Influence of Plasma Treatment Parameters on the Structural-Phase Composition, Hardness, Moisture-Resistance, and Raman-Enhancement Properties of Nitrogen-Containing Titanium Dioxide
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Analysis of the State of Nitrogen Plasma in a Discharge
3.2. Studies of the Structural-Phase and Elemental Composition
3.3. Study of Hardness, Moisture-Resistance, and Raman-Enhancement Properties
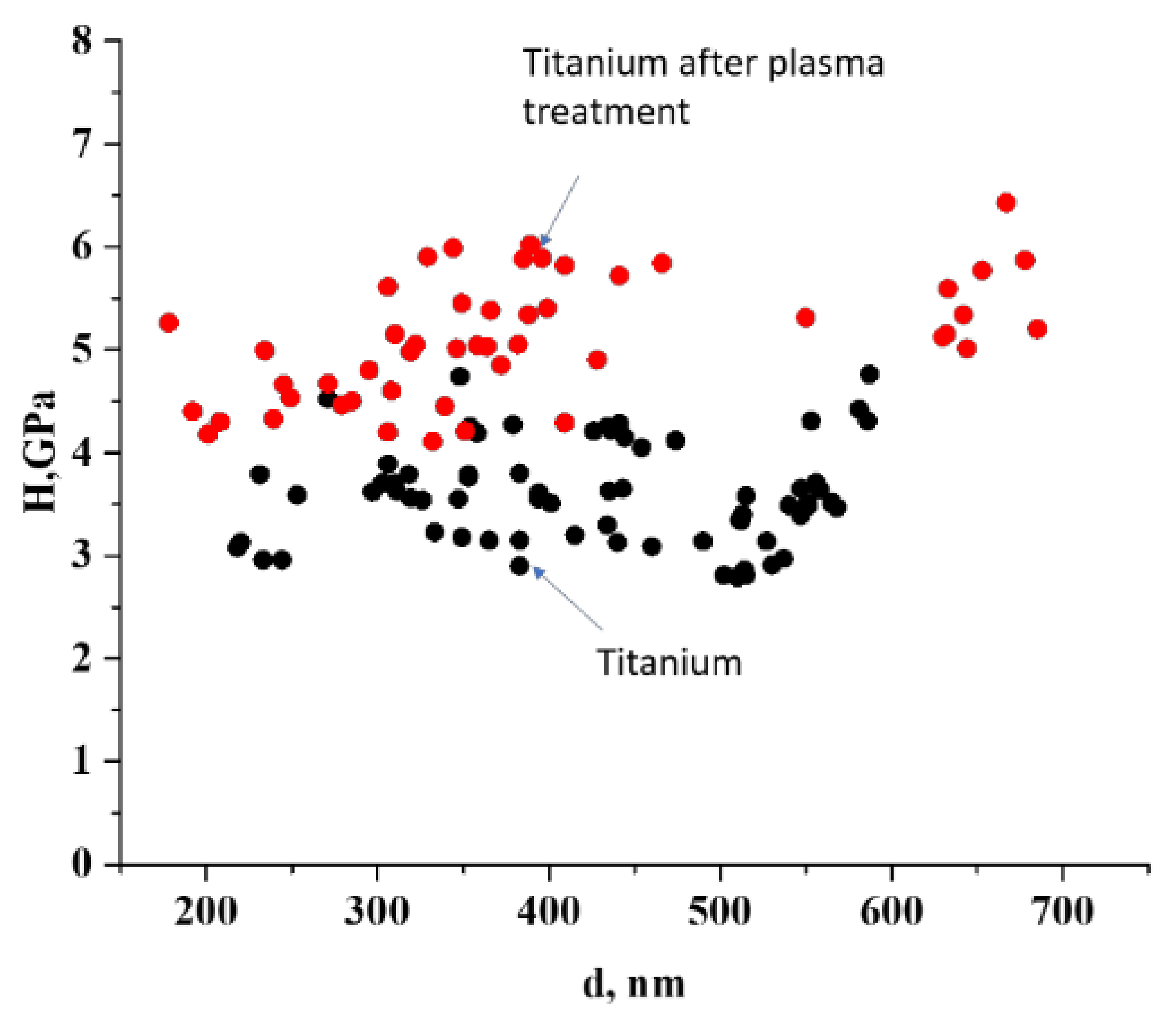
3.3.1. Microhardness
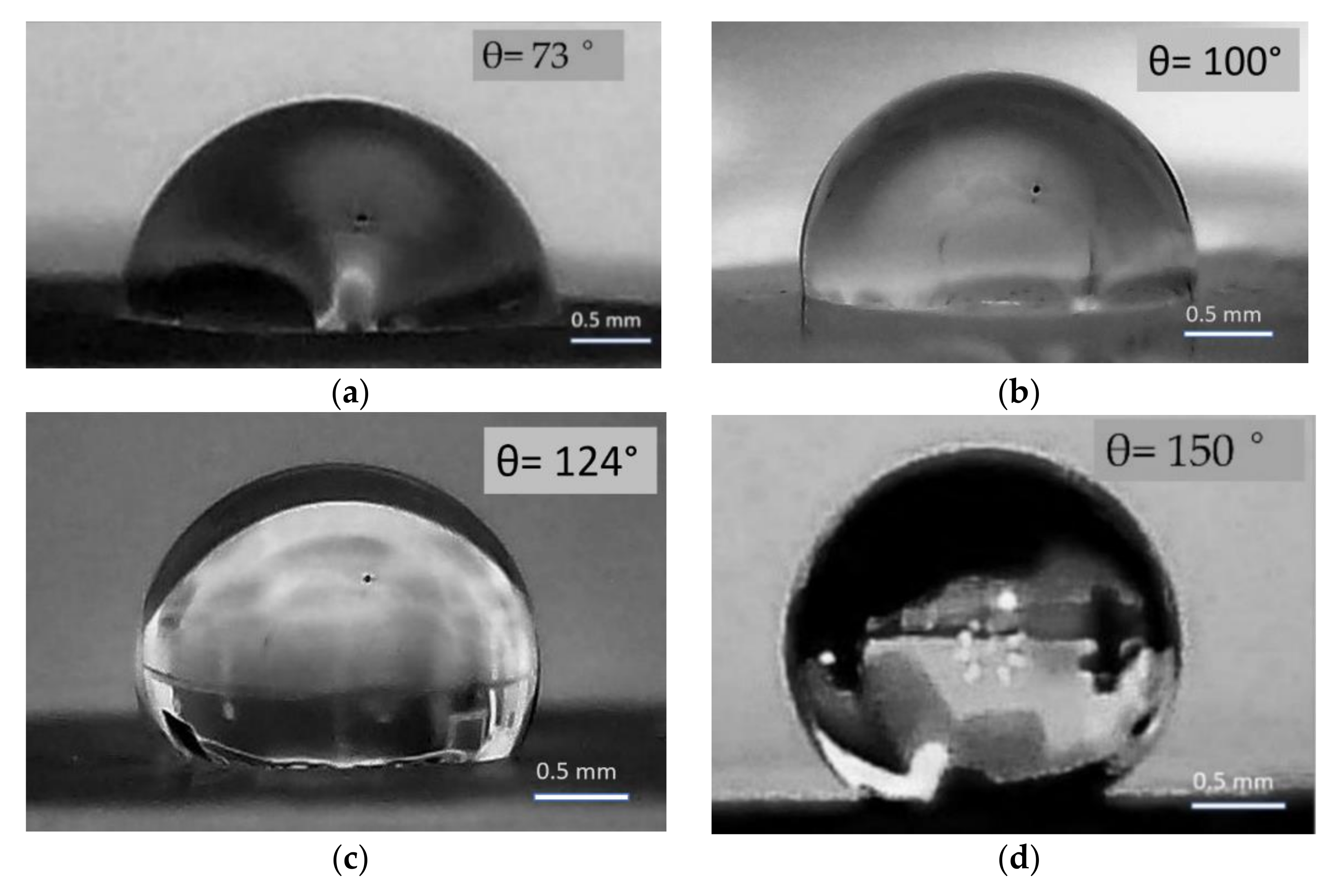
3.3.2. Moisture-Resistant Properties
3.3.3. Raman Active Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bickerton, R.J. Introduction to High Temperature Plasma Physics. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 1981, 300, 475–488. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, Q.; Li, M.; Sun, S.; Zhao, H.; Jin, S.; Wang, J. An overview of low-temperature plasma surface modification of carbon materials for removal of pollutants from liquid and gas phases. Plasma Process. Polym. 2021, 18, 3. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Li, L.; Liu, R.; Liu, Q.; Wu, Z.; Meng, X.; Fang, Y. Effects of Initial Microstructure on the Low-Temperature Plasma Nitriding of Ferritic Stainless Steel. Coatings 2022, 12, 1404. [Google Scholar] [CrossRef]
- Muslimov, A.; Orudzhev, F.; Gadzhiev, M.; Selimov, D.; Tyuftyaev, A.; Kanevsky, V. Facile Synthesis of Ti/TiN/TiON/TiO2 Composite Particles for Plasmon-Enhanced Solar Photocatalytic Decomposition of Methylene Blue. Coatings 2022, 12, 1741. [Google Scholar] [CrossRef]
- Muslimov, A.E.; Gadzhiev, M.K.; Kanevsky, V.M. Synthesis of Superhydrophobic Barium Hexaferrite Coatings with Low Magnetic Hardness. Materials 2022, 15, 7865. [Google Scholar] [CrossRef]
- Tabares, F.L.; Junkar, I. Cold Plasma Systems and Their Application in Surface Treatments for Medicine. Molecules 2021, 26, 1903. [Google Scholar] [CrossRef]
- Ye, Z.; Zhao, L.; Nikiforov, A.; Giraudon, J.; Chen, Y.; Wang, J.; Tu, X. A review of the advances in catalyst modification using nonthermal plasma: Process, Mechanism and pplications. Adv. Colloid Interface Sci. 2020, 308, 102755. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Mazur, M.; Indyka, J.; Jurkowska, A.; Kalisz, M.; Domanowski, P.; Kaczmarek, D.; Domaradzki, J. Mechanical and structural properties of titanium dioxide deposited by innovative magnetron sputtering process. Mater. Sci.-Pol. 2015, 33, 660–668. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Konaka, Y.; Ohtsu, N. Effects of alcoholic solvents on the structure of anodized TiO2 layer grown in nitrate electrolyte. Surf. Coat. Technol. 2020, 387, 125424. [Google Scholar] [CrossRef]
- Kaczmarek, D.; Domaradzki, J.; Wojcieszak, D.; Prociow, E.; Mazur, M.; Placido, F.; Lapp, S. Hardness of Nanocrystalline TiO2 Thin Films. J. Nano Res. 2012, 18–19, 195–200. [Google Scholar] [CrossRef]
- Dubrovinsky, L.S.; Dubrovinskaia, N.A.; Swamy, V.; Muscat, J.; Harrison, N.M.; Ahuja, R.; Holm, B.; Johansson, B. Materials science. The hardest known oxide. Nature 2001, 410, 653–654. [Google Scholar] [CrossRef] [PubMed]
- Zhecheva, A.; Malinov, S.; Sha, W. Titanium alloys after surface gas nitriding. Surf. Coat. Technol. 2006, 201, 2467–2474. [Google Scholar] [CrossRef]
- Simonsen, M.E.; Li, Z.; Søgaard, E.G. Influence of the OH groups on the photocatalytic activity and photoinduced hydrophilicity of microwave assisted sol–gel TiO2 film. Appl. Surf. Sci. 2009, 255, 8054–8062. [Google Scholar] [CrossRef]
- Muslimov, A.E.; Asvarov, A.S.; Shabanov, N.S. The Effect of Topographic Defects on the Superhydrophobic Properties of Coatings Based on ZnO. Tech. Phys. Lett. 2020, 46, 954–957. [Google Scholar] [CrossRef]
- Shirolkar, M.M.; Phase, D.; Sathe, V.; Rodríguez-Carvajal, J.; Choudhary, R.J.; Kulkarni, S.K. Relation between crystallinity and chemical nature of surface on wettability: A study on pulsed laser deposited TiO2 thin films. J. Appl. Phys. 2011, 109, 123512. [Google Scholar] [CrossRef]
- Kongsong, P.; Taleb, A.; Masae, M.; Jeenarong, A.; Hansud, P.; Khumruean, S. Effect of nitrogen doping on the photocatalytic activity and hydrophobic property of rutile TiO2 nanorods array. Surf. Interface Anal. 2018, 50, 1271–1277. [Google Scholar] [CrossRef]
- Kadir, M.; Renata, N.; Gulzhan, B.; Balaussa, A.; Aliya, A.; Vladimir, S. SERS-active substrates based on Ag-coated TiO2 nanotubes and nanograss. Phys. E Low-Dimens. Syst. Nanostruct. 2023, 145, 115499. [Google Scholar] [CrossRef]
- Mandrile, L.; Giovannozzi, A.M.; Sacco, A.; Martra, G.; Rossi, A.M. Flexible and Transparent Substrates Based on Gold Nanoparticles and TiO2 for in Situ Bioanalysis by Surface-Enhanced Raman Spectroscopy. Biosensors 2019, 9, 145. [Google Scholar] [CrossRef]
- Li, C.; Yang, W.; Liu, L.; Sun, W.; Li, Q. In situ growth of TiO2 on TiN nanoparticles for non-noble-metal plasmonic photocatalysis. RSC Adv. 2016, 6, 72659–72669. [Google Scholar] [CrossRef]
- Naik, G.V.; Schroeder, J.L.; Ni, X.; Kildishev, A.V.; Sands, T.D.; Boltasseva, A. Titanium nitride as a plasmonic material for visible and near-infrared wavelengths. Opt. Mater. Express 2012, 2, 478. [Google Scholar] [CrossRef]
- Chang, J.; Chen, T.P.; Li, X.D.; Liu, Y.C.; Liu, Y.; Yang, H.Y. Investigation of localized surface plasmon resonance of TiN nanoparticles in TiNxOy thin films. Opt. Mater. Express 2016, 6, 2422. [Google Scholar] [CrossRef]
- Isakaev, E.K.; Sinkevich, O.A.; Tyuftyaev, A.S.; Chinnov, V.F. Investigation of low-temperature plasma generator with divergent channel of the output electrode and some applications of this generator. High Temp. 2010, 48, 97–125. [Google Scholar] [CrossRef]
- Ochkin, V.N. Spektroskopiya Nizkotemperaturnoi Plazmy (Spectroscopy of Low-Temperature Plasma); Fizmatlit: Moscow, Russia, 2010. [Google Scholar]
- Yuan, Y.; Lee, T.R. Surface Science Techniques; Bracco, G., Holst, B., Eds.; Springer Series in Surface Sciences; Springer: Berlin/Heidelberg, Germany, 2013; Volume 51, pp. 3–34. [Google Scholar] [CrossRef]
- Dresvin, S.V. (Ed.) Physics and Technology of Low-Temperature Plasma; Atomizdat: Moscow, Russia, 1972. (In Russian) [Google Scholar]
- Korshunov, O.V.; Kavyrshin, D.I.; Chinnov, V.F. Kinetics of the processes in a nitrogen plasma flow with carbon admixture. High Temp. 2020, 58, 671–680. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Mynuddin, M. Kinetic Modelling of Atmospheric Pressure Nitrogen Plasma. Am. J. Mod. Phys. 2018, 7, 185–193. [Google Scholar] [CrossRef]
- Challagulla, S.; Tarafder, K.; Ganesan, R.; Roy, S. Structure sensitive photocatalytic reduction of nitroarenes over TiO2. Sci. Rep. 2017, 7, 8783. [Google Scholar] [CrossRef] [PubMed]
- Chalastara, K.; Guo, F.; Elouatik, S.; Demopoulos, G.P. Tunable Composition Aqueous-Synthesized Mixed-Phase TiO2 Nanocrystals for Photo-Assisted Water Decontamination: Comparison of Anatase, Brookite and Rutile Photocatalysts. Catalysts 2020, 10, 407. [Google Scholar] [CrossRef]
- Han, H.; Bai, R. Buoyant Photocatalyst with Greatly Enhanced Visible-Light Activity Prepared through a Low Temperature Hydrothermal Method. Ind. Eng. Chem. Res. 2009, 48, 2891–2898. [Google Scholar] [CrossRef]
- Bernard, M.; Deneuville, A.; Thomas, O.; Gergaud, P.; Sandstrom, P.; Birch, J. Raman spectra of TiN/AlN superlattices. Thin Solid Films 2000, 380, 252–255. [Google Scholar] [CrossRef]
- György, E.; Pérez del Pino, A.; Serra, P.; Morenza, J.L. Surface nitridation of titanium by pulsed Nd:YAG laser irradiation. Appl. Surf. Sci. 2002, 186, 130–134. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, J.; Chen, F.; Anpo, M. Synthesis and Characterization of Nitrogen-Doped TiO2 Nanophotocatalyst with High Visible Light Activity. J. Phys. Chem. C 2007, 111, 6976–6982. [Google Scholar] [CrossRef]
- Broitman, E. Indentation Hardness Measurements at Macro-, Micro-, and Nanoscale: A Critical Overview. Tribol. Lett. 2016, 65, 23. [Google Scholar] [CrossRef]
- Sinani, A.B.; Dynkin, N.K.; Lytvinov, L.A. Sapphire hardness in different crystallographic directions. Bull. Russ. Acad. Sci. Phys. 2009, 73, 1380. [Google Scholar] [CrossRef]
- Kofstad, P.; Anderson, P.B.; Krudtaa, O.J. Oxidation of titanium in the temperature range 800–1200 °C. J. Less Common Met. 1961, 3, 89–97. [Google Scholar] [CrossRef]
- Novikov, V.; Stepanov, N.; Zherebtsov, S.; Salishchev, G. Structure and Properties of High-Entropy Nitride Coatings. Metals 2022, 12, 847. [Google Scholar] [CrossRef]
- Zhukov, V.P.; Shein, I.R. Ab initio thermodynamic characteristics of the formation of oxygen vacancies, and boron, carbon, and nitrogen impurity centers in anatase. Phys. Solid State 2018, 60, 37–48. [Google Scholar] [CrossRef]
- Balajka, J.; Hines, M.A.; DeBenedetti, W.J.I.; Komora, M.; Pavelec, J.; Schmid, M.; Diebold, U. High-affinity adsorption leads to molecularly ordered interfaces on TiO2 in air and solution. Science 2018, 361, 786–789. [Google Scholar] [CrossRef]
- De Nicolai, S.H.A.; Rodrigues, P.R.P.; Agostinho, S.M.L.; Rubim, J.C. Electrochemical and spectroelectrochemical (SERS) studies of the reduction of methylene blue on a silver electrode. J. Electroanal. Chem. 2002, 527, 103–111. [Google Scholar] [CrossRef]
- Toropov, N.A.; Leonov, N.B.; Vartanyan, T.A. Influence of Silver Nanoparticles Crystallinity on Localized Surface Plasmons Dephasing Times. Phys. Status Solidi 2017, 255, 1700174. [Google Scholar] [CrossRef]
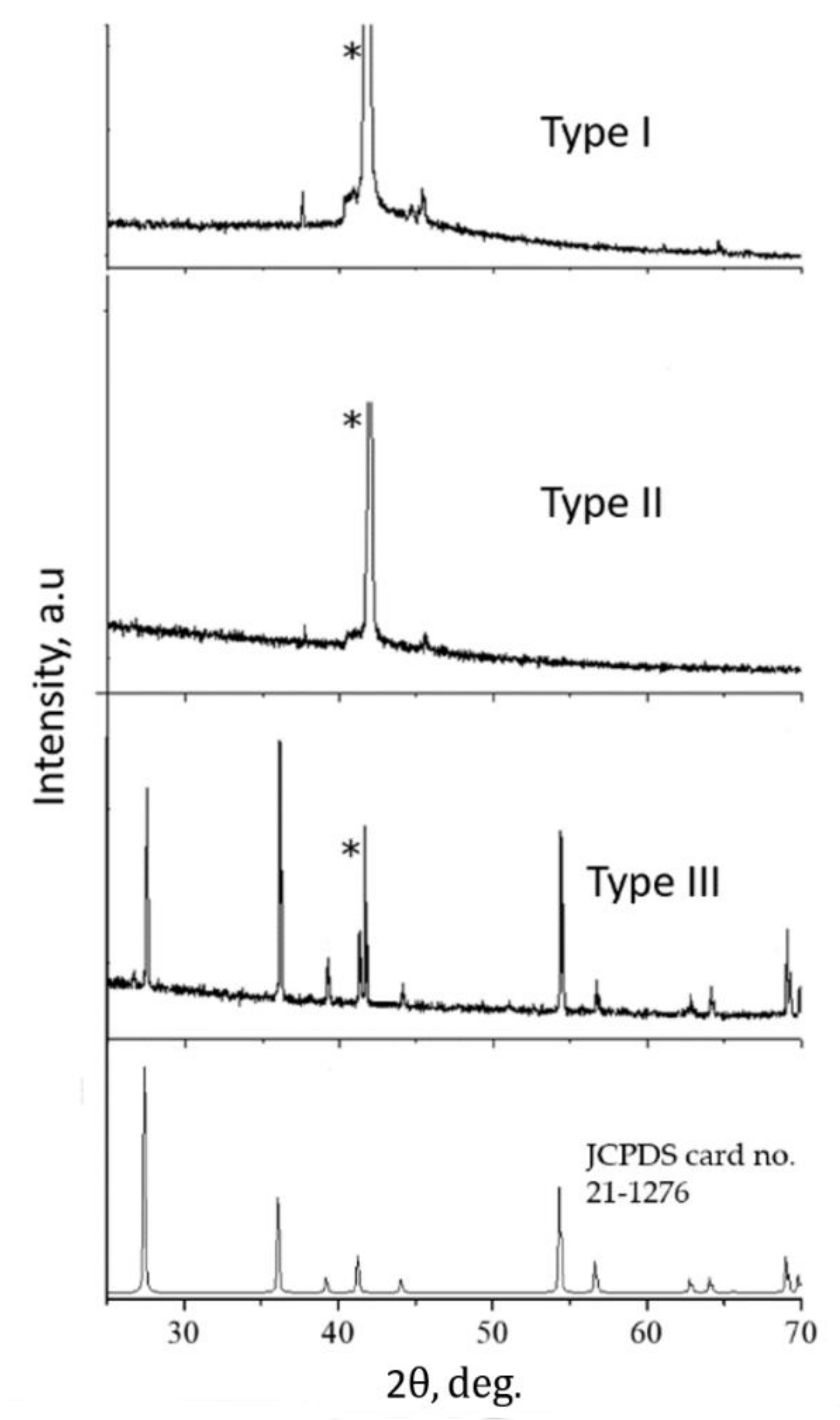
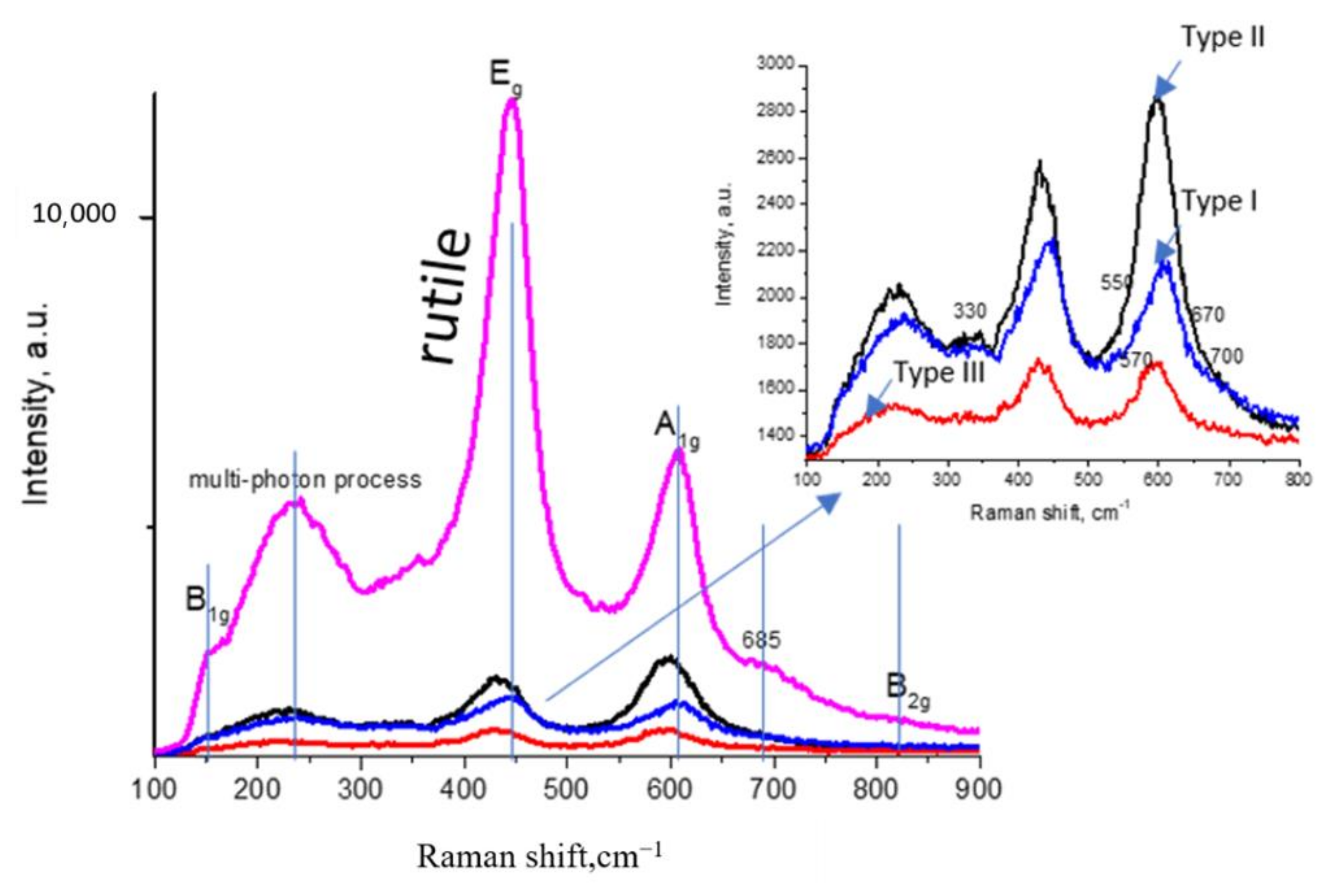
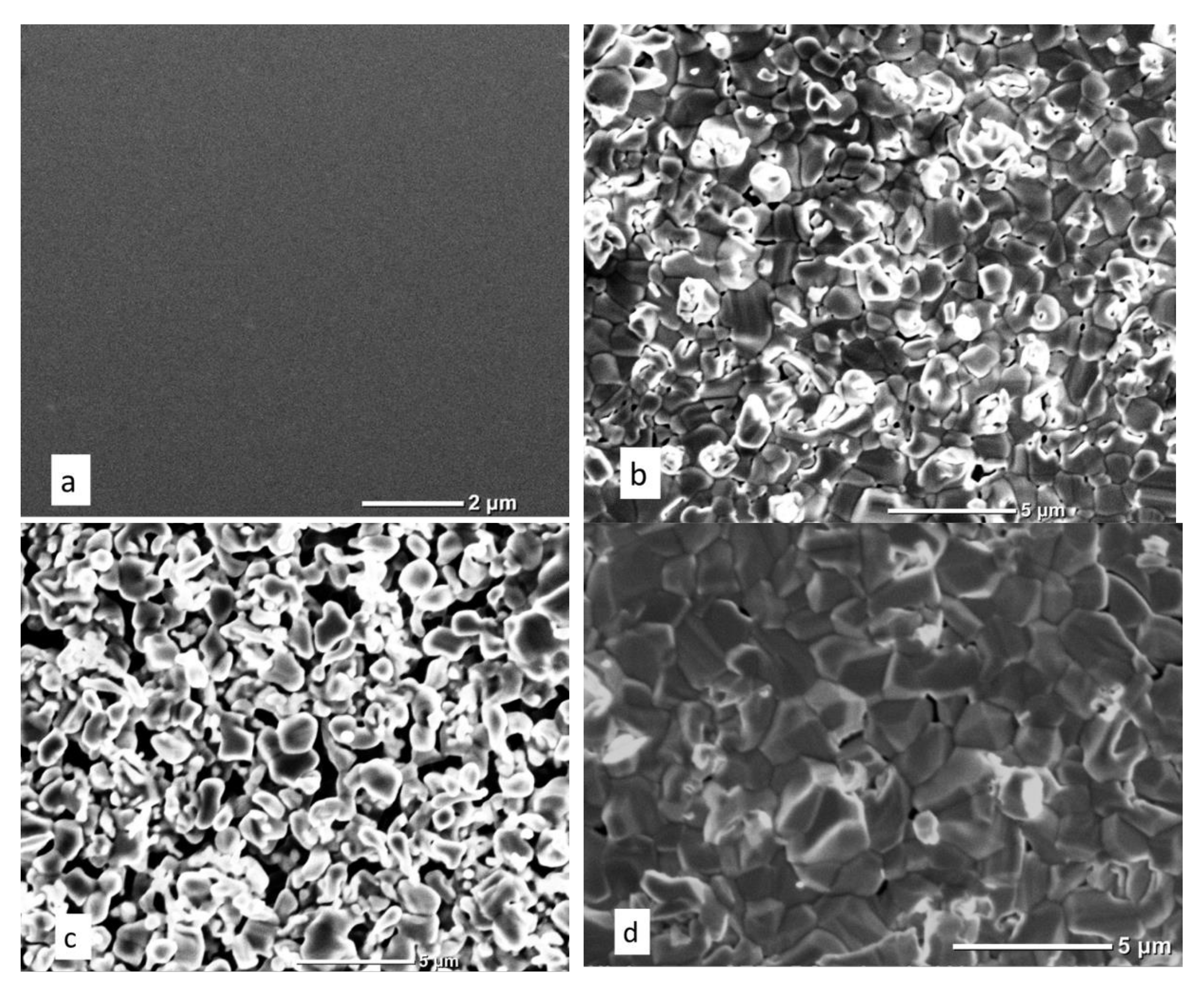
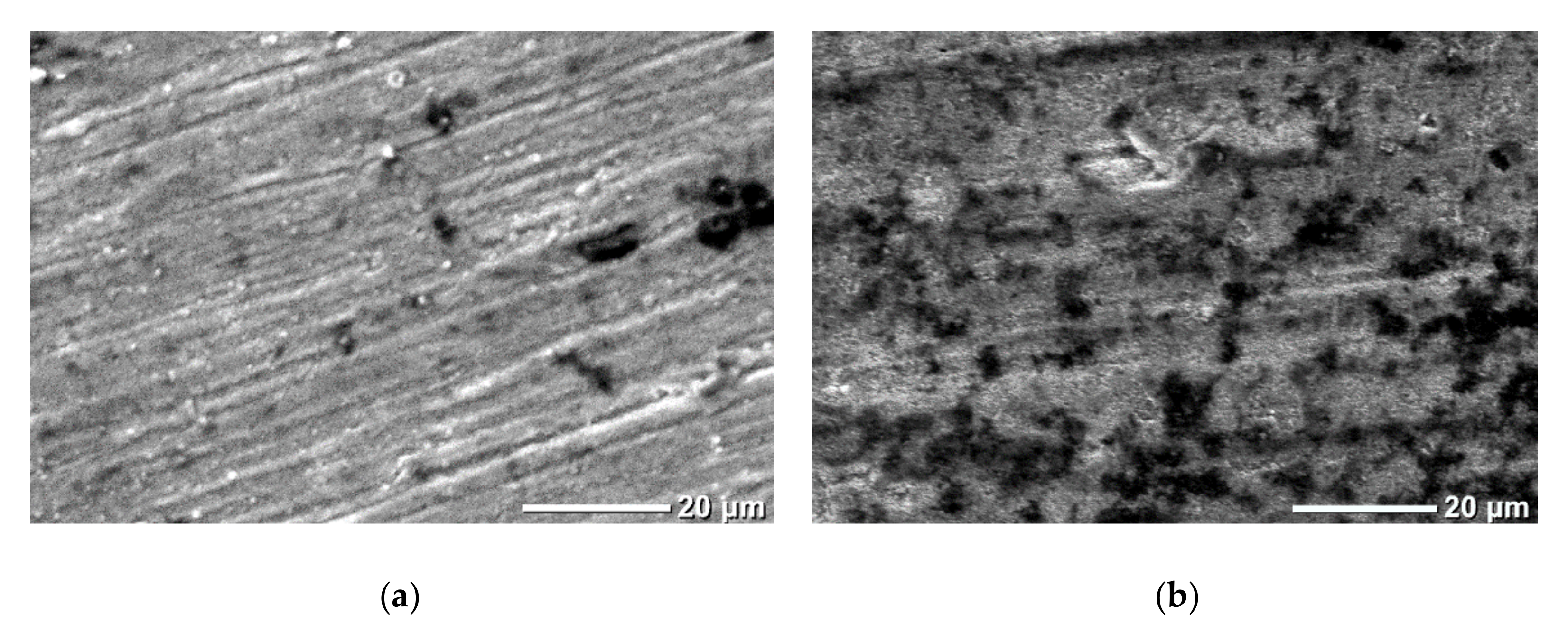

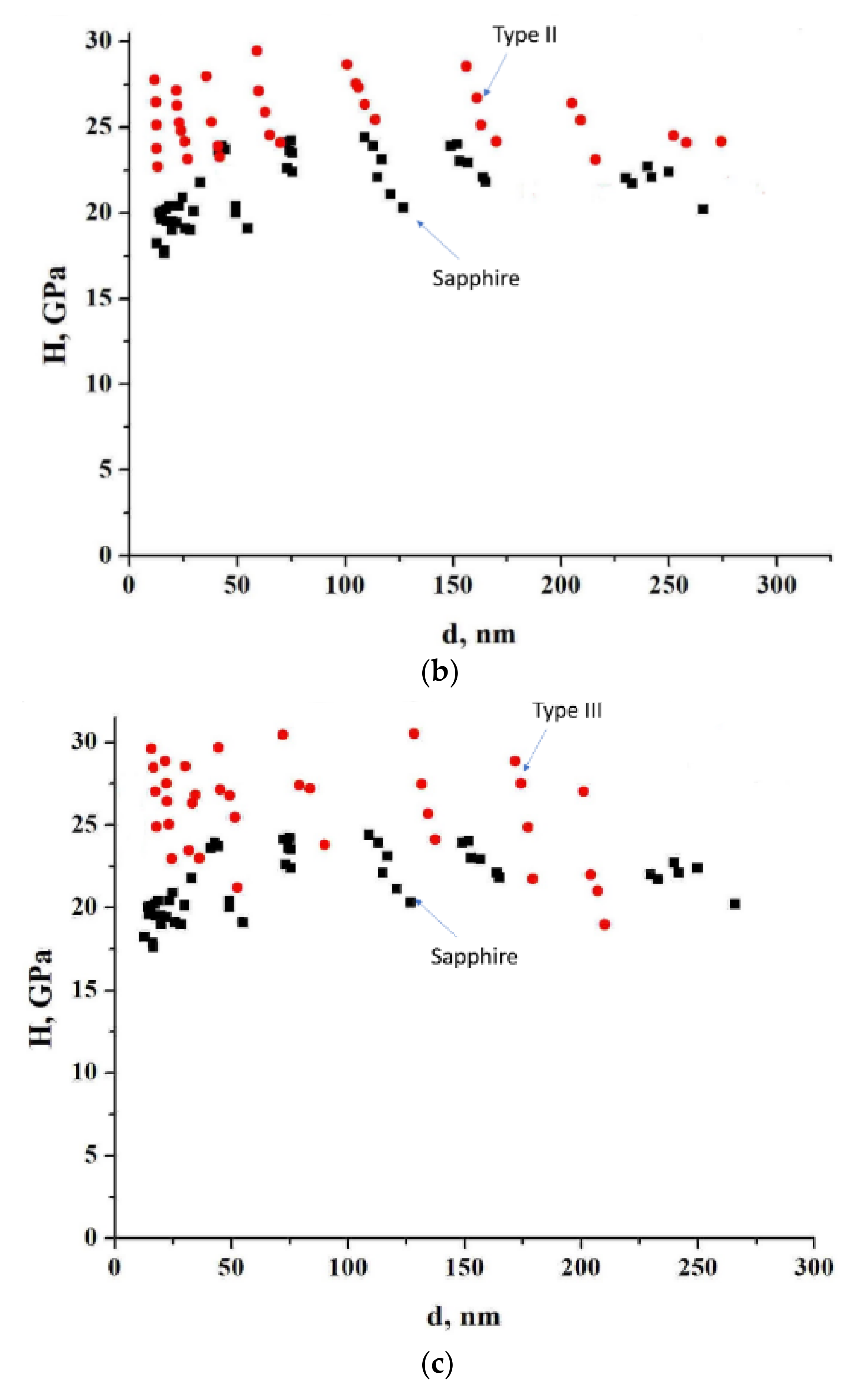

| Type I | Type II | Type III | |
|---|---|---|---|
| N/Ti | 0.22 | 0.51 | 0.52 |
| Sample | Type I | Type II | Type III |
|---|---|---|---|
| Hardness, GPa | 23.9 | 26.0 | 27.2 |
| Sample | pristine titanium | treated titanium | |
| Hardness, GPa | 3.5 | 5.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muslimov, A.E.; Gadzhiev, M.K.; Kanevsky, V.M. Influence of Plasma Treatment Parameters on the Structural-Phase Composition, Hardness, Moisture-Resistance, and Raman-Enhancement Properties of Nitrogen-Containing Titanium Dioxide. Materials 2022, 15, 8514. https://doi.org/10.3390/ma15238514
Muslimov AE, Gadzhiev MK, Kanevsky VM. Influence of Plasma Treatment Parameters on the Structural-Phase Composition, Hardness, Moisture-Resistance, and Raman-Enhancement Properties of Nitrogen-Containing Titanium Dioxide. Materials. 2022; 15(23):8514. https://doi.org/10.3390/ma15238514
Chicago/Turabian StyleMuslimov, Arsen E., Makhach Kh. Gadzhiev, and Vladimir M. Kanevsky. 2022. "Influence of Plasma Treatment Parameters on the Structural-Phase Composition, Hardness, Moisture-Resistance, and Raman-Enhancement Properties of Nitrogen-Containing Titanium Dioxide" Materials 15, no. 23: 8514. https://doi.org/10.3390/ma15238514
APA StyleMuslimov, A. E., Gadzhiev, M. K., & Kanevsky, V. M. (2022). Influence of Plasma Treatment Parameters on the Structural-Phase Composition, Hardness, Moisture-Resistance, and Raman-Enhancement Properties of Nitrogen-Containing Titanium Dioxide. Materials, 15(23), 8514. https://doi.org/10.3390/ma15238514











