Deep Eutectic Solvent for Facile Synthesis of Mn3O4@N-Doped Carbon for Aqueous Multivalent-Based Supercapacitors: New Concept for Increasing Capacitance and Operating Voltage
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Carbon Precursor
2.2. Carbon Material Synthesis
2.3. Characterization of Carbon Material
2.4. Electrode Preparation and Electrochemical Measurements
3. Results and Discussions
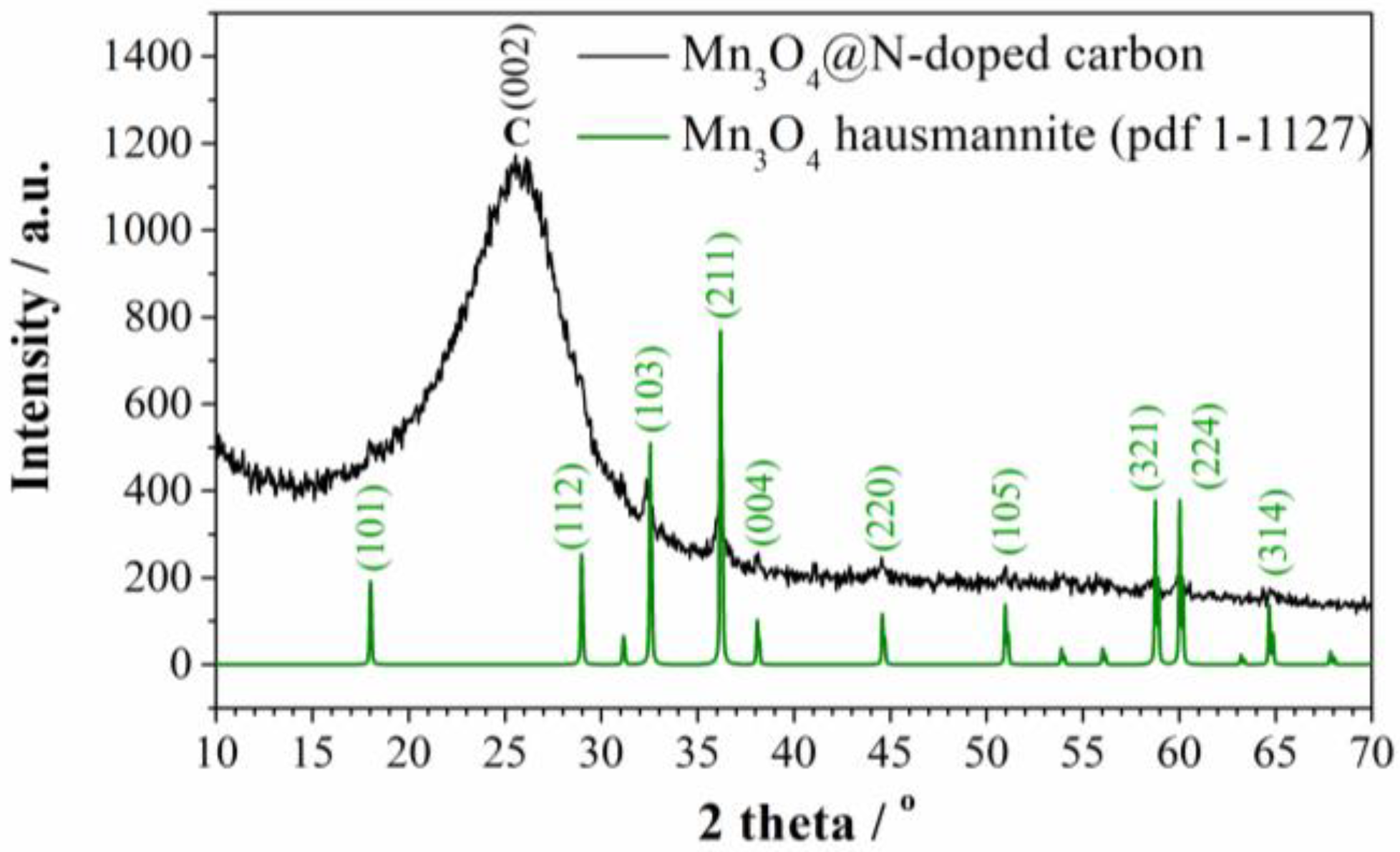
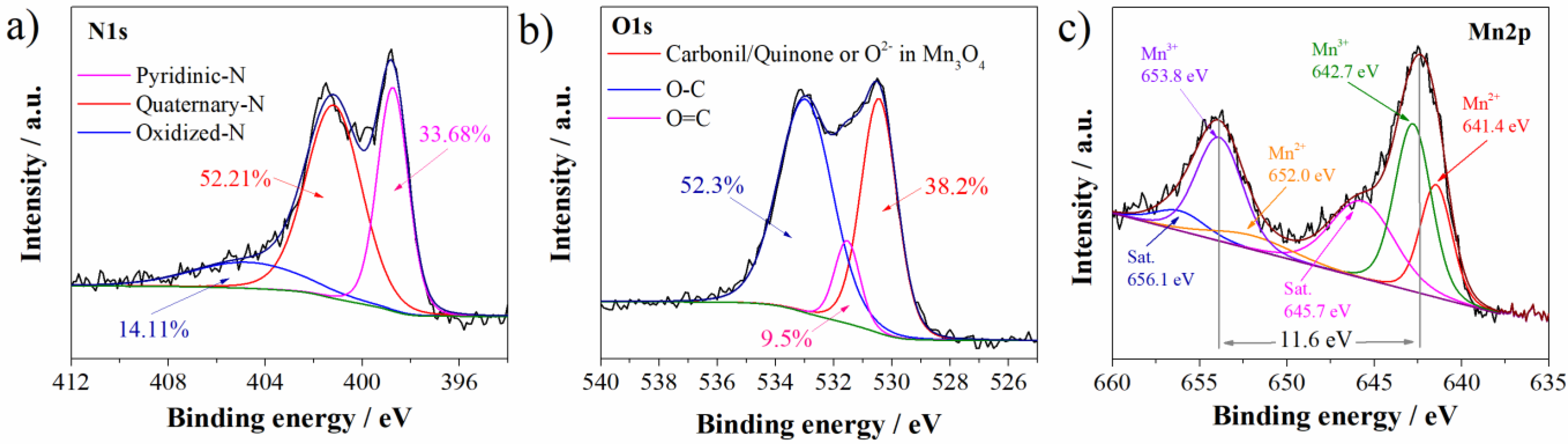
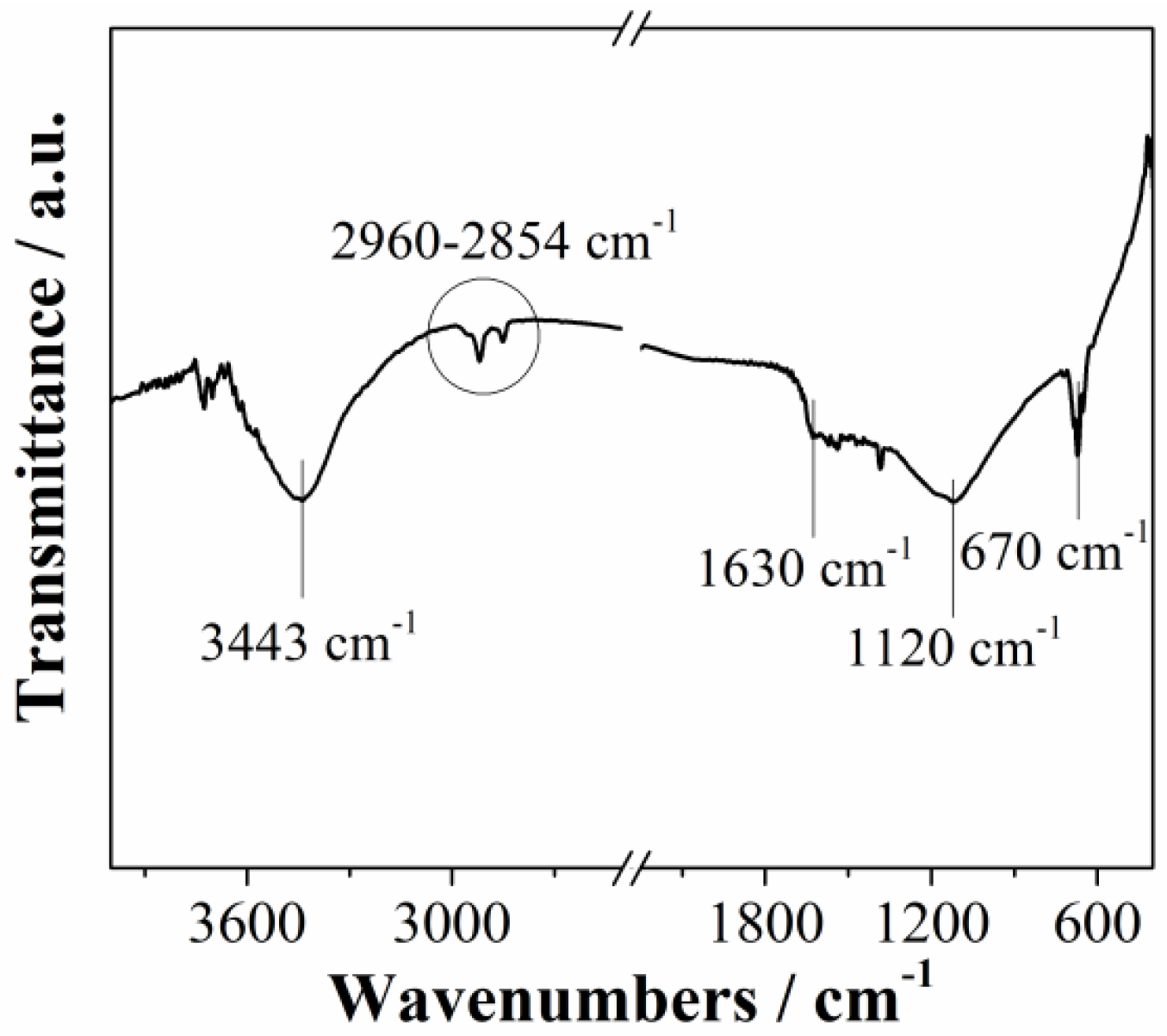
3.1. Characterization of Carbon Material
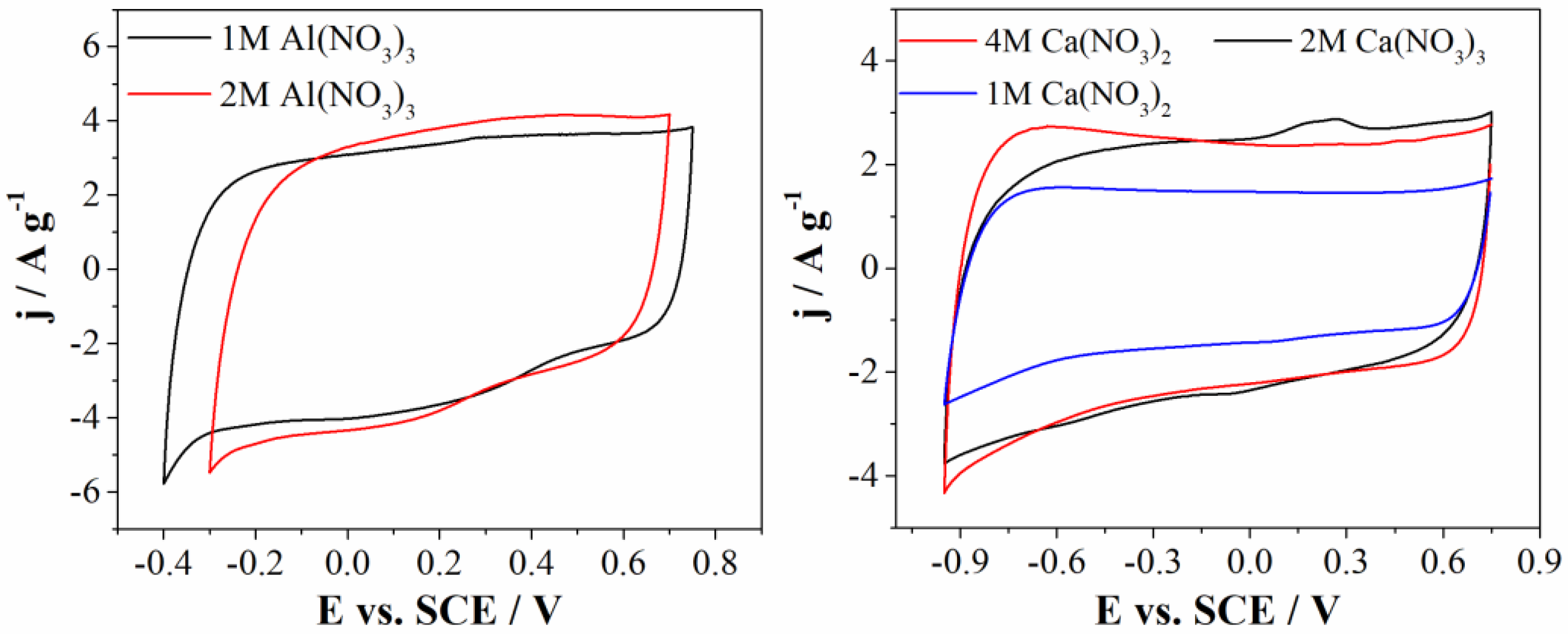
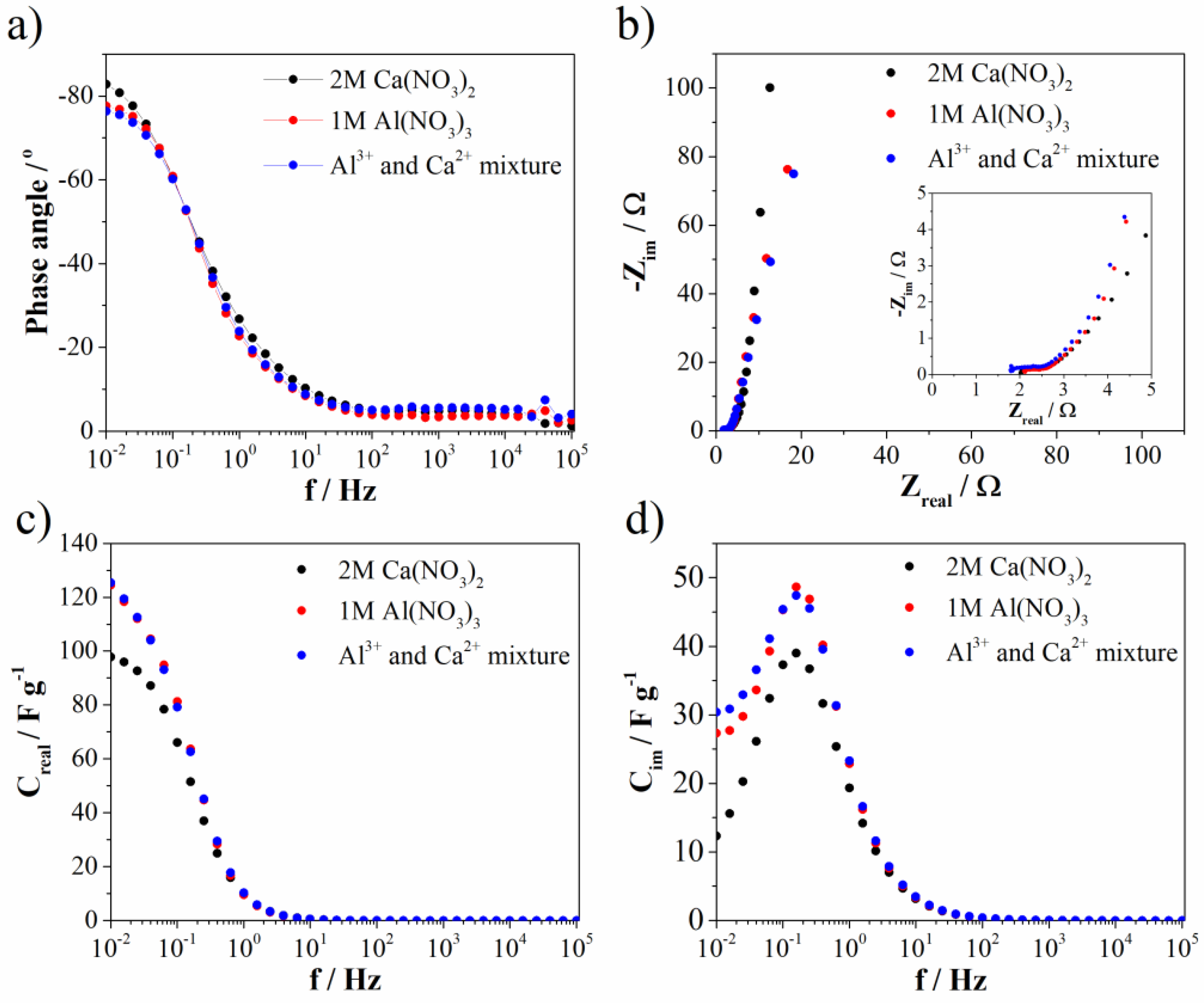
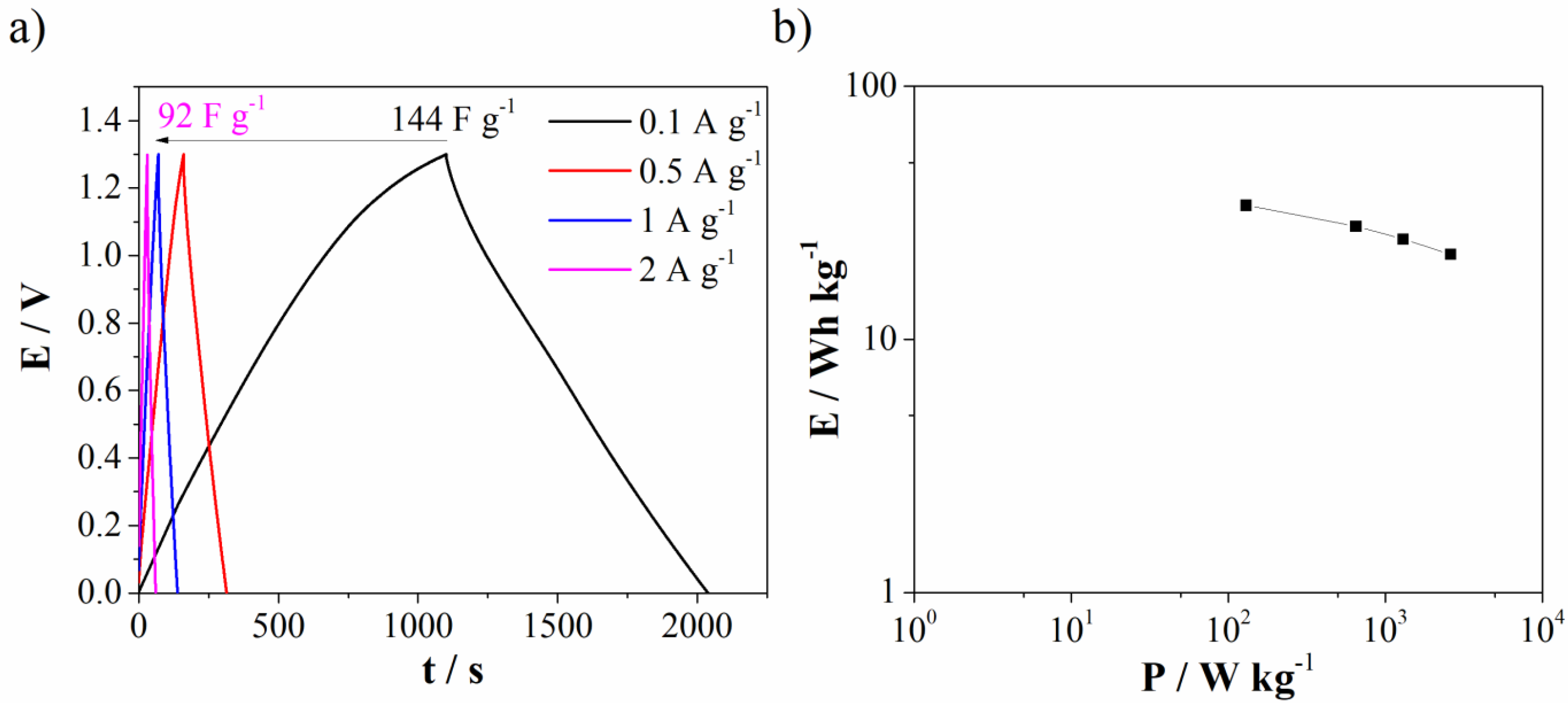
3.2. Electrochemical Investigation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhilip Kumar, R.; Nagarani, S.; Sethuraman, V.; Andra, S.; Dhinakaran, V. Investigations of Conducting Polymers, Carbon Materials, Oxide and Sulfide Materials for Supercapacitor Applications: A Review. Chem. Pap. 2022, 2022, 3371–3385. [Google Scholar] [CrossRef]
- Abdullin, K.A.; Gabdullin, M.T.; Kalkozova, Z.K.; Nurbolat, S.T.; Mirzaeian, M. Efficient Recovery Annealing of the Pseudocapacitive Electrode with a High Loading of Cobalt Oxide Nanoparticles for Hybrid Supercapacitor Applications. Nanomaterials 2022, 12, 3669. [Google Scholar] [CrossRef] [PubMed]
- Worsley, E.A.; Margadonna, S.; Bertoncello, P. Application of Graphene Nanoplatelets in Supercapacitor Devices: A Review of Recent Developments. Nanomaterials 2022, 12, 3600. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, L.; Huang, L.; Maximov, M.Y.; Jin, M.; Zhang, Y.; Wang, X.; Zhou, G. Biomass-Derived Oxygen and Nitrogen Co-Doped Porous Carbon with Hierarchical Architecture as Sulfur Hosts for High-Performance Lithium/Sulfur Batteries. Nanomaterials 2017, 7, 402. [Google Scholar] [CrossRef]
- Perumal, S.; Kishore, S.C.; Atchudan, R.; Sundramoorthy, A.K.; Alagan, M.; Lee, Y.R. Sustainable Synthesis of N/S-Doped Porous Carbon from Waste-Biomass as Electroactive Material for Energy Harvesting. Catalysts 2022, 12, 436. [Google Scholar] [CrossRef]
- Thomas, B.; Sain, M.; Oksman, K. Sustainable Carbon Derived from Sulfur-Free Lignins for Functional Electrical and Electrochemical Devices. Nanomaterials 2022, 12, 3630. [Google Scholar] [CrossRef]
- Costa, M.; Shi, X.; Fang, L.; Peng, H.; Deng, X.; Sun, Z. Metal-Organic Framework-Derived NiSe Embedded into a Porous Multi-Heteroatom Self-Doped Carbon Matrix as a Promising Anode for Sodium-Ion Battery. Nanomaterials 2022, 12, 3345. [Google Scholar]
- Lao, J.; Lu, Y.; Fang, S.; Xu, F.; Sun, L.; Wang, Y.; Zhou, T.; Liao, L.; Guan, Y.; Wei, X.; et al. Organic Crosslinked Polymer-Derived N/O-Doped Porous Carbons for High-Performance Supercapacitor. Nanomaterials 2022, 12, 2186. [Google Scholar] [CrossRef]
- Zdolšek, N.; Rocha, R.P.; Krstić, J.; Trtić-Petrović, T.; Šljukić, B.; Figueiredo, J.L.; Vujković, M.J. Electrochemical Investigation of Ionic Liquid-Derived Porous Carbon Materials for Supercapacitors: Pseudocapacitance versus Electrical Double Layer. Electrochim. Acta 2019, 298, 541–551. [Google Scholar] [CrossRef]
- Zdolšek, N.; Vujković, M.; Metin, Ö.; Brković, S.; Jocić, A.; Dimitrijević, A.; Trtić-Petrović, T.; Šljukić, B. Boosting Electrocatalysis of Oxygen Reduction and Evolution Reactions with Cost-Effective Cobalt and Nitrogen-Doped Carbons Prepared by Simple Carbonization of Ionic Liquids. Int. J. Hydrogen Energy 2022, 47, 14847–14858. [Google Scholar] [CrossRef]
- Milikić, J.; Oliveira, R.C.P.; Tapia, A.; Santos, D.M.F.; Zdolšek, N.; Trtić-Petrović, T.; Vraneš, M.; Šljukić, B. Ionic Liquid-Derived Carbon-Supported Metal Electrocatalysts as Anodes in Direct Borohydride-Peroxide Fuel Cells. Catalysts 2021, 11, 632. [Google Scholar] [CrossRef]
- Bijesh, P.; Selvaraj, V.; Andal, V. A Review on Synthesis and Applications of Nano Metal Oxide/Porous Carbon Composite. Mater. Today Proc. 2022, 55, 212–219. [Google Scholar] [CrossRef]
- Yang, H.; Wang, C.; Zhu, W.; Zhang, X.; Li, T.; Fan, J. A Sustainable Strategy for Solid-Phase Extraction of Antiviral Drug from Environmental Waters by Immobilized Hydrogen Bond Acceptor. Nanomaterials 2022, 12, 1287. [Google Scholar] [CrossRef] [PubMed]
- Pariiska, O.; Mazur, D.; Cherchenko, K.; Kurys, Y.; Koshechko, V.; Pokhodenko, V. Efficient Co-N-C Electrocatalysts for Oxygen Reduction Derived from Deep Eutectic Solvents. Electrochim. Acta 2022, 413, 140132. [Google Scholar] [CrossRef]
- Guo, J.; Yin, X.; Wang, T.; Feng, J.; Zeng, P.; Wu, D. N/B Codoped Porous Carbon Electrode and Electrolyte Derived from Amino Acid Based Deep Eutectic Solvent for High Capacitive Performance. J. Electroanal. Chem. 2021, 903, 115840. [Google Scholar] [CrossRef]
- Ge, X.; Gu, C.; Wang, X.; Tu, J. Deep Eutectic Solvents (DESs)-Derived Advanced Functional Materials for Energy and Environmental Applications: Challenges, Opportunities, and Future Vision. J. Mater. Chem. A 2017, 5, 8209–8229. [Google Scholar] [CrossRef]
- Wang, M.; Li, C.; Zhou, M.; Xia, Z.; Huang, Y. Natural Deep Eutectic Solvent Assisted Synthesis and Applications of Chiral Carbon Dots. Green Chem. 2022, 24, 6696–6706. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A Review of Electrolyte Materials and Compositions for Electrochemical Supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar]
- Zhang, D.; She, W.; Wang, J.; Gao, S.; Yang, B.; Men, X.; Wang, K.; Han, Z.; Chen, X. High-Specific-Energy Aqueous Supercapacitor with Extended Operating Potential Using Trichloroacetic Acid Electrolyte. J. Power Sources 2022, 527, 231211. [Google Scholar] [CrossRef]
- Gezović, A.; Mišurović, J.; Milovanović, B.; Etinski, M.; Krstić, J.; Grudić, V.; Dominko, R.; Mentus, S.; Vujković, M.J. High Al-Ion Storage of Vine Shoots-Derived Activated Carbon: New Concept for Affordable and Sustainable Supercapacitors. J. Power Sources 2022, 538, 231561. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, Q.; Li, J. Review And Prospect of Mn3O4-Based Composite Materials For Supercapacitor Electrodes. ChemistrySelect 2020, 5, 10407–10423. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Wang, Q.; Wang, T. Facile Synthesis of Porous Mn3O4 Nano crystal–Graphene Nanocomposites for Electrochemical Supercapacitors. Eur. J. Inorg. Chem. 2012, 2012, 628–635. [Google Scholar] [CrossRef]
- Sun, L.; Song, G.; Sun, Y.; Fu, Q.; Pan, C. One-Step Construction of 3D N/P-Codoped Hierarchically Porous Carbon Framework in-Situ Armored Mn3O4 Nanoparticles for High-Performance Flexible Supercapacitors. Electrochim. Acta 2020, 333, 135496. [Google Scholar] [CrossRef]
- Yan, B.L.; Jun, D.; Wang, J.; Yang, T.; Mao, X.H. A Simplified Electrophoretic Deposition Route for Sandwiched Structure-Based Mn3O4/G Composite Electrodes as High-Capacity Anodes for Lithium-Ion Batteries. J. Alloys Compd. 2022, 905, 164121. [Google Scholar] [CrossRef]
- Vujković, M.; Matović, L.; Krstić, J.; Stojmenović, M.; Đukić, A.; Babić, B.; Mentus, S. Mechanically Activated Carbonized Rayon Fibers as an Electrochemical Supercapacitor in Aqueous Solutions. Electrochim. Acta 2017, 245, 796–806. [Google Scholar] [CrossRef]
- Mu, T.; Huang, J.; Liu, Z.; Han, B.; Li, Z.; Wang, Y.; Jiang, T.; Gao, H. Synthesis and Characterization of Polyether Structure Carbon Nitride. J. Mater. Res. 2004, 19, 1736–1741. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A.; Antonietti, M. A Detailed View on the Polycondensation of Ionic Liquid Monomers towards Nitrogen Doped Carbon Materials. J. Mater. Chem. 2010, 20, 6746–6758. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Functional Carbon Materials From Ionic Liquid Precursors. Macromol. Chem. Phys. 2012, 213, 1132–1145. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, M.; Fu, Z.; Xu, X.; Wang, C.; Yuan, L.; Tang, Y. From Hierarchically Porous Carbon to Mn3O4/Carbon Composites for High Voltage Aqueous Supercapacitors. J. Power Sources 2021, 485, 229111. [Google Scholar] [CrossRef]
- Huang, Q.; Zhong, X.; Zhang, Q.; Wu, X.; Jiao, M.; Chen, B.; Sheng, J.; Zhou, G. Co3O4/Mn3O4 Hybrid Catalysts with Heterointerfaces as Bifunctional Catalysts for Zn-Air Batteries. J. Energy Chem. 2022, 68, 679–687. [Google Scholar] [CrossRef]
- Rouxhet, P.G.; Genet, M.J. XPS Analysis of Bio-Organic Systems. Surf. Interface Anal. 2011, 43, 1453–1470. [Google Scholar] [CrossRef]
- Burbano, A.A.; Gascó, G.; Paz-Ferreiro, J.; Méndez, A. Catalytic Activity of Carbon Materials in the Oxidation of Minerals. Catalysts 2022, 12, 918. [Google Scholar] [CrossRef]
- AlHumaidan, F.S.; Rana, M.S.; Vinoba, M.; Rajasekaran, N.; AlHenyyan, H.Y.; Ali, A.A. Synthesizing Few-Layer Carbon Materials from Asphaltene by Thermal Treatment. Diam. Relat. Mater. 2022, 129, 109316. [Google Scholar] [CrossRef]
- Chauhan, A.; Naseem, S.; Bahadur, J.; Singh, B.R.; Shoeb, M.; Husain, S.; Khan, W. Structural and Electrochemical Properties of GO/Mn3O4 Nanocomposite. J. Mater. Sci. Mater. Electron. 2021, 32, 3894–3902. [Google Scholar] [CrossRef]
- Carneiro-Neto, E.B.; Lopes, M.C.; Pereira, E.C. Simulation of Interfacial PH Changes during Hydrogen Evolution Reaction. J. Electroanal. Chem. 2016, 765, 92–99. [Google Scholar] [CrossRef]
- Keshari, A.S.; Dubey, P. Sucrose-Assisted One Step Hydrothermal Synthesis of MnCO3/Mn3O4 Hybrid Materials for Electrochemical Energy Storage. Electrochim. Acta 2022, 402, 139486. [Google Scholar] [CrossRef]
- Liu, Y.; He, G.; Jiang, H.; Parkin, I.P.; Shearing, P.R.; Brett, D.J.L. Cathode Design for Aqueous Rechargeable Multivalent Ion Batteries: Challenges and Opportunities. Adv. Funct. Mater. 2021, 31, 2010445. [Google Scholar] [CrossRef]
- Xie, B.; Chen, Y.; Yu, M.; Zhang, S.; Lu, L.; Shu, Z.; Zhang, Y. Phosphoric Acid-Assisted Synthesis of Layered MoS2/Graphene Hybrids with Electrolyte-Dependent Supercapacitive Behaviors. RSC Adv. 2016, 6, 89397–89406. [Google Scholar] [CrossRef]
- Huang, Z.H.; Liu, T.Y.; Song, Y.; Li, Y.; Liu, X.X. Balancing the Electrical Double Layer Capacitance and Pseudocapacitance of Hetero-Atom Doped Carbon. Nanoscale 2017, 9, 13119–13127. [Google Scholar] [CrossRef]
- Li, W.; Xu, A.; Zhang, Y.; Yu, Y.; Liu, Z.; Qin, Y. Metal-Organic Framework-Derived Mn3O4 Nanostructure on Reduced Graphene Oxide as High-Performance Supercapacitor Electrodes. J. Alloys Compd. 2022, 897, 162640. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, X.; Zhao, J.; Zheng, Y.; Xie, H.; Zhang, Z.; Wang, S.; Xu, Q.; Fu, Q.; Zhang, T. High Performance Flower-Like Mn3O4/RGO Composite for Supercapacitor Applications. J. Electroanal. Chem. 2022, 910, 116170. [Google Scholar] [CrossRef]
- Luo, H.; Zheng, L.; Lei, L.; Zhang, D.; Wu, J.; Yang, J. Supercapacitive Behavior of Mesoporous Carbon CMK-3 in Calcium Nitrate Aqueous Electrolyte. Korean J. Chem. Eng. 2014, 31, 712–718. [Google Scholar] [CrossRef]
- Stojmenović, M.; Vujković, M.; Matović, L.; Krstić, J.; Dukić, A.; Dodevski, V.; Živković, S.M.; Mentus, S. Complex Investigation of Charge Storage Behavior of Microporous Carbon Synthesized by Zeolite Template. Microporous Mesoporous Mater. 2016, 228, 94–106. [Google Scholar] [CrossRef]
- Zhao, H.; Xing, B.; Zhang, C.; Huang, G.; Yu, J.; Jiang, Z.; Qu, X.; Wu, X.; Cao, Y.; Zhang, C. MnOX–Modified Corrugated Carton–Derived Hierarchical Porous Carbon with Ultrafast Kinetics Behaviour for High–Performance Symmetric Supercapacitors. J. Alloys Compd. 2020, 848, 156423. [Google Scholar] [CrossRef]
- Kavitha, E.R.; Meiyazhagan, S.; Yugeswaran, S.; Balraju, P.; Suresh, K. Electrochemical Prospects and Potential of Hausmannite Mn3O4 Nanoparticles Synthesized through Microplasma Discharge for Supercapacitor Applications. Int. J. Energy Res. 2021, 45, 7038–7056. [Google Scholar] [CrossRef]





| C | O | N | Mn | |
|---|---|---|---|---|
| Composition/at. % | 89.83 | 3.98 | 5.66 | 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdolšek, N.; Perović, I.; Brković, S.; Tasić, G.; Milović, M.; Vujković, M. Deep Eutectic Solvent for Facile Synthesis of Mn3O4@N-Doped Carbon for Aqueous Multivalent-Based Supercapacitors: New Concept for Increasing Capacitance and Operating Voltage. Materials 2022, 15, 8540. https://doi.org/10.3390/ma15238540
Zdolšek N, Perović I, Brković S, Tasić G, Milović M, Vujković M. Deep Eutectic Solvent for Facile Synthesis of Mn3O4@N-Doped Carbon for Aqueous Multivalent-Based Supercapacitors: New Concept for Increasing Capacitance and Operating Voltage. Materials. 2022; 15(23):8540. https://doi.org/10.3390/ma15238540
Chicago/Turabian StyleZdolšek, Nikola, Ivana Perović, Snežana Brković, Gvozden Tasić, Miloš Milović, and Milica Vujković. 2022. "Deep Eutectic Solvent for Facile Synthesis of Mn3O4@N-Doped Carbon for Aqueous Multivalent-Based Supercapacitors: New Concept for Increasing Capacitance and Operating Voltage" Materials 15, no. 23: 8540. https://doi.org/10.3390/ma15238540
APA StyleZdolšek, N., Perović, I., Brković, S., Tasić, G., Milović, M., & Vujković, M. (2022). Deep Eutectic Solvent for Facile Synthesis of Mn3O4@N-Doped Carbon for Aqueous Multivalent-Based Supercapacitors: New Concept for Increasing Capacitance and Operating Voltage. Materials, 15(23), 8540. https://doi.org/10.3390/ma15238540






