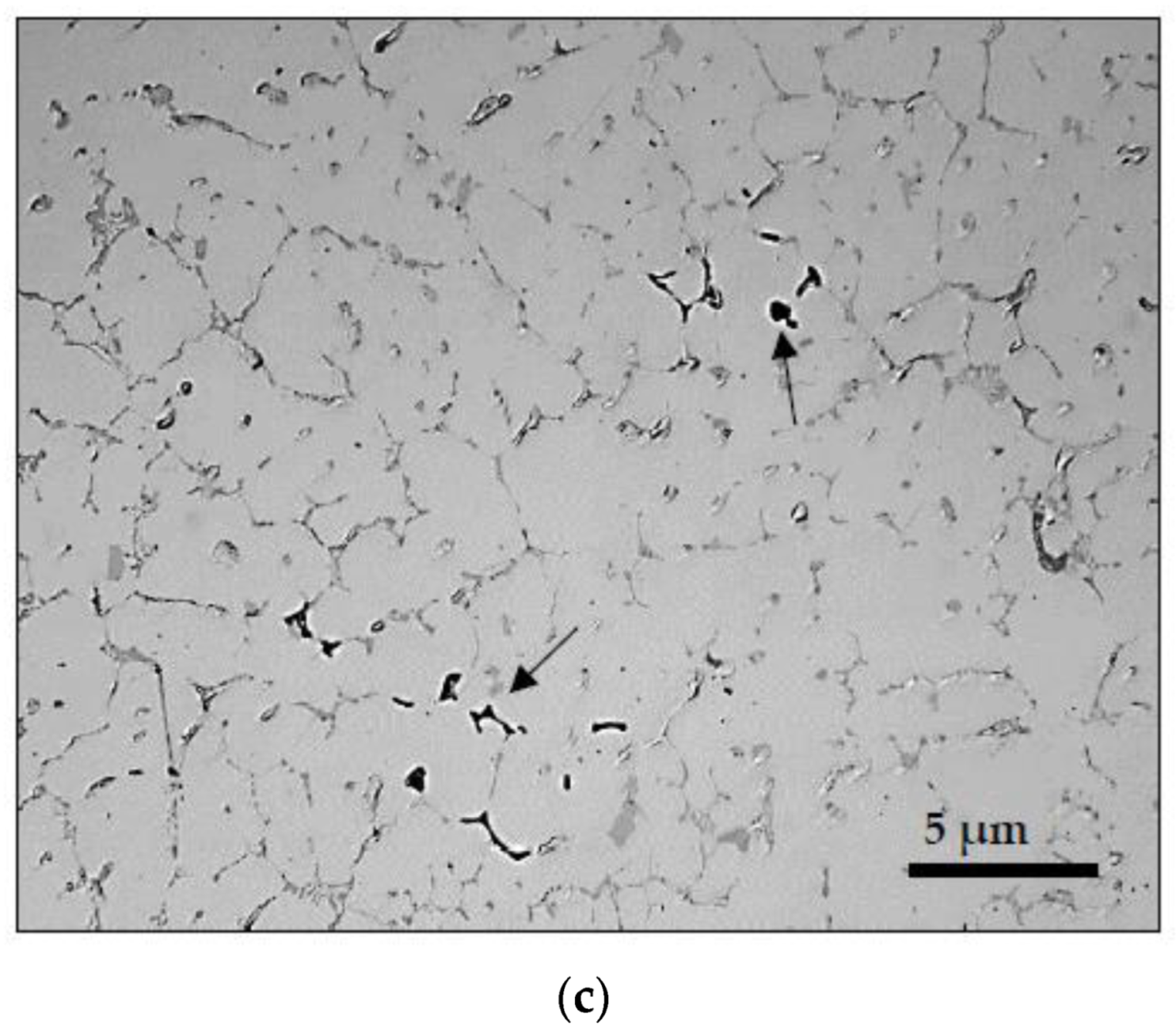
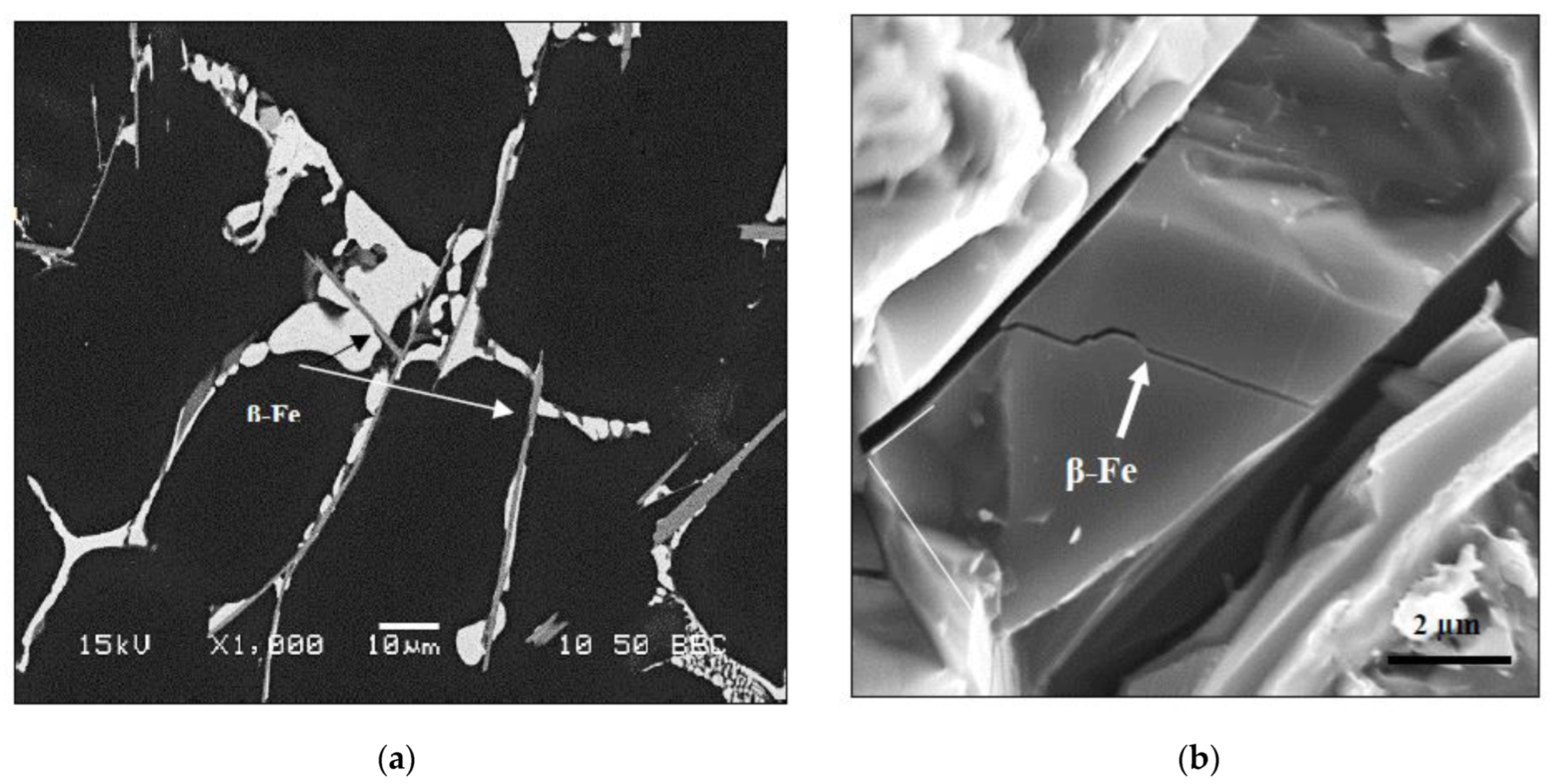
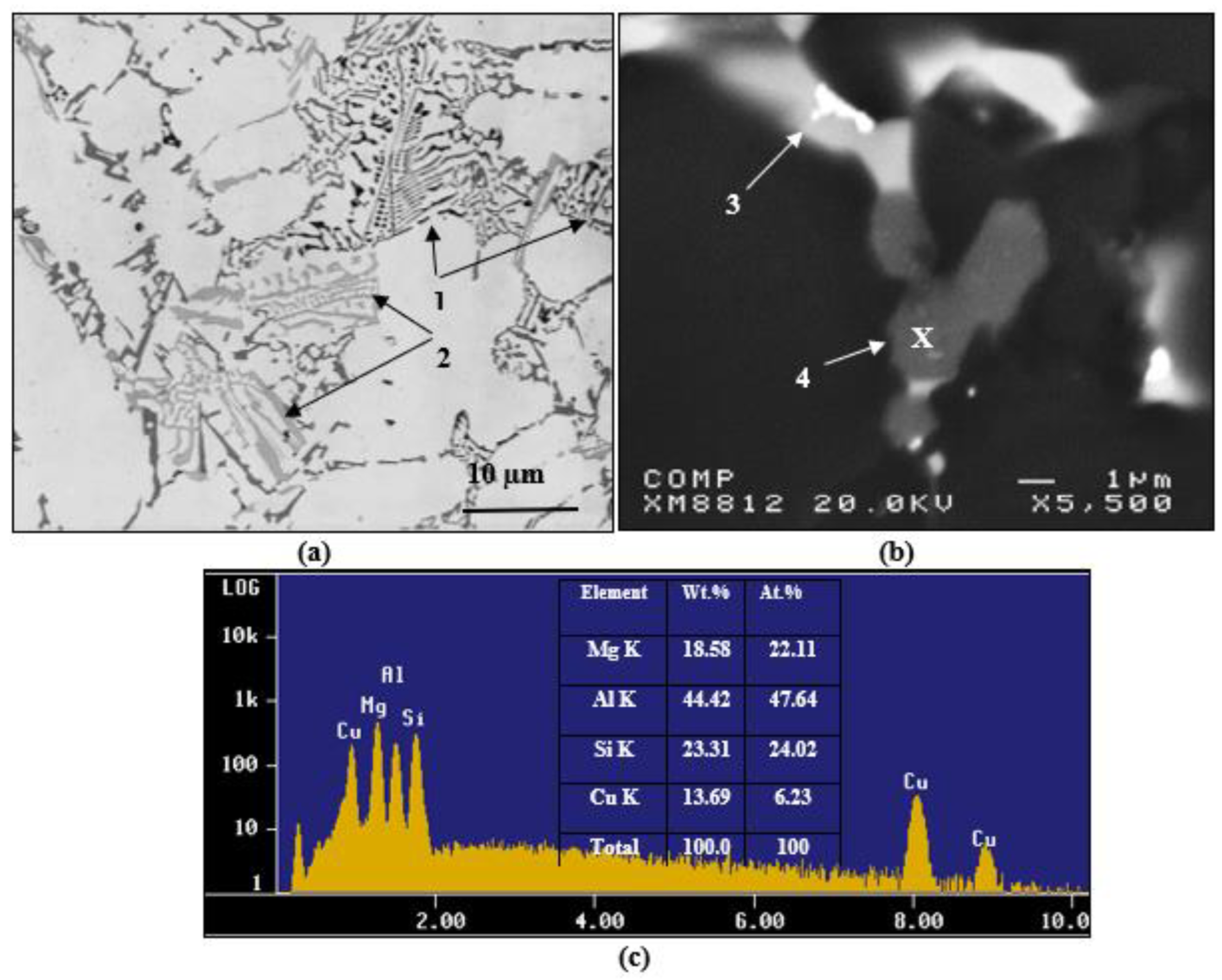
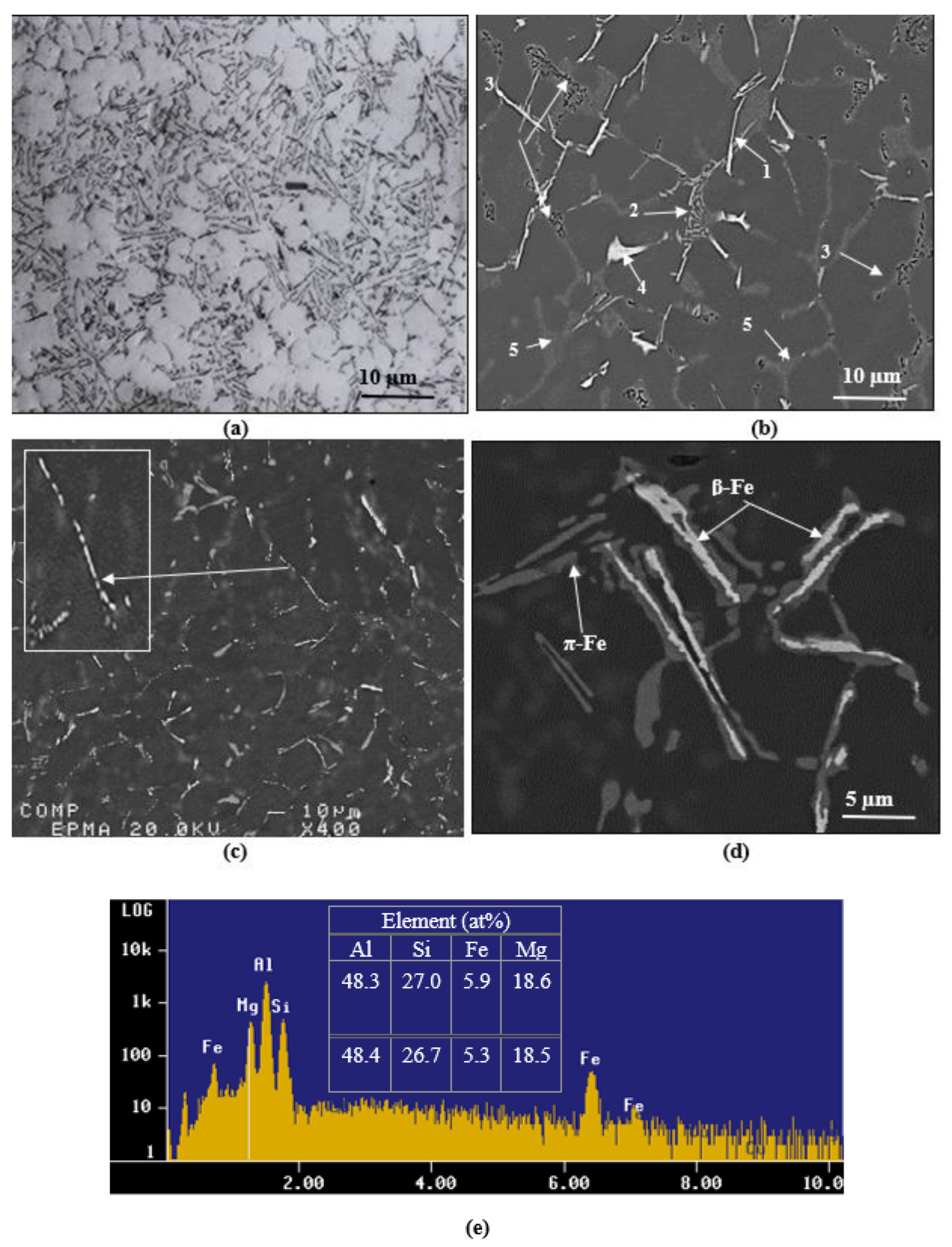
3.2. Macrostructure and Microstructure
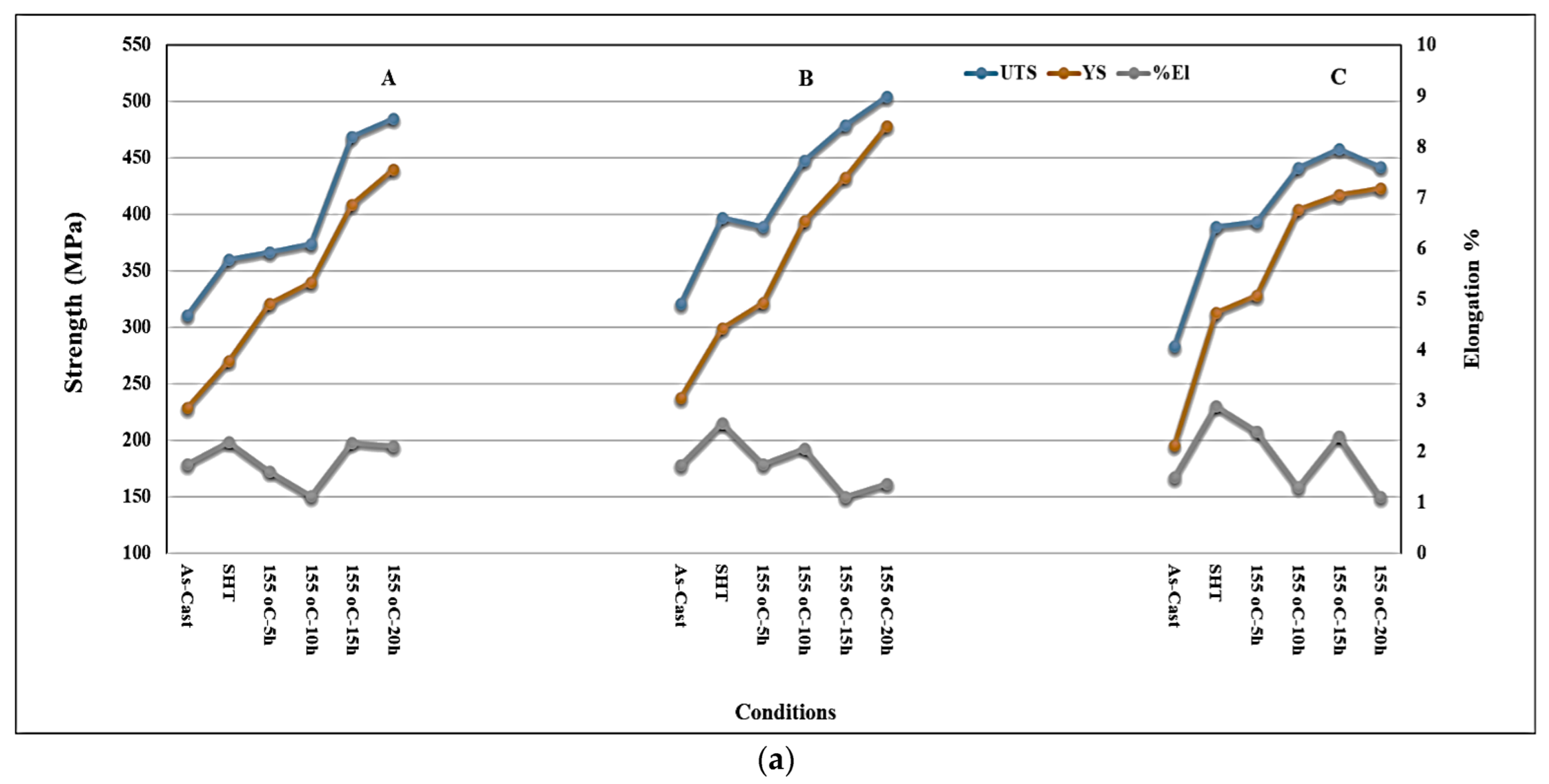
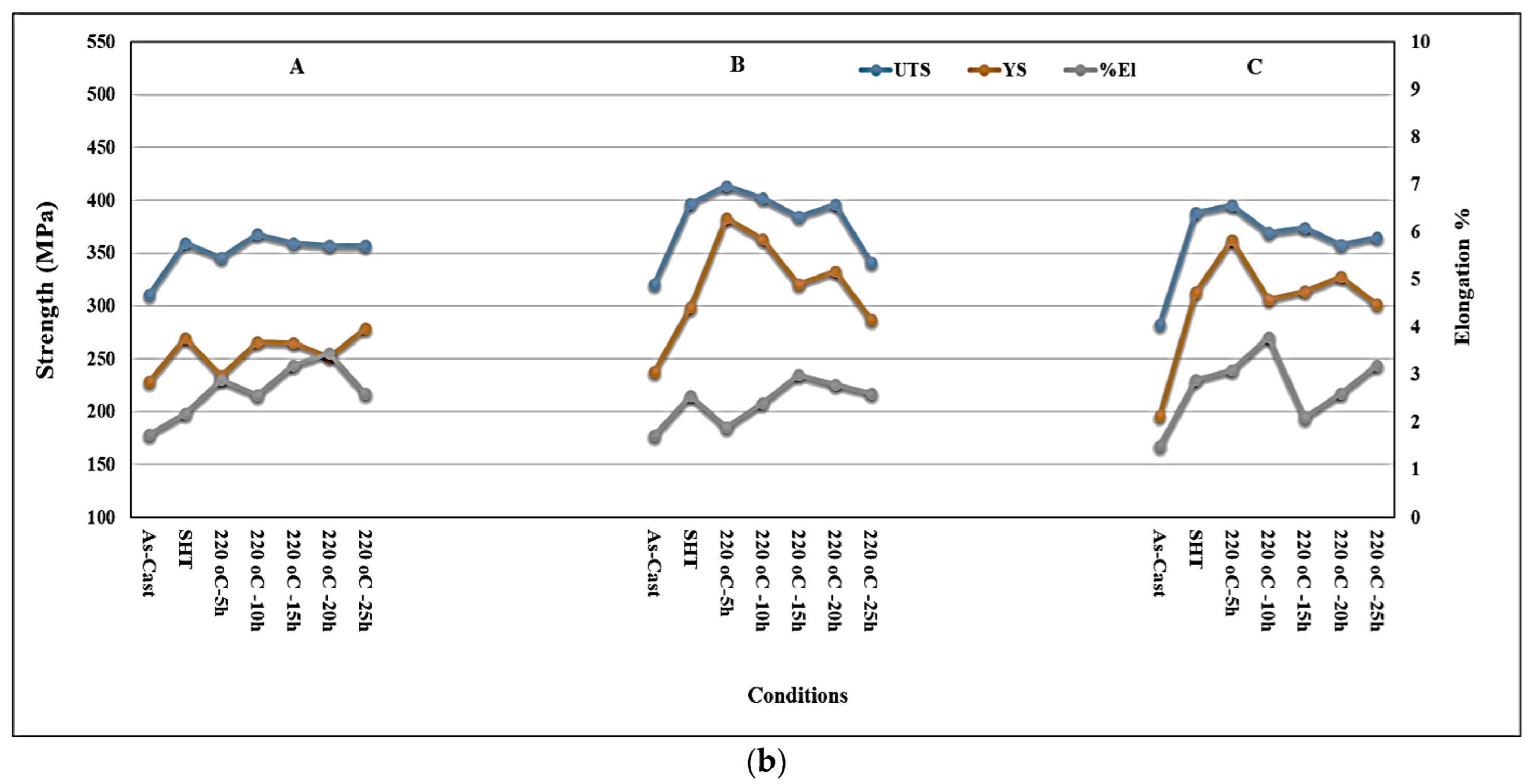
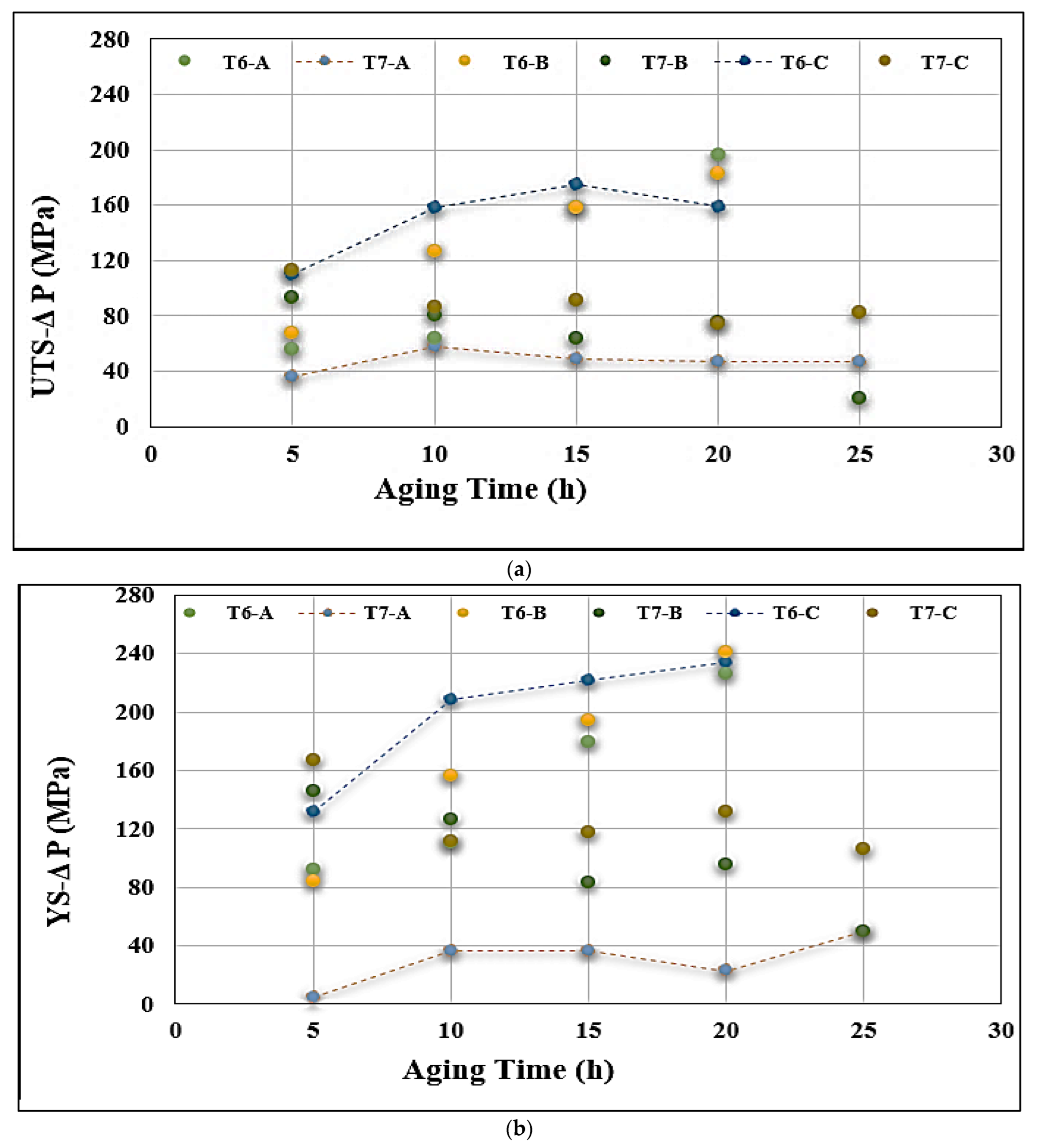
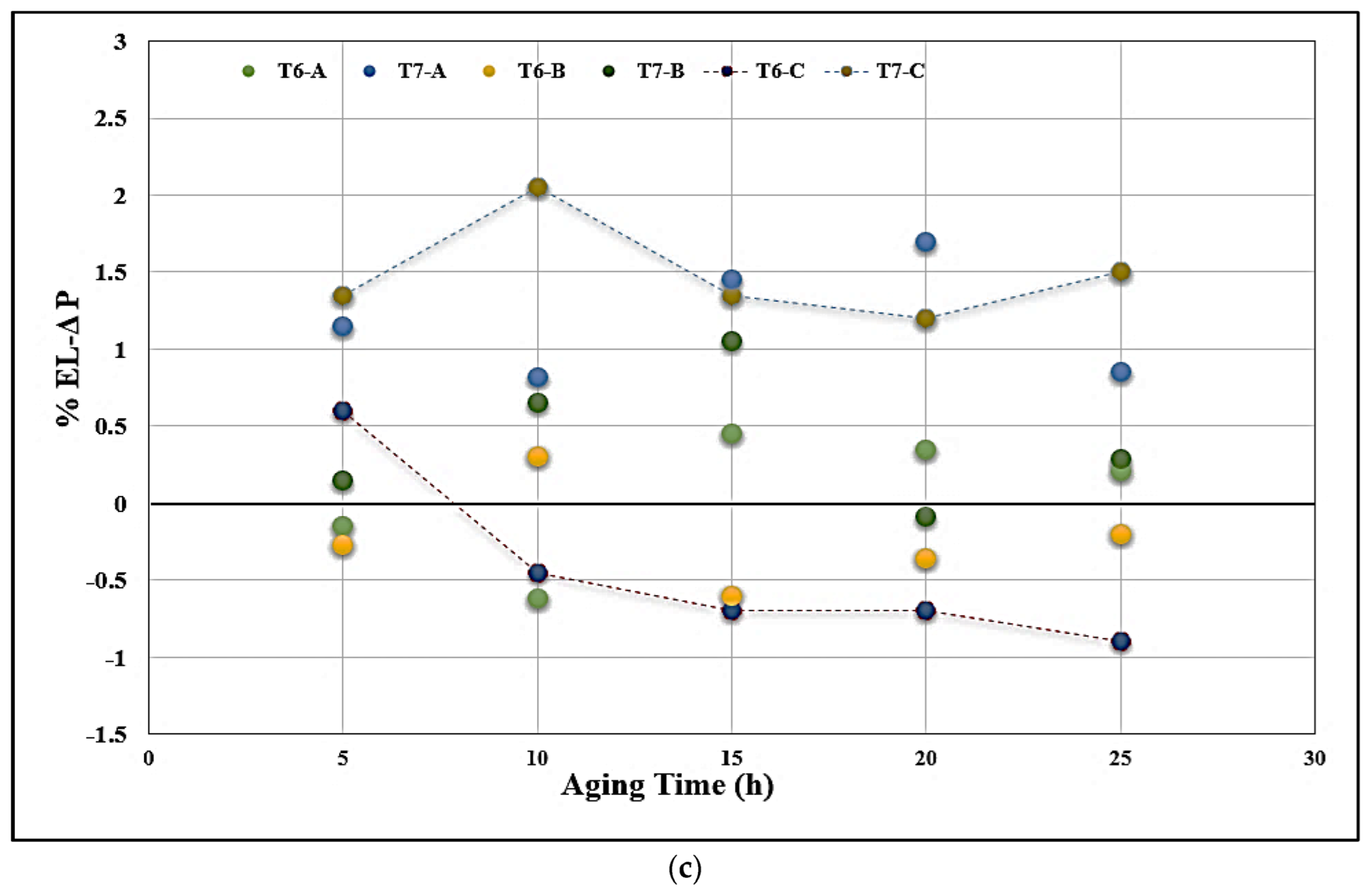
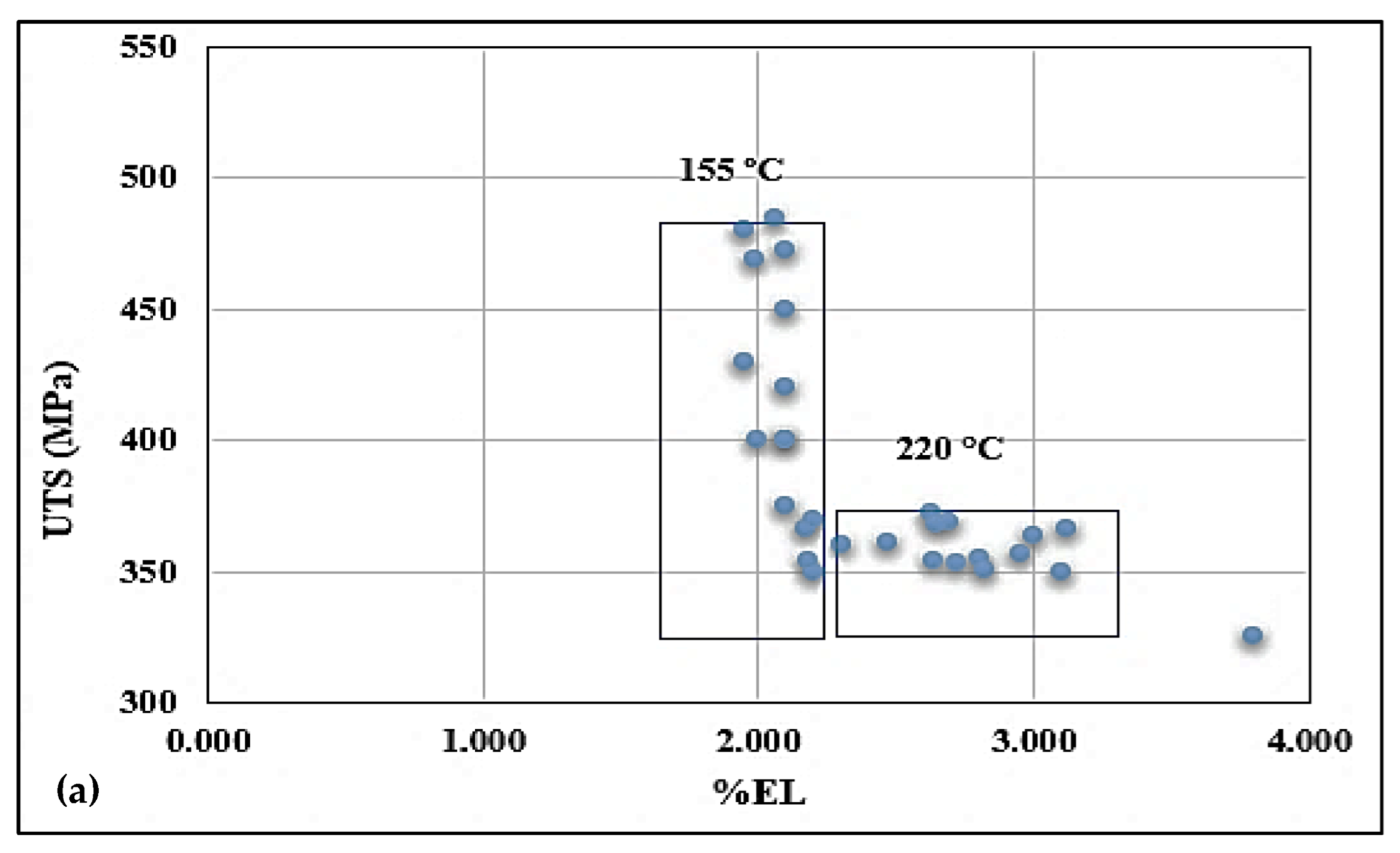
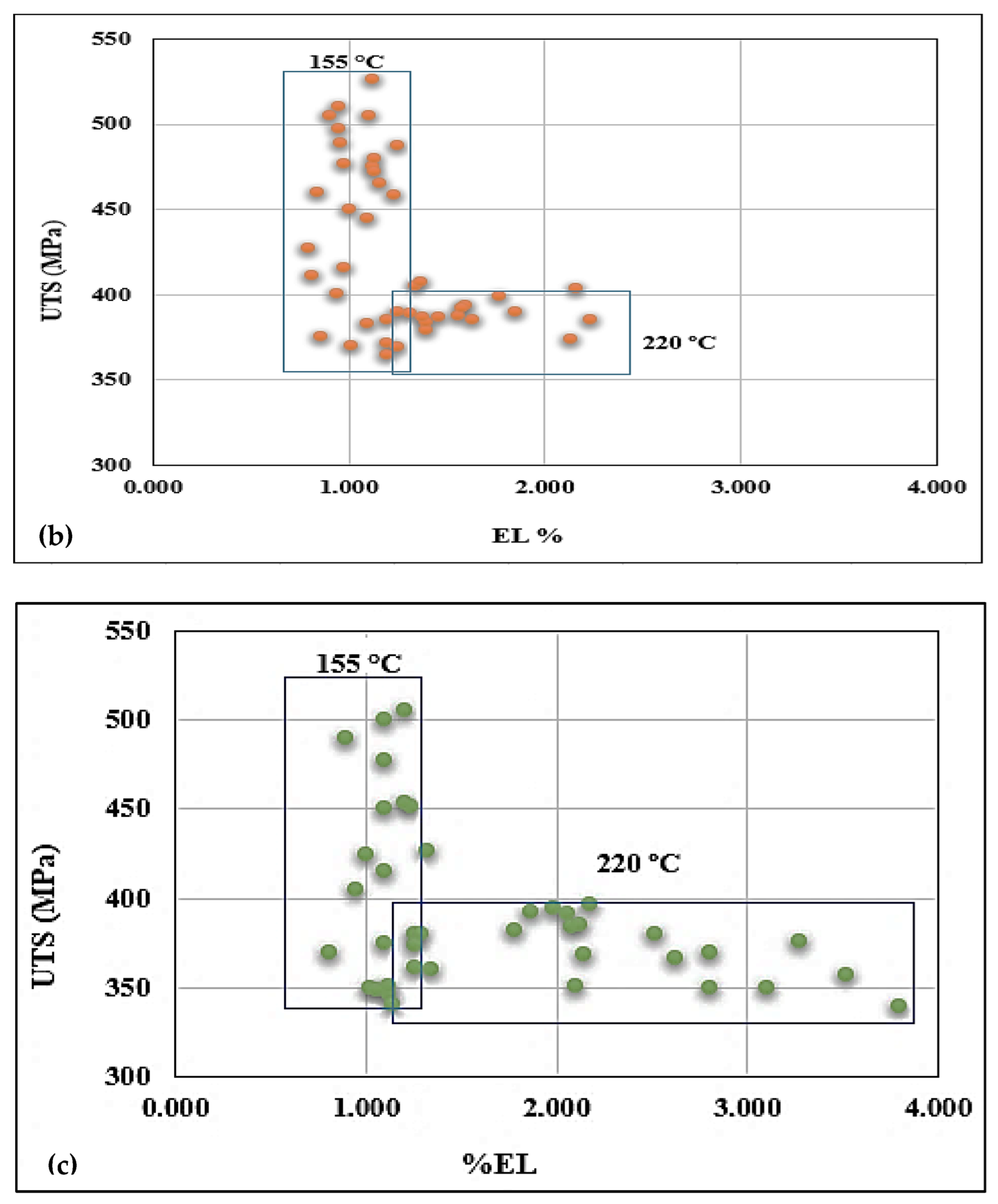
3.3. Tensile Properties and Fractography
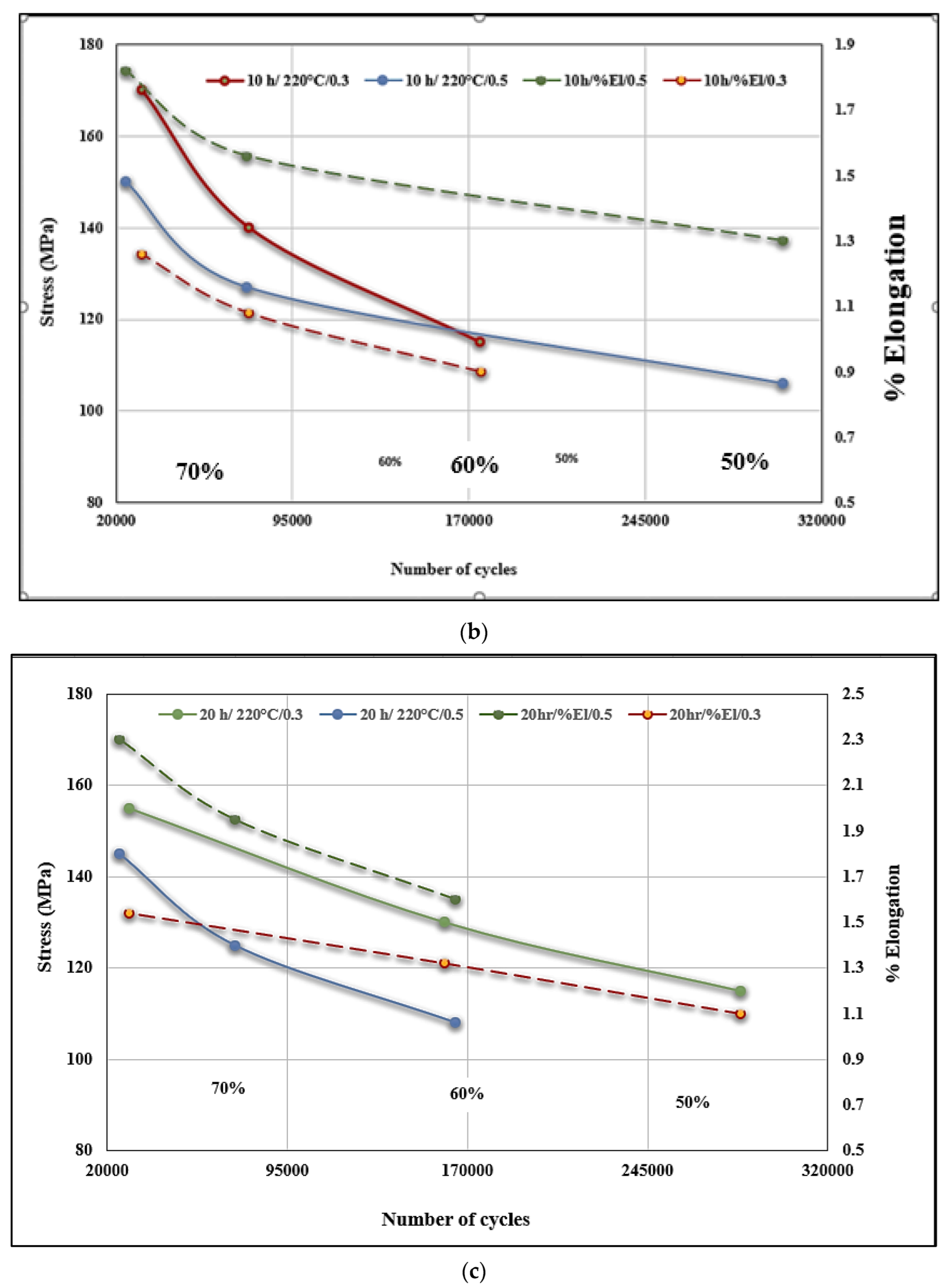
3.4. Fatigue Behavior
4. Conclusions
- At a high Mg content in 380 alloys, i.e., 0.5%, the Q–Al5Mg8Si6Cu2 phase precipitates in the form of large particles at the edges of the Al2Cu phase particles instead of growing out of the (Al–Al2Cu) eutectic structure. In addition, its precipitation temperature is higher than that of the Al2Cu formation temperature.
- Both Mg2Si and π-phase precipitate in a relatively large volume fraction and size.
- The use of the slope of the solidification curve in the mushy zone for measuring the solidification rate is not accurate due to the presence of several irregularities caused by the precipitation of different phases in this zone. It is suggested that the solidification time should be used instead.
- Low-pressure die casting using a cold chamber and small samples would result in a solidification rate of the order of about 800 °C/s corresponding to a dendrite arm spacing of 3–5 µm, compared to 85 µm for castings solidified at a slow rate (~0.35 °C/s).
- Solution heat treatment at 495 °C for 8 h causes the transformation of β-Fe to π-Fe that leads to a thinning of the β-Fe platelets with the appearance of multiple irregularities on the surface of the Fe platelets.
- Alloys containing 0.5% Mg offer tensile properties somewhat lower than those obtained from 0.3% Mg-containing alloys. However, the 0.5% Mg alloy aged at 155 °C provides a maximum contribution to the strength of the base alloy containing 0.06% Mg which offered minimum contribution under the same aging condition (a base alloy in the as-cast condition was used as a reference alloy).
- Quality index charts plotted for the three studied alloys revealed significantly different plots when the alloys were aged at 155 °C or 220 °C.
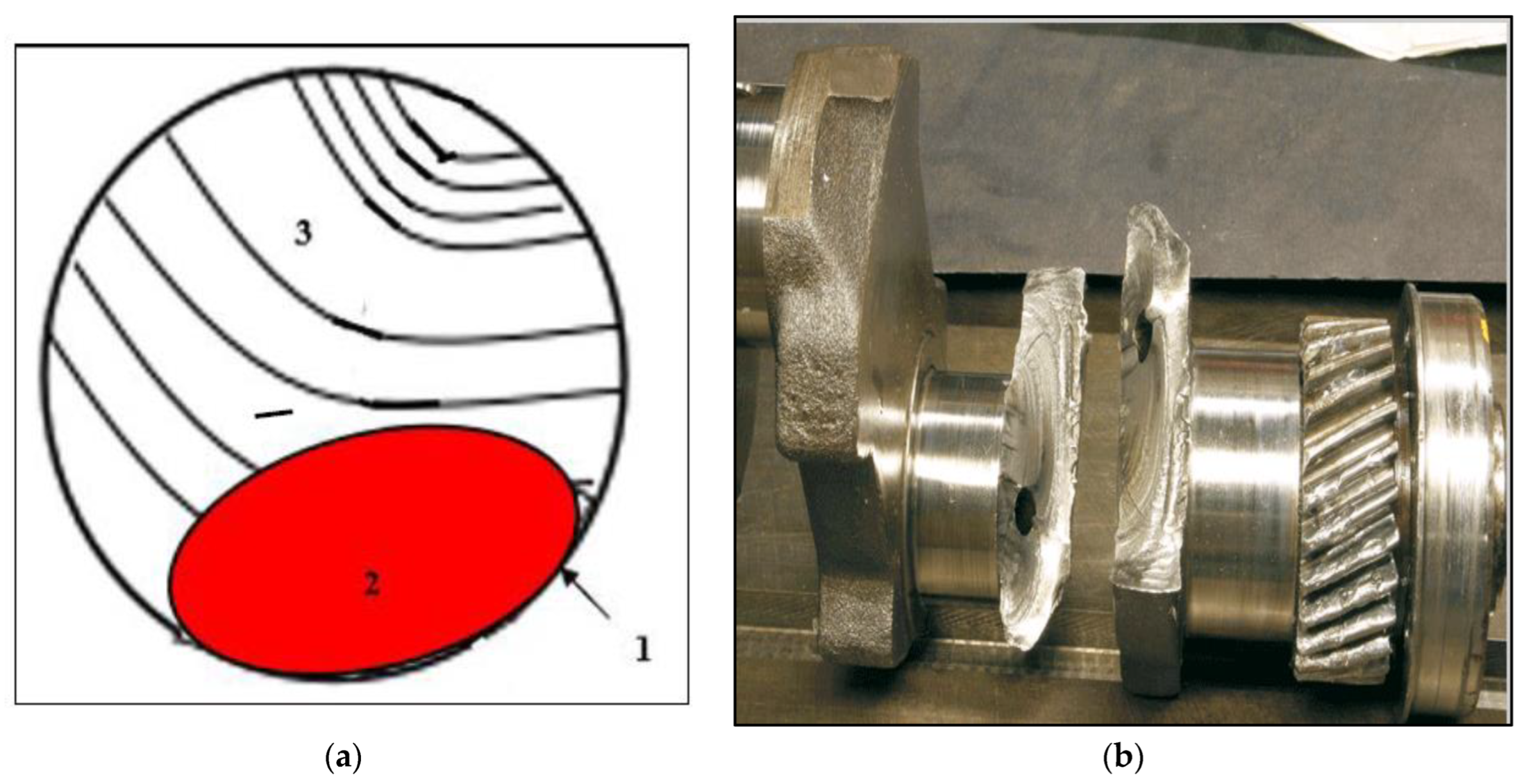
- The fracture of fatigue-tested samples was found to be initiated by imperfections at the outer surfaces of the tested bars.
Author Contributions
Funding
Institutional Review Board Statements
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Makhlouf, M.M.; Apelian, D. Aluminium die casting alloys: Alloy composition, microstructure, and properties-performance relationships. Int. Mater. Rev. 1995, 40, 221–238. [Google Scholar] [CrossRef]
- Wang, B.; Bourne, G.R.; Korenyi-Both, A.L.; Monroe, A.K.; Midson, S.P.; Kaufman, M.J. Method to evaluate the adhesion behavior of aluminum-based alloys on various materials and coatings for lube-free die casting. J. Mater. Process. Technol. 2016, 237, 386–393. [Google Scholar] [CrossRef]
- Li, D.; Cui, C.; Wang, X.; Wang, Q.; Chen, C.; Liu, S. Microstructure evolution and enhanced mechanical properties of eutectic Al–Si die cast alloy by combined alloying Mg and La. Mater. Des. 2016, 90, 820–828. [Google Scholar] [CrossRef]
- Yıldırım, M.; Özyürek, D. The effects of Mg amount on the microstructure and mechanical properties of Al–Si–Mg alloys. Mater. Des. 2013, 51, 767–774. [Google Scholar] [CrossRef]
- Gowri, S.V.; Samuel, F.H. Effect of magnesium on the solidification behavior of two aluminum die casting alloys of the type AlSiCu3. Giess.-Prax. 1996, 19–20. [Google Scholar]
- Samuel, A.M.; Samuel, F.H.; Doty, H.W.; Gowri, S.V. Influence of strontium and aluminum oxides on porosity formation in Al-Si castings. In Proceedings of the 122nd Metalcasting Congress, Fort Worth, TX, USA, 3–5 April 2018; American Foundry Society: Fort Worth, TX, USA, 2018. [Google Scholar]
- Samuel, F.H.; Samuel, A.M.; Liu, H. Effect of magnesium content on the ageing behaviour of water-chilled Al-Si-Cu-Mg-Fe-Mn (380) alloy castings. J. Mater. Sci. 1995, 30, 2531–2540. [Google Scholar] [CrossRef]
- Wan, L.; Hu, Z.; Wu, S.; Liu, X. Mechanical properties and fatigue behavior of vacuum-assist die cast AlMgSiMn alloy. Mater. Sci. Eng. A 2013, 576, 252–258. [Google Scholar] [CrossRef]
- Hartlieb, M. Aluminum alloys for structural die cast. Die Cast. Eng. 2013, 40–43. Available online: www.diecasting.org/dce (accessed on 1 December 2022).
- Anilchandra, A.R.; Arnberg, L.; Bonollo, F.; Fiorese, E.; Timelli, G. Evaluating the Tensile Properties of Aluminum Foundry Alloys through Reference Castings—A Review. Materials 2017, 10, 1011. [Google Scholar] [CrossRef]
- Adamane, A.R.; Arnberg, L.; Fiorese, E.; Timelli, G.; Bonollo, F. Influence of injection parameters on the porosity and tensile properties of high-pressure die cast Al-Si alloys: A review. Int. J. Metalcast. 2015, 9, 43–53. [Google Scholar] [CrossRef]
- Gunasegaram, D.R.; Finnin, B.R.; Polivka, F.B. Melt flow velocity in high pressure die casting:its effect on microstructure and mechanical properties in an Al–Si alloy. Mater. Sci. Technol. 2007, 23, 847–856. [Google Scholar] [CrossRef]
- Jiao, X.Y.; Wang, J.; Liu, C.F.; Guoa, Z.P.; Tong, G.D.; Mac, S.L.; Bi, Y.; Zhang, Y.F.; Xiong, S.M. Characterization of high-pressure die-cast hypereutectic Al-Si alloys based on microstructural distribution and fracture morphology. J. Mater. Sci. Technol. 2019, 35, 1099–1107. [Google Scholar] [CrossRef]
- Grosselle, F.; Timelli, G.; Bonollo, F. DOE applied to microstructural and mechanical properties of Al-Si-Cu-Mg casting alloys for automotive applications. Mater. Sci. Eng. A 2010, 527, 3536–3545. [Google Scholar] [CrossRef]
- Garb, C.; Leitner, M.; Grün, F. Fatigue strength assessment of AlSi7Cu0.5Mg T6W castings supported by computed tomography microporosity analysis. Procedia Eng. 2016, 160, 53–60. [Google Scholar] [CrossRef]
- Lin, C.; Chen, H.; Zeng, L.; Wu, S.; Fang, X. Microstructures and Properties of V-Modified A380 Aluminum Alloy Produced by High Pressure Rheo-Squeeze Casting with Compound Field Treatment. Metals 2021, 11, 587. [Google Scholar] [CrossRef]
- Isakov, M.; Rantalainen, O.; Saarinen, T.; Lehtovaara, A. Large-Scale Fatigue Testing Based on the Rotating Beam Method. Exp. Tech. 2022. [Google Scholar] [CrossRef]
- Murakami, Y. Effect of size and geometry of small defects on the fatigue limit. Met. Fatigue (Second Ed.) 2019, 39–59. [Google Scholar] [CrossRef]
- Wang, J.; Jiao, X.Y.; Xie, H.L.; Denga, B.; Xiong, S.M. Crack configuration feature and fracture surface difference for high pressure die casting hypereutectic Al-Si alloys in high cycle fatigue. Int. J. Fatigue 2021, 153, 106469. [Google Scholar] [CrossRef]
- Samantha, A.; Seffens, R.J.; Das, H.; Guzman, A.D.; Rooswndaal, T.J.; Garcia, D.; Song, M.; Grant, G.J.; Jana, J. Microstructure-refinement-driven enhanced tensile properties of high-pressure die-cast A380 alloy through friction stir processing. J. Manuf. Process. 2022, 78, 352–362. [Google Scholar] [CrossRef]
- Kolahdooz, A. Investigation of the hardness improvement for Al-A380 alloy using the controlled atmosphere in the mechanical stirring casting method. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2019, 233, 225–233. [Google Scholar] [CrossRef]
- Hu, X.; Fang, L.; Zhou, J.; Zhang, X.; Hu, H. Characterization and kinetic modeling of secondary phases in squeeze cast Al alloy A380 by DSC thermal analysis. China Foundry 2017, 14, 98–107. [Google Scholar] [CrossRef]
- Nafisi, S.; Emadi, D.; Shehata, M.T.; Ghomashchi, R. Effects of electromagnetic stirring and superheat on the microstructural characteristics of Al-Si-Fe alloy. Mater. Sci. Eng. A 2006, 432, 71–83. [Google Scholar] [CrossRef]
- Graf, A. Aluminum alloys for lightweight automotive structures. In Materials, Design and Manufacturing for Lightweight Vehicles; Elsevier: Amsterdam, The Netherlands, 2021; pp. 97–123. [Google Scholar] [CrossRef]
- Nakata, K.; Kim, Y.G.; Fujii, H.; Tsumura, T.; Komazaki, T. Improvement of mechanical properties of aluminum die casting alloy by multi-pass friction stir processing. Mater. Sci. Eng. A 2006, 437, 274–280. [Google Scholar] [CrossRef]
- Istrate, B.; Munteanu, C.; Geanta, V.; Baltatu, S.; Focsaneanu, S.; Earar, K. Microstructural analysis of biodegradable Mg-0.9Ca-1.2Zr alloy. Mater. Sci. Eng. 2016, 147, 012033. [Google Scholar] [CrossRef]
- Salahshoor, M.; Guo, Y. Biodegradable Orthopedic Magnesium-Calcium (MgCa) Alloys, Processing, and Corrosion Performance. Materials 2012, 5, 135–155. [Google Scholar] [CrossRef]
- Grant, N.J. Rapid Solidification Processing; Mehrabian, R., Kear, B.H., Cohen, M., Eds.; Claitor Publishing Division: Baton Rouge, LA, USA, 1970; p. 230. [Google Scholar]
- Hao, J.; Yu, B.; Bian, J.; Chen, B.; Wu, H.; Li, W.; Li, Y.; Li, R. Calculation Based on the Formation of Mg2Si and Its Effect on the Microstructure and Properties of Al–Si Alloys. Materials 2021, 14, 6537. [Google Scholar] [CrossRef] [PubMed]
- Malekan, M.; Dayani, D.; Mir, A. Thermal analysis study on the simultaneous grain refinement and modification of 380.3 aluminum alloy. J. Therm. Anal. Calorim. 2014, 115, 393–399. [Google Scholar] [CrossRef]
- Irizalp, S.G.; Saklakoglu, N. Effect of Fe-rich intermetallics on the microstructure and mechanical properties of thixoformed A380 aluminum alloy. Eng. Sci. Technol. Int. J. 2014, 17, 58–62. [Google Scholar] [CrossRef]
- Rajabi, M.; Vahidi, M.; Simchi, A.; Davami, P. Effect of rapid solidification on the microstructure and mechanical properties of hot-pressed Al-20Si-5Fe alloys. Mater. Charact. 2009, 60, 1370–1381. [Google Scholar] [CrossRef]
- Samuel, A.M.; Samuel, F.H. Effect of alloying elements and dendrite arm spacing on the microstructure and hardness of an Al-Si-Cu-Mg-Mg-Fe-Mn (380). J. Mater. Sci. 1995, 301, 1698–1708. [Google Scholar] [CrossRef]
- Narayanan, L.A.; Samuel, F.H.; Gruzleski, J.E. Crystallization behavior of iron-containing intermetallic compounds in 319 aluminum alloy. Metall. Mater. Trans. 1994, 25A, 1761–1773. [Google Scholar] [CrossRef]
- Ji, S.; Yang, W.W.; Wang, Y.; Fan, Z. Effect of Mg level on the microstructure and mechanical properties of die-cast Al–Si–Cu alloys. Mater. Sci. Eng. A 2015, 642, 340–350. [Google Scholar]
- Buchanan, K.; Colas, K.; Ribis, J.; Lopez, A.; Garnier, J. Precipitation behavior in an Al–Cu–Mg–Si alloy during ageing. Acta. Mater. 2017, 132, 209–221. [Google Scholar] [CrossRef]
- Bäckerud, L.; Chai, G.; Tamminen, J. (Eds.) Solidification Characteristics of Aluminum Alloys, Vol. 2: Foundry Alloys; AFS/SKANALUMINIUM: Des Plaines, IL, USA, 1990. [Google Scholar]
- Garza Elizondo, G.H.; Elsharkawi, E.A.; Samuel, A.M.; Doty, H.W.; Samuel, F.H. Effects of Alloying Elements Additions on Ambient Temperature Performance of Al–Si–Cu–Mg Base Alloys. Int. J. Met. 2021, 15, 1385–1401. [Google Scholar] [CrossRef]
- Elsharkawi, E.A. Effets Des Parametres Metallurgiques Sur la Decomposition des Phases π-AlFeMgSi Dans les Alliages Al-Si-Mg et son Influence sur les Proprietes Mecaniques. Ph.D. Thesis, UQAC, Chicoutimi, QC, Canada, 2011. [Google Scholar]
- Andrade, N.R.; Gruzleski, J.E.; Samuel, F.H.; Valtierra, S.; Doty, H.W. Age-Hardening Precipitates in Cast 319 Aluminum Alloys. In International Symposium on Aluminium: From Raw Materials to Applications, Proceedings of the 45th Annual Conference of Metallurgists of CIM, Montreal, QC, Canada, 1–4 October 2006; The Metallurgy and Materials Society of The Canadian Institute of Mining, Metallurgy and Petroleum (MetSoc of CIM): Montreal, QC, Canada, 2006; pp. 104–114. [Google Scholar]
- Drouzy, M.; Jacob, S.; Richard, M. Interpretation of Tensile Results by Means of Quality Index and Probable Yield Strength. AFS Int. Cast Met. J. 1980, 5, 43–50. [Google Scholar]
- Tiryakioğlu, M. Relationship between Defect Size and Fatigue Life Distributions in Al-7 Pct Si-Mg Alloy Castings. Metall Mater. Trans. A 2009, 40, 1623–1630. [Google Scholar] [CrossRef]
- Zhu, S.P.; Ai, Y.; Liao, D.; Correia, J.A.F.O.; de Jesus, A.M.P.; Wang, Q. Recent advances on size effect in metal fatigue under defects: A review. Int. J. Fract. 2021, 234, 21–43. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Z.; Tan, C.; Ito, M.; Pan, P.; Wang, X. Rotating Bending Fatigue Microscopic Fracture Characteristics and Life Prediction of 7075-T7351Al Alloy. Metals 2018, 8, 210. [Google Scholar] [CrossRef]









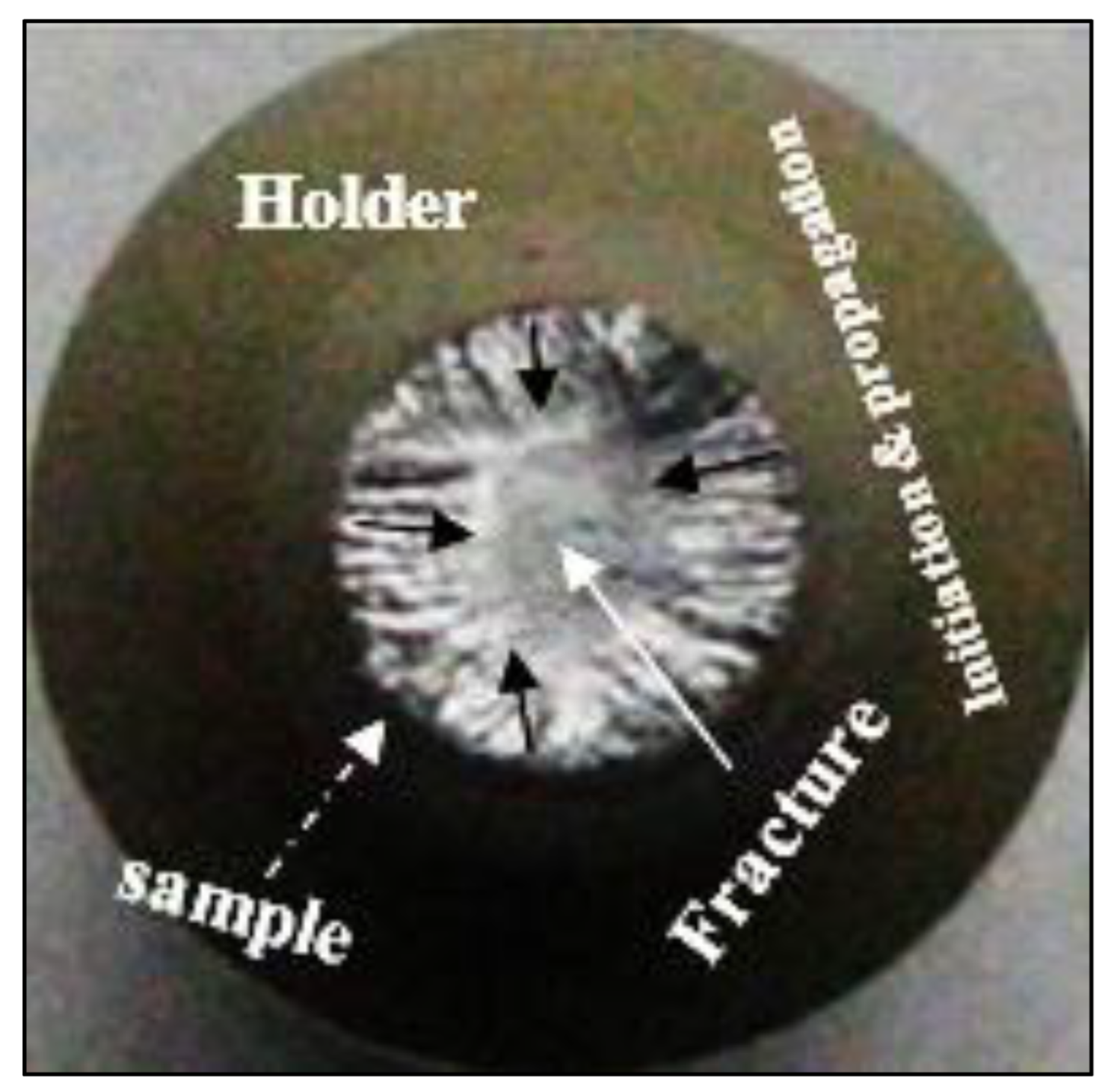
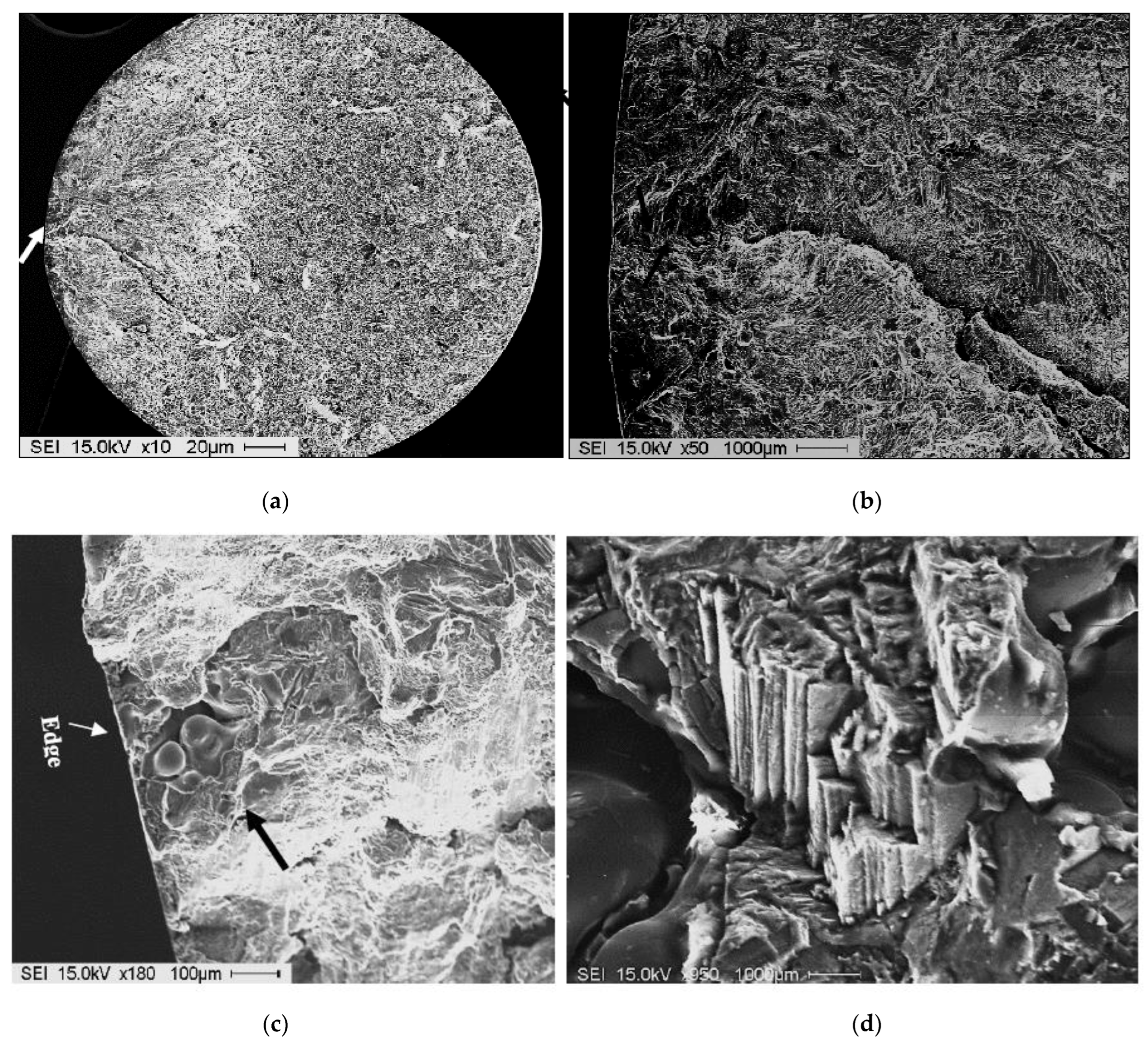
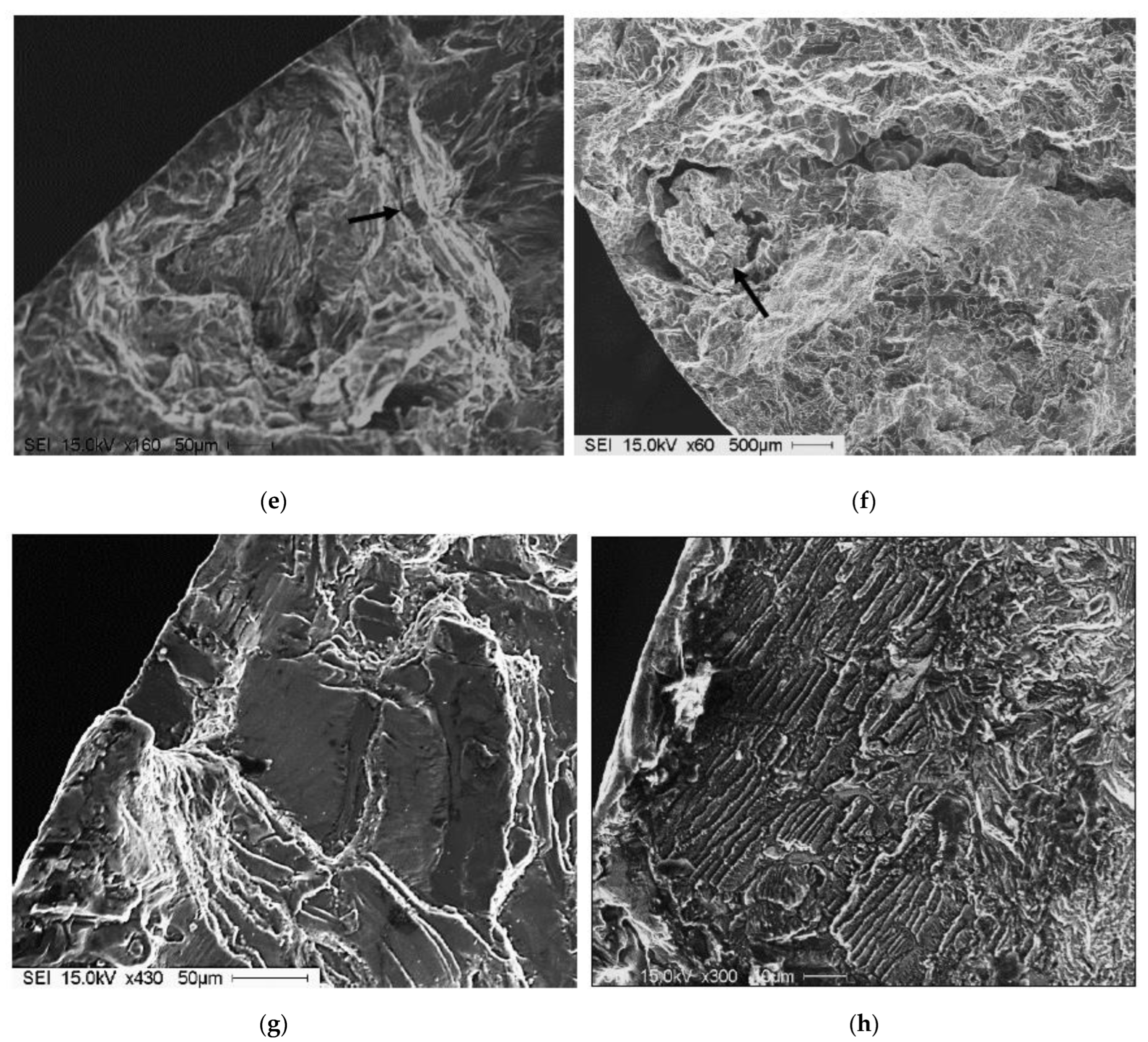
| AA No. | Commercial Designation | Cu | Zn | Fe | Si | Mn | Mg | Cr | Ni | Ti |
|---|---|---|---|---|---|---|---|---|---|---|
| 2081 | 108 | 3.5 4.5 | 1.0 | 0.9 | 2.5 3.50 | 0.50 | 0.10 | 0.35 | 0.25 | |
| 296.1 | B295.0/ B195 | 4.0 5.0 | 0.50 | 0.9 | 2.0 3.0 | 0.35 | 0.05 | 0.35 | 0.25 | |
| 319.1 | 319 | 3.0 4.0 | 1.0 | 0.8 | 5.5 6.5 | 0.50 | 0.10 | 0.35 | 0.25 | |
| 319.1 sr | 3.0 4.0 | 1.0 | 0.9 | 5.5 6.5 | 0.50 | 0.25 0.40 | 0.35 | 0.25 | ||
| 355.1 | 355 | 1.0 1.5 | 0.35 | 0.50 | 4.5 5.5 | 0.50 | 0.45 0.6 | 0.25 | 0.25 | |
| 356.1 | 356 | 0.25 | 0.35 | 0.50 | 6.5 7.5 | 0.35 | 0.25 0.40 | 0.25 | ||
| A360.1 | A360 | 0.6 | 0.40 | 1.0 | 9.0–10.0 | 0.35 | 0.45–0.6 | 0.50 | ||
| A380.1 | A380 | 3.0–4.0 | 2.9 | 1.0 | 7.5–9.5 | 0.5 | 0.10 | 0.50 | ||
| B380.1 | 3.0–4.0 | 0.9 | 1.0 | 7.5–9.5 | 0.5 | 0.10 | 0.50 | |||
| 383.1 | 383 | 2.0–3.0 | 2.9 | 1.0 | 9.5–11.5 | 0.5 | 0.10 | 0.30 | ||
| 383–1 | 2.0–3.0 | 0.90 | 1.0 | 9.5–11.5 | 0.5 | 0.10 | 0.30 | |||
| 384.1 | 384 | 3.0–4.5 | 2.9 | 1.0 | 10.5–12.0 | 0.5 | 0.10 | 0.50 | ||
| A384.1 | 3.0–4.5 | 0.9 | 1.0 | 10.5–12.0 | 0.5 | 0.10 | 0.50 | |||
| B390.1 | 4.0–5.0 | 1.4 | 1.0 | 16.0–18.0 | 0.5 | 0.50–0.65 | 0.10 | 0.20 | ||
| A413.1 | 13 | 1.0 | 0.40 | 1.0 | 11.0–13.0 | 0.35 | 0.10 | 0.50 | ||
| C443.1 | 43 | 0.6 | 0.40 | 1.1 | 4.5 6.0 | 0.35 | 0.10 | 0.50 | ||
| 706.1 | 603 Tern. 6 | 0.20 | 2.73 3.3 | 0.6 | 0.20 | 0.40 0.6 | 1.6 1.8 | 0.20 0.40 | 0.25 | |
| 712.2 | 0712.0/ | 0.25 | 6.0 6.5 | 0.4 | 0.15 | 0.10 | 0.50 0.65 | 0.40 0.60 | 0.15 0.25 | |
| 713.1 | 613 | 0.40 1.0 | 7.0 8.0 | 0.8 | 0.25 | 0.6 | 0.25 0.50 | 0.35 | 0.15 | 0.25 |
| Alloy | Si | Cu | Fe | Mg | Mn | Zn | Ti | Zn | Cr | Cr | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | 9.5 | 3.5 | - | - | - | - | - | -- | -- | -- | Bal |
| A380.1 | 9.18 | 3.22 | 1.01 | 0.06 | 0.15 | 2.29 | 0.02 | 2.3 | 0.06 | 0.04 | Bal |
| Alloys * | Elements (wt%) | |||||
|---|---|---|---|---|---|---|
| Cu | Fe | Mg | Mn | Zn | Ti | |
| A | 3.22 | 1.01 | 0.06 | 0.16 | 2.28 | 0.02 |
| B | 3.22 | 1.01 | 0.33 | 0.16 | 2.28 | 0.02 |
| C | 3.22 | 1.01 | 0.55 | 0.16 | 2.28 | 0.02 |
| Reaction No. | Temperature (◦C) | Reaction Details | |||
|---|---|---|---|---|---|
| Experimental Alloy | Alloy A * | Alloy B | Alloy C | ||
| 1 | 580 | 572.7 | 572.9 | 570.4 | Formation of α-Al network |
| 2 | 567.7 | 567.7 | 564.6 | Precipitation of β-Fe phase | |
| 3 | 563 | 563.8 | 561.9 | 560.6 | Formation of (Al+Si) eutectic |
| 4 | 536 | 539.5 | 537.6 | Partial transformation of β-Fe → Al8Mg3FeSi6 phase | |
| 5 | 513.8 | 508.8 | 508.3 | Precipitation of Mg2Si phase | |
| 6 | 475.5 | 509.13/480 | 505.1 | Formation of Q-Al5Mg8Cu2Si6 phase | |
| 7 | 500 | 485 | 490 | 488 | Formation of (Al + Al2Cu) eutectic |
| 8 | 475 | 460.1 | 470.0 | 470.2 | End of solidification |
| UTS (MPa) | YS (MPa) | %El |
|---|---|---|
| 605 | 210 | 1.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samuel, A.M.; Zedan, Y.; Elsharkawi, E.A.; Abdelaziz, M.H.; Samuel, F.H. The Role of Mg Content and Aging Treatment on the Tensile and Fatigue Properties of Die-Cast 380 Alloy. Materials 2022, 15, 8844. https://doi.org/10.3390/ma15248844
Samuel AM, Zedan Y, Elsharkawi EA, Abdelaziz MH, Samuel FH. The Role of Mg Content and Aging Treatment on the Tensile and Fatigue Properties of Die-Cast 380 Alloy. Materials. 2022; 15(24):8844. https://doi.org/10.3390/ma15248844
Chicago/Turabian StyleSamuel, Agnes M., Yasser Zedan, Ehab A. Elsharkawi, Mohamed H. Abdelaziz, and Fawzy H. Samuel. 2022. "The Role of Mg Content and Aging Treatment on the Tensile and Fatigue Properties of Die-Cast 380 Alloy" Materials 15, no. 24: 8844. https://doi.org/10.3390/ma15248844
APA StyleSamuel, A. M., Zedan, Y., Elsharkawi, E. A., Abdelaziz, M. H., & Samuel, F. H. (2022). The Role of Mg Content and Aging Treatment on the Tensile and Fatigue Properties of Die-Cast 380 Alloy. Materials, 15(24), 8844. https://doi.org/10.3390/ma15248844








