1. Introduction
As a promising alternative to traditional fossil energy, geothermal energy plays an important role in providing thermal energy for humans. The extraction of geothermal energy is an important measure to achieve “carbon neutrality” [
1]. Geothermal well power generation is the most promising way to use geothermal resources at high temperatures (above 150 °C). At present, China’s geothermal energy extraction is dominated by geothermal resources of about 180–220 °C [
2]. According to the characteristics of geothermal resources, the main method is to produce hot water or steam to generate electricity. In order to ensure the mining performance of geothermal wells, the mechanical properties of the cementitious materials at high temperatures should be satisfied [
3].
High-alite Class G cement is currently widely used as the cementitious material for oil wells. Alite cement, as a basic cementitious material, consumes a large amount of energy and emits a large amount of greenhouse gases during manufacturing. Even if the cement industry only accounts for approximately 7.4% of total CO
2 emissions [
4], it still has a large influence. As a kind of Portland cement, high belite cement (HBC) takes belite as the dominant mineral and increases its content to more than 30%. The advantage of HBC is that the low energy consumption and low-emission component, C
2S, in the clinker replaces the high energy consumption and high-emission C
3S as the main mineral component, and the calcination temperature of 1250 °C is also lower than the 1450 °C required for alite cement [
5]. Compared to Portland cement, HBC can reduce energy by 16%, and the total CO
2 emissions during cement production can be reduced by 10% [
6]. HBC has advantages over alite cement due to its long-term strength and durability and is often used in dams and roads [
7,
8]. The shortcoming of HBC is its low early strength, but a high-temperature service environment can change this status [
7,
9].
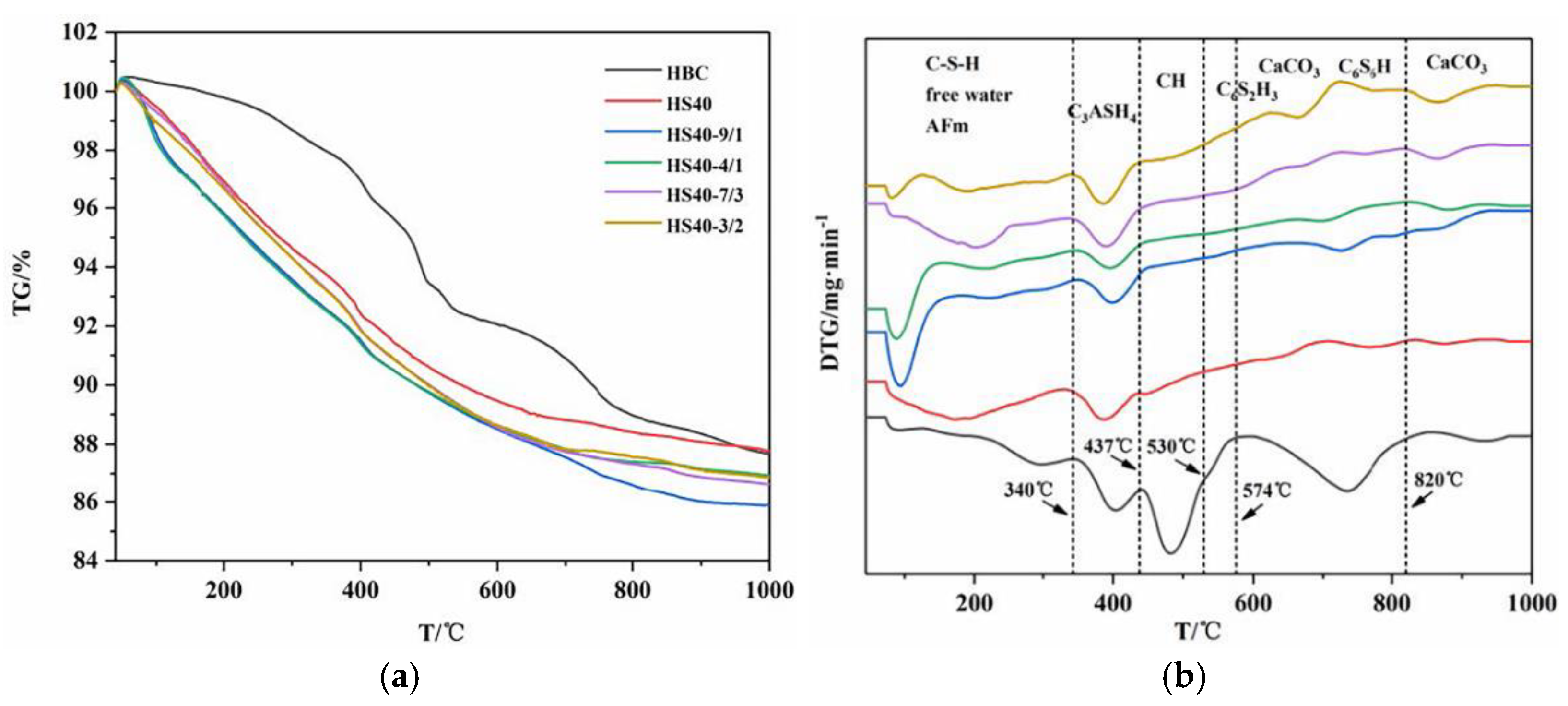
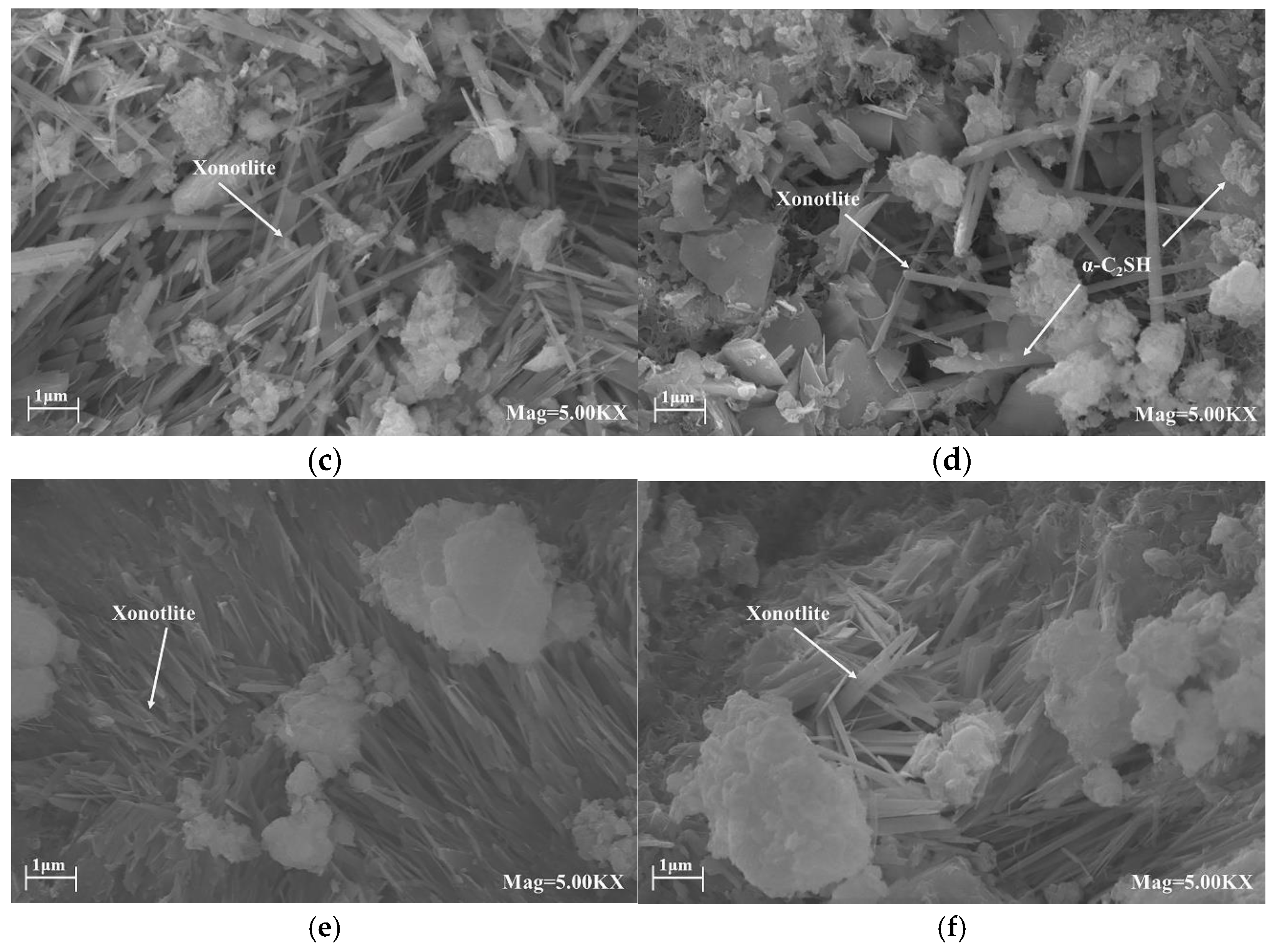
For Portland cement, unstable hydration products are formed under high-temperature hydrothermal conditions. When the temperature exceeds 110 °C, the C-S-H gel phase initially formed by Portland cement hydration is transformed into hydrated dicalcium silicate crystals (α-C
2SH) [
10]. When the temperature rises further, α-C
2SH transforms into jaffeite (C
6S
2H
3) [
11]. These two crystals have low strength and high permeability, and the increase in density causes the cement to shrink and stay. Larger pores will eventually lead to a decrease in compressive strength and an increase in permeability. Therefore, it is necessary to add some supplementary cementitious materials (SCMs) to Portland cement to improve its thermal stability.
SCMs often have a similar chemical composition to Portland cement and have gelling activity. In recent years, considering environmental protection factors, an increasing number of researchers have added waste materials to Portland cement as SCMs, such as slag, fly ash, and red mud [
12,
13,
14], which can appropriately decrease costs while reducing environmental pollution and land resource occupation by these solid waste materials [
15].
Red mud is the tailing produced during the production of alumina from bauxite [
16]. Its appearance is due to its hematite content, which varies and appears reddish brown or grey [
17,
18]. At present, the main red mud treatment methods used in the world are the ocean discharge method, the lagoon method, and the dry accumulation method. The world accumulative reserves of red mud have exceeded 4 billion tons, but due to the very limited use of red mud in the industry, stock is constantly increasing, and the utilization rate is extremely low [
19] The greatest impact on the environment from the disposal and storage of red mud is the contamination of soil and water bodies by residual liquids and suspensions [
20,
21].
Red mud contains SiO
2, A1
2O
3, Fe
2O
3, CaO, and silicate minerals necessary for the mass production of cement, as well as a certain amount of amorphous aluminosilicate substances, which can react with water to hydrate [
22,
23,
24,
25]. In addition, the aluminosilicate in red mud undergoes a pozzolanic reaction under the action of Ca(OH)
2 released during cement hydration and is a potentially active cementitious material [
15]. Because of its high calcium content, Bayer-process red mud can be used as a raw material for cement production and can also be used as SCMs in cement [
26,
27,
28].
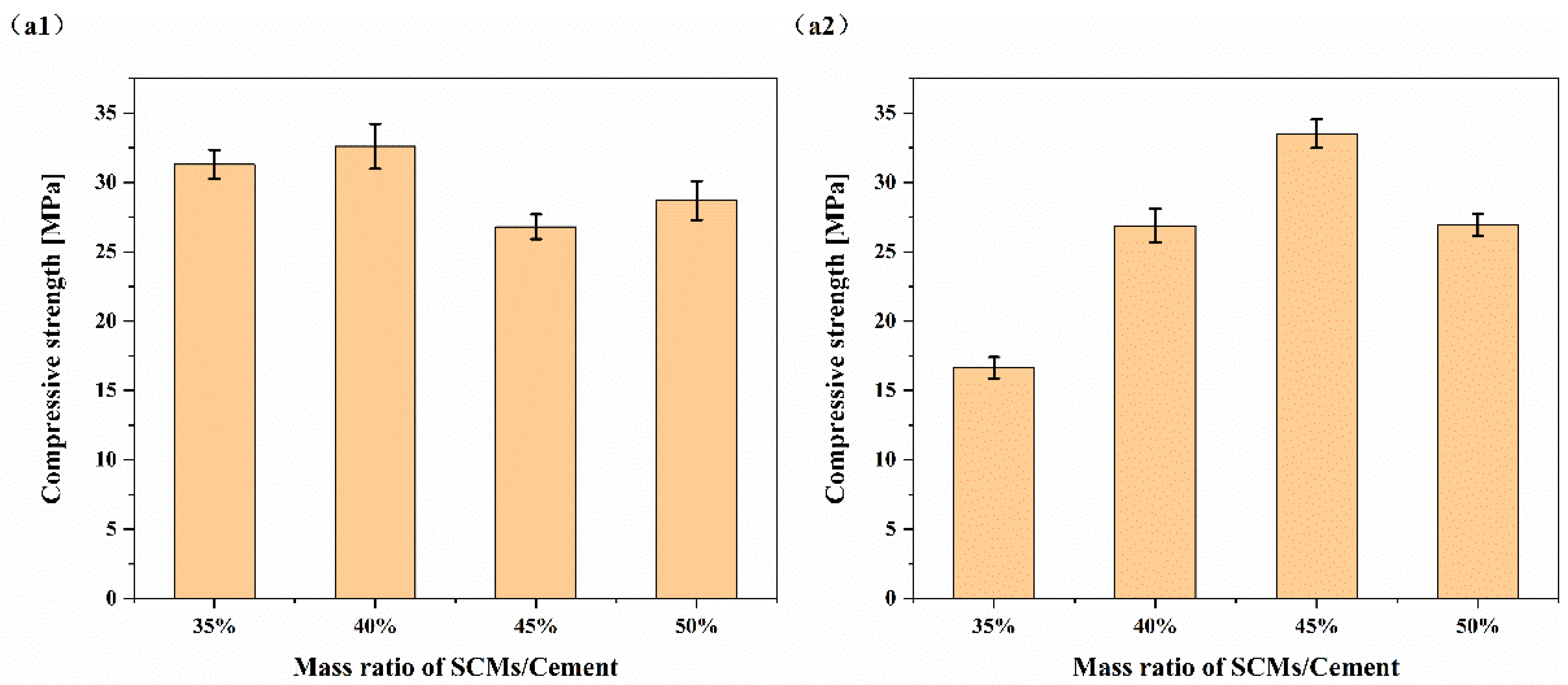
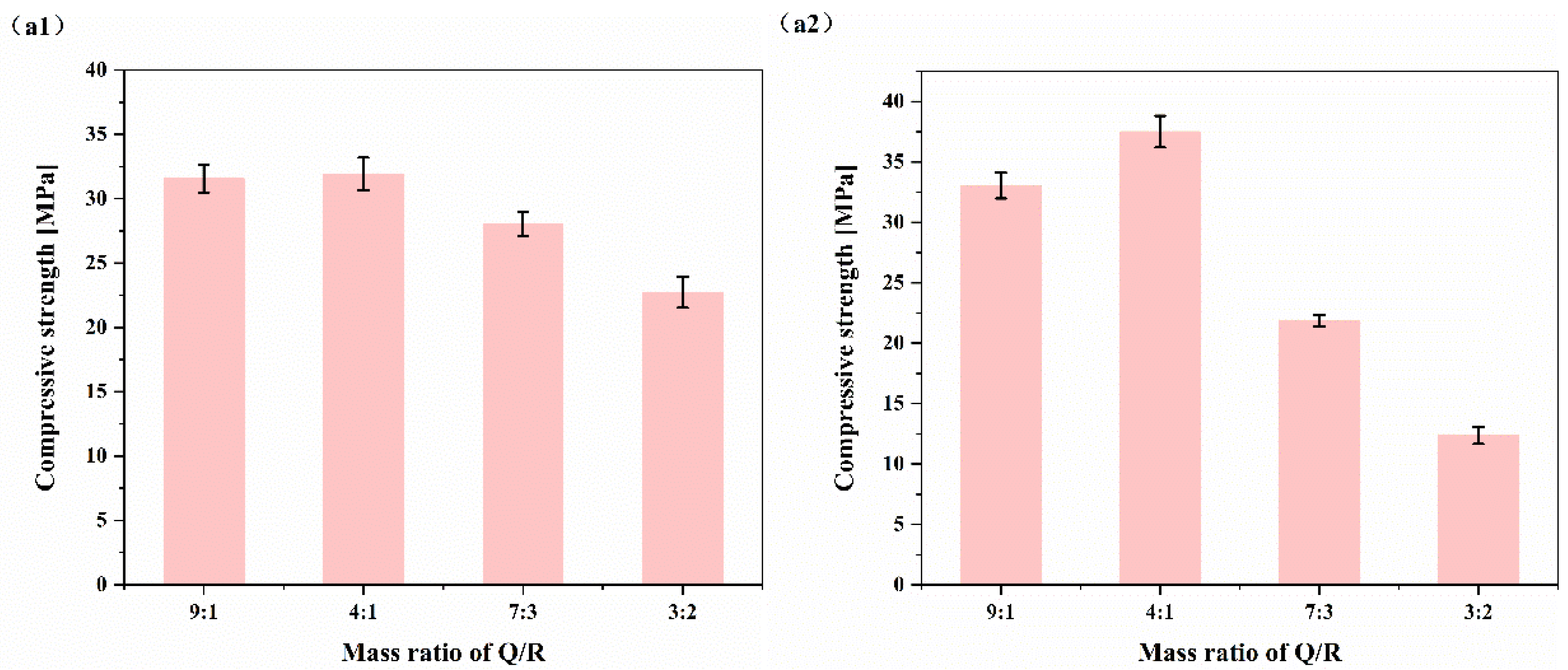
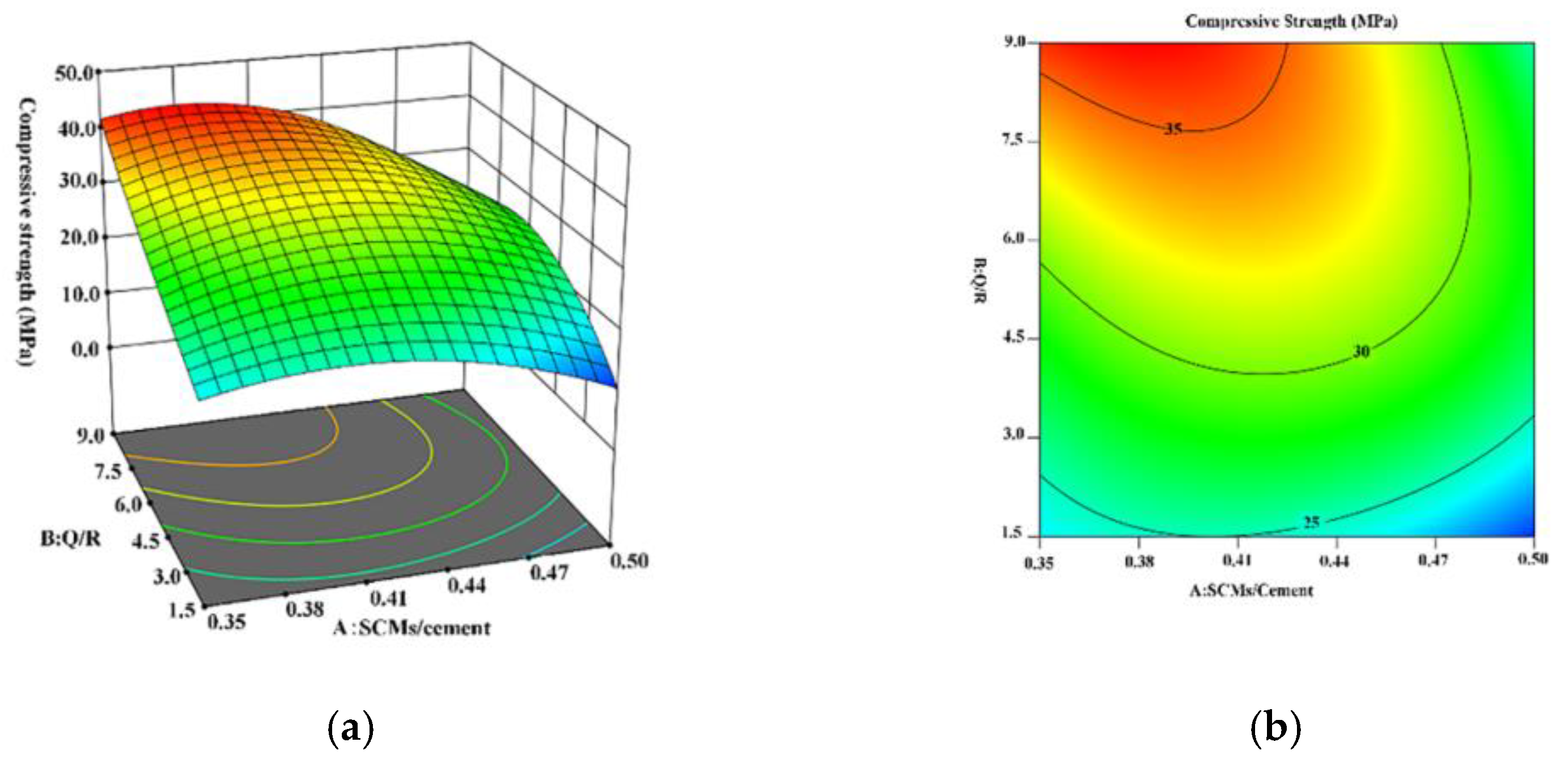
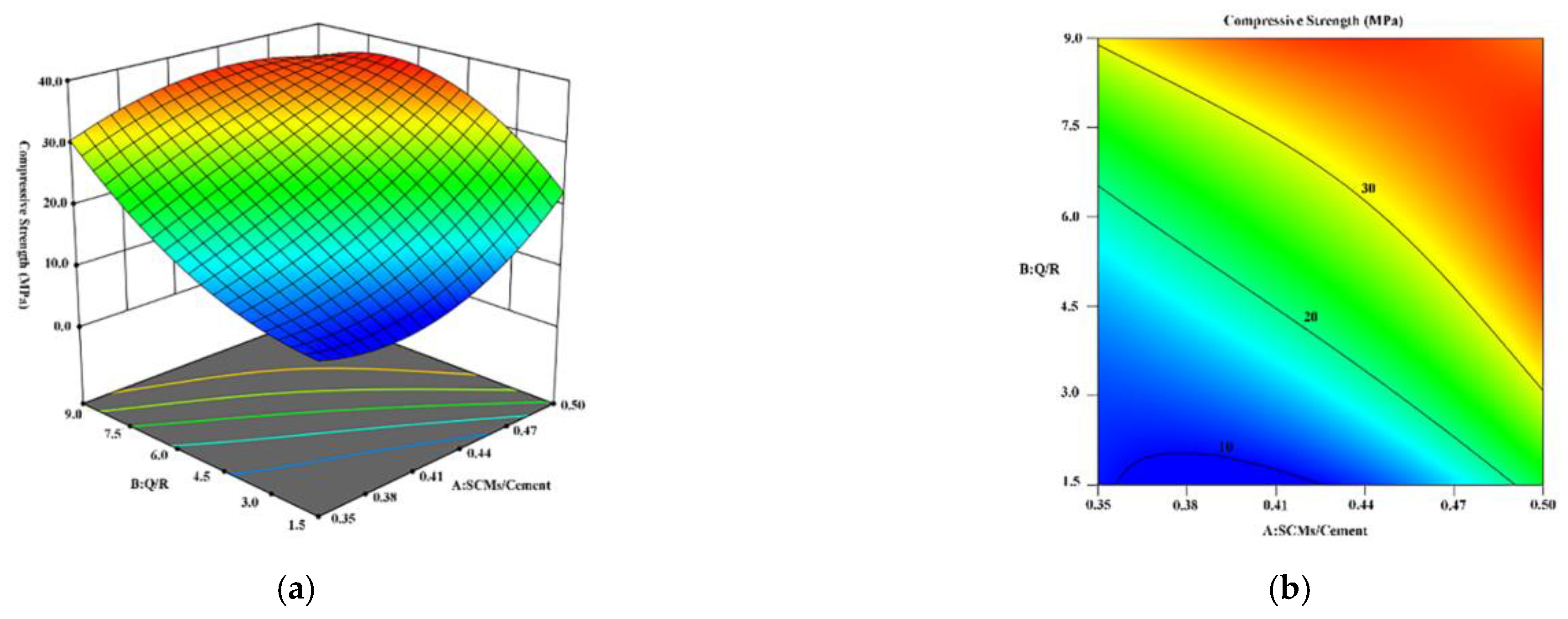
Therefore, this study used HBC as a cementitious material and quartz sand and Bayer-process red mud as SCMs to explore the influence of SCMs on the strength of HBC at high temperatures and study the microscopic mechanism of the hydration reaction between SCMs and HBC. Based on the response surface method (RSM), the incorporation ratio of SCMs and the composition ratio of SCMs are optimized. By adding SCMs composed of quartz sand and red mud to HBC, it is possible to effectively decrease waste and reduce energy consumption while ensuring stable cement performance. This study provides a new way to solve the development of cementing slurry in geothermal wells, and also provides a certain experimental and theoretical basis. In addition, it provides a new development method to solve the problem of solid waste red mud piling up and realize the rational application of red mud resources.
2. Materials and Methods
2.1. Materials
Table 1 shows the mineral composition of HBC and red mud used in the experiment. The HBC came from Leshan Jiahua Special Cement Co., Ltd. The content of C
2S in the HBC clinker increased to 36.97%, while the content of C
3S decreased to 37.87%. The red mud was obtained from the Bayer process. The mineral composition of the red mud is listed in
Table 2. From the table, it can be seen that the Bayer-process red mud contained cement-like components. Among them, the contents of silicon and aluminum were higher, the content of SiO
2 reached 27.63%, the content of Al
2O
3 reached 26.44%, and the main component of the red mud was aluminosilicate. The quartz sand came from Sichuan Huaxi Group Co., Ltd., with a size of 150 mesh. The SiO
2 content in the silica sand used in this experiment was greater than 96%.
The particle size of the HBC ranged from 0.18 to 126 μm, and the D50 was 12.23 μm. The particle size range of the red mud was 1~158 μm, and the D50 was 3.619 μm. The particle size range of the quartz sand was 0.89~158 μm, and the D50 was 46.407 μm. The particle size distribution of the material was measured by a laser particle size analyzer (Mastersizer 2000 laser particle size analyzer, Malvern Instrument Co., Ltd., Malvern, UK).
The red mud was dried using a GW300-PLC frequency conversion roller heating furnace (Qingdao Tongchun Petroleum Instrument Co., Ltd., Qingdao, China) at 105 °C for 2 days to remove most of the water, and then placed in a 60 °C electric air box (101-2 type, Beijing Zhongxingweiye Instrument Co., Ltd., Beijing, China) to remain dry.
The additives used in the experiment included a fluid loss agent (G33S) and dispersant (SXY-2). G33S is a polymer-modified material made of polyamide, low-molecular-weight polyamide, and hydroxypolycarboxylic acid, which is soluble in water. SXY-2 is a polymer formed by the coagulation of formaldehyde and acetone (Cheng et al., 2018).
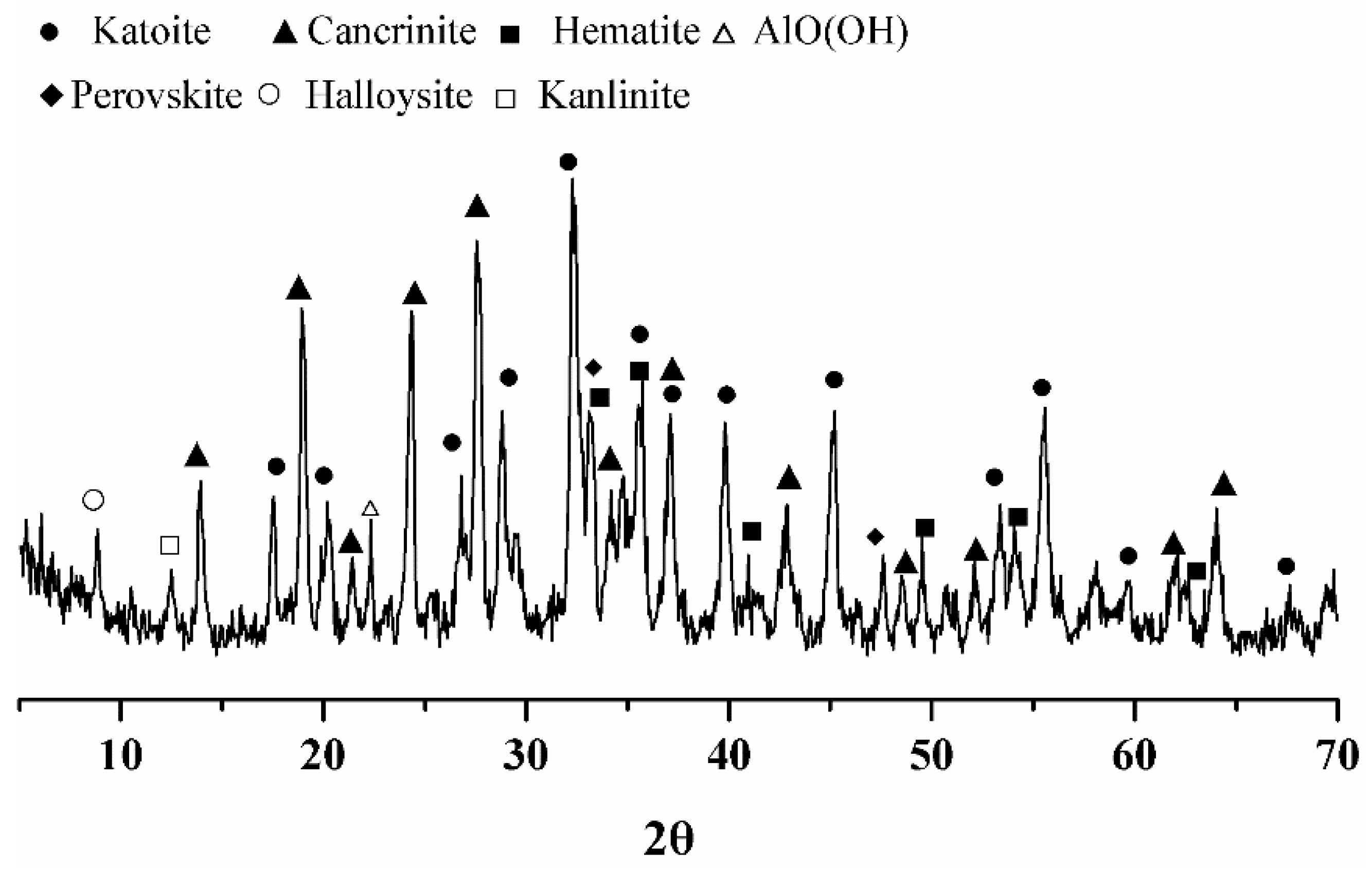
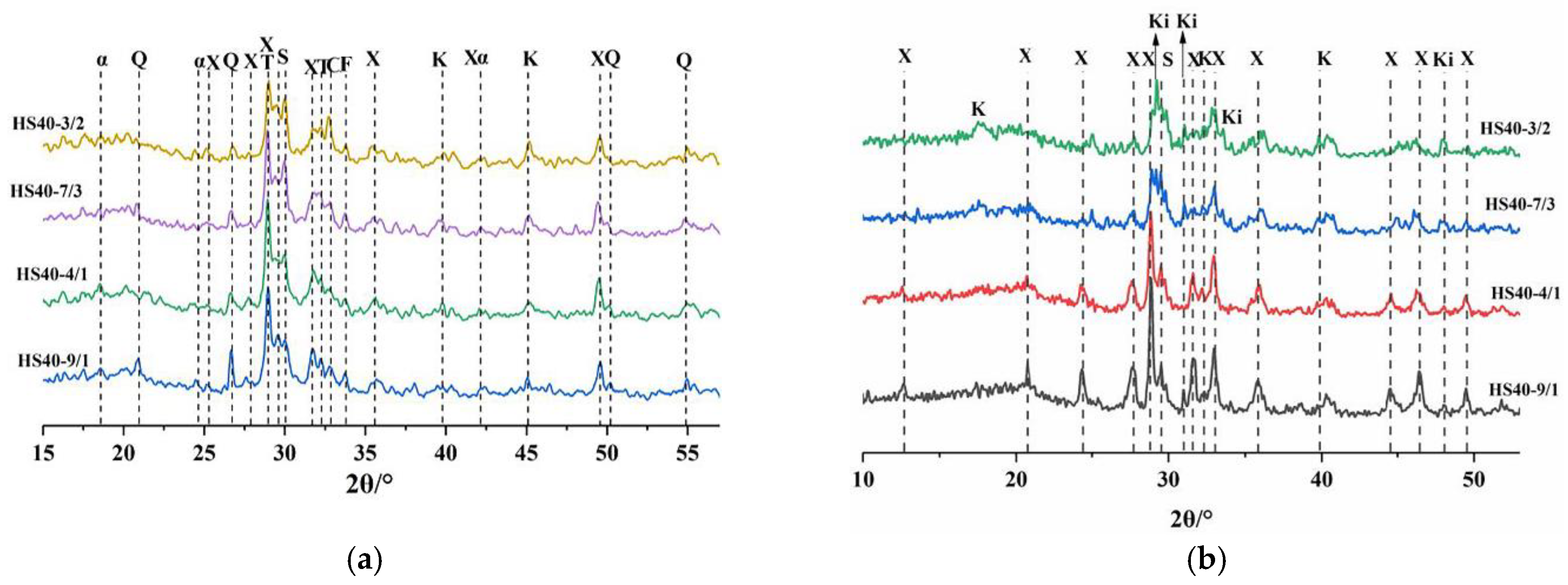
The phase composition of Bayer red mud was obtained by X-ray diffraction analysis, as shown in
Figure 1. It can be seen in the figure that the main mineral composition of the red mud was katoite (Ca
3Al
2(SiO
4)(OH)
8, C
3A
SH
4, ICSD: 38-0368), cancrinite (Na
6Ca
2Al
6Si
6O
24(CO
3)
2·2H
2O, ICSD: 46-1332), hematite (Fe
2O
3, ICSD: 33-0664), perovskite (CaTiO
3, ICSD: 22-0153), halloysite (Al
2Si
2O
5(OH)
4·2H
2O, ICSD: 29-1489), kaolinite (Al
2Si
2O
5(OH)
4, ICSD: 14-0164), and AlO(OH) (ICSD: 05-0355) [
12,
29]. Most of the red mud was aluminosilicate material, of which cancrinite is an unstable material. Cancrinite and katoite were the main active mineral components in the red mud.
2.2. Methods
2.2.1. Cement Slurry Preparation
The specific mix proportions of the cement slurry are shown in
Table 3 and
Table 4. The mixing procedure was conducted according to the API RP 10B-2 standard, in which a premixture was thoroughly and uniformly mixed with cement and dry additives.
2.2.2. The Compressive Strength Test
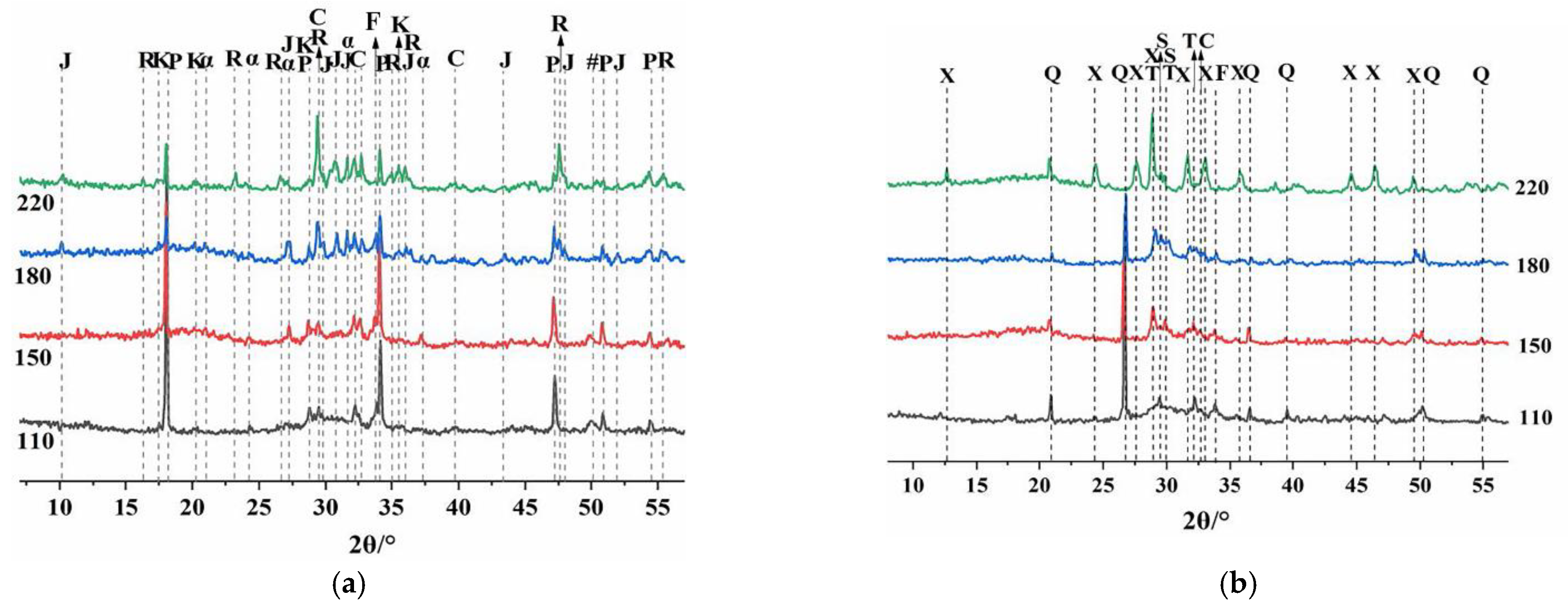
Compressive strength tests were performed on the samples that had been cured in a high-temperature and high-pressure curing kettle at 110, 150, 180, and 220 °C for 7 days. According to the API RP 10B-2 standard, the specimens used for the compressive strength tests were cubes with 50-mm sides, and the tests were conducted with an electronically controlled hydraulic testing machine (TYE-300B, Wuxi Jianyi Instrument & Machinery Co., Ltd., Wuxi, China) operated with a load rate of 1200 ± 100 N/s.
2.2.3. Characterization
X-ray diffraction (XRD) with Cu Kα radiation (DX-1000, V = 30 kV, I = 25 mA) was used to collect the sample data. The measurements were performed with a step size of 0.02° and a scan rate of 2°/min in the scan range of 5–70° 2 theta (2θ).
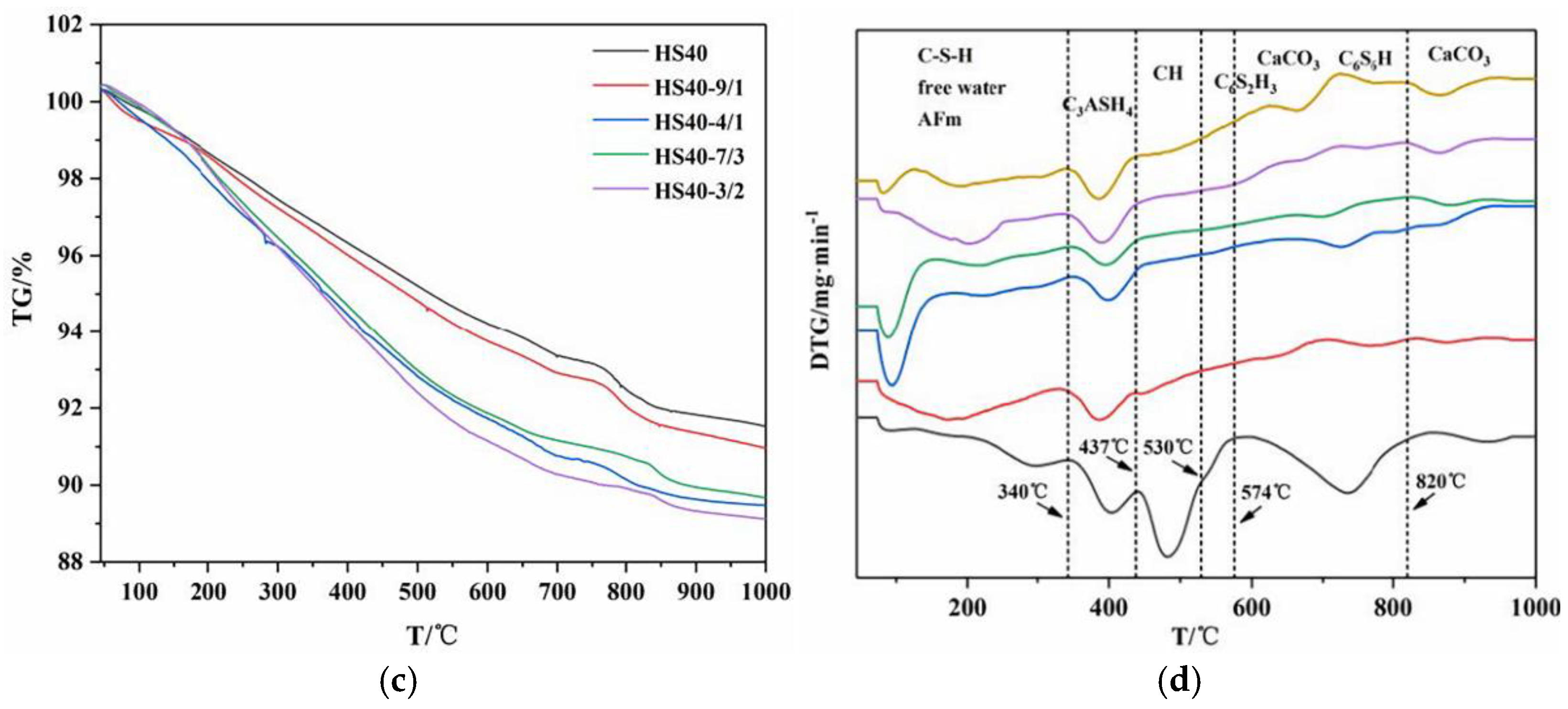
The weight loss of the samples after drying was measured by thermogravimetric analysis (TGA/SDTA85/e, METTLER TOLEDO, Greifensee, Switzerland). The temperature ranged from 105 to 800 °C. The heating rate was 10 °C/min. The protective gas was nitrogen with a flow rate of 50 mL/min.
Environmental scanning electron microscopy (ESEM) (Model Quanta 450, FEI, Morristown, NJ, USA) was used to assess the morphology of the samples. To minimize electric charging during the measurement, the samples were sputtered with a fine layer of gold.
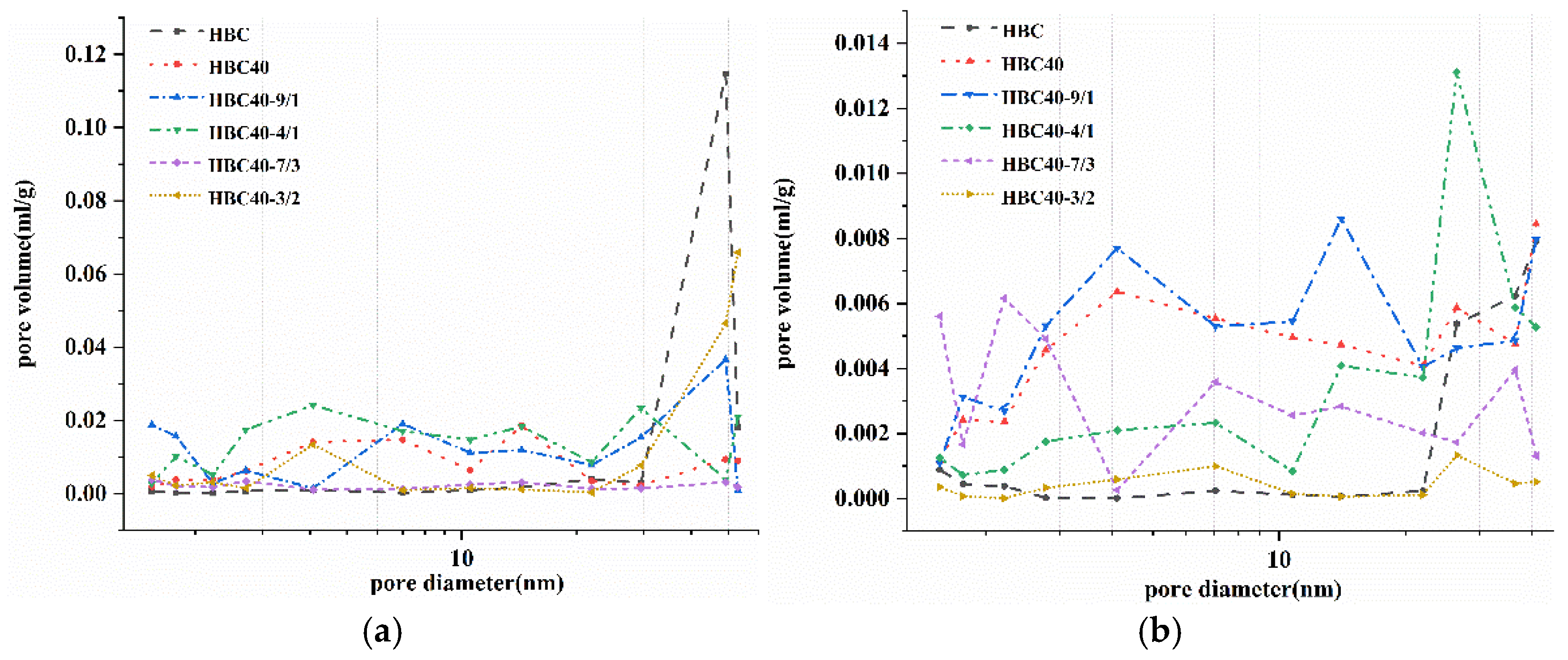
The pore size distribution of the sample was measured by a specific surface area and pore size distribution instrument (F-Sorb3400, Beijing Jin Aipu Technology Co., Ltd., Beijing, China).