Effect of an Anaerobic Fermentation Process on 3D-Printed PLA Materials of a Biogas-Generating Reactor
Abstract
1. Introduction
- To assess PLA use for the development of a bioreactor for the testing of anaerobic digestion processes in a mesophilic temperature regime (i.e., 30 to 37 °C). The large variety of PLA applications and the fact that such materials are manufactured using renewable resources define the impact of this work.
- To determine if this material is suitable for such applications, considering the parameter impact and residence time of substrate, as well as of the produced biogas inside the vessel.
- To assess the capability of the main considered method to perform these investigations, Optical Coherence Tomography (OCT).
2. Materials and Methods
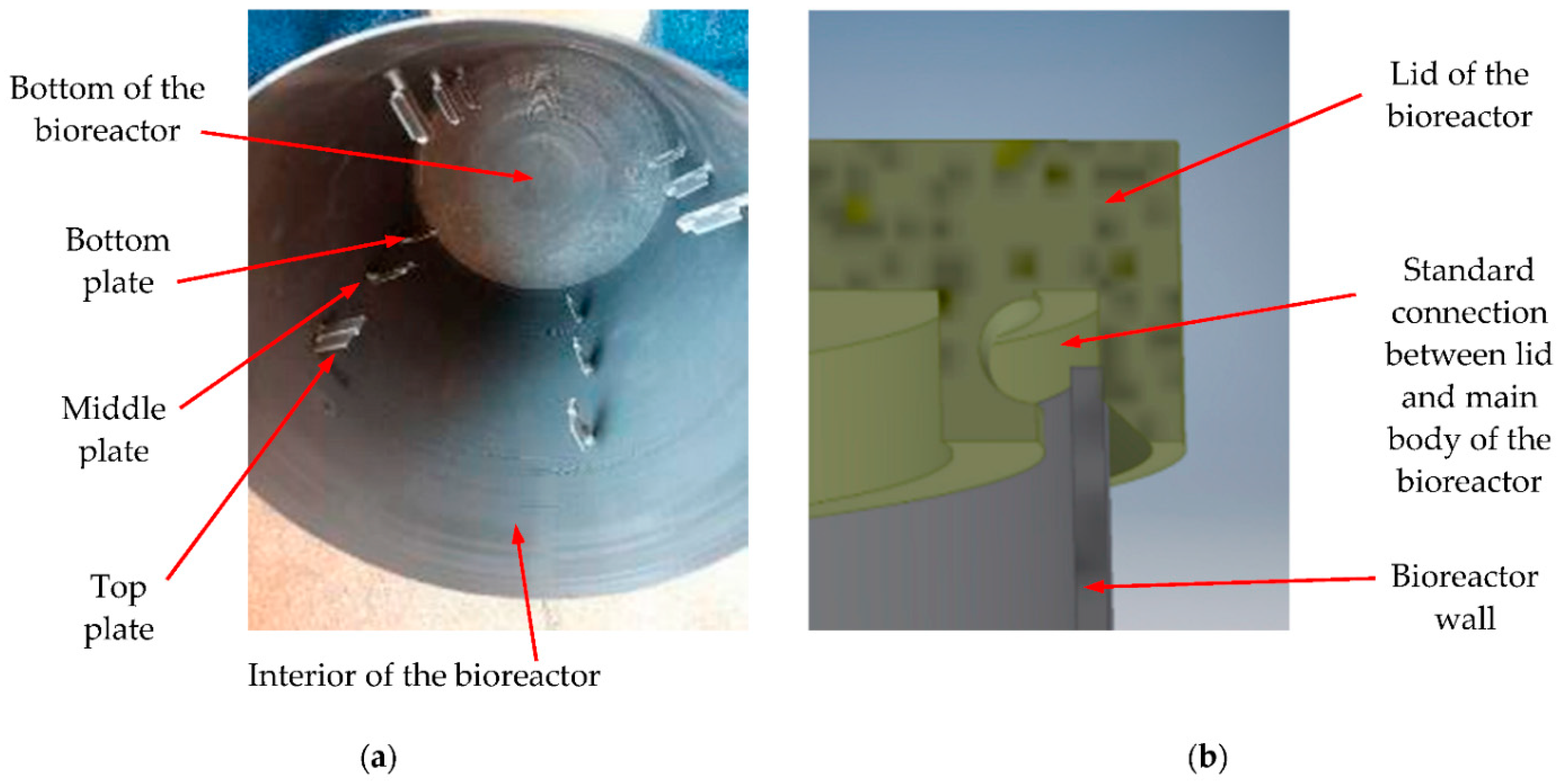
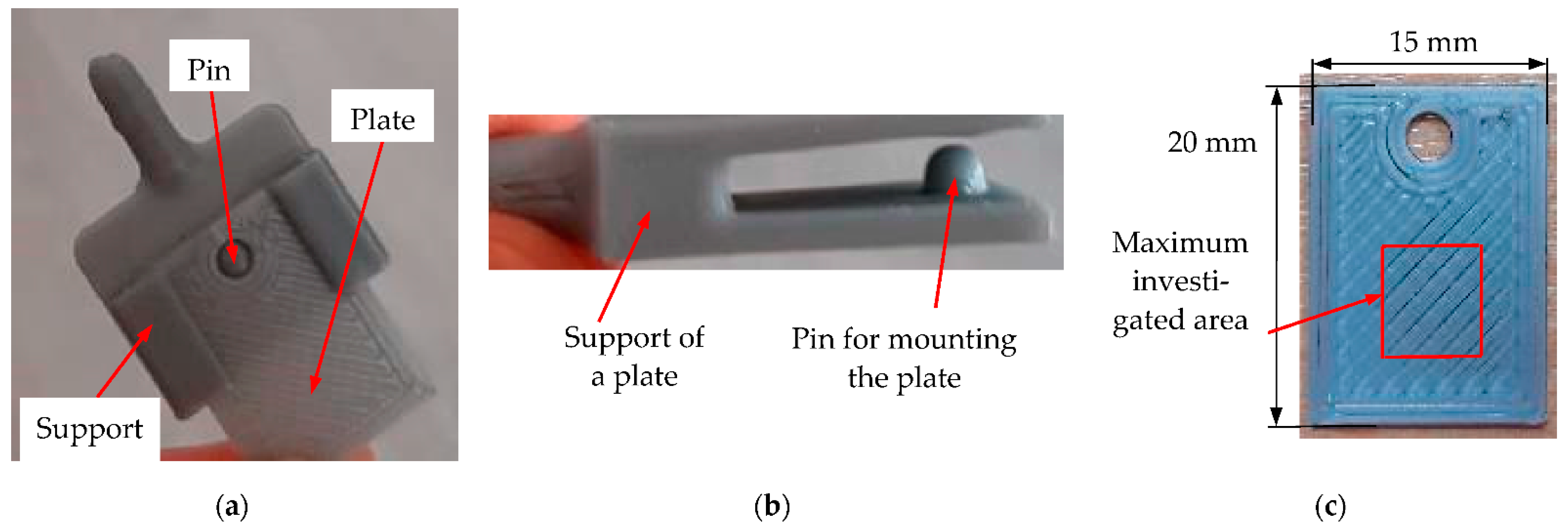
2.1. Biogas-Generating Reactor and Testing Plates
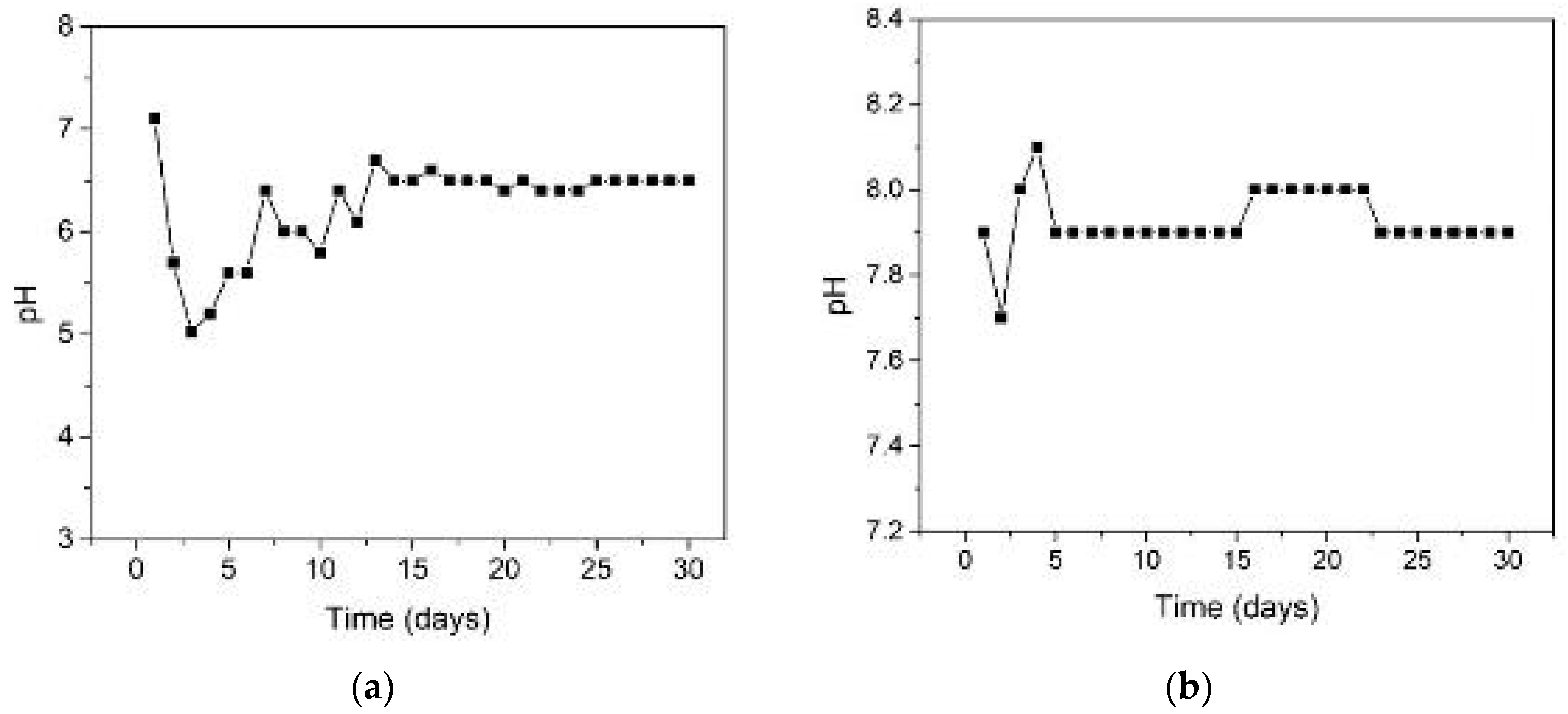
2.2. Biogas-Generating Process
2.3. Imaging Methods
3. Results
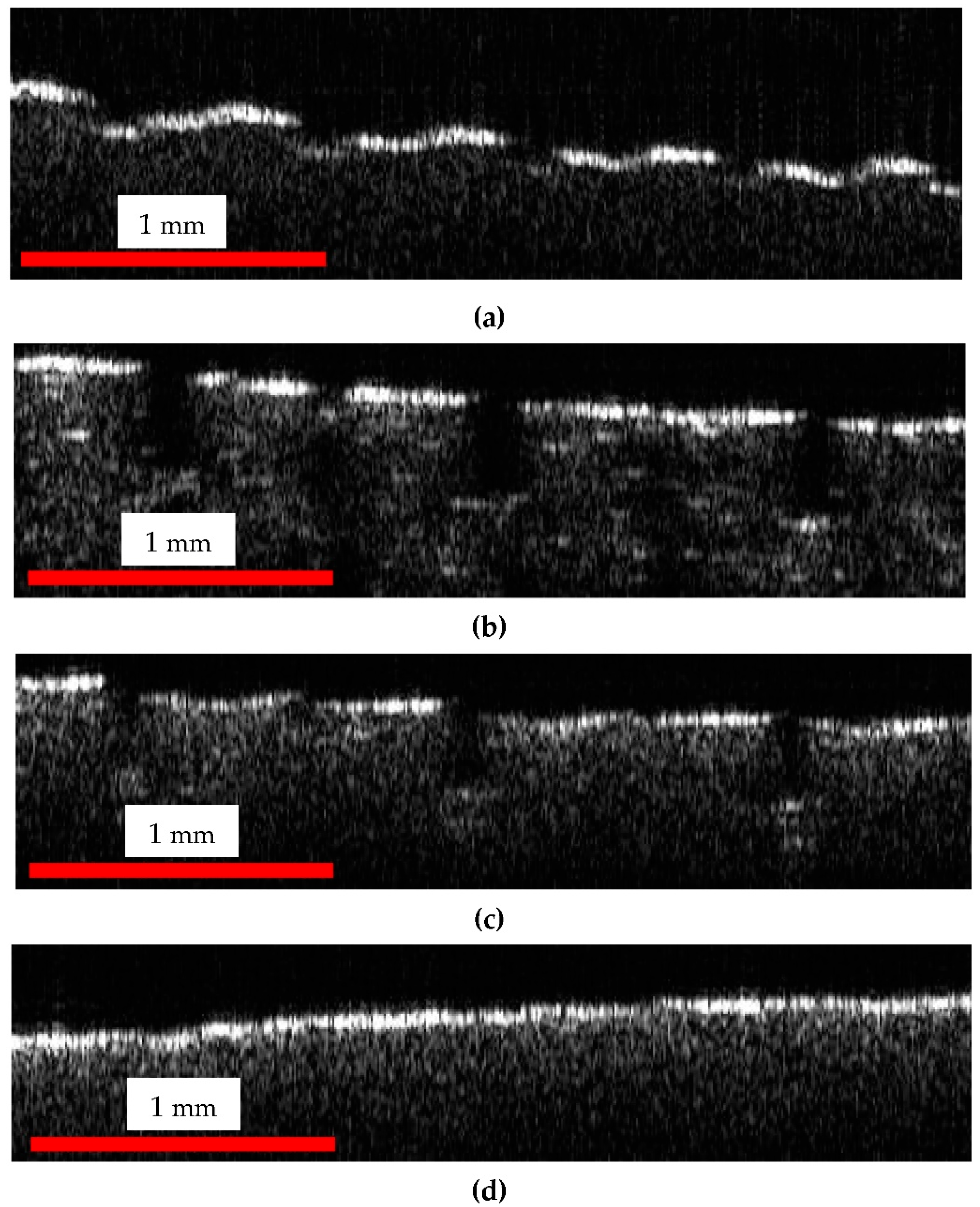
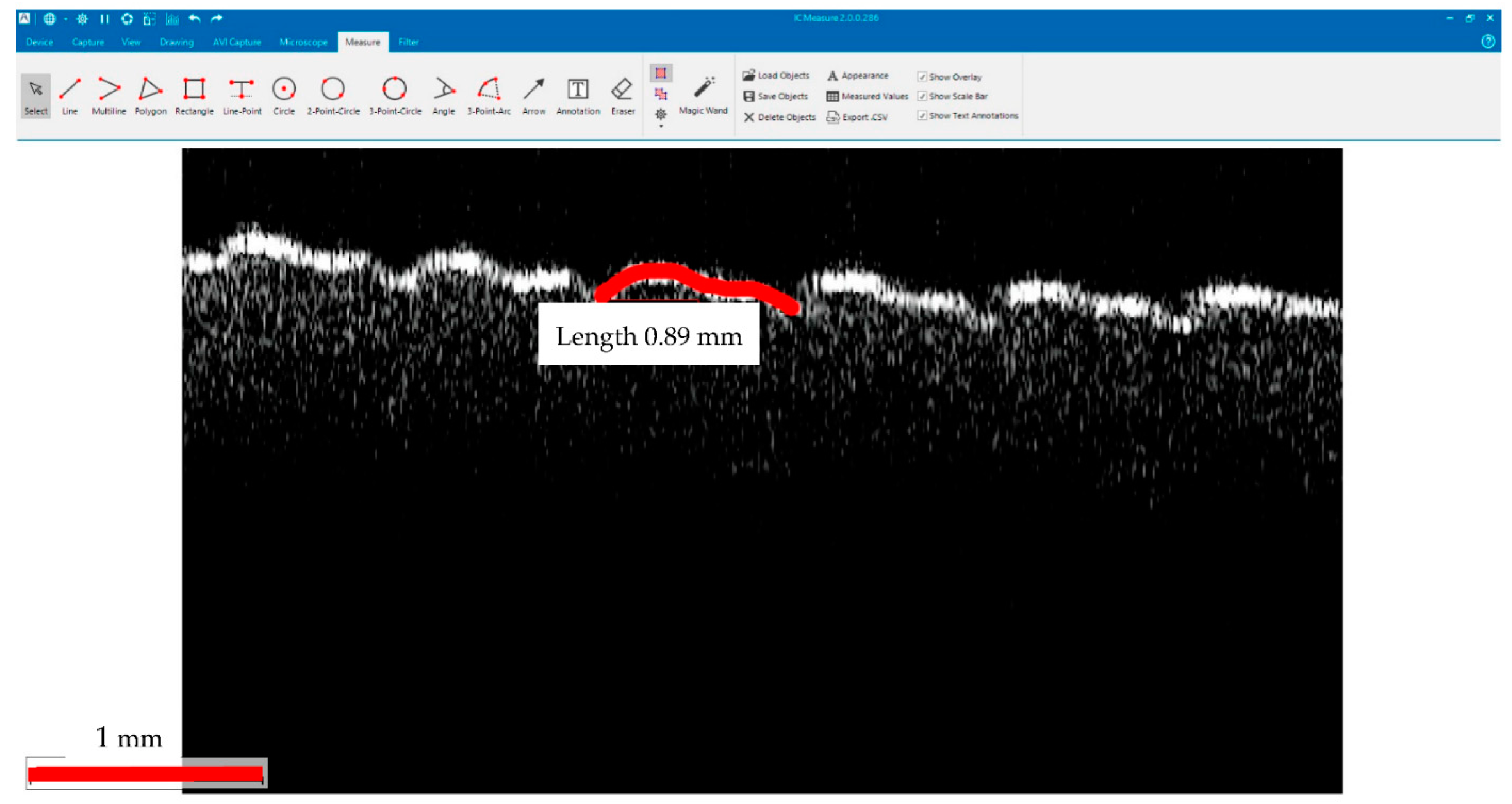
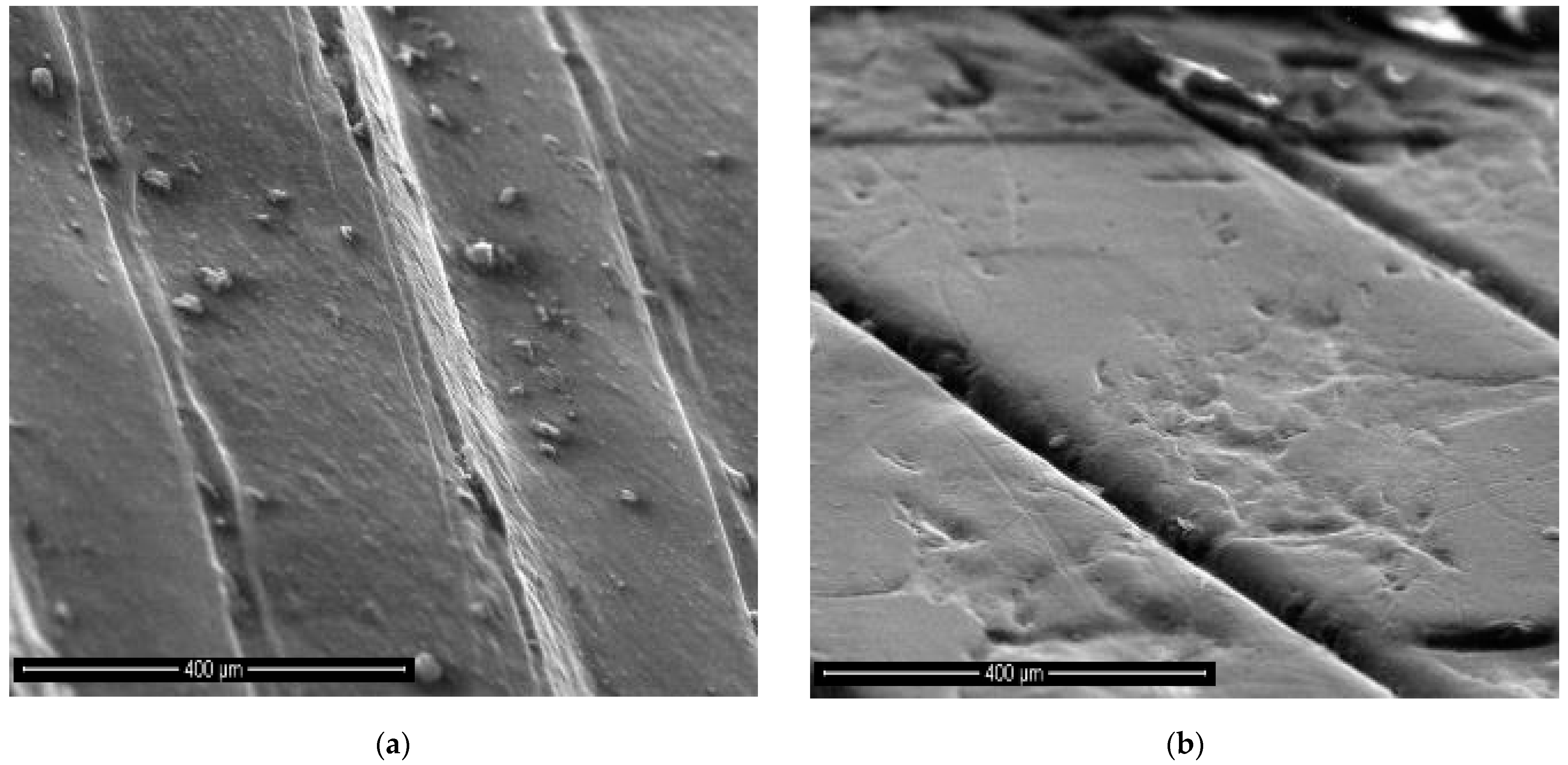
OCT and SEM Images
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ofoefule, A.U.; Uzodinma, E.O.; Onukwuli, O.D. Comparative study of the effect of different pretreatment methods on biogas yield from water Hyacinth (Eichhornia crassipes). Int. J. Phys. Sci. 2010, 4, 535–539. [Google Scholar]
- Veeken, A.; Kalyuzhnyi, S.; Scharff, H.; Hamelers, B. Effect of pH and VFA on hydrolysis of organic solid waste. J. Environ. Eng. 2001, 4, 1076–1081. [Google Scholar] [CrossRef]
- Gerardi, M.H. The Microbiology of Anaerobic Digesters; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Zinder, S.H. Physiological Ecology of Methanogens. In Methanogenesis: Ecology, Physiology; Ferry, J.G., Ed.; Chapman & Hall: New York, NY, USA, 1994. [Google Scholar]
- Gogina, E.; Gulshin, I. Simultaneous denitrification and nitrification in the lab-scale oxidation ditch with low C/N ratio. Proc. Eng. 2015, 117, 107–113. [Google Scholar]
- Gogina, E.S.; Ruzhitskaya, O.A.; Yantsen, O.V. Investigation of the processes of nitrification and denitrification in wastewater treatment. Adv. Mater. Res. 2014, 919–921, 2145–2148. [Google Scholar] [CrossRef]
- Singh, R.; Mandal, S.K.; Jain, V.K. Development of mixed inoculum for methane enriched biogas production. Indian J. Microbiol. 2010, 50 (Suppl. S1), 26–33. [Google Scholar] [CrossRef]
- Romano, R.T.; Zhang, R.; Teter, S.; McGarvey, J.A. The effect of enzyme addition on anaerobic digestion of Jose Tall Wheat Grass. Bioresour. Technol. 2009, 100, 4564–4571. [Google Scholar] [CrossRef]
- Agyeman, F.O.; Tao, W.D. Anaerobic co-digestion of food waste and dairy manure: Effects of food waste particle size and organic loading rate. J. Environ. Manag. 2014, 133, 268–274. [Google Scholar] [CrossRef]
- Scherbakov, V.; Gogina, E.; Schukina, T.; Kuznetsova, N.; Makisha, N.; Poupyrev, E. Calculation of biogas facilities for recycling of organic sewage sludge of breeding factories. Int. J. Appl. Eng. Res. 2015, 10, 44353–44356. [Google Scholar]
- Kulakov, A.A.; Lebedeva, E.A. Development of Engineering Solutions concerning Modernization of Wastewater Treatment Facilities Basedon Technology Simulation. Water Treat. 2011, 12, 10–19. [Google Scholar]
- Gogina, E.; Makisha, N. Information technologies in view of complex solution of waste water problems. Appl. Mech. Mater. 2014, 587–589, 636–639. [Google Scholar] [CrossRef]
- Makisha, N. Waste water and biogas—Ecology and economy. Proc. Eng. 2016, 165, 1092–1097. [Google Scholar] [CrossRef]
- Den, W.; Sharma, V.K.; Lee, M.; Nadadur, G.; Varma, R.S. Lignocellulosic Biomass Transformations via Greener Oxidative Pretreatment Processes: Access to Energy and Value-Added Chemicals. Front. Chem. 2018, 6, 141. [Google Scholar] [CrossRef] [PubMed]
- De Lemos, C.A. Anaerobic Reactors; IWA Publishing: London, UK, 2007. [Google Scholar]
- Begum, L. Advanced Processes and Technologies for Enhanced Anaerobic Digestion; Green Nook Press: Toronto, ON, Canada, 2014. [Google Scholar]
- Penumakala, P.; Santo, J.; Thomas, A. A critical review on the fused deposition modeling of thermoplastic polymer composites. Compos. Part B 2020, 201, 108336. [Google Scholar] [CrossRef]
- Deshmukh, K.; Houkan, M.T.; AlMaadeed, M.A.; Sadasivuni, K.K. Introduction to 3D and 4D printing technology: State of the art and recent trends. 3d 4d Print. Polym. Nano Mater. 2020, 1–24. [Google Scholar] [CrossRef]
- Qian, T.T.; Dong, L.; Tian, X.J.; Liu, C.M.; Wang, H.M. Microstructure of TA2/TA15 graded structural material by laser additive manufacturing process. Trans. Nonferrous Metals Soc. 2014, 24, 2729–2736. [Google Scholar] [CrossRef]
- Du, Y.; Chen, J.; Meng, Q.; Dou, Y.; Xu, J.; Biplab, P.; Per, E. Flexible ternary carbon black/ Bi2Te3 based alloy/polylactic acid thermoelectric composites fabricated by additive manufacturing. J. Mater. 2020, 6, 293–299. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, D.; Zaera, R.; Arias, A. A hyperelastic-thermoviscoplastic constitutive model for semi-crystalline polymers: Application to PEEK under dynamic loading conditions. Int. J. Plast. 2017, 88, 27–52. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, D.; Garzon-Hernandez, S.; Arias, A. A new constitutive model for polymeric matrices: Application to biomedical materials. Compos. Part B 2018, 139, 117–129. [Google Scholar] [CrossRef]
- Kinvi-Dossou, G.; Matadi Boumbimba, R.; Bonfoh, N.; Garzon-Hernandez, S.; Garcia- Gonzalez, D.; Gerard, P.; Arias, A. Innovative acrylic thermoplastic composites versus conventional composites: Improving the impact performances. Compos. Struct. 2019, 217, 1–13. [Google Scholar] [CrossRef]
- Barba, D.; Arias, A.; Garcia-Gonzalez, D. Temperature and strain rate dependences on hardening and softening behaviours in semi-crystalline polymers: Application to PEEK. Int. J. Solids Struct. 2020, 182, 205–207. [Google Scholar] [CrossRef]
- Farrington, D.W. Poly (lactic acid) fibres. Biodegradable and Sustainable Fibres; Blackburn, R.S., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2005; pp. 191–220. [Google Scholar]
- Henton, D.; Gruber, P. Polylactic acid Technology. In Natural Fibres, Biopolymer, and Biocomposites; Amar, K., Manjusri, M., Lawrence, T., Eds.; CRC Press: Cambridge, UK, 2005; pp. 527–579. [Google Scholar]
- Okada, M. Chemical syntheses of biodegradable polymers. Prog. Polym. Sci. 2002, 27, 87–133. [Google Scholar] [CrossRef]
- Bhatia, A. Compatibility of biodegradable poly (lactic acid) (PLA) and poly (butylene succinate) (PBS) blends for packaging application. Korea–Aust. Rheol. J. 2007, 19, 125–131. [Google Scholar]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Ganster, J.; Fink, H.-P. Novel cellulose fibre reinforced thermoplastic materials. Cellulose 2006, 13, 271–280. [Google Scholar] [CrossRef]
- Bajpai, P.K.; Singh, I.; Madaan, J. Comparative studies of mechanical and morphological properties of poly (lactic acid) and polypropylene based natural fiber composites. J. Reinf. Plast. Compos. 2012, 31, 1712–1724. [Google Scholar] [CrossRef]
- Oksman, K.; Skrifvars, M.; Selin, J.-F. Natural fibres as reinforcement in poly lactic acid (PLA) composites. Compos. Sci. Technol. 2003, 63, 1317–1324. [Google Scholar] [CrossRef]
- García, M.; Garmendia, I.; Garcia, J. Influence of natural fiber type in ecocomposites. J. Appl. Polym. Sci. 2008, 107, 2994–3004. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef]
- Choma, M.A.; Sarunic, M.V.; Yang, C.; Izatt, J.A. Sensitivity advantage of swept-source and Fourier-domain optical coherence tomography. Opt. Express 2003, 11, 2183–2189. [Google Scholar] [CrossRef]
- Drexler, W.; Liu, M.; Kumar, A.; Kamali, T.; Unterhuber, A.; Leitgeb, R.A. Optical coherence tomography today: Speed, contrast, and multimodality. J. Biomed. Opt. 2014, 19, 071412. [Google Scholar] [CrossRef]
- Mehreen, A.; Duker, J.S. Optical coherence tomography–current and future applications. Curr. Opin. Ophthalmol. 2013, 24, 213–221. [Google Scholar]
- Hsieh, Y.-S.; Ho, Y.-C.; Lee, S.-Y.; Chuang, C.-C.; Tsai, J.-c.; Lin, K.-F.; Sun, C.-W. Dental Optical Coherence Tomography. Sensors 2013, 13, 8928–8949. [Google Scholar] [CrossRef] [PubMed]
- Erdelyi, R.-A.; Duma, V.-F.; Sinescu, C.; Dobre, G.M.; Bradu, A.; Podoleanu, A. Dental Diagnosis and Treatment Assessments: Between X-rays Radiography and Optical Coherence Tomography. Materials 2020, 13, 4825. [Google Scholar] [CrossRef]
- Erdelyi, R.-A.; Duma, V.-F.; Sinescu, C.; Dobre, G.M.; Bradu, A.; Podoleanu, A. Optimization of X-ray Investigations in Dentistry using Optical Coherence Tomography. Sensors 2021, 21, 4554. [Google Scholar] [CrossRef]
- Gambichler, T.; Jaedicke, V.; Terras, S. Optical coherence tomography in dermatology: Technical and clinical aspects. Arch. Derm. Res. 2011, 303, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, A.; Canavesi, C.; Hayes, A.; Tankam, P.; Duma, V.-F.; Santhanam, A.; Thompson, K.P.; Rolland, J.P. MEMS-based handheld scanning probe for distortion-free images in Gabor-Domain Optical Coherence Microscopy. Opt. Express 2016, 24, 13365–13374. [Google Scholar] [CrossRef] [PubMed]
- Adler, D.C.; Chen, Y.; Huber, R.; Schmitt, J.; Connolly, J.; Fujimoto, J.G. Three-dimensional endomicroscopy using optical coherence tomography. Nat. Photonics 2007, 1, 709–716. [Google Scholar] [CrossRef]
- Kang, W.; Wang, H.; Pan, Y.; Jenkins, M.W.; Isenberg, G.A.; Chak, A.; Atkinson, M.; Agrawal, D.; Hu, Z.; Rollins, A.M. Endoscopically guided spectral-domain OCT with double-balloon catheters. Opt. Express 2010, 18, 17364–17372. [Google Scholar] [CrossRef]
- Thrane, L.; Jørgensen, T.M.; Jørgensen, M.; Krebs, F.C. Application of optical coherence tomography (OCT) as a 3-dimensional imaging technique for roll-to-roll coated polymer solar cells. Sol. Energy Mater. Sol. Cells 2012, 97, 181–185. [Google Scholar] [CrossRef]
- Wiesauer, K.; Sanchis Dufau, A.D.; Gotzinger, E.; Pircher, M.; Hitzenberger, C.K.; Stifter, D. Non-destructive quantification of internal stress in polymer materials by polarisation sensitive optical coherence tomography. Acta Mater. 2005, 53, 2785–2791. [Google Scholar] [CrossRef]
- Czajkowsk, J.; Prykari, T.; Alarousu, E.; Palosaari, J.; Myllyla, R. Optical Coherence Tomography as a Method of Quality Inspection for Printed Electronics Products. Opt. Rev. 2010, 17, 257–262. [Google Scholar] [CrossRef]
- Hutiu, G.; Duma, V.-F.; Demian, D.; Bradu, A.; Podoleanu, A.G. Surface imaging of metallic material fractures using optical coherence tomography. Appl. Opt. 2014, 53, 5912–5916. [Google Scholar] [CrossRef]
- Hutiu, G.; Duma, V.-F.; Demian, D.; Bradu, A.; Podoleanu, A.G. Assessment of Ductile, Brittle, and Fatigue Fractures of Metals Using Optical Coherence Tomography. Metals 2018, 8, 117. [Google Scholar] [CrossRef]
- Liang, H.; Cid, M.G.; Cucu, R.G.; Dobre, G.M.; Podoleanu, A.G.; Pedro, J.; Saunders, D. En-face optical coherence tomography—A novel application of non-invasive imaging to art conservation. Opt. Express 2005, 13, 6133–6144. [Google Scholar] [CrossRef] [PubMed]
- Maria, M.; d’Hont, L.; Anisimov, A.; Stiols-Witlox, M.; Groves, R. Three-dimensional spectral measurements of paint samples using optical coherence tomography. Proc. SPIE 2021, 11784, 117840. [Google Scholar]
- Lu, C.D.; Kraus, M.F.; Potsaid, B.; Liu, J.J.; Choi, W.; Jayaraman, V.; Cable, A.E.; Hornegger, J.; Duke, J.S.; Fujimoto, J.G. Handheld ultrahigh speed swept source optical coherence tomography instrument using a MEMS scanning mirror. Biomed. Opt. Express 2014, 5, 293–311. [Google Scholar] [CrossRef]
- Duma, V.-F.; Dobre, G.; Demian, D.; Cernat, R.; Sinescu, C.; Topala, F.I.; Negrutiu, M.L.; Hutiu, G.; Bradu, A.; Podoleanu, A.G. Handheld scanning probes for optical coherence tomography. Rom. Rep. Phys. 2015, 67, 1346–1358. [Google Scholar]
- Monroy, G.L.; Won, J.; Spillman, D.R.; Dsouza, R.; Boppart, S.A. Clinical translation of handheld optical coherence tomography: Practical considerations and recent advancements. J. Biomed. Opt. 2017, 22, 121715. [Google Scholar] [CrossRef]
- Podoleanu, A.; Bradu, A. Master–slave interferometry for parallel spectral domain interferometry sensing and versatile 3D optical coherence tomography. Opt. Express 2013, 21, 19324–19338. [Google Scholar] [CrossRef]
- Duma, V.-F. Laser scanners with oscillatory elements: Design and optimization of 1D and 2D scanning functions. Appl. Math. Model. 2019, 67, 456–476. [Google Scholar] [CrossRef]
- Xu, Q.; Qin, J.; Ko, J.H. Municipal solid waste landfill performance with different biogascollection practices: Biogas and leachate generations. J. Clean. Prod. 2019, 222, 446–454. [Google Scholar] [CrossRef]
- Chukwunonso, C.A.; Baroutian, S. Decentralized anaerobic digestion systems for increased utilization of biogas from municipal solid waste. Renew. Sustain. Energy Rev. 2018, 90, 982–991. [Google Scholar]
- Miguel, E.M.; Udaeta, G.A.; de Medeiros, S.; da Silva, V.O.; Galvao, L.C.R. Basic and procedural requirements for energy potential from biogas of sewage treatment plants. J. Environ. Manag. 2019, 236, 380–387. [Google Scholar]
- Chunfeng, S.; Run, L.; Yingxin, Z.; Ruying, L.; Degang, M.; Yasuki, K. Assessment of four sewage sludge treatment routes with efficientbiogas utilization and heat integration. Process. Saf. Environ. 2019, 126, 205–213. [Google Scholar]
- Kurahashi, K.; Kimura, C.; Fujimoto, Y.; Tokumoto, H. Value-adding conversion and volume reduction of sewage sludge by anaerobic co-digestion with crude glycerol. Bioresour. Technol. 2017, 232, 119–125. [Google Scholar] [CrossRef]
- Oliverira, J.V.; Duarte, T.; Costa, J.C.; Cavaleiro, A.J.; Pereira, M.M.A. Improvement of Biomethane Production from Sewage Sludge in Co-digestion with Glycerol and Waste Frying Oil, Using a Design of Experiments. Bioenergy Res. 2018, 11, 763–771. [Google Scholar] [CrossRef]
- Aguilar, F.A.; Adriana, L.; Juantorena, A.U.; Santos, A.S.; Pantoja, L.A.; Sebastian, P.J. Optimization of Hydrogen Yield from the Anaerobic Digestion of Crude Glycerol and Swine Manure. Catalysts 2019, 9, 316. [Google Scholar]
- Marti-Herrero, J.; Soria-Castellon, G.; Diaz de Basurto, A.; Alvarez, R.; Chemisana, D. Biogas from a full scale digester operated in psychrophilic conditions and fed only with fruit and vegetable waste. Renew. Energy 2019, 133, 676–684. [Google Scholar] [CrossRef]
- Tirado Garcia, D.; Garcia Gonzalez, S.; Garzon Hernandez, A.; Rusinek, G.; Robles, J.M.; Martinez Tarifa, A. Conductive 3D printed PLA composites: On the interplay of mechanical, electrical and thermal behaviours. Compos. Struct. 2021, 265, 1–9. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Oksiuta, Z.; Jalbrzykowski, M.; Mystkowska, J.; Romanczuk, E.; Osiecki, T. Mechanical and Thermal Properties of Polylactide (PLA) Composites Modified with Mg, Fe, and Polyethylene (PE) Additives. Polymers 2020, 12, 2939. [Google Scholar] [CrossRef] [PubMed]
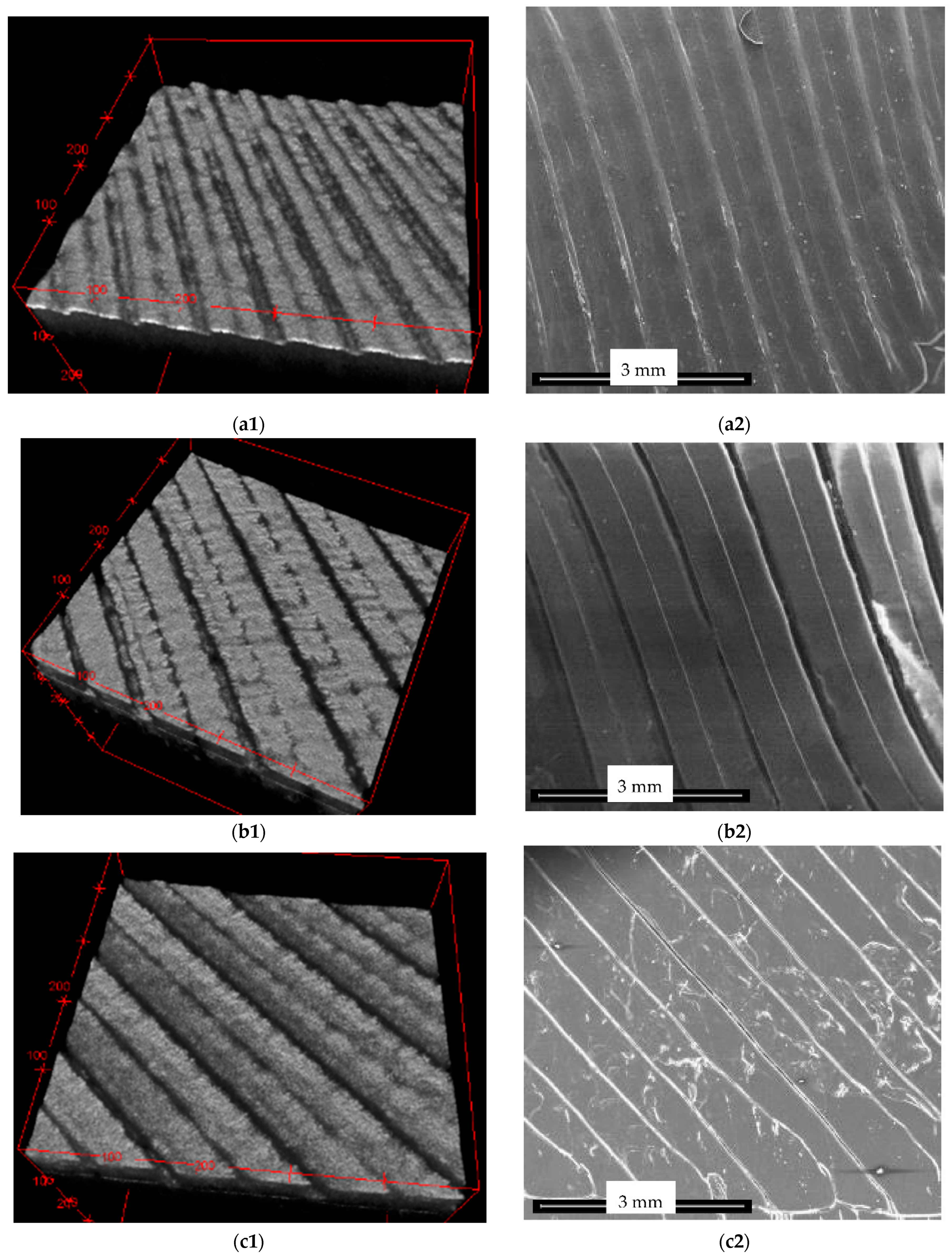


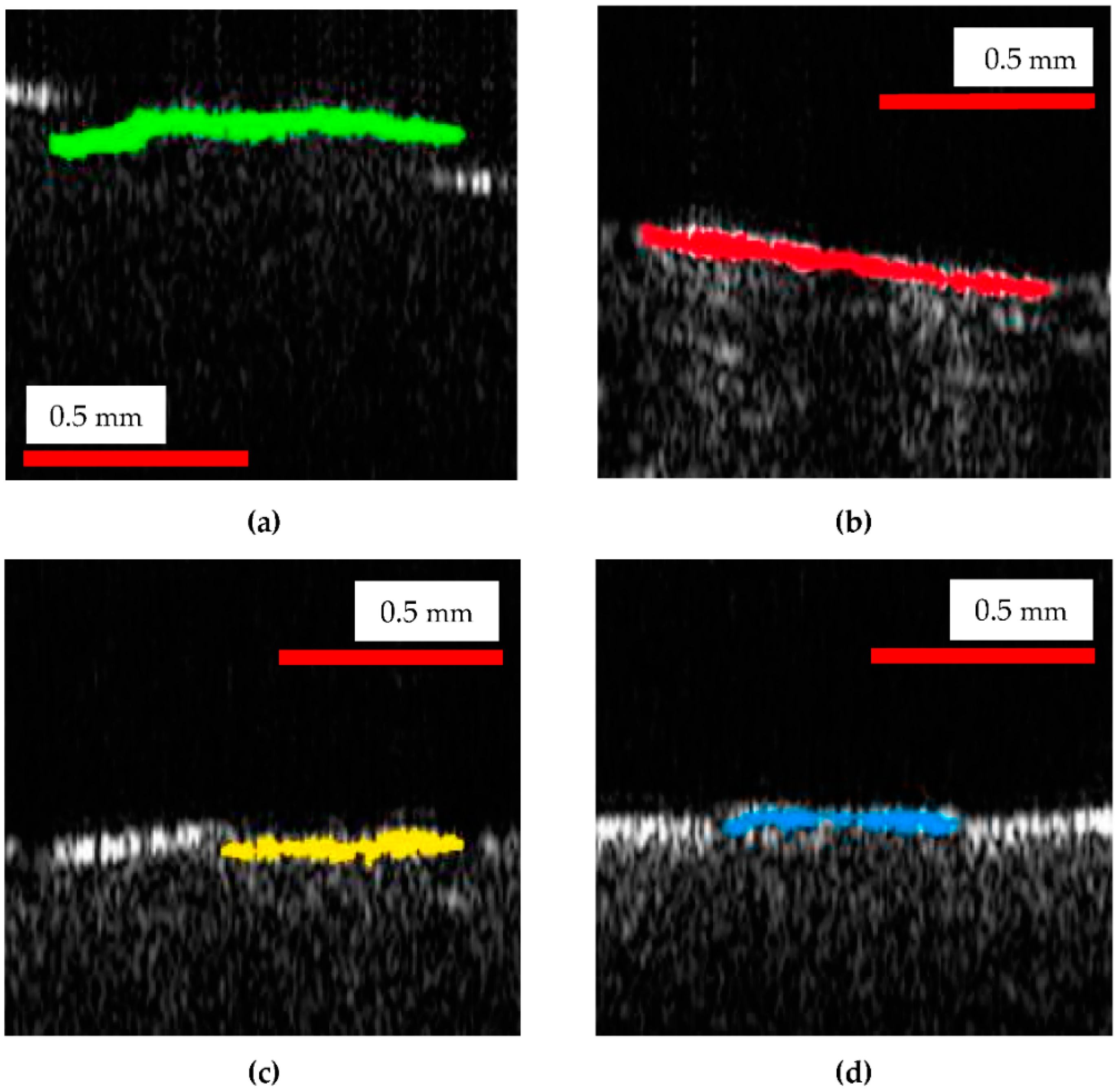
| Sample | Reference (mm) | Top (mm) | Middle (mm) | Bottom (mm) |
|---|---|---|---|---|
| Measured values of the colored contours marked in Figure 8 | 0.91 | 0.82 | 0.59 | 0.56 |
| 1 | 0.7 | 0.64 | 0.6 | |
| 0.81 | 0.65 | 0.56 | 0.61 | |
| 0.84 | 0.69 | 0.53 | 0.49 | |
| 0.88 | 0.86 | 0.61 | 0.62 | |
| 0.98 | 0.84 | 0.63 | 0.59 | |
| 0.91 | 0.8 | 0.57 | 0.52 | |
| 0.83 | 0.84 | 0.63 | 0.54 | |
| 0.9 | 0.83 | 0.68 | 0.58 | |
| 0.89 | 0.78 | 0.59 | 0.57 | |
| 0.86 | 0.81 | 0.55 | 0.61 | |
| 0.92 | 0.69 | 0.59 | 0.5 | |
| 0.84 | 0.85 | 0.65 | 0.62 | |
| 0.81 | 0.76 | 0.61 | 0.58 | |
| 0.95 | 0.74 | 0.57 | 0.61 | |
| 0.87 | 0.81 | 0.6 | 0.53 | |
| 0.98 | 0.73 | 0.59 | 0.57 | |
| 1 | 0.8 | 0.62 | 0.55 | |
| 0.93 | 0.79 | 0.58 | 0.59 | |
| 0.89 | 0.76 | 0.61 | 0.6 | |
| Mean (mm) | 0.9 | 0.78 | 0.6 | 0.57 |
| Mean Absolute Deviation (mm) | 0.048 | 0.05 | 0.028 | 0.031 |
| Standard Deviation (mm) | 0.059 | 0.059 | 0.036 | 0.038 |
| Standard Error of the Mean (mm) | 0.0130 | 0.0131 | 0.0078 | 0.0085 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cioabla, A.; Duma, V.-F.; Mnerie, C.; Erdelyi, R.-A.; Dobre, G.M.; Bradu, A.; Podoleanu, A. Effect of an Anaerobic Fermentation Process on 3D-Printed PLA Materials of a Biogas-Generating Reactor. Materials 2022, 15, 8571. https://doi.org/10.3390/ma15238571
Cioabla A, Duma V-F, Mnerie C, Erdelyi R-A, Dobre GM, Bradu A, Podoleanu A. Effect of an Anaerobic Fermentation Process on 3D-Printed PLA Materials of a Biogas-Generating Reactor. Materials. 2022; 15(23):8571. https://doi.org/10.3390/ma15238571
Chicago/Turabian StyleCioabla, Adrian, Virgil-Florin Duma, Corina Mnerie, Ralph-Alexandru Erdelyi, George Mihai Dobre, Adrian Bradu, and Adrian Podoleanu. 2022. "Effect of an Anaerobic Fermentation Process on 3D-Printed PLA Materials of a Biogas-Generating Reactor" Materials 15, no. 23: 8571. https://doi.org/10.3390/ma15238571
APA StyleCioabla, A., Duma, V.-F., Mnerie, C., Erdelyi, R.-A., Dobre, G. M., Bradu, A., & Podoleanu, A. (2022). Effect of an Anaerobic Fermentation Process on 3D-Printed PLA Materials of a Biogas-Generating Reactor. Materials, 15(23), 8571. https://doi.org/10.3390/ma15238571










