Research on Spheroidization of Tungsten Powder from Three Different Raw Materials
Abstract
1. Introduction
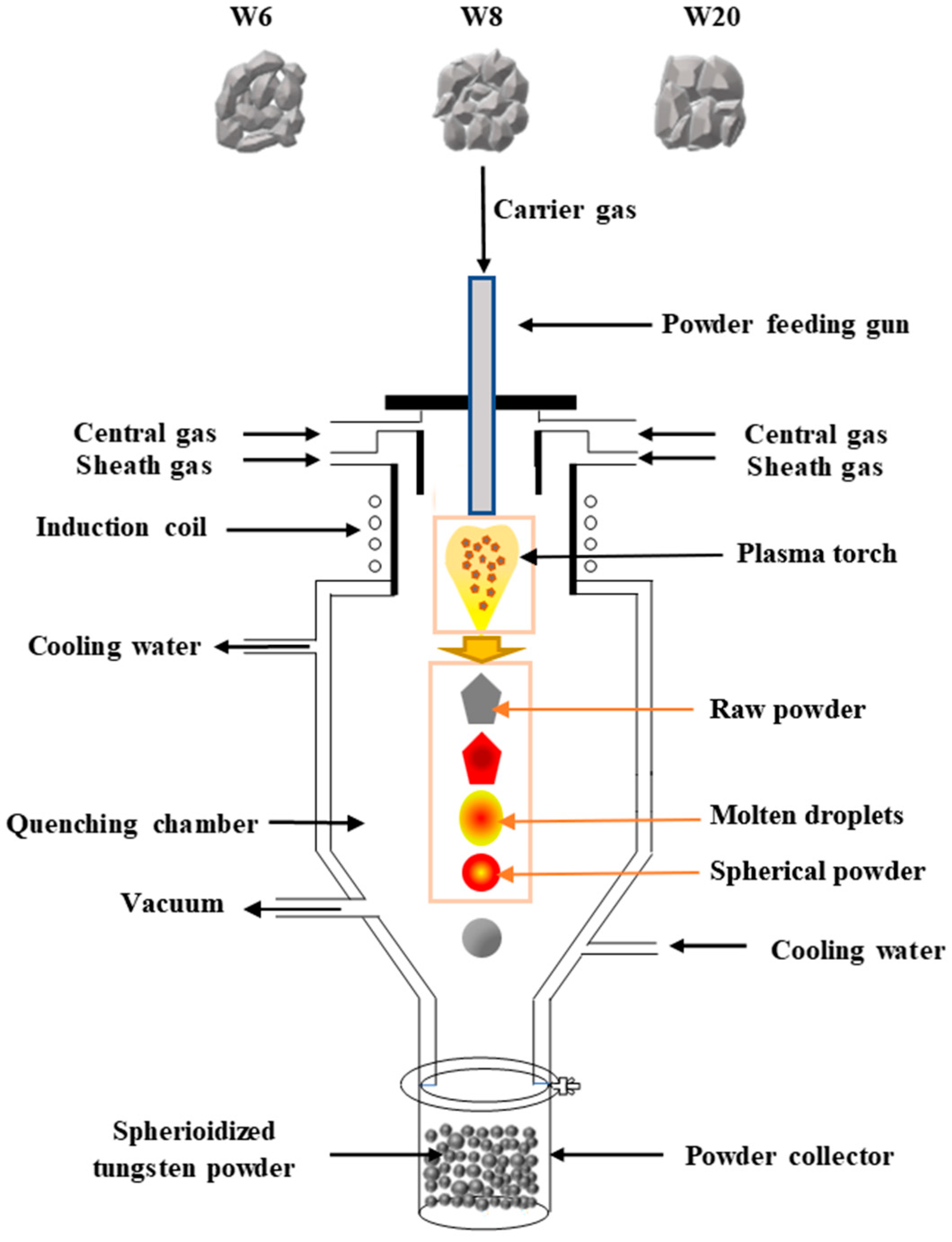
2. Materials and Methods
2.1. Experiment
2.2. Characterization
2.3. Characterization of the Precursor Powder
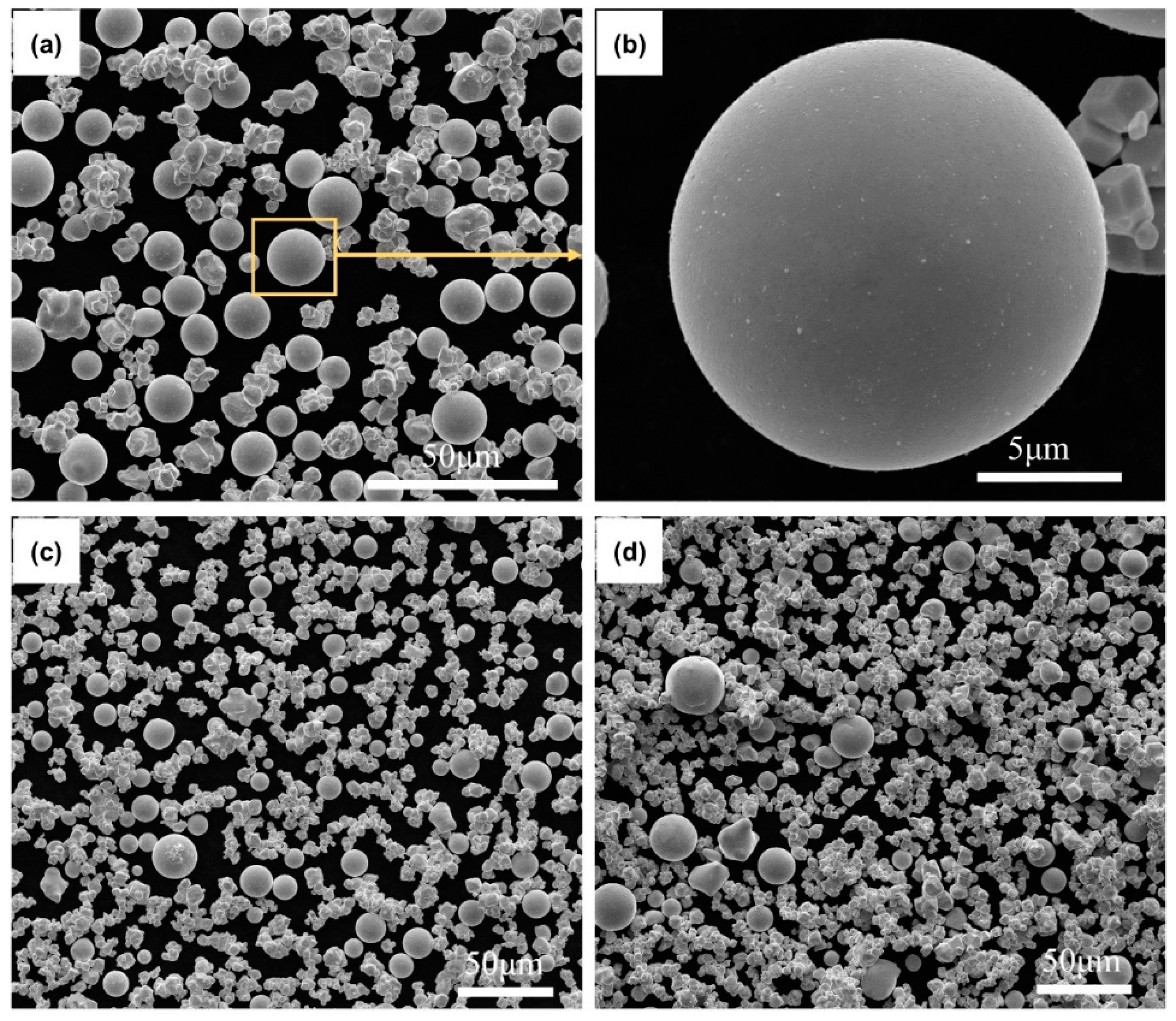
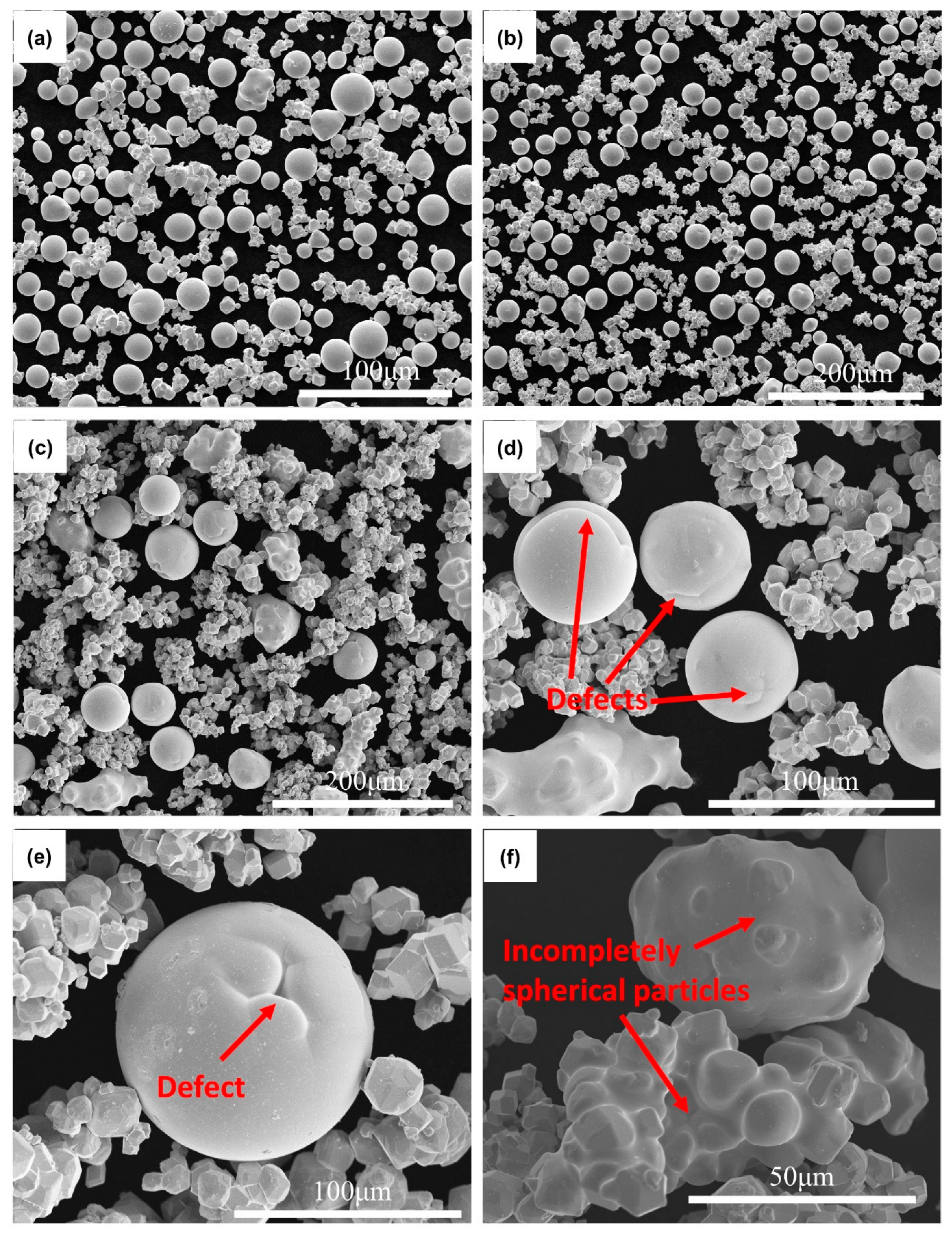
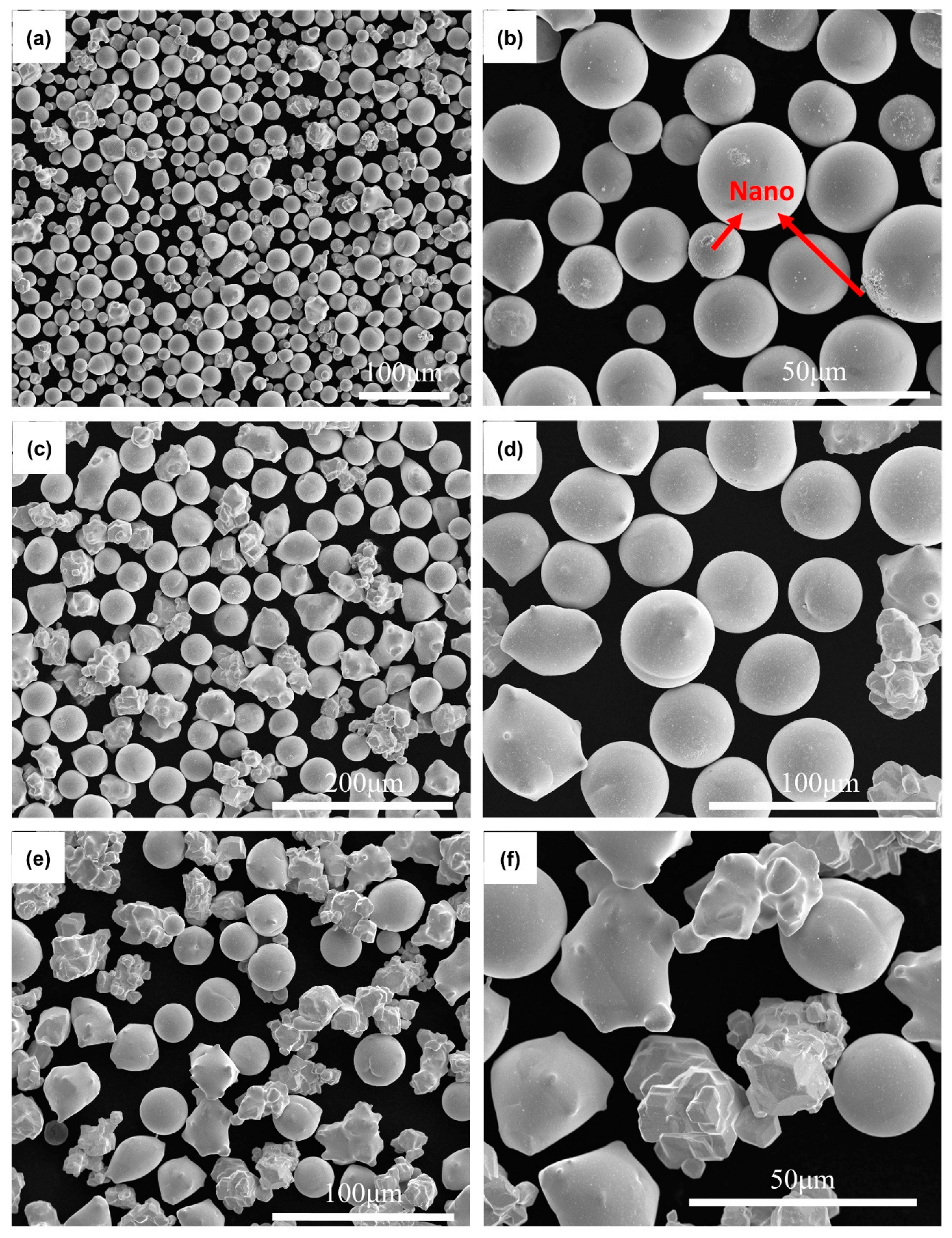
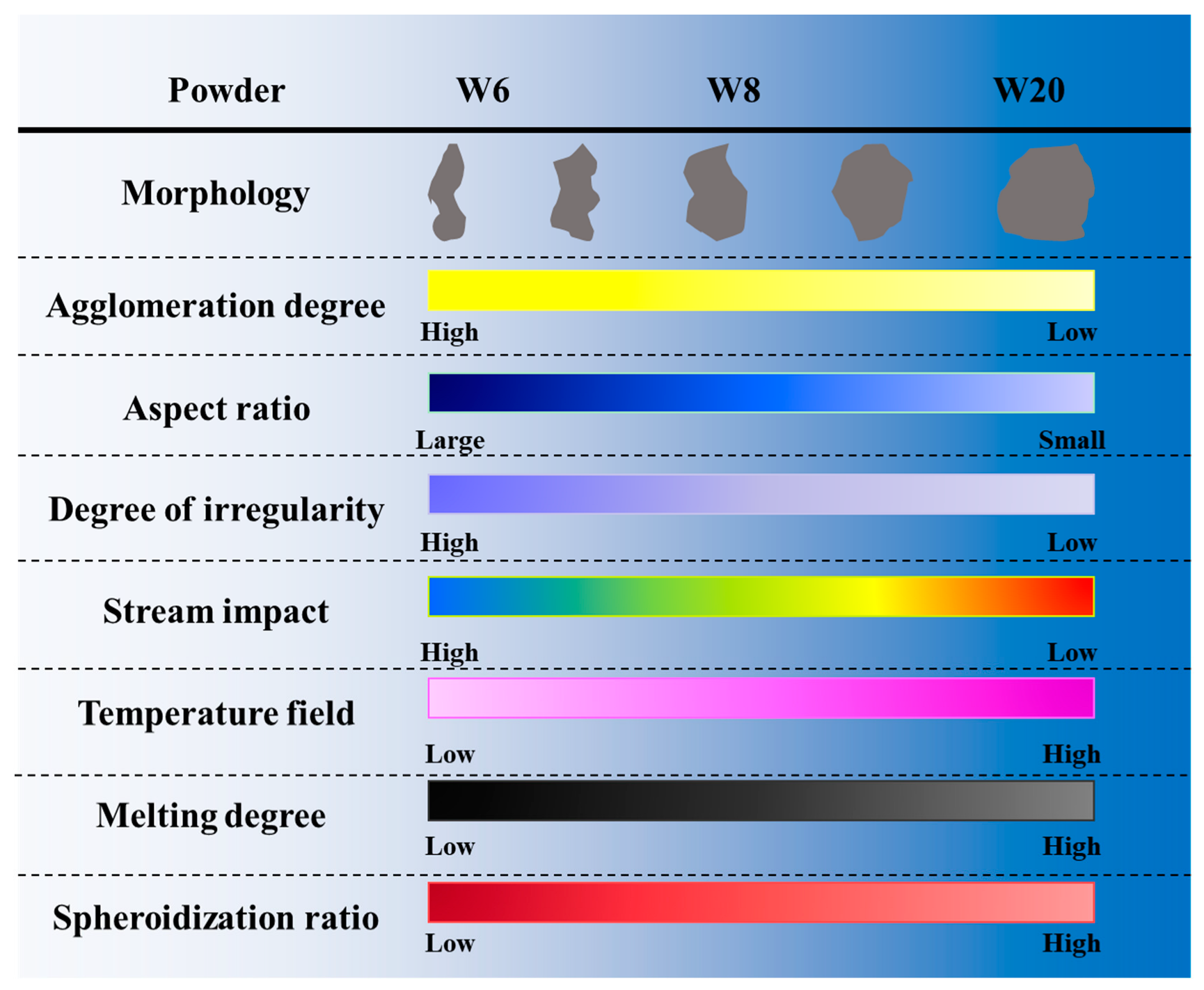
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, L.; Kear, B. Low temperature carburization of high surface area tungsten powders. Nanostructured Mater. 1995, 5, 555–569. [Google Scholar] [CrossRef]
- Piotter, V.; Zeep, B.; Norajitra, P.; Ruprecht, R.; von der Weth, A.; Hausselt, J. Development of a powder metallurgy process for tungsten components. Fusion Eng. Des. 2008, 83, 1517–1520. [Google Scholar] [CrossRef]
- Blagoeva, D.; Opschoor, J.; van der Laan, J.; Sârbu, C.; Pintsuk, G.; Jong, M.; Bakker, T.; Pierick, P.T.; Nolles, H. Development of tungsten and tungsten alloys for DEMO divertor applications via MIM technology. J. Nucl. Mater. 2013, 442, S198–S203. [Google Scholar] [CrossRef]
- Ryu, T.; Sohn, H.; Hwang, K.S.; Fang, Z.Z. Chemical vapor synthesis (CVS) of tungsten nanopowder in a thermal plasma reactor. Int. J. Refract. Met. Hard Mater. 2009, 27, 149–154. [Google Scholar] [CrossRef]
- Tong, J.; Lu, X.; Liu, C.; Pi, Z.; Zhang, R.; Qu, X. Numerical simulation and prediction of radio frequency inductively coupled plasma spheroidization. Appl. Therm. Eng. 2016, 100, 1198–1206. [Google Scholar] [CrossRef]
- Han, C.; Na, H.; Kim, Y.; Choi, H. In-situ synthesis of tungsten nanoparticle attached spherical tungsten micro-powder by inductively coupled thermal plasma process. Int. J. Refract. Met. Hard Mater. 2015, 53, 7–12. [Google Scholar] [CrossRef]
- Murugan, K.; Chandrasekhar, S.; Joardar, J. Nanostructured α/β-tungsten by reduction of WO3 under microwave plasma. Int. J. Refract. Met. Hard Mater. 2011, 29, 128–133. [Google Scholar] [CrossRef]
- Li, B.-S.; Marrow, T.; Armstrong, D. Measuring the brittle-to-ductile transition temperature of tungsten–tantalum alloy using chevron-notched micro-cantilevers. Scr. Mater. 2020, 180, 77–82. [Google Scholar] [CrossRef]
- Han, C.; Fang, Q.; Shi, Y.; Tor, S.B.; Chua, C.K.; Zhou, K. Recent Advances on High-Entropy Alloys for 3D Printing. Adv. Mater. 2020, 32, e1903855. [Google Scholar] [CrossRef] [PubMed]
- Vrancken, B.; Ganeriwala, R.K.; Matthews, M.J. Analysis of laser-induced microcracking in tungsten under additive manufacturing conditions: Experiment and simulation. Acta Mater. 2020, 194, 464–472. [Google Scholar] [CrossRef]
- Deprez, K.; Vandenberghe, S.; Van Audenhaege, K.; Van Vaerenbergh, J.; Van Holen, R. Rapid additive manufacturing of MR compatible multipinhole collimators with selective laser melting of tungsten powder. Med. Phys. 2013, 40, 012501. [Google Scholar] [CrossRef]
- Lu, X.; Liu, C.-C.; Zhu, L.-P.; Qu, X.-H. Influence of process parameters on the characteristics of TiAl alloyed powders by fluidized bed jet milling. Powder Technol. 2014, 254, 235–240. [Google Scholar] [CrossRef]
- Wang, C.; Tan, X.; Du, Z.; Chandra, S.; Sun, Z.; Lim, C.; Tor, S.; Wong, C. Additive manufacturing of NiTi shape memory alloys using pre-mixed powders. J. Mater. Process. Technol. 2019, 271, 152–161. [Google Scholar] [CrossRef]
- Xie, J.; Lu, H.; Lu, J.; Song, X.; Wu, S.; Lei, J. Additive manufacturing of tungsten using directed energy deposition for potential nuclear fusion application. Surf. Coat. Technol. 2021, 409, 126884. [Google Scholar] [CrossRef]
- Chen, H.; Wei, Q.; Zhang, Y.; Chen, F.; Shi, Y.; Yan, W. Powder-spreading mechanisms in powder-bed-based additive manufacturing: Experiments and computational modeling. Acta Mater. 2019, 179, 158–171. [Google Scholar] [CrossRef]
- Thornton, C.; Ning, Z. A theoretical model for the stick/bounce behaviour of adhesive, elastic-plastic spheres. Powder Technol. 1998, 99, 154–162. [Google Scholar] [CrossRef]
- Tolias, P.; Riva, G.; De Angeli, M.; Ratynskaia, S.; Daminelli, G.; Lungu, C.; Porosnicu, C. Adhesive force distributions for tungsten dust deposited on bulk tungsten and beryllium-coated tungsten surfaces. Nucl. Mater. Energy 2018, 15, 55–63. [Google Scholar] [CrossRef]
- Ratynskaia, S.; Vignitchouk, L.; Tolias, P. Modelling of dust generation, transport and remobilization in full-metal fusion reactors. Plasma Phys. Control. Fusion 2022, 64, 044004. [Google Scholar] [CrossRef]
- Li, J.; Hao, Z.; Shu, Y.; He, J. Fabrication of spherical Ti–6Al–4V powder for additive manufacturing by radio frequency plasma spheroidization and deoxidation using calcium. J. Mater. Res. Technol. 2020, 9, 14792–14798. [Google Scholar] [CrossRef]
- Zhang, H.; Bai, L.; Hu, P.; Yuan, F.; Li, J. Single-step pathway for the synthesis of tungsten nanosized powders by RF induction thermal plasma. Int. J. Refract. Met. Hard Mater. 2012, 31, 33–38. [Google Scholar] [CrossRef]
- Zhu, H.; Tong, H.; Cheng, C.; Liu, N. Study on behaviors of tungsten powders in radio frequency thermal plasma. Int. J. Refract. Met. Hard Mater. 2017, 66, 76–82. [Google Scholar] [CrossRef]
- Boulos, M.I. The Role of Transport Phenomena and Modeling in the Development of Thermal Plasma Technology. Plasma Chem. Plasma Process. 2016, 36, 3–28. [Google Scholar] [CrossRef]
- Boulos, M.I. New frontiers in thermal plasmas from space to nanomaterials. Nucl. Eng. Technol. 2012, 44, 1–8. [Google Scholar] [CrossRef]
- Zeng, B.; Wang, J.; Fan, H.-Y.; Chang, H. Effect of central gas velocity and plasma power on the spheroidizing copper powders of radio frequency plasma. Vacuum 2020, 174, 109195. [Google Scholar] [CrossRef]
- Wang, J.-J.; Hao, J.-J.; Guo, Z.-M.; Wang, Y.-M. Preparation of spherical tungsten and titanium powders by RF induction plasma processing. Rare Met. 2014, 34, 431–435. [Google Scholar] [CrossRef]
- Li, R.; Qin, M.; Liu, C.; Huang, H.; Lu, H.; Chen, P.; Qu, X. Injection molding of tungsten powder treated by jet mill with high powder loading: A solution for fabrication of dense tungsten component at relative low temperature. Int. J. Refract. Met. Hard Mater. 2017, 62, 42–46. [Google Scholar] [CrossRef]
- Hao, Z.; Fu, Z.; Liu, J.; Zhu, X.; Zhou, F.; Shu, Y.; Yi, J.; He, J. Spheroidization of a granulated molybdenum powder by radio frequency inductively coupled plasma. Int. J. Refract. Met. Hard Mater. 2019, 82, 15–22. [Google Scholar] [CrossRef]
- Haider, A.; Levenspiel, O. Drag coefficient and terminal velocity of spherical and nonspherical particles. Powder Technol. 1989, 58, 63–70. [Google Scholar] [CrossRef]
- Kumar, S.; Na, H.; Selvarajan, V.; Lee, C. Influence of metal powder shape on drag coefficient in a spray jet. Curr. Appl. Phys. 2008, 9, 678–682. [Google Scholar] [CrossRef]
- Pfender, E. Particle behavior in thermal plasmas. Plasma Chem. Plasma Process. 1989, 9, 167S–194S. [Google Scholar] [CrossRef]
- Jiang, X.-L.; Boulos, M. Induction plasma spheroidization of tungsten and molybdenum powders. Trans. Nonferrous Met. Soc. China 2006, 16, 13–17. [Google Scholar] [CrossRef]
- Yu, C.; Zhou, X.; Wang, D.; VAN Linh, N.; Liu, W. Study on the RF inductively coupled plasma spheroidization of refractory W and W-Ta alloy powders. Plasma Sci. Technol. 2017, 20, 014019. [Google Scholar] [CrossRef]

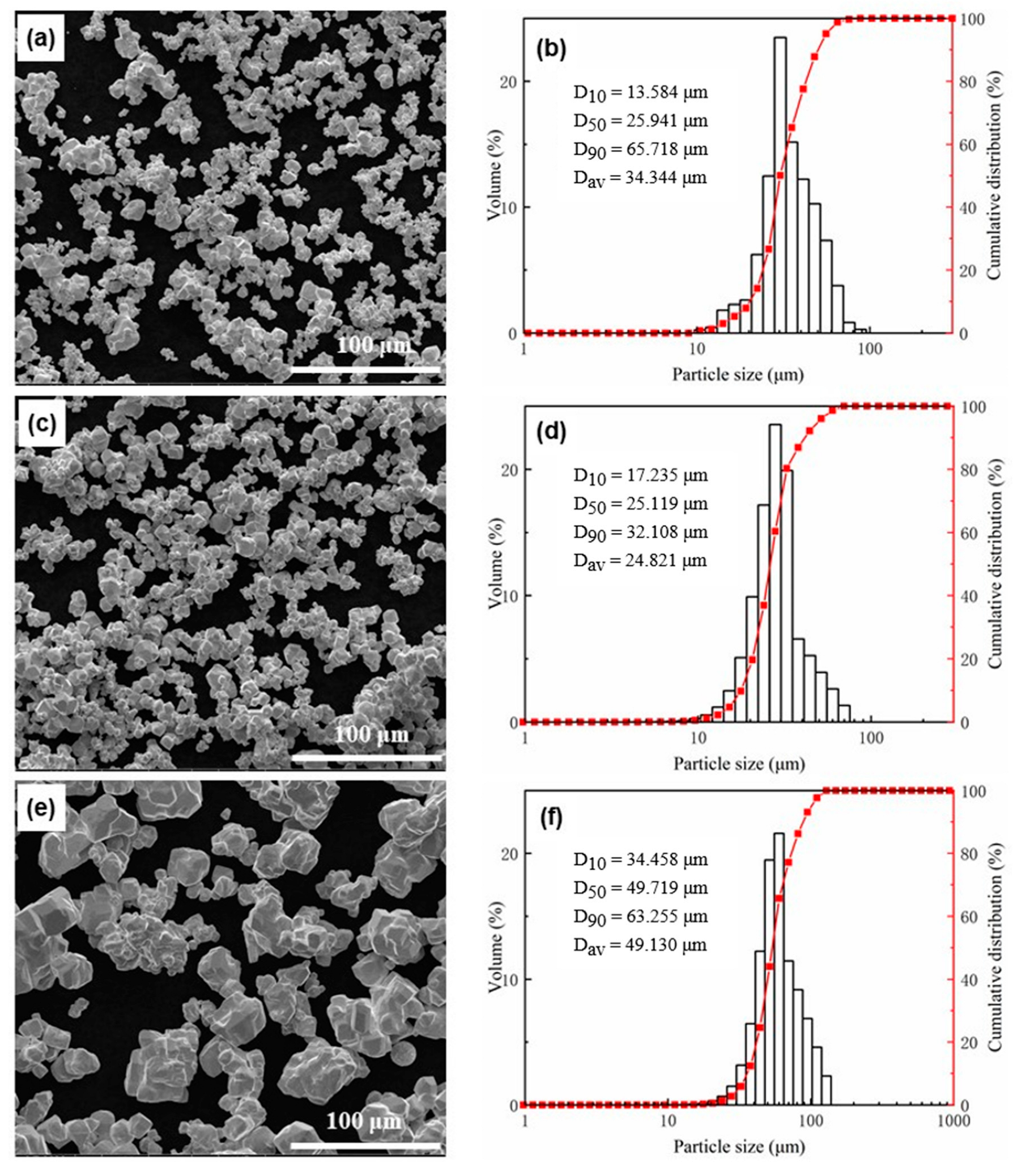
| Powder | Specific Surface Area (m2/g) | Primary Particle Size (μm) | Average Laser Particle Size (μm) | Agglomeration Coefficient |
|---|---|---|---|---|
| W6 | 0.049 | 6.28 | 34.344 | 5.47 |
| W8 | 0.039 | 7.95 | 24.821 | 3.12 |
| W20 | 0.016 | 19.9 | 49.130 | 2.47 |
| Powder | Apparent Density/g·cm−3 | Hall Flow Time/s·(50 g)−1 |
|---|---|---|
| W6 | 3.91 | - |
| W8 | 4.64 | - |
| W20 | 6.11 | 11.21 |
| Powder | Apparent Density/g·cm−3 | Hall Flow Time/s·(50 g)−1 |
|---|---|---|
| WS6 | 4.84 | 19.52 |
| WS8 | 6.22 | 16.15 |
| WS20 | 9.36 | 6.28 |
| Powder | Specific Surface Area m2/g | Primary Particle Size/μm | Average Laser Particle Size/μm | Agglomeration Coefficient |
|---|---|---|---|---|
| WS6 | 0.029 | 10.871 | 36.092 | 3.32 |
| WS8 | 0.040 | 7.769 | 24.091 | 3.10 |
| WS20 | 0.019 | 16.396 | 26.105 | 1.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Hou, X.; Hao, Z.; Wang, P.; Shu, Y.; He, J. Research on Spheroidization of Tungsten Powder from Three Different Raw Materials. Materials 2022, 15, 8449. https://doi.org/10.3390/ma15238449
Zhang X, Hou X, Hao Z, Wang P, Shu Y, He J. Research on Spheroidization of Tungsten Powder from Three Different Raw Materials. Materials. 2022; 15(23):8449. https://doi.org/10.3390/ma15238449
Chicago/Turabian StyleZhang, Xiuqing, Xuchu Hou, Zhenhua Hao, Pei Wang, Yongchun Shu, and Jilin He. 2022. "Research on Spheroidization of Tungsten Powder from Three Different Raw Materials" Materials 15, no. 23: 8449. https://doi.org/10.3390/ma15238449
APA StyleZhang, X., Hou, X., Hao, Z., Wang, P., Shu, Y., & He, J. (2022). Research on Spheroidization of Tungsten Powder from Three Different Raw Materials. Materials, 15(23), 8449. https://doi.org/10.3390/ma15238449






